Abstract
Objectives
We sought to synthesize data on systemic arthroprosthetic cobaltism, a recently described syndrome that results from wear or corrosion of chrome-cobalt hip components.
Methods
We conducted a systematic literature review to identify all reported cases of systemic arthroprosthetic cobaltism. To assess the epidemiologic link between blood cobalt levels (B[Co]), we developed a symptom scoring tool that evaluated 9 different symptom categories and a category of medical utilization.
Results
We identified 25 patients reported between 2001 and 2014 with a substantial increase in case reports over the past 3 years. Symptoms were diverse and involved the hip (84%), cardiovascular system (60%), audiovestibular system (52%), peripheral motor-sensory system (48%), thyroid (48%), psychological functioning (32%), visual system (32%), and the hematological, oncological, or immune system (20%). The mean latency from implantation to presentation or revision was 41 months (range, 9–99 months). The mean B[Co] was 324 μg/L and 4 patients had levels less than 20 μg/L. The B[Co] but not blood chromium level was highly associated with a quantitative measure of overall symptom severity (r2, 0.81; P < 0.001). Mean B[Co] and symptom scores were substantially higher in patients with revisions of failed ceramic-on-ceramic prostheses than those with primary metal-on-metal prostheses.
Conclusions
Systemic arthroprosthetic cobaltism is an increasingly recognized complication of wear or corrosion of chrome-cobalt hip implants, may involve a large number of organ systems, and may occur with relatively low B[Co]. There is an urgent need to better define the overall scope of the problem and to develop screening and management strategies.
Key Words: arthroplasty, cobalt, cobaltism, hip, hip replacement
The ill effects of ingestion or inhalation of cobalt on the peripheral and central nervous system, heart, and thyroid were extensively catalogued in 1981.1 Historically, iatrogenic cobaltism resulted from oral cobalt chloride treatment of anemia, vocational cobaltism from exposure to cobalt power, and “beer drinkers' ” cardiomyopathy from a cobalt foam–stabilizing additive. This formerly esoteric malady is important because millions of hips implanted over the past decade use chrome-cobalt components. It was thought that metal-on-metal articular surfaces would be protected from contact and wear by fluid film lubrication.2 Analysis of explanted metal-on-metal hips suggests that this protective mechanism rarely occurs in vivo.3 Rather, metal-on-metal hips can wear or corrode, resulting in periprosthetic metallosis and hypercobaltemia (blood cobalt level, B[Co], >1 μg/L). Metal-on-metal hips comprising a third of American hips implanted over the past decade use chrome-cobalt femoral and acetabular articular surfaces and may have a particularly high risk for generating chrome-cobalt metallosis.4,5 A recent survey of 498 patients implanted with metal-on-metal hips found that one-third had B[Co] of more than 4 μg/L, with periprosthetic complications that increased as B[Co] increased.6
Subjects without abnormal cobalt exposure have a mean B[Co] of 0.3 μg/L, and 95% have a value of less than 0.6 μg/L.7,8 The biologic exposure threshold for B[Co] is 1 μg/L, and an end-of-work-week value of 1 or greater indicates potentially unsafe vocational exposure.9 Patients with well-functioning metal-on-metal hip resurfacings have a mean B[Co] of 2 μg/L, and a level greater than 3 μg/L in a patient with a unilateral metal-on-metal hip indicates a degree of periprosthetic chrome-cobalt metallosis that is likely to incite a periprosthetic adverse reaction to metallic debris.10,11
Neurocobaltism and cardiac cobaltism occur when cobalt impairs mitochondrial metabolism resulting in cellular dysfunction or death.1,12,13 Studies in rabbits have found that the neurons in the cochlea and retina have the greatest susceptibility to oxidative stress and the greatest likelihood of dying after experimentally induced cobaltism.13 Different mechanisms may explain the effects of cobalt or chromium on mood and cognition. Acute cobalt exposure increases neural activity and can result in seizures.1,14 Because cobalt may deposit preferentially in the myocardium and pericardial fluid, cobaltism may have predominant or only cardiac symptoms.15–18 Because hip replacement often occurs among elderly patients, age-associated decreases in renal cobalt clearance may contribute to increased cobalt levels in some patients.19
The published peer-reviewed literature contains an increasing number of case reports of systemic cobaltism after hip arthroplasty. We present a review of all cases resulting from metal-on-metal primary arthroplasties or revised arthroplasties with chrome-cobalt femoral heads to define symptom presentation, identify basic characteristics of affected patients, and determine if symptom severity correlates with cobalt level or measures of cobalt exposure.
METHODS
Search Strategy
We conducted a systematic search of PubMed for manuscripts published at any point in the past through June 2014 with no language or geographic restrictions and no specified start date using the following terms: (“cobalt” OR “cobaltemia” OR “cobaltic” OR “cobaltism”) AND (“hip” OR “acetabulum” OR “total hip arthroplasty” OR “case report” OR “cohort”).
This search yielded 2318 manuscripts whose titles and abstracts were screened for potential inclusion (Fig. 1). We also screened references of retrieved full-text manuscripts. As a last informal check on our search strategy, we conducted a nonsystematic review of EBSCOhost and Google Scholar using the terms “hip replacement” OR “hip arthroplasty” OR “total hip arthroplasty” OR “cobaltism” OR “cobalt” OR “case report” OR “arthroprosthetic cobaltism.” The goal of this search was to determine if any manuscripts were identified that obviously should be included. Neither of these 2 additional search strategies yielded additional manuscripts.
FIGURE 1.
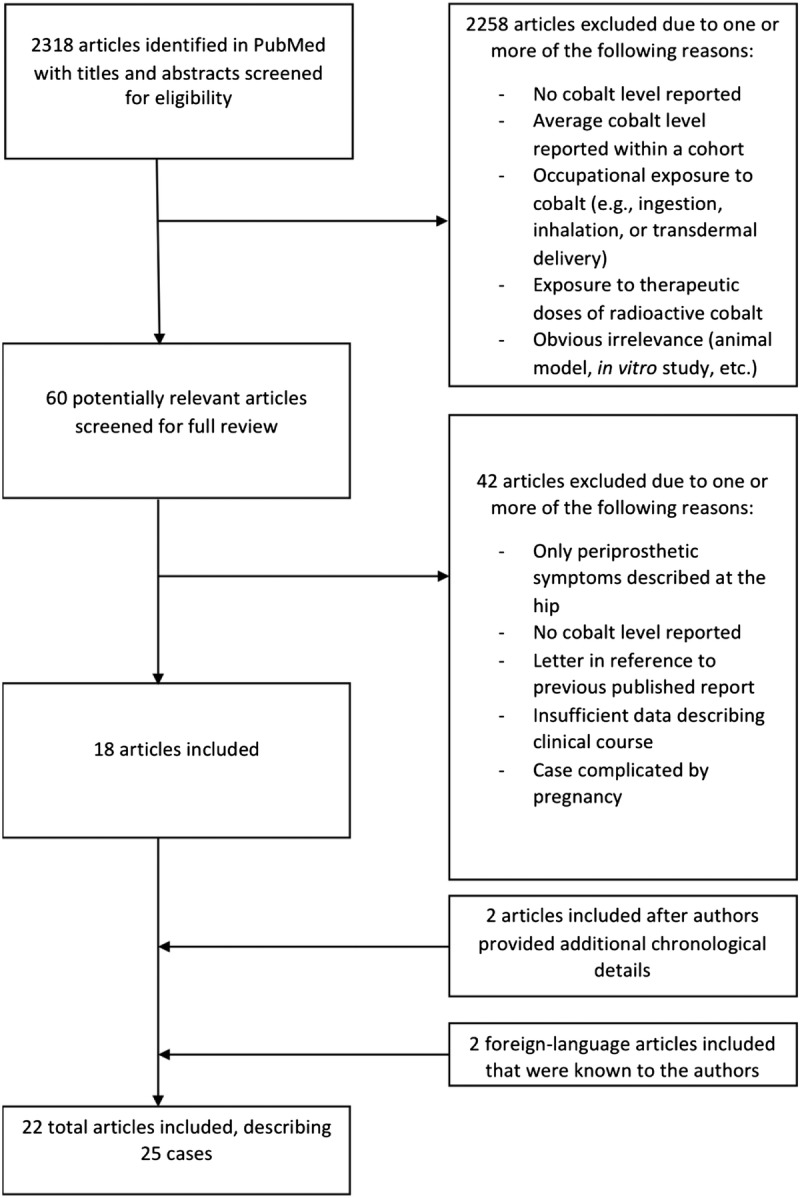
Flow chart for a systematic review of systemic cobaltism after wear or corrosion of chrome-cobalt hip implants.
Inclusion criteria included human studies with reported systemic illness, a surgical history of hip arthroplasty with at least one chrome-cobalt component at the bearing surface, and a reported blood or serum cobalt level greater than 7 μg/L. The selected blood cobalt level cutoff was that used by the United Kingdom Medicines and Healthcare Products Regulatory Agency as indicative of a bearing whose wear may induce periprosthetic tissue damage.20 In addition, we only included cases with at least 2 of 3 details of the patient's clinical course: latency to illness, latency to revision, or latency to hip symptoms. Patients who had isolated periprosthetic pseudotumors, neuropathies associated with local inflammation or masses (e.g., femoral or sciatic neuropathy from pseudotumor), and elevated C-reactive protein or erythrocyte sedimentation rate in isolation were not considered to have systemic illness, and their case reports were not included.
Exclusion criteria included occupational exposure to cobalt (e.g., ingestion, inhalation, or transdermal delivery), exposure to therapeutic doses of radioactive cobalt, and multipatient studies for which measured cobalt levels could not be associated with a specific patient's clinical symptoms. In addition, cases were excluded if the patient had had a diagnosis of a clear underlying disorder that explained all of their systemic symptoms (e.g., Hashimoto disease in a case of thyroid dysregulation). Although we did not limit our search to English language manuscripts, we did not include one manuscript for which we could not obtain a suitable translation.21 We also excluded one report of periprosthetic cobaltism during pregnancy to avoid confounding by the numerous symptoms that can complicate pregnancy.22
We independently reviewed all 60 potentially relevant manuscripts. Where disagreement on inclusion occurred, the reviewers met and developed a consensus opinion on inclusion. This process led to inclusion of 18 manuscripts. Two manuscripts that were initially excluded23,24 were included after the authors were contacted, and they provided previously missing chronological details. We included 2 additional manuscripts known previously to one of the authors,15,25 which may not have been identified in our search because of their publication in Dutch and Italian, respectively. Although the manuscript by Megaterio et al15 did not include blood cobalt levels, we elected to include it because it was the first case report of arthroprosthetic cobaltism and the patient reportedly had significant increases in blood and urine cobalt levels. In total, we included 22 manuscripts,14–16,23–41 one of which reported 3 patients,37 three of which reported 2 patients,32,38,39 and 2 occasions where descriptions of the same patient were reported twice.29,34,40,41 This led to results for 25 unique patients.
Data Abstraction and Synthesis
We independently abstracted data using a standard data abstraction form for all 22 relevant manuscripts (Fig. 1). In the 2 instances where a case was described in more than one publication, we used all sources to create the most complete clinical history. One of the current study authors authored 2 case reports included in the current review39,40; clinical histories of patients described in these reports were supplemented by additional data not published in the original manuscripts.
All 25 cases were scored using a cobaltism scoring rubric developed by the authors (Table 1). This rubric was not designed for clinical management purposes but rather for the epidemiological purpose of determining if blood cobalt concentrations correlated with clinical severity. Within organ systems, medical specialists reviewed the scoring tool (for example, a cardiologist for the cardiovascular symptoms, a psychiatrist for psychiatric symptoms, etc). We independently scored each patient. Where discrepancies occurred, these were resolved through consensus. To arrive at a final summary cobaltism score, we added together the scores for each symptom category.
TABLE 1.
Scoring Tool Developed to Determine Symptom Severity of Arthroprosthetic Cobaltism

Elevations of blood cobalt are likely not constant throughout the period of hip implantation. Poorly positioned metal-on-metal hips are prone to edge loading resulting in accelerating rates of wear as articular incongruity increases.3 Because of this issue, ideally, cobalt exposure would be based on frequent monitoring of B[Co] for the duration of the prosthetic placement. Practically speaking, most patients had a single prediagnosis or peridiagnosis cobalt level available from the time of diagnosis. Consequently, we assessed 3 measures of exposure: highest B[Co], latency from prosthetic placement to revision, and the product of these 2 variables. As a control analysis, we also assessed blood chromium levels as an exposure variable.
In addition to descriptive analyses, we conducted linear regression analysis to determine the relationship between exposure measures and clinical outcomes. Evaluated clinical outcomes included overall symptom score and scores within symptom categories. An adjusted model included patients' age and sex. All analyses were conducted with SPSS (IBM, SPSS Statistics, version 19, 2010).
RESULTS
We identified 25 patients with reported systemic arthroprosthetic cobaltism after wear or corrosion of chrome-cobalt hip implants. Sixteen patients (64%) were men, and mean age was 56 years (median, 54 years; range, 39–75 years). Seven of the patients resided in the United States (5 from Alaska), 5 in Australia, 4 in Germany, 3 each in Italy and The Netherlands, and 1 each in Canada, the Czech Republic, and Japan. The number of reported cases increased over time, with 1 case reported during 2001, 1 during 2006, 2 cases during 2009, 3 during 2010, 2 during 2011, 6 each during 2012 and 2013, and 4 through the first half of 2014.
Hip symptoms were identified in 21 patients (84%). Because we only included cases with systemic manifestations, all patients also had symptoms from one of the categories in Table 1 other than hip and medical utilization, most commonly constitutional and cardiovascular symptoms (Table 2). The mean number of systemic symptom categories involved was 3.6 (median, 4; range, 1–7).
TABLE 2.
Frequency of Symptoms Among 25 Patients With Reported Systemic Arthroprosthetic Cobaltism
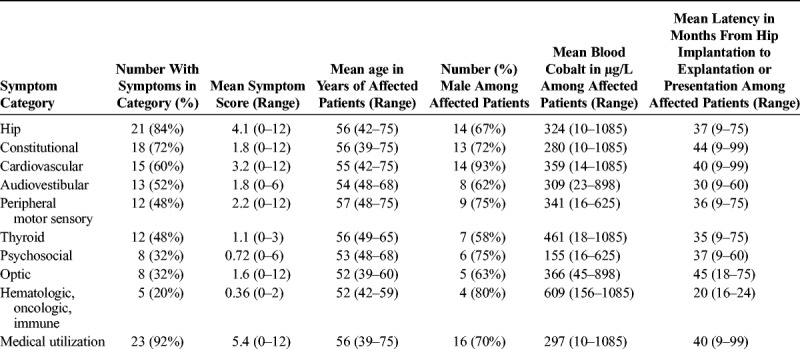
All but one included patient (18) had at least one recorded B[Co] with the mean peak level equal to 324 μg/L (range, 10–1085 μg/L). Of the 24 patients with available peak B[Co], 4 (17%) had a level of less than 20 μg/L and 11 (46%) had a level of less than 100 μg/L. Of 21 persons with blood chromium levels (B[Cr]) available, the mean was 57 μg/L (range, 4.1–249 μg/L). Five patients (24%) had a B[Cr] level of less than 20 μg/L, and 20 (91%) had a B[Cr] level of less than 100 μg/L. Twenty-two patients had information on the latency from the original insertion of a chrome-cobalt component to revision (if this was documented) or presentation (if revision latency was not documented), with a mean of 41 months (range, 9–99 months). One patient had a latency of less than 1 year, 6 (27%) from 1 to less than 2 years, 2 (9%) from 2 to less than 3 years, 5 (23%) from 3 to less than 4 years, and 8 (36%) for 4 years or greater.
Twenty patients (80%) had documentation of hip explantation. Reporting of postexplantation symptoms was nonsystematic and incomplete, making analysis impossible. However, for 19 of the patients, the authors noted that at least some of the symptoms improved; the 20th patient died of cobalt cardiomyopathy 2 months after hip resection,29 the only death reported among the cases we identified. Despite improvement, for all patients where this was noted, at least some symptoms attributable to cobaltism persisted after explantation of the cobalt-containing prosthesis.
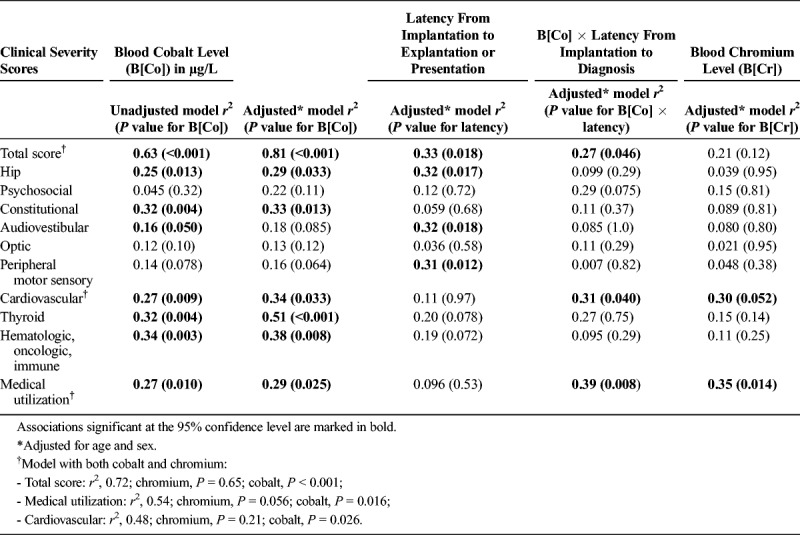
During linear regression analysis, the peak B[Co] was associated with the total symptom score on unadjusted and adjusted analyses (Fig. 2 and Table 3). The symptom category most strongly associated with B[Co] was thyroid abnormality. Latency from implantation to explantation or presentation was less strongly associated with total symptom score than peak B[Co]. However, for the individual categories of hip, audiovestibular, and peripheral motor sensory, latency predicted severity better than B[Co]. B[Cr] was not associated with total symptom score and infrequently associated with individual symptom categories.
FIGURE 2.
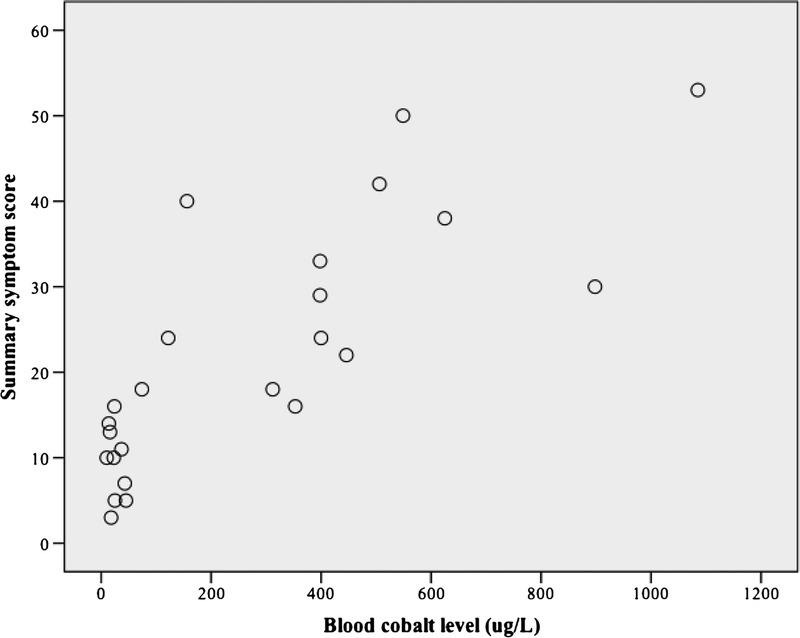
Scatter plot of the association between blood cobalt level and a summary clinical symptom score in patients with systemic cobaltism after wear or corrosion of chrome-cobalt hip implants.
TABLE 3.
Unadjusted and Adjusted R2 Values for the Association Between Exposures and Clinical Severity Scores Overall and Within Organ Systems
Sixteen of the 25 patients had a metal-on-metal primary arthroplasty, whereas 9 had a chrome-cobalt revision femoral head after fracture of a primary ceramic-on-ceramic arthroplasty. The mean B[Co] among the 9 patients with revision surgery was 613 μg/L (range, 398–1085 μg/L) compared with 104 μg/L (range, 10–398 μg/L) among the remaining patients (P < 0.001; Student 2-tailed t test). Similarly, the mean symptom score was 35 (range, 22–53) among those with revision surgery and 15 (range, 3–40) among the remaining patients (P < 0.001); those with revision surgery had a symptom score significantly higher at the 95% confidence level than other patients for hip symptoms (6.9 versus 2.6; P = 0.033), audiovestibular involvement (3.3 versus 1.0; P = 0.039), peripheral motor-sensory involvement (4.8 versus 0.69; P = 0.030), and thyroid disease (2.0 versus 0.63; P = 0.003).
DISCUSSION
Our study identified 25 cases of reported arthroprosthetic cobaltism that occurred since the initial report during 2001. Case reports have accelerated recently and have occurred from 8 countries. Despite the relatively widespread distribution, all but 2 US cases have originated from Alaska, suggesting the true scope of the problem has gone largely unreported. This likely has resulted from a combination of several factors including the slow onset, the protean symptoms that may mimic many different conditions, lack of uniform screening strategies for at-risk patients, and lack of awareness of arthroprosthetic cobaltism among surgeons and primary care physicians.
An additional difficulty is that hip replacement surgery often occurs in elderly patients or patients with underlying conditions, making it difficult to associate symptoms with cobaltism. In part to address this issue in our review, we developed a symptom scoring tool, which confirmed a strong association between B[Co] but not B[Cr] and symptom severity. This association differed by individual symptom category, with the strongest association between B[Co] and thyroid abnormalities and the weakest with optic abnormalities. This finding may indicate that not all symptoms in the case reports actually resulted from arthroprosthetic cobaltism. It may also indicate that presentation in any particular individual is modified by a variety of factors including underlying illness, age, activity level, and type of physical activities in which the patient engages.
Chrome-cobalt femoral head placement after revision of a fractured ceramic head led to higher B[Co] levels and more severe symptoms than primary metal-on-metal arthroplasty. The mechanism is likely to be due to abrasion of the metal surface by retained ceramic fragments. This was suggested in one case history in which the pieces of the fractured ceramic head were reconstructed and found to be incomplete.14 Whereas use of a chrome-cobalt head for revision of a failed ceramic-on-ceramic hip likely identifies a particularly high-risk group, the substantial symptoms among those with metal-on-metal primary arthroplasty indicates screening and management strategies need to be developed for both groups.
The American Academy of Orthopedic Surgeons upgraded monitoring guidelines for patients with metal-on-metal hips in 2014.10 These recommendations focus primarily on local adverse reactions to metallic debris and advise B[Co] levels and cross-sectional hip imaging for patients presenting with pain, noise, or swelling at the hip. Going further is Britain's national Medicines and Healthcare Products Regulatory Agency, which in 2012 issued a medical device alert (MDA).20 This medical device alert recommends B[Co] determination for most patients implanted with metal-on-metal hips and notes that a rising level or abnormal hip imaging should prompt consideration for revision surgery even in asymptomatic patients. Perhaps most comprehensively, Australia's independent, not-for-profit group NPS MedicineWise recommends annual B[Co] determination for all patients implanted with metal-on-metal hips (increasing to every 3 months if levels rise), surveillance for periprosthetic complications by a surgeon, and evaluation for constitutional, psychological, neurologic, and cardiovascular manifestations of cobaltism by the patient's primary provider.42 This approach could potentially avert the substantial systemic morbidity documented in the case reports presented here as well as lead to earlier identification of the pseudotumors that can result in progressive periprosthetic tissue damage and complicate revision surgery.43,44 In this respect, it is worth noting that 4 of the 25 patients did not have hip symptoms; and thus, using this as a sole screening criterion for further evaluation may delay identification of systemic manifestations.
Complicating matters is that the B[Co] level at which risk for adverse outcomes increases is poorly defined. Whereas the mean B[Co] level among reported cases was relatively high (324 μg/L), 17% had a level of less than 20 μg/L and almost half had a level of less than 100 μg/L. These data suggest that at a minimum, B[Co] level greater than 10 μg/L should trigger additional evaluation, but the scope of this evaluation remains undefined. For example, evaluation could include hip radiographs to assess component fixation and position and to assess for bone loss; cross-sectional hip imaging to assess the condition of periprosthetic soft tissue45,46; and audiograms, echocardiograms, and thyroid function studies. Additionally, patients with metal-on-metal hips might benefit from baseline assessment of various organ systems. The use of these approaches will depend on the prevalence of cobaltism in the total population of persons with metal-on-metal hips, their average symptom severity and the proportion with severe symptoms, as well as the economic benefit of routine screening at varying levels of intensity versus waiting until patients become symptomatic. Unfortunately, none of this information is known.
CONCLUSIONS
Future work should be done to define the prevalence and burden of arthroprosthetic cobaltism and the cost-effectiveness of various screening and management strategies. In addition to targeted and systematic studies, another strategy to facilitate these goals is the establishment of joint registries in the United States and other countries similar to those used in many European countries. In the meantime, surgeons and general practitioners should receive training on the potential risk for cobaltism associated with wear or corrosion of chrome-cobalt hip implants. A validated symptom scoring tool would facilitate clinical screening. Finally, it seems prudent to assess B[Co] yearly in patients with chrome-cobalt–containing hip prostheses and to develop a management plan when levels greater than 10 μg/L are identified.
Footnotes
Conflicts of interest and funding sources: The study was funded by the Alaska Arthroplasty Initiative, which in turn was funded entirely by a grant from Providence Alaska Medical Center. SST was the originator and is the chairperson of the board of AAI and was a full participant in the current project. None of the authors report any conflicts of interest in conducting the evaluation or writing the manuscript. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
REFERENCES
- 1.Smith IC, Carson BL. Volume 6-Cobalt an Appraisal of Environmental Exposure. 1st ed Ann Arbor, MI: Ann Arbor Science; 1981. [Google Scholar]
- 2.Isaac GH, Siebel T, Schmalzried TP, et al. Development rationale for an articular surface replacement: a science-based evolution. Proc Inst Mech Eng [H]. 2006;220:253–268. [DOI] [PubMed] [Google Scholar]
- 3.Currier JH, McHugh DJ, Tower DR, et al. Gouge features on metal-on-metal hip bearings can result from high stresses during rim contact. Tribology International. 2012;63:89–96. [Google Scholar]
- 4.Bozic KJ, Kurtz S, Lau E, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620. [DOI] [PubMed] [Google Scholar]
- 5.Tower S. Arthroprosthetic cobaltism: identification of the at-risk patient. Alaska Med. 2010;52:28–32. [PubMed] [Google Scholar]
- 6.Van Der Straeten C, Van Quickenborne D, Pennynck S, et al. Systemic toxicity of metal Ions in a metal-on-metal hip arthroplasty population. Bone Joint J. 2013;95-B(suppl 34):187. [Google Scholar]
- 7.Alimonti A, Bocca B, Mannella E, et al. Assessment of reference values for selected elements in a healthy urban population. Ann Ist Super Sanità. 2005;41:181–187. [PubMed] [Google Scholar]
- 8.Hodnett D, Wood DM, Raja K, et al. A healthy volunteer study to investigate trace element contamination of blood samples by stainless steel venipuncture needles. Clin Toxicol (Phila). 2012;50:99–107. [DOI] [PubMed] [Google Scholar]
- 9.American Conference of Governmental Industrial Hygienists. 2012 threshold limit values (TLVs) and biological exposure indices (BEIs). Appendix B. ACGIH, Cincinnati, Ohio. Available at: http://www.nsc.org/news_resources/studentportal/CaseStudy/FIH%206e%20Appendix%20B.pdf. Accessed September 23, 2014.
- 10.Kwon YM, Lombardi AV, Jacobs JJ, et al. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96:e4. [DOI] [PubMed] [Google Scholar]
- 11.Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93:164–171. [DOI] [PubMed] [Google Scholar]
- 12.Rona G, Chappel CI. Pathogenesis and pathology of cobalt cardiomyopathy. Recent Adv Stud Cardiac Struct Metab. 1973;2:407–422. [PubMed] [Google Scholar]
- 13.Catalani S, Rizzetti MC, Padovani A, et al. Neurotoxicity of cobalt. Hum Exp Toxicol. 2012;31:421–437. [DOI] [PubMed] [Google Scholar]
- 14.Oldenburg M, Wegner R, Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J Arthroplasty. 2009;24:825 e15–825 e20. [DOI] [PubMed] [Google Scholar]
- 15.Megaterio S, Galetto F, Alossa E, et al. Effetti a distanza del rilascio di ioni metallo in usura della testa protesica: presentazione di un caso. GIOT. 2001;27:173–175. [Google Scholar]
- 16.Pelclova D, Sklensky M, Janicek P, et al. Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin Toxicol (Phila). 2012;50:262–265. [DOI] [PubMed] [Google Scholar]
- 17.Jones DA, Lucas HK, O'Driscoll M, et al. Cobalt toxicity after McKee hip arthroplasty. J Bone Joint Surg Br. 1975;57:289–296. [PubMed] [Google Scholar]
- 18.Frustaci A, Magnavita N, Chimenti C, et al. Marked elevation of myocardial trace elements in idiopathic dilated cardiomyopathy compared with secondary cardiac dysfunction. J Am Coll Cardiol. 1999;33:1578–1583. [DOI] [PubMed] [Google Scholar]
- 19.Guzzo TJ, Drach GW. Major urologic problems in geriatrics: assessment and management. Med Clin North Am. 2011;95:253–264. [DOI] [PubMed] [Google Scholar]
- 20.Medicines and Healthcare Products Regulatory Agency. Medical Device Alert, MDA/2012/036; 25 June 2012. Available at: http://www.mhra.gov.uk/home/groups/dts-bs/documents/medicaldevicealert/con155767.pdf. Last accessed September 23, 2014.
- 21.Brodner W, Grohs JG, Bitzan P, et al. Serum cobalt and serum chromium level in 2 patients with chronic renal failure after total hip prosthesis implantation with metal-metal gliding contact. Z Orthop Ihre Grenzgeb. 2000;138:425–429. [DOI] [PubMed] [Google Scholar]
- 22.Fritzsche J, Borisch C, Schaefer C. Case report: high chromium and cobalt levels in a pregnant patient with bilateral metal-on-metal hip arthroplasties. Clin Orthop Relat Res. 2012;470:2325–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connell E, Mead N, Fesniak H. Idiopathic cardiomyopathy following metal-on-metal hip arthroplasty: the new fact of “beer drinker's cardiomyopathy”. Eur J Cardiovasc Med. 2013;2:210–211. [Google Scholar]
- 24.Giampreti A, Lonati D, Locatelli CA. Chelation in suspected prosthetic hip-associated cobalt toxicity. Can J Cardiol. 2014;30:e13. [DOI] [PubMed] [Google Scholar]
- 25.Van Quickenborne D, Van Der Straeten C, De Smet KA. Hearing loss in a bilateral hip resurfacing. Tijdschr voor Geneeskunde. 2012;68. [Google Scholar]
- 26.Allen LA, Ambardekar AV, Devaraj KM, et al. Clinical problem-solving. Missing elements of the history. N Engl J Med. 2014;370:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apel W, Stark D, Stark A, et al. Cobalt-chromium toxic retinopathy case study. Doc Opthalmol. 2013;126:69–78. [DOI] [PubMed] [Google Scholar]
- 28.Dahms K, Sharkova Y, Heitland P, et al. Cobalt intoxication diagnosed with the help of Dr House. Lancet. 2014;383:574. [DOI] [PubMed] [Google Scholar]
- 29.Gilbert CJ, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29:639 e1–639 e2. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda T, Takahashi K, Kabata T, et al. Polyneuropathy caused by cobalt-chromium metallosis after total hip replacement. Muscle Nerve. 2010;42:140–143. [DOI] [PubMed] [Google Scholar]
- 31.Machado C, Appelbe A, Wood R. Arthroprosthetic cobaltism and cardiomyopathy. Heart Lung Circ. 2012;21:759–760. [DOI] [PubMed] [Google Scholar]
- 32.Mao X, Wong AA, Crawford RW. Cobalt toxicity—an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194:649–651. [DOI] [PubMed] [Google Scholar]
- 33.Ng SK, Ebneter A, Gilhotra JS. Hip-implant related chorio-retinal cobalt toxicity. Ind J Ophthalmol. 2013;61:35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzetti MC, Liberini P, Zarattini G, et al. Loss of sight and sound. Could it be the hip? Lancet. 2009;373:1052. [DOI] [PubMed] [Google Scholar]
- 35.Roessler PP, Witt F, Efe T, Schmitt J. Arthroprosthetic cobaltism and pseudotumour also occur in patients with small diameter femoral ball head metal-on-metal total hip arthroplasties. Br Med J Case Rep. 2014: e203362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steens W, Loehr JF, von Foerster G, et al. Chronic cobalt poisoning in endoprosthetic replacement. Orthopade. 2006;35:860–864. [DOI] [PubMed] [Google Scholar]
- 37.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty. J Bone Joint Surg. 2010;92:2847–2851. [DOI] [PubMed] [Google Scholar]
- 38.Tower SS. Arthroprosthetic cobaltism associated with metal on metal hip implants. Br Med J. 2012;344:e430. [DOI] [PubMed] [Google Scholar]
- 39.van Lingen CP, Etterna HB, Timmer JR, et al. Clinical manifestations in ten patients with asymptomatic metal-on-metal hip arthroplasty with very high cobalt levels. Hip Internat. 2013;23:441–444. [DOI] [PubMed] [Google Scholar]
- 40.Pazzaglia UE, Apostoli P, Congiu T, et al. Cobalt, chromium and molybdenum ions kinetics in the human body: data gained from a total hip replacement with massive third body wear of the head and neuropathy by cobalt intoxication. Arch Orthop Trauma Surg. 2011;131:1299–1308. [DOI] [PubMed] [Google Scholar]
- 41.Ziwiel MG, Brandt JM, Overgaard CB, et al. Fatal cardiomyopathy after revision total hip replacement for fracture of a ceramic liner. Bone Joint J. 2013;95-B:31–37. [DOI] [PubMed] [Google Scholar]
- 42.NPS MedicineWise. Monitoring for potential toxicity in patients with metal-on-metal hip. Published 21 September 2012. Available at: http://www.nps.org.au/publications/health-professional/health-news-evidence/2012/metal-hip-replacement-toxicity. Accessed September 23, 2014.
- 43.de Steinger RN, Miller LN, Prosser GH, et al. Poor outcome of revised resurfacing hip arthroplasty. Acta Orthop. 2010;81:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graves SE, Rothwell A, Tucker K, et al. A multinational assessment of metal-on-metal bearings in hip replacement. J Bone Joint Surg Am. 2011;93(Suppl 3):43–47. [DOI] [PubMed] [Google Scholar]
- 45.Lombardi AV, Jr, Barrack RL, Berend KR, et al. The Hip Society: algorithmic approach to diagnosis and management of metal-on-metal arthroplasty. J Bone Joint Surg Br. 2012;94-B(Suppl 11):14–18. [DOI] [PubMed] [Google Scholar]
- 46.Kwon YM, Jacobs JJ, MacDonald SJ, et al. Evidence-based understanding of management perils for metal-on-metal hip arthroplasty patients. J Arthroplasty. 2012;27(Suppl 8):20–25. [DOI] [PubMed] [Google Scholar]


