Supplemental digital content is available in the text.
Key Words: margins of excision, multidetector computed tomography, neoplasm invasiveness, pancreatic ductal carcinoma, pancreatic neoplasms
Abstract
Objective
To quantitatively assess the probability of tumor resection based on measurements of tumor contact with the major peripancreatic vessels.
Methods
This is a retrospective cohort study of pancreatic cancer patients treated between January 2001 and December 2015 in a single academic comprehensive cancer center. Radiographic measurements of the circumferential degree and length of solid tumor contact with major peripancreatic vessels were obtained from diagnostic pancreatic protocol computed tomography images and tested for correlation with tumor resection and margin status.
Results
Of 294 patients analyzed, 113 (38%) were resected, with 71 (63%) with negative margins. Based on the individual measurements of vascular involvement, a resectability scoring system (RSS) was created. The RSS correlated strongly with resection (P < 0.0001) and R0 resection (P < 0.0001) probabilities. Moreover, the RSS correlated with overall survival (P < 0.0001) and metastasis-free survival (P < 0.0001), being able to substratify resectable (P = 0.022) and unresectable patients (P = 0.014) into subgroups with different prognosis based on RSS scores.
Conclusions
Based on a comprehensive and systematic quantitative approach, we developed a scoring system that demonstrated excellent accuracy to predict tumor resection, surgical margin status, and prognosis.
A pancreaticoduodenectomy (Whipple procedure) has a mortality rate of less than 2.5% in experienced tertiary oncologic centers, but morbidity due to postsurgical complications can reach rates higher than 30%.1,2 Moreover, unsuccessful resections put patients at risk of unnecessary morbidity while also delaying the delivery of systemic therapy. For this reason, an accurate preoperative staging of pancreatic cancer (PCA) is essential to select patients most likely to benefit from surgery.
Improvements in cross-sectional imaging such as the development of the multidetector computed tomography (MDCT) with optimized high-resolution protocols with postprocessing features such as 3-dimensional volume rendering and multiplanar reformations have allowed more precise characterization of the anatomic relationship between the tumor and blood vessels.3–5
Multiple systems for classifying PCA resectability exist, resulting in a lack of consensus.6–12 Moreover, potential resectability is highly subjective and can vary from surgeon to surgeon, making it difficult to compare surgical outcomes across different institutions.
In an effort to reduce this variability, this study was undertaken to define a set of objective criteria by which to classify and quantify tumor involvement of major peripancreatic vessels in patients with PCA.
MATERIALS AND METHODS
Data Collection and Study Population
This study is a retrospective, single-institution analysis based on data from patients diagnosed with PCA treated at Stanford Cancer Institute between January 2001 and December 2015. Demographic, pathologic, radiologic, and treatment-related information was retrieved from medical records after institutional review board approval. Patients were excluded if they had any of the following characteristics that could bias the decision regarding resectability: (1) presence of distant metastasis at diagnosis or found at the time of laparotomy; (2) considered to be medically inoperable for poor performance status, severe acute or chronic comorbidities, or patient's refusal of surgery; (3) absence of a pancreatic protocol computed tomography (CT); (4) presence of a vascular malformation or anatomic variation of the celiac axis/common hepatic artery (CA/CHA), superior mesenteric artery (SMA), or superior mesenteric vein/portal vein (SMV/PV); or (5) unavailable or inadequately documented follow-up.
CT Scanning Technique
After the patient cohort was selected, the first diagnostic CT image with a pancreatic protocol was assessed. Our pancreatic CT protocol involves a dual-phase image acquisition using a breath-hold during the late arterial phase (35–40 seconds after onset of iodine-contrast intravenous injection) and the portal venous phase (60–70 seconds after onset of intravenous injection). Images were obtained using either 16- or 64-multidetector-row CT scanners (LightSpeed VCT [General Electric Healthcare Systems, Waukesha, Wis] or SOMATOM Sensation 64 [Siemens Healthineers, Erlangen, Germany]). The slice thickness of the images was 0.625, 1.00, or 1.25 mm.
Postprocessing of the CT Data
Postprocessing of the MDCT data was performed using dedicated workstations (Volume Viewer and Advantage Windows 3.1; General Electric Healthcare Systems) by specialized, trained CT technologists in our 3-dimensional imaging laboratory. Multiplanar coronal and sagittal images were obtained at 1-mm intervals. In addition, curved planar reformations (CPRs) were performed of all major peripancreatic vessels. Once the CPR images were obtained, additional analysis was performed on Aquarius Intuition software (TeraRecon, Inc, Foster City, Calif) for the intent of this research.
Objective Measurement of Tumor Resectability
Utilizing the vascular CPR images, we measured the circumferential degree of tumor contact in an axial slice perpendicular to the vessel, at the point of maximum encasement by the tumor, and the longitudinal length of solid tumor contact along the centerline track of the vessel. Both of these metrics were assessed for the SMA, CA/CHA, and SMV/PV for each patient by a radiation oncologist specifically trained and supervised by 2 abdominal diagnostic radiologists, both of whom with expertise in PCA imaging. Based on the measured tumor-vessel interface, patients were classified as resectable, borderline resectable, or unresectable according to the most recent National Comprehensive Cancer Network (NCCN) and MD Anderson Cancer Center (MDACC) resectability criteria.6,9
Statistical Analysis
Demographic and clinical characteristics of patients were summarized using means, medians, ranges, and proportions, as appropriate. Kaplan-Meier curves and medians with 95% confidence interval (CI) limits calculated using Greenwood's formula were used to calculate and summarize time-to-event outcomes. Logistic regression models were employed for binary outcomes analysis. Proportions were tested using χ2 or Fisher exact test. One-way analysis of variance was used for the analysis of means. All tests performed were 2-sided with an α level of 0.05, and these analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Recursive partitioning trees were generated using the rpart package version 4.1-11 in the R Statistical Software version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria). A logistic regression was fit to the data, and then the value of each predictor (arterial or venous score) was found that corresponded to a specific threshold for the probability of undergoing tumor resection and that resection having negative margins.
RESULTS
Patient Characteristics
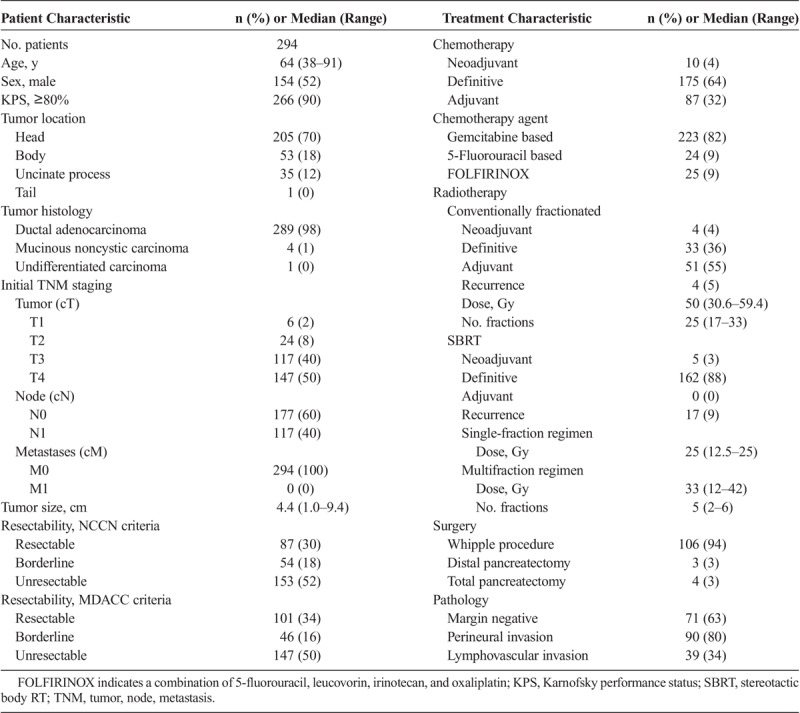
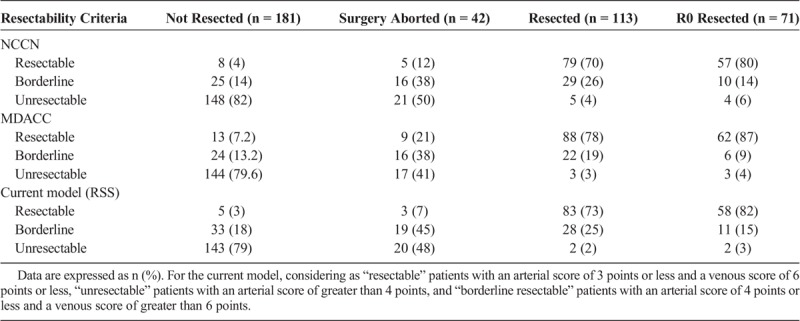
A total of 399 patients with PCA consecutively treated were initially identified, of which 105 were excluded, mostly for metastatic disease at diagnosis or severe comorbidities precluding tumor resection, yielding 294 cases of PCA used in the final analysis. Median follow-up was 14 months (range, 1–116 months). One hundred forty-seven patients (50%) presented with tumor contact with the SMA or CA, being classified as stage cT4 per the seventh edition of the American Joint Committee on Cancer tumor, node, metastasis classification.13 Pancreaticoduodenectomies were performed by a team of surgeons highly experienced on this type of malignancy. Adjuvant treatment consisted most commonly of gemcitabine-based systemic therapy followed or not by external radiation therapy (RT) concurrently with capecitabine. For borderline resectable and unresectable patients, treatment consisted of a combination of folinic acid, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) or a gemcitabine-based systemic therapy, followed mostly by stereotactic body RT in the absence of systemic progression. Only 10 patients received neoadjuvant chemotherapy or neoadjuvant RT. For 37 patients (13%), the pancreatic protocol CT used for resectability assessment was obtained soon after the start of chemotherapy (Table 1).
TABLE 1.
Patient and Treatment Characteristics
Resection Outcomes
Ultimately, 113 patients (38%) underwent tumor resection, of which 71 (63%) were resected with negative surgical margins (R0). In contrast, 42 (37%) had positive margins, 31 of which (74%) were retroperitoneal or at the vascular groove of the specimen. Of the resected cases, 35 (31%) underwent vein resection and reconstruction. Forty-two patients (23%) underwent attempted resection but were found to be unresectable at surgery due to locally advanced disease. Of the 54 cases classified as borderline resectable by the NCCN criteria, 29 (54%) were resected. Of the 46 cases classified as borderline resectable by the MDACC criteria, 22 (48%) were resected (P = 0.56).
Radiographic Measures of Degree and Length of Tumor-Vessel Contact
Tumor contact with the SMV/PV was present in 240 (82%) of the patients, SMA in 141 (48%), and with the CA/CHA in 110 (37%) patients. Among the 45 patients without any major peripancreatic vessel contact, 44 (98%) were resected, with 34 (75%) with negative margins. Superior mesenteric vein/PV–only involvement was present in 66 patients, and 47 (71%) of these were resected. Most commonly, patients presented with involvement of 2 vessels, the SMV/PV + SMA in 64 cases and the SMV/PV + CA/CHA in 41. Of the 69 patients who had tumor involvement of all 3 major vessels, none were able to undergo resection (see Supplemental Tables 1 and 2, http://links.lww.com/MPA/A720, which show descriptive statistics of measurements of degree and length of vessel involvement).
On univariate analysis, the degree of tumor contact with the SMA (P < 0.001), CA/CHA (P < 0.001), and SMV/PV (P < 0.001) all correlated with the probability of tumor resection. Similarly, the length of longitudinal contact with the SMA (P < 0.001), CA/CHA (P < 0.001), and SMV/PV (P < 0.001) also correlated with the probability of resection.
We created multivariate models to determine if degree or length of tumor contact, and with what vessels, correlated with resectability. We observed that SMA degree of encasement (P < 0.001), CA/CHA degree of encasement (P < 0.001), and SMV/PV degree of encasement (P = 0.036) were all significant for probability of resectability, while length of SMV/PV involvement was nearly significant (P =0.058). (see Supplemental Table 3, http://links.lww.com/MPA/A720, which demonstrates multivariate models testing a correlation of peripancreatic vessels tumor involvement with resectability).
Resectability Scoring System
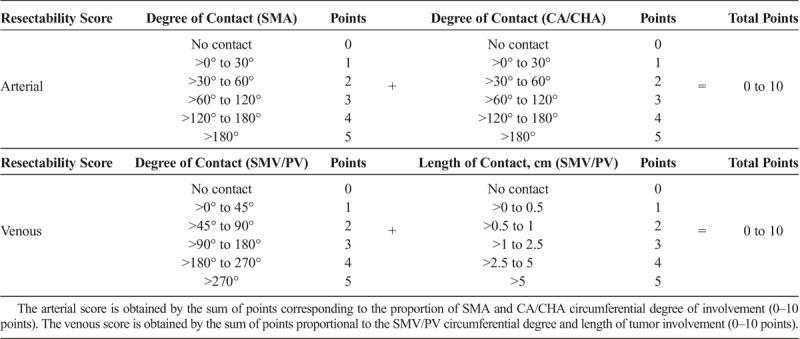
Based on the results from the multivariate models, we developed a resectability scoring system (RSS) composed of 2 factors: an arterial score, based on the circumferential degree of tumor contact with the SMA and CA/CHA, and a venous score, based on degree and length of SMV/PV involvement. Zero to 5 points were allocated to the SMA and to the CA/CHA, proportional to the circumferential degree of tumor contact, and summed to obtain the final arterial score (0–10 points). Similarly, up to 5 points were designated for SMV/PV degree and length of involvement and summed to obtain the final venous score (0–10 points) (Table 2).
TABLE 2.
Resectability Scoring System for Preoperative Radiographic Assessment of PCA
The arterial score was strongly correlated with the probability of tumor resection (odds ratio [OR], 0.44; 95% CI, 0.37–0.52; P < 0.001) and resection with negative margins (OR, 0.46; 95% CI, 0.36–0.57; P < 0.001). The venous score was also found to be highly correlated with the probability of resection (OR, 0.66; 95% CI, 0.59–0.73; P < 0.001) and resection with negative margins (OR, 0.68; 95% CI, 0.62–0.75; P < 0.001) (Fig. 1).
FIGURE 1.
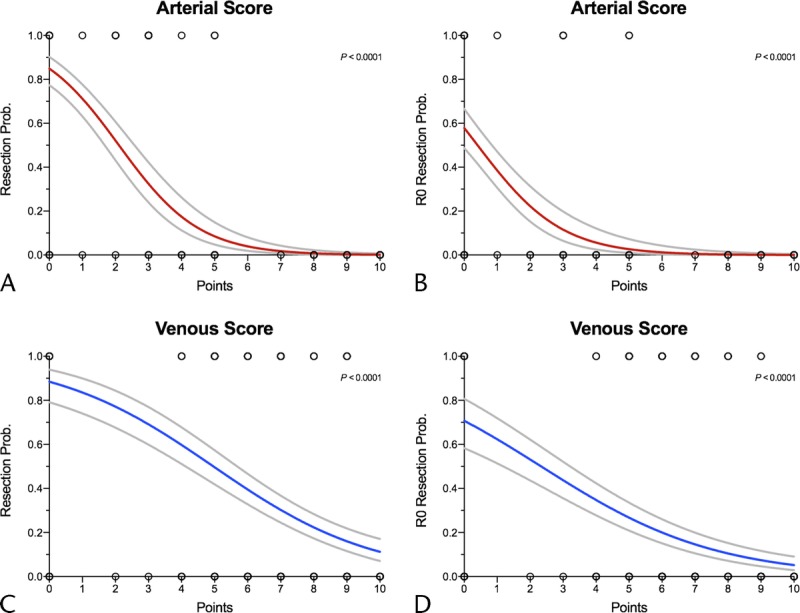
Logistic regression graphs for the probability of (A) resection and (B) resection with negative margins based on arterial score and the probability of (C) resection and (D) resection with negative margins based on venous score. Red and blue curves = predicted resectability, light gray curves = 95% CI limits.
Mean values of arterial and venous scores were 5.96 and 7.68 for unresected patients, 1.07 and 5.31 for resected patients, and 0.32 and 3.06 for those resected with negative surgical margins, respectively (P < 0.001).
To combine the predictive information from both the arterial and the venous scores into a single model, we used Classification and Regression Tree analysis, a statistical tool that employs machine-learning methods that recursively partitions the data into matched groups, helping to determine the most “relevant” (based on explanatory power) variables in a particular data set, as well as the best cutoff points to further stratify new subgroups within each variable. Using this tool, we created a decision tree model that estimates the probability of resection as well as resection with negative margins based on arterial and venous scores (Fig. 2).
FIGURE 2.
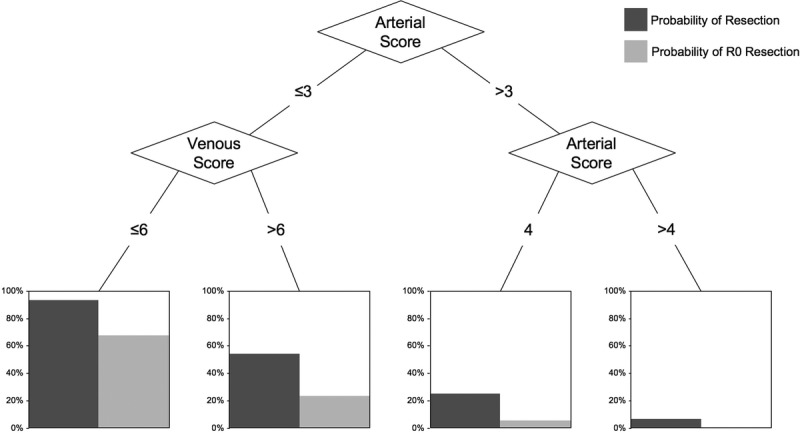
Decision tree model showing tumor resection probabilities based on final resectability scores.
Of the 87 cases considered to be resectable on preoperative assessment by the NCCN resectability criteria, 79 were resected (positive predicted value [PPV], 90.8%; negative predicted value [NPV], 96.7%; accuracy, 94.5%). Utilizing the MDACC resectability criteria, 101 cases were preoperatively categorized as resectable, of which 88 were eventually resected (PPV, 87.1%; NPV, 97.9%; accuracy, 93.5%). Since the proposed RSS is not a binary tool, but rather a scoring that offers probabilities of resection proportional to the amount of vessel involvement, in order to compare our model to the NCCN and MDACC criteria, we defined as “unresectable” patients with an arterial score of greater than 4 points, and “resectable” those patients with an arterial score of 3 points or less plus a venous score of 6 points or less. Our model demonstrated a PPV of 94.3%, an NPV of 98.6%, and accuracy of 97%. By using our model, fewer cases preoperatively considered to be resectable would have undergone an aborted pancreaticoduodenectomy (n = 3; 3%), compared with the NCCN (n = 5; 6%) and MDACC (n = 9; 9%) criteria (MDACC vs RSS, P = 0.14; NCCN vs RSS, P = 0.49) (Table 3).
TABLE 3.
Final Surgical Outcomes and Measures of Accuracy of Each Resectability Criteria
Patients classified preoperatively as borderline resectable per the NCCN criteria (n = 54) could be stratified into subcategories with different probabilities of tumor resection using the RSS. Only 2 (12%) of 16 patients were resected among those with an arterial score of greater than 3 points, compared with 27 (71%) of 38 cases with 3 points or less in the arterial score (P < 0.001). Moreover, of 16 borderline resectable cases with an arterial score of 3 points or less combined with a venous score of 6 points or less, 15 (94%) were resected (P < 0.001).
The RSS correlated with the probability of venous resection and reconstruction among the resected cases (n = 113) (P < 0.001). Of the 61 patients with a venous score of less than 6 points, only 7 (11%) needed vascular reconstruction, whereas of 52 patients with a venous score of 6 or greater, 28 (54%) needed vascular reconstruction (P < 0.001).
Survival Outcomes
Median overall survival (OS) was 13 months (95% CI, 12–15 months) for unresected patients, 27 months (95% CI, 19–35 months) for resected patients, and 34 months (95% CI, 21–45 months) for those resected with negative margins (P < 0.001).
The preoperative RSS demonstrated a clear correlation with patient prognosis (P < 0.001). Median OS for patients with an arterial score of greater than 3 points was 13 months (95% CI, 11–14 months). For patients with an arterial score of 3 or less and a venous score of greater than 6 points, it was 18 months (95% CI, 13–26 months), and 28 months (95% CI, 19–36 months) for patients with arterial score of 3 or less and venous score of 6 points or less (P < 0.001) (Fig. 3A).
FIGURE 3.
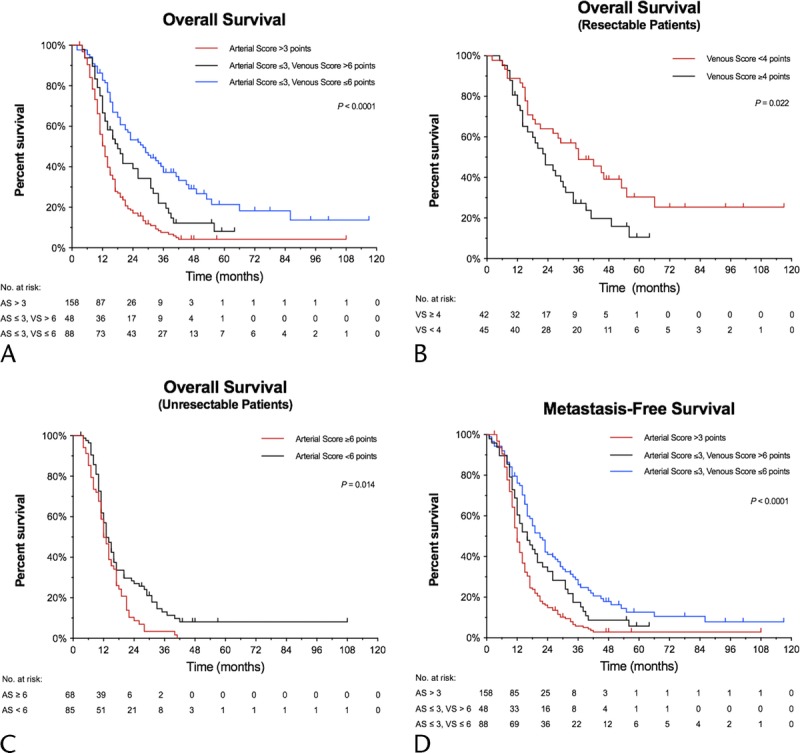
Kaplan-Meier estimates for OS according to (A) RSS, (B) RSS for patients preoperatively classified as resectable by the NCCN criteria, (C) RSS for patients preoperatively classified as unresectable by the NCCN criteria. D, Metastasis-free survival based on RSS. AS indicates arterial score; VS, venous score.
All patients preoperatively classified as resectable by NCCN criteria had an arterial score of 0 and could be further subclassified by the venous score, with those with 4 points or greater having a median OS of 23 months (95% CI, 14–31 months) versus 36 months (95% CI, 21–55 months) for patients with a venous score of less than 4 points (P = 0.022). Furthermore, the RSS predicted prognosis among the unresectable patients per NCCN criteria, with a median OS of 12 months (95% CI, 11–15 months) for unresectable patients with an arterial score of 6 points or greater and 13 months (95% CI, 12–16 months) for those with less than 6 points (P = 0.014) (Figs. 3B, C).
Resectability scoring system also correlated with distant metastasis-free survival, with a median interval of 12 months (95% CI, 11–13 months) for patients with an arterial score of greater than 3 points, 16 months (95% CI, 12–22 months) for patients with an arterial score of 3 or less and a venous score of greater than 6 points, and 21 months (95% CI, 16–27 months) for patients with arterial score of 3 or less and venous score of 6 points or less (P < 0.001) (Fig. 3D).
Other patient characteristics were tested for a correlation with prognosis including Karnofsky performance status (P = 0.13) and age at diagnosis (P = 0.67), but only tumor size (P < 0.001) was significant. On multivariate analysis with RSS, tumor size lost significance (P = 0.23), whereas RSS remained strongly correlated with survival (P < 0.001).
DISCUSSION
Clinical management of patients with PCA continues to pose a formidable challenge. Surgery with negative margins is the only curative treatment yet it is associated with significant morbidity with high recurrence rates. Therefore, accurately selecting patients likely to undergo R0 surgical resection is a critical goal of preoperative CT imaging.
Tumor resectability has been categorized by multiple different criteria in the literature based on the degree of arterial and/or venous involvement,6–12 with the 3 most widely used systems being those developed by the MDACC,6 the NCCN,9 and the Americas Hepato-Pancreato-Biliary Association/Society of Surgical Oncology/Society for Surgery of the Alimentary Tract.7,8 However, the use of subjective terms, such as “abutment,” “short-segment,” and “amenable to resection,” allows for ambiguity and disagreement among practitioners, especially when considering cases that may be classified as borderline resectable.
Other groups have utilized an array of different radiographic features, each of them suggesting distinctive resectability criteria with variable accuracies (see Supplemental Table 4, http://links.lww.com/MPA/A720, which includes different imaging scoring systems for the quantification of vessel involvement by tumor in PCA).14–21 Loyer et al14 categorized patients from type A to F according to specific tumor-vessel interface characteristics such as fat plane or pancreatic parenchyma interposition, and the shape of tumor contact (convex or concave), on the vessel wall and found that tumor type correlated with resectability. Lu et al15 divided the circumferential degree of vessel involvement into quarters, classifying patients from 0 to 4 points and found that this categorization correlated with resection probability, with tumors with less than 180° of vessel involvement being an appropriate threshold for resectability, yielding the lowest number of false-negatives and an acceptable number of false-positives. Klauss et al16 developed a scoring criterion based on length and degree of CA, SMA, SMV, PV, and splenic vein abutment, where points were added according to increasing amounts of involvement, reporting a sensitivity and specificity for resectability of 95.5% (21/22) and 100% (6/6), respectively.
To our knowledge, this is the first study to address PCA resectability, surgical margin status, and prognosis based on objective quantitative MDCT measures of degree and length of arterial and venous involvement incorporated into a single predictive model. The fact that our proposed RSS correlated with metastasis-free survival and OS suggests that the local extent of disease may be indicative of the biologic behavior of PCA. Although our data are the first to characterize and correlate these radiological findings in a comprehensive and quantitative manner, these findings are hypothesis generating, and the next steps include validation in an independent cohort.
There are a number of limitations of our study, such as its retrospective, single-institution nature, the fact that radiographic segmentation and analysis was carried out by a single investigator without a blinded second investigator for control, no control for surgeon experience, the evolution of imaging technology during the study period, and the reliance on high-resolution and specialized imaging processing.
In summary, we developed an objective scoring system that offers quantitative estimates of tumor resection probability and prognostic information based on measures of circumferential degree and length of vessel involvement by tumor. External validation is warranted.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the support of the My Blue Dots Foundation for this work and publication.
Footnotes
This research has not been supported by any funds or grants.
This work was presented at the 2018 Gastrointestinal Cancers Symposium/ASCO in San Francisco, CA, January 18 to 20, 2018.
D.T.C. has stocks on ViewRay, Inc, and received honoraria from Varian Medical Systems, Inc. The other authors declare no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Miedema BW, Sarr MG, van Heerden JA, et al. Complications following pancreaticoduodenectomy. Current management. Arch Surg. 1992;127:945–949; discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 2.Gouma DJ, van Geenen RC, van Gulik TM, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamm E, Charnsangavej C, Szklaruk J. Advanced 3-D imaging for the evaluation of pancreatic cancer with multidetector CT. Int J Gastrointest Cancer. 2001;30:65–71. [DOI] [PubMed] [Google Scholar]
- 4.Prokesch RW, Chow LC, Beaulieu CF, et al. Local staging of pancreatic carcinoma with multi-detector row CT: use of curved planar reformations initial experience. Radiology. 2002;225:759–765. [DOI] [PubMed] [Google Scholar]
- 5.Jeffrey RB. Pancreatic cancer: radiologic imaging. Gastroenterol Clin North Am. 2012;41:159–177. [DOI] [PubMed] [Google Scholar]
- 6.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. [DOI] [PubMed] [Google Scholar]
- 7.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. [DOI] [PubMed] [Google Scholar]
- 8.Abrams RA, Lowy AM, O'Reilly EM, et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751–1756. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Pancreatic adenocarcinoma (version 1.2017, February 24, 2017). Available at: https://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf. Accessed August 25, 2017. [DOI] [PubMed]
- 10.Appel BL, Tolat P, Evans DB, et al. Current staging systems for pancreatic cancer. Cancer J. 2012;18:539–549. [DOI] [PubMed] [Google Scholar]
- 11.Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155:977–988. [DOI] [PubMed] [Google Scholar]
- 13.Sobin LH, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumors. 7th ed Oxford, UK: Wiley-Blackwell; 2010. [Google Scholar]
- 14.Loyer EM, David CL, Dubrow RA, et al. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin-section CT. Abdom Imaging. 1996;21:202–206. [DOI] [PubMed] [Google Scholar]
- 15.Lu DS, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439–1443. [DOI] [PubMed] [Google Scholar]
- 16.Klauss M, Mohr A, von Tengg-Kobligk H, et al. A new invasion score for determining the resectability of pancreatic carcinomas with contrast-enhanced multidetector computed tomography. Pancreatology. 2008;8:204–210. [DOI] [PubMed] [Google Scholar]
- 17.Phoa SS, Reeders JW, Stoker J, et al. CT criteria for venous invasion in patients with pancreatic head carcinoma. Br J Radiol. 2000;73:1159–1164. [DOI] [PubMed] [Google Scholar]
- 18.Tran Cao HS, Balachandran A, Wang H, et al. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg. 2014;18:269–278; discussion 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada S, Fujii T, Takami H, et al. Evaluation and proposal of novel resectability criteria for pancreatic cancer established by the Japan Pancreas Society. Surgery. 2017;162:784–791. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Zeng MS, Zhou KR, et al. Pancreatic adenocarcinoma: the different CT criteria for peripancreatic major arterial and venous invasion. J Comput Assist Tomogr. 2005;29:170–175. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Zeng MS, Zhou KR, et al. Pancreatic adenocarcinoma: signs of vascular invasion determined by multi-detector row CT. Br J Radiol. 2006;79:880–887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


