The human immunodeficiency virus seroprevalence in Mycoplasma genitalium–infected females was significantly higher than in uninfected females, regardless of the presence or absence of other sexually transmitted infection pathogens.
Abstract
Background
Mycoplasma genitalium is associated with genital discharge syndrome, but limited prevalence data are available in South Africa. The prevalence rates of M. genitalium infection and human immunodeficiency virus (HIV) coinfection were determined in urogenital specimens collected from male and female patients presenting with genital discharge syndrome to a primary health care center in Johannesburg, South Africa from 2007 through 2014.
Methods
Genital specimens from 4731 patients were tested by a validated in-house multiplex real-time polymerase chain reaction assay for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, and M. genitalium. Sera were tested for HIV infection using the Determine HIV 1/2 and Unigold assays.
Results
The relative prevalence of M. genitalium in males and females was 8.9% and 10.6%, respectively. The prevalence of HIV infection in those infected with M. genitalium, without other sexually transmitted infections (STIs), was significantly higher than in those without M. genitalium infection (48.9% vs. 40.5%, P = 0.014). This significant difference in HIV seroprevalence was particularly observed among females in the study cohort.
Conclusions
The relative prevalence of M. genitalium and its association with prevalent HIV among females with vaginal discharge syndrome (VDS) calls for further research on the potential role of M. genitalium in the transmission and acquisition of HIV.
Mycoplasma genitalium is an emerging cause of genital discharge and has been implicated in male and female urogenital infections globally.1 More than 25 years after its initial isolation from men with non-gonococcal urethritis, data supporting the role of M. genitalium as an etiological agent of acute and persistent male non-gonococcal urethritis, has increased over the years.2 Mycoplasma genitalium has also been significantly associated with both cervicitis and pelvic inflammatory disease (PID) in women.1
The prevalence of M. genitalium infection varies widely in different countries. Studies conducted in Western Europe, North America and Australia, have estimated the prevalence of M. genitalium in men to range between 1% and 3.3%3,4 and among women to range between 1% and 6.4%.5 In a systematic review conducted by Baumann et al., the prevalence estimates of M. genitalium in the general population were reported to be low, ranging from 1.3% in countries with a high human development index to 3.9% in those with lower human development index.6 Prevalence estimates were highest in female commercial sex workers ranging from 13.2% in community-based samples to 26.3% in clinic-based samples.
In comparison with other sexually transmitted infections (STIs), there is a paucity of information on the prevalence of M. genitalium in South Africa.7–11 In a recent study conducted in South Africa, among adolescents and young adults with asymptomatic genital tract infections, the prevalence among females and males was 9.6% and 3.3%, respectively.10 In another study performed among asymptomatic people living with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS), the overall M. genitalium prevalence was 6.1%.11 Mycoplasma genitalium has been associated with HIV acquisition and coinfection in a number of studies.11–14 Investigators have documented that M. genitalium may be an independent risk factor for the acquisition of HIV in women in Zimbabwe and Uganda, respectively.12,13
Mycoplasma genitalium is a fastidious and slow growing STI pathogen, which makes it difficult to culture; therefore, molecular methods have been the predominant means of detecting M. genitalium in clinical specimens.1,15 The use of nucleic acid amplification tests, such as polymerase chain reaction (PCR)16 and transcription-mediated amplification (TMA)17 for the detection of M. genitalium DNA and 16S rRNA targets, respectively, has increased an understanding of the epidemiology of M. genitalium infection. The center for HIV and STIs at the National Institute for Communicable Diseases, in South Africa, has been conducting STI microbiological surveillance in Johannesburg since 2007. We describe trends in the prevalence of M. genitalium infection, and association with HIV infection, in patients presenting with genital discharge syndrome to a primary healthcare facility in Johannesburg, South Africa, spanning a period of 8 years (2007–2014).
MATERIALS AND METHODS
Study Participants
Consecutive patients presenting with male urethral syndrome (MUS) or VDS were enrolled, with written informed consent, as part of annual STI surveys at Alexandra Health Center in Johannesburg between 2007 and 2014. Alexandra Health Center is a community-orientated primary health care facility that provide services, such as HIV, AIDS, and tuberculosis treatment; STI syndromic treatment; and HIV counseling, testing, and prevention services. Demographic data were collected by a nurse-administered questionnaire. STI syndromes were treated according to national syndromic management guidelines.18 All patients were offered on-site HIV voluntary counseling and testing.
Clinical Specimens
Each year between 2007 and 2014, urine and endocervical swab specimens were collected from males and females, respectively. Additionally, a 10-mL whole blood sample was collected from each participant.
Laboratory Methodology
DNA extraction was undertaken with an automated DNA extraction method (X-tractor Gene; QIAGEN, Hilden, Germany). A validated in-house real-time multiplex PCR assay was performed to detect Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, and M. genitalium.19 Those DNA extracts with inconclusive M. genitalium results on the in-house PCR assay, were re-tested using a commercial M. genitalium real-time PCR assay (Sacace Biotechnologies, Como, Italy) targeting the DNA subunit B of M. genitalium.13 Human immunodeficiency virus screening was performed on sera (prepared from whole blood) by the use of two rapid tests, namely the DetermineHIV 1/2 assay (Abbott Laboratories, Japan) and Unigold™ assay (Trinity Biotech PLC, Ireland).
Statistical Analysis
Data were captured onto a study specific Microsoft Access database and exported into Stata 14.2 (Stata Corporation, College Station, TX), for analysis. Descriptive statistics were used to describe the prevalence of STI pathogens in MUS and VDS. The χ2test was used to test for the association of STI pathogens and HIV serostatus. A P value less than 0.05 was considered statistically significant. Univariable and multivariable piecewise logistic regressions with splines at each of the calendar years were used to determine demographic and clinical factors independently associated with M. genitalium infection over the defined period.
RESULTS
A total of 4731 specimens were obtained from patients presenting with genital discharge syndrome in the surveillance period 2007 to 2014. Of these, 2509 (53%) were from males and 2222 (47%) from females. The median age of both males and females at enrolment was 28 years (interquartile range, 24–32 and 23–34, respectively). At enrolment, a significantly higher percentage of females had a history of STI syndrome in the preceding 12 months compared to males (63.7% vs. 29.7%, P < 0.001). None of the demographic and clinical variables studied (ie, age, sex, year of specimen collection, history of previous STI in the preceding 1-year period, history of multiple sexual partners) was significantly associated with M. genitalium infection.
During the 8-year surveillance period, the overall relative prevalence of M. genitalium infections among males and females was 8.9% and 10.6%, respectively (Fig. 1). The highest prevalence in males was observed in the year 2007 (15.1%), and in females in 2013 (13.6%). Trend analysis was performed across all 8 years, which showed a statistically significant decline in the relative prevalence of M. genitalium infection in males (15.1% to 5.3%, P = 0.010). However, no such trend was observed among females in the 8-year period (P = 0.389) (Fig. 1). Our in-house multiplex real-time PCR also tested for other STI pathogens in the genital discharge specimens. In males, N. gonorrhoeae, C. trachomatis, and T. vaginalis were detected in 76.7%, 24.4%, and 5.4% of participants, respectively. The 8-year trend analyses for other STIs revealed a statistically significant upward trend for N. gonorrhoeae (P = 0.024) and C. trachomatis (P = 0.039) and a significant downward trend for T. vaginalis (P = 0.0007) in males. Among females, the prevalence of N. gonorrhoeae, C. trachomatis and T. vaginalis was 13.5%, 16.1%, and 22.7%, respectively (data not shown), with a statistically significant upward and downward trend for C. trachomatis (P = 0.021) and T. vaginalis (P = 0.031), respectively. However, trend analysis for N. gonorrhoeae, revealed an upward trend approaching statistical significance (P = 0.059).
Figure 1.
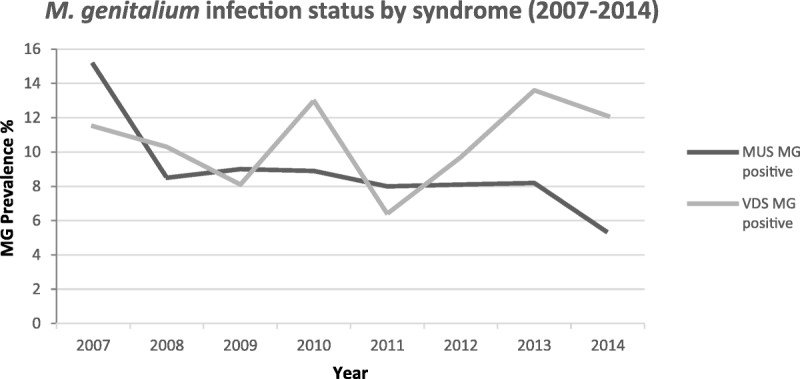
Prevalence of M. genitalium infection detected in symptomatic males (n = 222) and females (n = 231). MG, Mycoplasma genitalium; MUS, Male Urethral Syndrome.
The association of HIV seropositivity and M. genitalium infection status among males and females is depicted in Table 1. Over the 8-year period, in the absence of other STIs, the overall prevalence of HIV in those infected with M. genitalium, was significantly higher than in those without M. genitalium infection (48.9% vs. 40.5%, P = 0.014) (Table 1). A similar trend was observed in the presence of other STIs, whereby, the prevalence of HIV coinfection in those with M. genitalium, was significantly higher than in those without M. genitalium infection (49.6% vs. 40.5%, P = 0.006) (Table 1).
TABLE 1.
Association of HIV Coinfection With M. genitalium Among Males and Females (2007–2014)
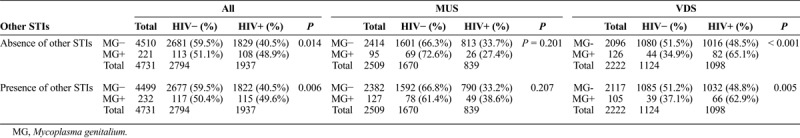
When stratified by gender, there was no significant difference in the prevalence of HIV-coinfection observed in males with or without M. genitalium, either in the presence (38.6% vs. 33.2%, P = 0.207) or absence (27.4% vs. 33.7%, P = 0.201) of other STIs. In females, the prevalence of HIV infection in those coinfected with M. genitalium was significantly higher than in those without M. genitalium infection, regardless of the presence (62.9% vs. 48.8%, P = 0.005) or absence (65.1% vs. 48.5%, P < 0.001) of other STIs (Table 1).
DISCUSSION
This study sought to assess trends in the relative prevalence of M. genitalium infection in a population of adult males and females seeking care at Alexandra primary health care facility in Johannesburg. A population with symptoms of genital discharge was chosen as a preferential STI target population to address the association of HIV infection in those infected with M. genitalium, with or without other STI pathogens. These findings were reviewed in the context of previously published data.
The relative prevalence of M. genitalium among females in our study, was higher than that reported in a study performed in Zimbabwe, where 7.0% of women with symptomatic vaginal discharge were infected with M. genitalium.20 This prevalence is comparable to that found in studies of high-risk women in Uganda (14%),21 Kenya (12.9%–16%),22,23 and the United States (11.3%).24 However, these rates were higher than those found in the general female population of other countries such as Denmark and Australia (3%–4%).4,5 The variation in M. genitalium prevalence across studies may be due to differences in study methods, study populations, specimen sampling methods, and diagnostic assays used.
In our study, the 8.9% M. genitalium prevalence in males was lower than the prevalence of 13.7% in males with visible symptoms of urethritis presenting to a family practitioner in Pretoria, South Africa,9 but similar to the prevalence of 9.0% reported among symptomatic males in Belgium.25 Over the 8-year period, there was a decline in the relative prevalence of M. genitalium from 15.1% to 5.3% in males. This decline may, in part, have been due to a statistical significant increase in the relative prevalence of other STI pathogens such as N. gonorrhoeae (69.3% to 80.1%, P = 0.024) and C. trachomatis (23.4% to 29.6%, P = 0.039), over the 8-year period (data not shown).
In South Africa, the total number of persons living with HIV increased from an estimated 5.09 million in 2007 to 7.52 million by 2018.26 Approximately one-fifth of South African women in the reproductive age group (15–49 years) is HIV positive.26 Our study showed that the prevalence of HIV infection among M. genitalium infected females was higher than the prevalence of HIV in M. genitalium uninfected females regardless of the absence and presence of other STIs. The high prevalence of HIV infection in M. genitalium infected females supports the idea of further research into this association. Infection with M. genitalium has been reported to lead to cervical inflammation, and it is possible that the infection also increases the risk of HIV-acquisition, as is the case in other STIs.27 Other African studies have reported a high prevalence of HIV coinfection in M. genitalium in both high and low risk women.28,29 In our M. genitalium infected cohort, there was no significant difference in the prevalence of HIV among those who were coinfected with other STIs versus those who had no STI coinfection (49.6% and 48.9%, respectively).
Studies of HIV-STI interactions have been conducted mostly on individuals not receiving antiretroviral therapy (ART). Less is known about the impact of STI coinfections on HIV shedding from individuals on ART. The extended life expectancy associated with ART and the potentially higher exposure of the HIV-infected individuals to STIs may increase the prevalence of STI coinfections, particularly with public awareness of the decreased infectiousness of HIV while on ART, leading to risk compensation with a consequential decrease in condom usage.30 A prospective study would be required to better understand the effects of coinfection with M. genitalium on HIV transmission.
Ciprofloxacin and doxycycline were the recommended syndromic treatment options for patients presenting with MUS and VDS, before 2008, but due to the rapid emergence of fluoroquinolone-resistant Neisseria gonorrhoeae within South Africa the treatment regimen was changed in 2008 to cefixime and doxycycline. Currently, the STI Syndromic Management Guidelines (2015) for South Africa incorporate the use of 1 g azithromycin, orally, as a single dose in the treatment of MUS and VDS. Therefore, before 2015, a 7-day doxycycline course was used to provide syndromic cover for M. genitalium infection; and since 2015, single-dose azithromycin therapy has been used. Using the same samples from this article, we have recently conducted a study on the prevalence of macrolide and fluoroquinolone resistance-associated mutations in M. genitalium among symptomatic patients (article in press).
Strengths of the study include the large number of participants enrolled and tracking the prevalence of M. genitalium over an 8-year surveillance period. The major limitation of this study is that patients were only recruited in one public health care center in Johannesburg; hence, the results may not be generalizable to other provinces or populations.
In conclusion, M. genitalium was found to represent an important microbial pathogen among patient presenting with genital discharge syndromes in Johannesburg. The significant association of M. genitalium with HIV among females with VDS calls for further research on the potential role of M. genitalium in the transmission and acquisition of HIV.
Footnotes
Acknowledgments: The authors would like to thank the clinical staff (Alex Vezi and Valencia Kekana) at the Alexandra Health Centre and the data management staff (Gloria de Gita and Dr Tendesayi Kufa-Chakezha) at the Centre for HIV and STIs (CHIVSTI/STI Section) at NICD for their assistance.
Conflicts of Interest: None declared.
Sources of Funding: This study was internally funded by the National Institute for Communicable Diseases (NICD), a division of the National Health Laboratory Service (NHLS).
Ethical approval: This study was granted ethical approval by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (Protocol No. M051024).
REFERENCES
- 1.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: From chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24:498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman T, Yossepowitch O, Samra Z, et al. Prevalence of Mycoplasma genitalium in men with urethritis and in high risk asymptomatic males in Tel Aviv: A prospective study. Int J STD AIDS 2017; 28:127–132. [DOI] [PubMed] [Google Scholar]
- 3.Jensen JS, Cusini M, Gomberg M, et al. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol 2016; 30:1650–1656. [DOI] [PubMed] [Google Scholar]
- 4.Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: A Danish nationwide retrospective survey. Clin Infect Dis 2014; 59:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker J, Fairley CK, Bradshaw CS, et al. Mycoplasma genitalium incidence, organism load, and treatment failure in a cohort of young Australian women. Clin Infect Dis 2013; 56:1094–1100. [DOI] [PubMed] [Google Scholar]
- 6.Baumann L, Cina M, Egli-Gany D, et al. Prevalence of Mycoplasma genitalium in different population groups: Systematic review and meta-analysis. Sex Transm Infect 2018; 94:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hay B, Dubbink JH, Ouburg S, et al. Prevalence and macrolide resistance of Mycoplasma genitalium in South African women. Sex Transm Dis 2015; 42:140–142. [DOI] [PubMed] [Google Scholar]
- 8.Le Roux MC, Ramoncha MR, Adam A, et al. Aetiological agents of urethritis in symptomatic South African men attending a family practice. Int J STD AIDS 2010; 21:477–481. [DOI] [PubMed] [Google Scholar]
- 9.le Roux MC, Hoosen AA. Quantitative real-time polymerase chain reaction for the diagnosis of Mycoplasma genitalium infection in South African men with and without symptoms of urethritis. Sex Transm Dis 2017; 44:17–20. [DOI] [PubMed] [Google Scholar]
- 10.Kaida A, Dietrich JJ, Laher F, et al. A high burden of asymptomatic genital tract infections undermines the syndromic management approach among adolescents and young adults in South Africa: Implications for HIV prevention efforts. BMC Infect Dis 2018; 18:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis DA, Chirwa TF, Msimang VM, et al. Urethritis/cervicitis pathogen prevalence and associated risk factors among asymptomatic HIV-infected patients in South Africa. Sex Transm Dis 2012; 39:531–536. [DOI] [PubMed] [Google Scholar]
- 12.Mavedzenge SN, Van Der Pol B, Weiss HA, et al. The association between Mycoplasma genitalium and HIV-1 acquisition in African women. AIDS 2012; 26:617–624. [DOI] [PubMed] [Google Scholar]
- 13.Vandepitte J, Weiss HA, Bukenya J, et al. Association between Mycoplasma genitalium infection and HIV acquisition among female sex workers in Uganda: Evidence from a nested case-control study. Sex Transm Infect 2014; 90:545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Costa FA, da Silva RC, Arruda LB, et al. Prevalence of Mycoplasma genitalium among HIV-infected men in São Paulo city detected by realtime polymerase chain reaction. Int J STD AIDS 2010; 21:23–25. [DOI] [PubMed] [Google Scholar]
- 15.Munoz JL, Goje OJ. Mycoplasma genitalium: An emerging sexually transmitted infection. Scientifica (Cairo) 2016; 2016:7537318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen JS, Bjornelius E, Dohn B, et al. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol 2004; 42:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munson E, Bykowski H, Munson KL, et al. Clinical laboratory assessment of Mycoplasma genitalium transcription-mediated amplification using primary female urogenital specimens. J Clin Microbiol 2016; 54:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The National Department of Health. Essential drug list and standard treatment guidelines—primary health care (PHC), 2nd ed. Pretoria, South Africa: The National Department of Health, 2003. [Google Scholar]
- 19.Mhlongo S, Magooa P, Muller EE, et al. Etiology and STI/HIV coinfections among patients with urethral and vaginal discharge syndromes in South Africa. Sex Transm Dis 2010; 37:566–570. [DOI] [PubMed] [Google Scholar]
- 20.Chirenje ZM, Dhibi N, Handsfield HH, et al. The Etiology of vaginal discharge syndrome in Zimbabwe: Results from the Zimbabwe STI Etiology study. Sex Transm Dis 2018; 45:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandepitte J, Weiss HA, Kyakuwa N, et al. Natural history of Mycoplasma genitalium infection in a cohort of female sex workers in Kampala, Uganda. Sex Transm Dis 2013; 40:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomih-Alakija A, Ting J, Mugo N, et al. Clinical characteristics associated with Mycoplasma genitalium among female sex workers in Nairobi, Kenya. J Clin Microbiol 2014; 52:3660–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lokken EM, Balkus JE, Kiarie J, et al. Association of recent bacterial vaginosis with acquisition of Mycoplasma genitalium. Am J Epidemiol 2017; 186:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balkus JE, Manhart LE, Jensen JS, et al. Mycoplasma genitalium infection in Kenyan and US women. Sex Transm Dis 2018; 45:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libois A, Hallin M, Crucitti T, et al. Prevalence of Mycoplasma genitalium in men with urethritis in a large public hospital in Brussels, Belgium: An observational, cross-sectional study. PLoS One 2018; 13:e0196217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.StatsSA. Mid-year population estimates: P0302. Available from: http://www.statssa.gov.za/. Accessed 23 July 2018.
- 27.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999; 75:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen CR, Nosek M, Meier A, et al. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis 2007; 34:274–279. [DOI] [PubMed] [Google Scholar]
- 29.Manhart LE, Mostad SB, Baeten JM, et al. High Mycoplasma genitalium organism burden is associated with shedding of HIV-1 DNA from the cervix. J Infect Dis 2008; 197:733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: Systematic review with implications for using HIV treatments for prevention. Sex Transm Infect 2011; 87:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]


