Abstract
Lymphatic filariasis (LF) is targeted for elimination by the year 2020. The Global Programme for Elimination of LF (GPELF) aims to achieve elimination by interrupting transmission through annual mass drug administration (MDA) of albendazole with ivermectin or diethylcarbamazine. The program has successfully eliminated the disease in 11 of the 72 endemic countries, putting in enormous efforts on systematic planning and implementation of the strategy. Mapping areas endemic for LF is a pre-requisite for implementing MDA, monitoring and evaluation are the components of programme implementation. This review was undertaken to assess how the mapping and impact monitoring activities have evolved to become more robust over the years and steered the LF elimination programme towards its goal. The findings showed that the WHO recommended mapping strategy aided 17 countries to delimit, plan and implement MDA in only those areas endemic for LF thereby saving resources. Availability of serological tools for detecting infection in humans (antigen/antibody assays) and molecular xenomonitoring (MX) in vectors greatly facilitated programme monitoring and evaluation in endemic countries. Results of this review are discussed on how these existing mapping and monitoring procedures can be used for re-mapping of unsurveyed and uncertain areas to ensure there is no resurgence during post-MDA surveillance. Further the appropriateness of the tests (Microfilaria (Mf)/antigenemia (Ag)/antibody(Ab) surveys in humans or MX of vectors for infection) used currently for post-MDA surveillance and their role in the development of a monitoring and evaluation strategy for the recently WHO recommended triple drug regimen in MDA for accelerated LF elimination are discussed.
Keywords: lymphatic filariasis, mass drug administration, mapping, monitoring, elimination, xenomonitoring, TAS (transmission-assessment survey)
Introduction
Lymphatic filariasis (LF) is one of the neglected tropical diseases (NTD) caused by three species of filarial worm: Wuchereria bancrofti, Brugia malayi, and Brugia timori. As of 1997, the disease was prevalent in 72 tropical and sub-tropical countries of the world.1 In 1997, the World Health Assembly2 declared elimination of LF as a public health problem. Subsequently, the World Health Organization (WHO) launched the Global Programme to Eliminate Lymphatic Filariasis (GPELF) in 2000 to achieve elimination by 2020.3 The elimination program has two components 1) interruption of transmission; and 2) morbidity management and disability prevention. For interruption of transmission, the strategy is annual single dose mass drug administration (MDA) of albendazole in combination with diethylcarbamazine (DEC) or ivermectin (IVM) to the LF endemic population. The program encompasses the following sequence of steps:4 1) map LF endemic areas (>1% of antigenemia (Ag), among 50–100 individuals (>15 years), from two communities of an endemic district);5 2) deliver MDA for a minimum of 5 years with an effective coverage of 65%; 3) conduct a transmission-assessment survey (TAS);4 4) conduct post-MDA surveillance; 5) develop a dossier that documents the achievement of elimination targets; and 6) independent validation of the claim that elimination criteria have been achieved.
Mapping the areas endemic for LF is the pre-requisite for the countries to plan and implement the elimination program. The next step is to implement MDA in endemic areas for a minimum of 5 years with an effective coverage of 65%. The program is to be monitored every 6 months post-MDA by assessing filarial infection (microfilaria [Mf]/Ag) in sentinel and spot check sites. If the prevalence of Mf in all the sentinel and in the randomly selected spot check sites are <1%, TAS is conducted to decide on stopping or continuing MDA. The TAS’s decision to stop or continue MDA is based on a pre-determined number of Ag positives below which transmission is expected to be interrupted and hence MDA can be stopped, if not MDA is continued for another two rounds. TAS is repeated twice at 2–3 year intervals (post-MDA surveillance) to assess if transmission interruption is sustained after cessation of MDA following TAS 1. After successfully passing all the three TASs, a dossier is submitted to the WHO, which validates the claim of elimination made by the country and once validated, WHO acknowledges LF elimination in that country. As of 2017, 51 of the 72 LF endemic countries have fully implemented MDA, and WHO acknowledged after post-MDA validation in 11 countries, that LF is no longer a public health problem.6
As GPELF is nearing its end game in many endemic countries it would be useful to do a comprehensive assessment of the mapping and monitoring activities used so far and to see how they been improvised to help in upscaling and assessing the impact of the program. Such a review would also be helpful to provide an insight on the methods that could be used in future for 1) remapping areas that are unsurveyed and not under MDA and areas bordering endemic districts; and 2) in identifying the appropriate sensitive tool that would detect ongoing transmission during post-MDA surveillance or validation phase. With this background, a systematic review of the literature on use of mapping and monitoring methods for LF elimination is undertaken.
Search criteria
All published articles in national and international peer-reviewed journals were searched. Searches were made in PUBMED MEDLINE database with key words like “lymphatic filariasis” in combination with “monitoring,” “mapping,” “infection,” “disease,” “MDA,” “antigeneamia,” “antibody,” “ICT,” “FTS,” “Wb123," “Mf” (or “microfilaria”), “vector infection,” and “xenomonitoring” using Boolean operators like “and” and “or.” Attempts were made to download the full articles and the back reference lists in these articles were searched for additional studies.
Eligibility criteria
For a study to be eligible for the review it should either have carried out mapping or monitored/measured impact evaluation with respect to MDA or both in terms of filarial infection (Mf/Ag/Ab) in humans or disease in humans or infection in vectors.
Results
The studies in this review were from the regions of Africa, Americas, Western Pacific, East Mediterranean and South-east Asia (as specified by WHO). The countries included from the African region were Benin, Botswana, Burkina Faso, Cameroon, Central African Republic, Côte d’Ivoire, Democratic Republic of the Congo, Ethiopia, Ghana, Malawi, Mozambique, Nigeria, Republic of the Congo, Rwanda, Senegal, Seychelles, Sierra Leone, Togo, Uganda, United Republic of Tanzania and Zambia; the studies in the American region were from Brazil and Haiti; the Western Pacific included studies from Papua New Guinea, Samoa, and American Samoa; Egypt and Sudan were the two countries from the East Mediterranean. The countries included from South East Asia were Bangladesh, India, Indonesia, Myanmar, Nepal, Sri Lanka, and Thailand. While in the African and East Mediterranean countries, where bancroftian filariasis is co-endemic with onchocerciasis (oncho), ivermectin plus albendazole were used for MDA, in the rest of the regions, where bancroftian filariasis is the only predominant form, DEC plus albendazole are being used.
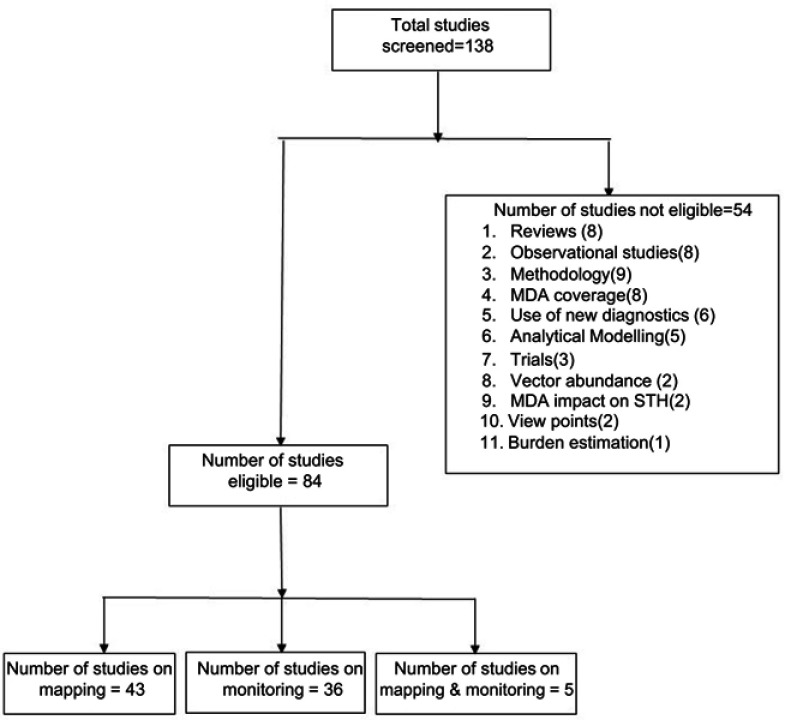
Up until 2018, a total of 138 papers had been published on mapping, and or monitoring of LF. Of these, 541,7–59 were not eligible (as per the inclusion criteria) and therefore were excluded from subsequent review (Figure 1). A total of 84 studies were eligible for this review. Of these, 43 were on mapping alone, 36 on monitoring alone and five both on mapping and monitoring aspects of LF.
Figure 1.
Selection of studies for the review.
Abbreviations: MDA, Mass Drug Administration; STH, Soil transmitted helminths.
The results of this review on mapping and monitoring of LF are presented in accordance with different phases of the program (pre-MDA, during MDA and post-MDA). While one would expect mapping to be undertaken mostly prior to implementation of MDA, it was observed that mapping was also done in the during- and post-MDA phases. As for monitoring, the studies included in this review assessed only the impact of MDA and not on coverage or compliance aspects of MDA.
Mapping
The majority (86%; 37/43) of the studies on mapping were conducted prior to implementation of MDA, mainly to delimit areas that required MDA. Of these 37 studies, 23 (62%) were from Africa, 5 (14%) from South East Asia, 6(16%) from the Americas and 3 (8%) from the Eastern Mediterranean region.
The WHO mapping guidelines5 for LF endemic areas (preferably an implementation unit (IU)/district) recommended a two stage sampling method of examining 50–100 individuals aged >15 years (conveniently sampled) for filarial antigen from two communities purposively selected from the IU/district. If the Ag prevalence is found to be ≥1%, the IU/district is considered for MDA.
Pre-MDA
The details of the pre-MDA mapping studies that were carried out to assess if MDA is required are given in Table 1. The review showed that most African countries used the WHO recommended mapping method for delimiting areas requiring MDA.60–68 The surveys were conducted among adults and or children aged 5–15 years across the studies spanning over countries. In addition to these surveys, a few studies used Mf surveys (current and historic data), key informant techniques69-78 for assessing LF endemicity. In four studies, Mf, Ag and or disease surveys were used to map areas requiring MDA.79–82
Table 1.
Details of the studies on mapping prior to MDA
| Study no. | WHO Region | Country | Author & year | Study area (no. of communities) | No. of districts requiring MDA | No. examined | Indicator | Age (years) | Findings |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Africa | Benin, Burkina Faso, Ghana and Togo | Gyapong et al 200260 | 401 rural or semi rural communities representing IU from these four countries. | Not mentioned | 20,050 persons | Ag prevalence | ≥15 | Spatial analysis with RAGFIL data were tested in the four countries Benin, Bukina Faso, Ghana and Togo. The models could closely predict the Mf prevalences at all sampled points, with low or zero prevalence for Togo, Benin and eastern Ghana. This study provided the information that LF was more widely spread than it was thought to be earlier |
| 2 | South East Asia | Nepal | Sherchand et al, 200361 | 37 districts of 57 endemic districts | 33 out of 37 districts found endemic and require MDA | 4,488 persons | Mf, Ag and disease prevalence | ≥15 | Mapping was carried out in 37 districts in 2001 Results showed that 89% (33/37) of the districts were endemic and 11 had an Ag prevalence of more than 20%. The study also suggested the cross-benefits of different national intervention programs, particularly against TB and leprosy. |
| 3 | Americas | Haiti | Madsen V.E Beau de rochars 200462 | Whole country (133 communes or districts) | 117 of 133 (87.9%) required MDA | 22,365 children | Ag prevalence | 6-11- | The study was carried out to see the geographical distribution of LF in Haiti using the Ag survey among children aged 6–11 years among 113 communes of the country in 2001 (Rochars et al, 2004). The results when put across on maps indicated that LF was more widespread and suggested that the entire country will have to be considered for LF elimination programme. |
| 4 | Africa | Malawi | Ngwira et al, 200763 | 35 villages of 23 districts | 29 villages required MDA | 2,913 persons | Ag prevalence | ≥15 | A national map was developed which incorporates data from surveys in Karonga, Chikwawa and Nsanje districts, carried out in 2000. There is a marked decline in prevalence with increasing altitude. Further analysis revealed a strong negative correlation (R2=0.7 p<0.001) between altitude and prevalence. These results suggest that the lake shore, Phalombe plain and the lower Shire valley will be priority areas for the Malawi LF elimination programme. Implications of these findings as regards implementing a national LF elimination program in Malawi are discussed. |
| 5 | Africa | Rwanda | Ruberanziza et al, 200964 | Five districts | No MDA required | 797 persons | Ag prevalence | >15 | A rapid community-based mapping of LF was carried out in five districts of Rwanda by Ag surveys showed that only one was positive was LF and therefore it was declared that LF was not a public health problem in Rwanda |
| 6 | East Mediterranean | Sudan | Sturrock et al, 200965 | 43 villages of Northern Bahr-el Ghazal State | As per results, MDA is not required | Not mentioned | Ag prevalence | All ages | In a total of 43 villages in state, Ag surveys were carried out. This study used an integrated survey design for NTDs (STH, LF, schistosomiasis) to guide their control programme in the large area of Sudan. This approach was proved to be practical and simplified by reducing the survey work and costs. The exercise provided evidence that none of the areas needed MDA, confirming the cost-effectiveness of integrated mapping methods. |
| 7 | Africa | Nigeria | Iboh et al, 201266 | 4 communities, Yakkurr Govt | 4 communities required MDA | 785 persons | Ag and disease prevalence | All ages | Yakurr people of Cross River state of Nigeria living in four communities were tested for MF and LF disease. It was found that Mf and disease prevalence were 6.1 and 0.3%. These results were used to by the Government to expand the distribution of albendazole in the LF endemic region |
| 8 | Africa | Zambia | Shawa et al, 201367 | Luangwa district | Prevalence was 8.6%, require MDA | 546 persons | Mf,Ag and Ab prevalence | ≥1 | A pre-control epidemiological study for assessing the prevalence of infection (both Ag/MF), disease, and transmission and human perception aspects of LF. Results drug administration be initiated to accelerate this positive trend of decline in LF transmission in the area |
| 9 | Africa | Zambia | Mwase et al, 201468 | 15 districts (14 endemic and 1 non-endemic) | 64 of 108 study sites required MDA | 9964 persons | Ag prevalence | All ages | This study carried out Ag surveys, collected remote sensing data and did ecological niche modelling for filarial vectors distribution in the study area. Integrating all the above, it was shown that for areas with Ag prevalence ≥5% and those with ≥15%, land cover and land surface temperature respectively were significant predictors. The maps produced based on these indicated widespread occurrence of LF in Zambia, and the Ministry of Health in Zambia initiated mass drug administration iin late 2012, and scaled up this activity across the country in the next few years. |
| 10 | Africa | Ethiopia | Rebello et al, 201569 | 658 districts with 1,315 communities | 75 of 658 districts with 89 communities require MDA | 1,30,166 persons | Ag prevalence | ≥15 | These are results of the integrated mapping of LF and Podoconiosis in seven regional states and two cities. Of the 658 districts surveyed, 75 were endemic for LF. Including the previous data on LF endemicity, a total of 112 districts require MDA |
| 11 | South East Asia | India | Sabesan et al, 200070 | 289 districts studied | 257 of 289 found endemic and require MDA | Not mentioned | Mf and disease prevalence | All ages | Data from published studies on 289 districts was analyzed and showed that LF was endemic in 257 districts of 289 in India, |
| 12 | Africa | Nigeria | Awolola et al, 200471 | Akinyele local government area | Key informant method | 95 KIs | Filarial disease | >15 | Results suggest that the data from key informants on filarial disease cases is a rapid method to delimit areas with filariasis |
| 13 | South East Asia | India | Chhotray et al, 200572 | 24 villages from 2 districts of Orissa | All 110 villages found endemic and require MDA | 7304 persons | Mf and disease prevalence | All ages | This was a baseline study in 24,110 villages that were surveyed for Ag and Mf in Puri/Ganjam districts and concluded that LF was widely distributed in the coastal districts of Orissa. |
| 14 | South East Asia | Bihar | Das et al 200673 | 2 villages of Patna distirct | MDA is required in 2 villages | 1872 persons | Mf and disease prevalence | All ages | Results showed that both villages were highly endemic with an Mf rate of 8.4% and a disease rate of 12%. Vector infection and infectivity rates were 14% and 8.2% respectively indicating on-going tranmsission in the study villages and that MDA is necessary. |
| 15 | South East Asia | India | Singh et al, 200674 | 7 villages of Patna district | All 7 villages found endemic and require MDA | 1878 persons | Mf prevalence | All ages | A baseline epidemiological study in rural areas of Patna district during pre-MDA period. A total of 7 villages were surveyed in from Patna district. Results showed that while Mf prevalnce was 6.2%, there was no infection in the vectors |
| 16 | Americas | Brazil | Bonfim et al, 2009a75 | Municipality of Jaboatão dos Guararapes, | Not mentioned | 23,673 persons | Mf prevalence | ≥1 | The Social deprivation index was useful in quantifying social inequalities thereby help in planning intervention. The strata constructed based on the index was helpful in indicating a risk gradient, with 74.9% of the microfilaremia cases situated in the high-risk stratum. |
| 17 | Americas | Brazil | Bonfim et al, 2009b76 | Municipality of Jaboata ˜o dos Guararapes, | Not mentioned | 23,673 persons | Mf prevalence | ≥1 | The socio-environmental composite risk index (SRI) was useful in mapping areas with higher risk of infection. As SRI could stratify spaces by using available official data, it is considered an important tool for use in the worldwide LF elimination program |
| 18 | Africa | Congo | Hope et al, 201177 | 10 provinces and six cities | Not mentioned | Not mentioned | Mf prevalence | All ages | As the country is co-endemic for both loiasis and Onchocerciasis, a new mapping approach termed as Micro-stratification Overlap Mapping (MOM) is proposed prior planning MDA for LF . The authors reproduced the map with historical data of LF, overlapped with the maps of loiasis and onchocerciasis and provided key information about the ecology and transmission of W. bancroftivectors in DRC. These maps were a useful resource for national LF programme in countries with co-endemicity as they provide information on areas with risk of serious adverse events and requires extra precautions or alternative intervention strategies |
| 19 | Americas | Brazil | Brandao et al, 201178 | 24 districts- 484 census tracts | 13 of 24 districts were hyperendemic and require MDA | 8670 children | Mf prevalence | <=18 | Mapping of filarial infection (Mf) and morbidity among children/adolescents was carried out prior to implementation of MDA. Results showed that transmission was intense among the pediatric population in 54% of the surveyed districts .Spatial analysis showed that the localities in which the populations most exposed to filarial transmission were concentrated. |
| 20 | Americas | Brazil | Brandão et al, 201579 | Municipality of Jaboata ˜o dos Guararapes, State of Pernambuco | Not applicable | 8670 children | Mf prevalence | <=18 | The results on the survey on children and adolescents in combination with SRI (Bonfim et al, 2009) showed that the localities where the children most exposed to filarial transmission are concentrated. This index precisely measured the relationship between social deprivation and the prevalence of infection among children and cna be used in control and elimination activities. |
| 21 | Africa | Sierra Leone | Koroma et al, 201280 | 14 health districts | 14 districts require MDA | 1982 persons | Mf and Ag prevalence | ≥15 | Ag (ICT) survey was carried out in 14 health districts showed LF was endemic nationwide and that preventive chemotherapy was justified across the country. These data provided information for the NTDCP to design and implement MDA and the basis for future monitoring and evaluation of the national LF elimination programme. |
| 22 | Africa | Ethiopia | Shiferaw et al 201181 | 125 villages of 112 districts | 34 of 112 districts require MDA | 11,685 persons | Ag and disease prevalence | All ages | Though the overall Ag prevalence was 3.7%, its distribution was found to be heterogeneous in these villages. Of these 112 districts 34 had prevalence rates more than 5% (Range: 4–20%) and these data were used to provide a tentative map of LF distribution in the study area. |
| 23 | Africa | Nigeria | Okorie et al, 201382 | 134 sites | Not applicable | 55,026 persons | Mf and Ag prevalence | All ages | This review collated and mapped all LF data in Nigeria, to assess the extent of co-endemicity with loasis and determine the risk and benefits of different intervention strategies. It is suggested that integrating LF activities with that of STH and distributions of ITN/LLINs may have significant impact on both loasis and f LF. |
| 24 | Africa | Cameroon | Djeunga et al,201583 | 120 districts | 106 of 120 (88.3%) require MDA | 26,586 persons | Mf and Ag prevalence | ≥5 | With a purpose of providing a quick and easy estimate on filarial endemicity status of the 90% of health districts in Cameroon and to obtain a country-wide map on LF, Ag survey was carried out in 120 health districts showed that 88% were eligible for MDA. (Ag prevalence:3.3% (95% CI: 3.0–3.7%), |
| 25 | Africa | Malawi | Ngwira et al, 200284 | 12 villages, Shire valley, Songwe river | 12 study villags were require MDA | 685 adults | Ag prevalence | ≥20 | Mapping of LF areas was carried out using Ag surveys in Karonga, Chikwawa and Nsanje districts. Results suggest that the lake shore, Phalombe plain and the lower Shire valley were found to be the priority areas for the Malawi LF elimination programme. The map so created showed that infection with W. bancrofti was more widespread than previously appreciated. |
| 26 | Africa | Uganda | Onapa et al, 200585 | 45 districts | 19 of 76 sites require MDA | 17,533 children | Ag prevalence | 5–19 | An Ag survey was carried out among school children (5–19 years) in 15 districts. The study suggested that screening of school children for Ag was a simpler and useful approach to mapping the geographical distribution of LF. |
| 27 | Eastern Mediterranean | Egypt | Hassan et al, 199886 | Nile delta area covering 201 villages of 11 district | Not mentioned | Not mentioned | Mf prevalence | All ages | The first study on spatial analysis that looked into clustering of Mf prevalences (up to 2 km) at community level and showed heterogeneous pattern in filariasis transmission.Correlation between low Mf prevalence and higher humidity, low temperature and low rainfall were significant. |
| 28 | Africa | Africa | Gyapong et al 200187 | 87 communities selected using 25x25km grid sampling method; 30 communities of these were at 50x50km grids | Not mentioned | Not mentioned | Ag prevalence | >15 | A spatial sampling grid of 50km interval between villages was used for sampling villages for rapid assessment of filariasis endemicity using Ag. This approach of mapping was recommended to capture the cross-border foci, which was found to exist. It was suggested that this method would provide the information for effective treatment planning. |
| 29 | Americas | Brazil | Medeiros et al, 201288 | 27 districts of Jaboatao dos Guararapes Municipality | 24 districts require MDA | 23,673 children/adolescents | Mf prevalence | 1–18 | The data collected in an earlier study (Brandao et al, 2011), was used to identify the areas with high infection foci, applying kernel density approach on household level data. This method rapidly detected areas with highest concentration of infected cases and assisted the programme towards planning, monitoring, and surveillance of filariasis elimination activities. Infection in children/adolescents in combination with SRI showed that the localities where the children most exposed to filarial transmission were clustered and precisely measured the relationship between social deprivation and the prevalence of infection among children. |
| 30 | Africa | Ethiopia | Sime et al, 201489 | 659 districts with 1,315 communities | 75 of 659 districts require MDA | 1,29,959 persons | Ag prevalence | >=15 | The two studies demonstrated that an integrated nationwide mapping of podoconiosis and LF in 659 districts with 1315 communities showed that this approach was feasible, cost effective and expanded geographical coverage and rapidly made available the data for decision makers. |
| 31 | East Mediterranean | Sudan | Finn et al, 201290 | 14 counties from 2 states | 11 out of 14 counties require MDA | 3,980 persons | Mf prevalence | >=16 | This one is the extension of the integrated mapping exercise (Sturrock et al, 2009) to three states of Sudan. In this study, three of the NTDs schistosomiasis, loasis and LF were mapped at county level. One to three sites with LF disease cases within the counties were sampled purposively and 250 individuals ≥16 years were examined for Ag(ICT). Results indicated only in two states the prevalence of Ag was >2% and these were identified for MDA. |
| 32 | Africa | Uganda | Stensgaard et al, 201191 | Primary schools all over the country | Not mentioned | 17,533 children | Ag prevalence | 5-19 | A geo-statistical model was applied on the data collected in an earlier study (Onap et al, 2005) and predicted the LF and malaria prevalence in unsurveyed locations and determined the extent of geographical overlap of the two diseases. The model predicted areas with hyper-endemic W. bancrofti transmission and this was acclaimed to provide a better informed platform for integrated control by the health authorities of Uganda |
| 33 | Africa | Africa | Slater et al, 201292 | Africa | Not mentioned | Not mentioned | Not available | Not mentioned | Combining correlative spatial modelling approaches with mechanistic models linking climate envrionmental /population to parasite transmission provide a useful solution of improving spatial predictions |
| 34 | Africa | Africa | Slater et al.201393 | Africa | Not mentioned | Not mentioned | Not available | Not mentioned | A generalized linear spatial model fitted to the data on infection from published articles on Lf infection in Africa showed that the predicted LF prevalence to be highly heterogenous across Africa |
| 35 | Africa | Sub-saharan Africa | Moraga et al, 201594 | Eastern, Middle, Northern and Western | Not applicable | Not mentioned | Ag prevalence | All ages | Mapping of LF was carried by predicting LF prevalence by integrated application of geostatistical and mathematical models. Mf data from 1,145 surveys conducted between 1950 and 2000 and Ag data from the Ag surveys conducted in from 1990 to 2000 were used for model building and predictions. The predictions showed that LF transmission is highly heterogeneous and the maps are expected to guide intervention, monitoring and surveillance strategies as countries progress towards LF elimination. |
| 36 | Africa | Ethiopia, Tanzania | Gass et al, 201795 | Ethiopia-45 districts, Tanzania-11 districts |
Three of 45 districts in ethiopiarequire MDA | 40,868 children | Ag prevalence | 9–14 | A new tool for confirmatory mapping developed of LF and validated it against the mapping tool by WHO in 45 districts of Ethiopia and 10 MDA naïve districts of Tanzania. By avoiding unnecessary MDA in 52 districts, the confirmatory mapping strategy is estimated to have saved a total of $9,293,219. Particularly in low prevalence setting, this new tool was shown to have the potential to save time, money, resources and avoid unnecessary treatments. With the 2020 elimination targets on the horizon, the confirmatory mapping tool may prove to be particularly useful for “shrinking the map“ and conserving resources for use in areas where they are needed most. |
| 37 | Africa | Ethiopia | Sime et al, 201596 | 45 districts | 3 districts require MDA | 18,254 persons | Ag prevalence | 9–14 |
Abbreviations: LF, Lymphatic filariasis; DEC, Diethylcarbamazine; IVM, Ivermectin; Ag, Antigeneamia; Mf, Microfilaraemia; Ab, Antibody; MDA, Mass drug administration; ICT, Immunochromatographic Test; EU, Evaluation unit.
In Rwanda, mapping showed that there was only one Ag positive case in all five districts that were surveyed and this helped in declaring that LF was not a public health problem and therefore MDA was deferred thereby saving money and other humane efforts.63
In the countries of the African region, starting with rapid assessment procedures (Ag survey and key informant interviews on LF symptoms) for delimiting areas endemic for LF, the progress made on mapping of LF has been tremendous. A few studies mapped the distribution of LF61,62,66,83,84 using Mf and or Ag surveys and showed that the LF was more widely spread than it was previously thought in those countries. Few studies went further to carry out spatial analysis of the filarial prevalence data collected for mapping through a spatial sampling grid with 50 km distance between sampled locations and predicted LF prevalence at 25 km distance.60,77,85–87
Studies that carried out mapping of LF in Oncho co-endemic area helped the program to produce LF distribution maps80,82 with areas having Ag rates >1% and recommended inclusion of albendazole with the monotherapy of ivermectin to aid elimination of LF in these areas.65 Subsequently, as the countries in the African region were known to be co-endemic for NTDs like loasis, soil transmitted helminthic diseases, schistosomiasis and podoconiosis, attempts were made to map all these under one roof and this paved the way for an integrated mapping approach to understand the extent of distribution of diseases, and implementing control programs. Integrated mapping of LF with podoconiosis led to efficient use of resources and helped in rapidly delimiting large geographical areas. This approach was popular among the health staff in Ethiopia.68,88 A large NTD survey integrating mapping for LF, schistosomiasis and STH in three states of Sudan89 established that an estimated population of 1.2 and 1.4 million individuals were eligible for regular MDA with preventive chemotherapy (PCT) to treat STH and schistosomiasis respectively, and that 1.3 million individuals residing in Central- and Eastern Equatoria required MDA for LF. This integrated mapping approach cut costs and saved time with the same man-power efforts.64 Yet another approach called Micro stratification overlapped mapping (MOM) in Congo76 helped the program authorities to take extra precautions in handling serious adverse events that may take place due to co-endemicity of LF and loasis. In Nigeria, a similar approach using historical data helped in producing a series of maps to assist in maximizing existing interventions, cost effective usage of resources as the LF elimination programme scaled up.81
Countries such as Ghana, Uganda and India used the historical data (Mf, Ag), for predicting LF prevalence in the uncertain and un-surveyed areas using geo-statistical models67,69,90–93 and showed the heterogeneous distribution of LF for the respective countries, indicating the possibility on the risk of occurrence of LF even in the probably non-endemic areas. Three studies from India used Mf prevalence to identify districts for MDA.71–73 Mapping data along with social deprivation index (SDI) and socio-environmental composite risk index (SRI) were used in Haiti to specifically map LF risk in urban areas74,75,78 and to see clustering of infection. Two studies tested a confirmatory mapping tool against the WHO recommended mapping strategy mainly to assess whether those areas in Ethiopia and Tanzania94,95 with uncertain endemicity (one Ag positive among 200 adults; 45 in Ethiopia and 11 in Tanzania) needed to be included for MDA. This tool tested a sample of 320 children aged 9–14 years, from 30 schools selected based on probability proportional to estimated population size in a Woreda (a district) to find areas requiring MDA. The results showed that while only 3 out of 45 in Ethiopia and none of the 11 from Tanzania required MDA, thereby saving time and the resources by preventing MDA in areas where there was no evidence of transmission.
During MDA
Mapping during MDA (Table 2) was useful in re-assessing the geographic limits of LF and identifying new LF endemic areas that were either not surveyed, or classified as uncertain or non-endemic earlier.96–98 It was observed that in India, 113 of the 190 districts, which were not surveyed earlier and not included for MDA, were identified to be having varying risk of transmission. As the elimination goal is nearing, the National Vector-Borne Disease Control Programme (NVBDCP) is in the process of validating these findings before bringing these districts under MDA net. Mf and entomological surveys conducted in the sentinel villages of the Gampha district of Sri Lanka after 5 rounds of MDA showed ongoing transmission99 and recommended continuation of further rounds of MDA. In another study in Malawi that had already undergone 5 rounds of MDA for LF elimination is co-endemic for oncho and malaria, it was felt that the intervention programmes against these diseases could have impacted the transmission of LF. Therefore, to identify the high-risk areas of LF, a multiple intervention score map (MISM), that is based on weighted sum of individual intervention scores was used. This score was useful in identifying areas with high and low coverage of LF impacting interventions. It was shown that those areas with low coverage with high baseline LF prevalence were identified and considered to be with high risk of transmission or re-emergence.100 Another study101 attempted morbidity mapping to estimate lymphedema burden in Malawi after 5 rounds of MDA, observed that as there was no standardized method for collection of morbidity data, the morbidity burden due to LF could be grossly underestimated.
Table 2.
Details of the studies on mapping LF during and post-MDA
| Study no. | Region | Country | Author & year | Study area (no. of communities) | Indicator | Age (years) | Findings |
|---|---|---|---|---|---|---|---|
| 1 | Africa | Ghana | Gyapong et al, 200197 | 87 communities | Ag and hydrocele prevalence | ≥15 | The grid sampling methodology with Ag prevalence was a rapid method to assess the distribution of filariasis. Variogram analysis showed the presence of spatial dependency among the Mf prevalences of communities spaced at 25 km and 50 km grids. Prevalence contours predicted the infection prevalence in areas where data was not collected. |
| 2 | South East Asia | Nepal | Dhimal et al, 201198 | Five administrative areas | MX | Not relevant | This study was carried out in endemic districts that had completed at least five rounds of MDA to define the geographical limits of the endemic zone of LF in high mountain areas. Results showed that LF cases were found in higher altitudes up to 1800 m asl and LF vectors at 2100 m asl. The study recommends MX to rule out any transmission at this altitude. |
| 3 | South East Asia | India | Chand et al, 201699 | 12 villages from four districts of Madhya Pradesh | Mf and vector infection prevalence | All ages | In India where MDA is currently on-going, Mf and mosquito surveys were carried out in the district considered non-endemic and not under MDA. Mf prevalence varied between 3.2 and 11.2%; vector infection rate: 2–13% and infectivity rate: 1.3–3.6%. The authors suggest accurate mapping of areas is essential to initiate MDA in the bordering areas of filarial endemic districts and areas as population migration may be high. |
| 4 | South East Asia | Sri lanka | Wijegunawardana et al, 2012100 | One district, Gampaha | Mf and vector infection prevalence (MX) | >3 years | The study assessed the current status of LF after five rounds of MDA in two sentinel and one spot check site using GIS. While the Mf prevalence ranged between 0.5% and 3.4% in sentinel sites, vector infection by xenomonitoring ranged between 0 and 32.4%, indicating that transmission is on-going and therefore may need to intensify further rounds of MDA. |
| 5 | Africa | Malawi | Stanton et al, 2014101 | Six LF-Oncho co-endemic districs | Ag prevalence | All ages | This study quantified the geographical extent of LF (MDA), Oncho (MDA), Bednets and IRS interventions impacting LF and produced a multiple intervention score map(MISM–weighted sum of individual intervention score). Districts were classified into four groups based on baseline LF prevalence and MISM. High coverage areas included the LF-onchocerciasis endemic areas in the southern region of the country and areas along the shores of Lake Malawi, where malaria vector control had been prioritised. Three districts with high baseline LF prevalence measures but low coverage of multiple interventions were identified and considered to be most at risk of ongoing transmission or re-emergence. In this analysis, six districts were identified as priority districts for additional interventions, with a further five being identified as having low prevalence and high intervention coverage, suggesting that they should be prioritised for post-MDA surveillance and move to the elimination phase |
| 6 | Africa | Malawi | Smith et al, 2014102 | Chikwawa district hospital catchment area | Chronic symptoms (lymphoedema) | 22–90 | Mapping of LF disease cases was done after five rounds of MDA showed that lymphoedema cases were nearer Shire river. Authors suggest that there is a need to develop new LF morbidity identification and surveillance approaches to ensure that morbidity management strategies are effectively targeted. |
Abbreviations: LF, Lymphatic filariasis; DEC, Diethylcarbamazine; IVM, Ivermectin; Ag, Antigeneamia; Mf, Microfilaraemia; Ab, Antibody; MDA, Mass drug administration; ICT, Immunochromatographic Test; MX, Molecular Xenomontoring.
Monitoring
A total of 36 studies have monitored filarial infection during the period of MDA implementation and also during post-MDA surveillance. Of them, 19 were during MDA implementation, 15 during post-MDA, one study reported monitoring of both pre-MDA and during MDA, and another study about monitoring in all the three phases, (pre, during and post MDA) of elimination programme.
During MDA
Of the 19 studies conducted during MDA phase, 6, 7, 3, 2 and 1 were from African, Southeast Asian, Western Pacific, Americas and eastern Mediterranean regions respectively (Table 3). In eleven studies, the impact of MDA had been measured in terms of reduction in Mf Ag or Ab or filarial disease prevalence102-112 in humans. Seven studies, two from India,113,114 two from Tanzania,115,116 one each from Haiti,117 Nigeria,118 Samoa,119 and Egypt120 monitored infection (xenomonitoring/dissection) in vectors in addition to infection in humans (MF/Ag/Ab).
Table 3.
Details of the studies in which monitoring was done during MDA
| Study no. | Region | Country | Author & year | No. of districts or villages or sites | No. of people tested | Age (years) | Indicator | Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Americas | Haiti | Hochberg et al, 2006103 | Five sites | 3,781 persons | All ages | Ag prevalence | In this study, the researchers had explored the occurrence of systemic symptoms (fever, dizziness, headache, pruritus and myalgias) after the 5th round of MDA and its relation to filarial infection (Ag using ICT). Those who returned to the distribution posts seeking treatment of symptoms after the MDA were interviewed. Results showed that majority of the people reporting systemic and scrotal symptoms did not have detectable Ag levels at the time of reporting. The authors concluded that given the low doses of DEC and albendazole used in the MDA, it is less likely that the reported symptoms were caused by drug-related side effects. However, they queried whether treating such symptoms as a part of surveillance would affect MDA coverage and that it would vary from country to country. |
| 2 | South East Asia | India | Mukhopadhyay et al, 2007104 | Two urban areas and six rural villages of East godavari district | 5,056 persons | All ages | Mf, disease and vector infection prevalence | East Godavari district in Andhra Pradesh, India had undergone six rounds of MDA from 1999 to 2005. Mf and entomological surveys were conducted in six villages and 2 towns of the district. The Mf and disease rates were 4.4% and 2.4% respectively. The vector infection and infectivity rates were 3.6 and 0,4% respectively. It stressed on the need to develop strategies to implement MDA more effectively by increasing the drug compliance. |
| 3 | South East Asian | India | Yuvaraj et al, 2008105 | 15 villages, Villupuram district | Not mentioned | >15 | Hydrocele prevalence | This study was conducted during a long term community based trial to measure the effectiveness of MDA with DEC alone or Ivermectin alone where the coverage under MDA ranged between 54–75%. Cross-sectional clinical surveys were carried out before and after seven rounds of MDA. After seven rounds, hydrocele prevalence had declined from the pre-intervention level of 20.5–5.1% (P<0.05) in the DEC arm, from 23.9% to 10.4% (P<0.05) in the ivermectin arm and from 20.4% to 10.9% (P<0.05) in the placebo arm, equivalent to reductions of 75.3%, 56.6% and 46.6%, respectively. However there was only a marginal decrease in lymphoedema in both arms. After the seventh MDA, there was a statistically significant decline in hydrocele prevalence in all other age groups in the communities treated with DEC. The impact was relatively less in ivermectin arm. Repeated DEC administration has the potential to prevent incidence of new hydrocele cases and may resolve the manifestation at least in a proportion of affected people. |
| 4 | Western Pacific | Samoa | Joseph et al 2011106 | Five villages | 2,474 persons | All ages & ≤10 | Mf, Ag and Ab prevalence | In Samoa, inspite of 6 rounds of MDA, post treatment survey showed persistent Ag in one of the islands. Mf, Ag and Ab surveys (CELISA kit) were carried out to assess if transmission had interrupted in this island with persistent infection and in another island where transmission had interrupted. Results showed the need for strengthened control effects in these areas. The area, which was declared LF free was found to be endemic based on Ab survey in children. It concluded that more studies were required to validate the use of CELISA as a tool for assessing transmission. |
| 5 | Western Pacific | Papua New Guinea | Tisch et al, 2011107 | Dreikikir district of East Sepik Province | Not mentioned | ≥5 years | Disease prevalence | This study measured the impact of an MDA trial (with single dose of DEC alone vs single dose of DEC plus ivermectin) after 4 rounds on acute filariasis morbidity(AFM) using a questionnaire. The annual incidence of AFM decreased by 20% during the year following the first MDA and by 49–61% in subsequent years relative to pre-MDA rates. AFM rates did not differ according to microfilaremic status or density (categorized as 0, 1–9, 10–99 and 100 microfilaria per ml) during any other study periods. Risk factor analysis showed that age, residence, Mf positivity, not taking MDA drugs were at greater risk of developing AFM. The authors concluded that the incidence of AFM in this population decreased to 57% of the pre-treatment level after 2 annual MDAs in areas of moderate transmission and by 61% in areas of high transmission irrespective of the drugs used for MDA. This rapid decrease in AFM incidence was sustained over the entire 5-year surveillance period, thereby highlighting the potential of MDA to alleviate this feature of LF morbidity. |
| 6 | South East Asia | India | Shriram et al, 2014108 | Five islands of Andaman and Nicobar islands | 2,561 persons | All ages | Mf prevalence | Transmission Assessment surveys (TAS) to decide on stopping or continuing MDA was carried out in a lone foci of infection, Nancowry group of Islands. It was found that inspite of 6 rounds of MDA, Mf prevalence was 3.3%. The authors had suggested mass distribution of DEC medicated salt as an adjunct to hasten elimination of infection in these islands. |
| 7 | South East Asia | Indonesia | Dewi et al, 2015109 | Three communities (one non endemic, one passed TAS, one failed TAS) |
1,543 children | 6–7 | Ab prevalence | This study provided the epidemiological support for the use of Ab levels to determine “critical cut-off thresholds” in Brugia spp. areas. Results from districts which followed the current WHO guidance for mapping, MDA, and implementing TAS, while providing Ab profiles of treated and untreated populations under programmatic settings, supported the choice of Ab prevalence in the 6 and 7yo group in TAS for stopping MDA decisions. |
| 8 | Africa | Ethiopia | Endeshaw et al, 2015110 | Three villages | 774 persons | ≥2 | Ag prevalence | It assessed the impact of 7 years of annual MDA with IVM monotherapy for oncho on LF and showed that it did not interrupt LF transmission. If LF was to be targeted along with oncho in the control program, albendazole should be added and treatment coverage should be improved. |
| 9 | South East Asia | Bangladesh | Hafiz et al, 2015111 | 30 villages of Nilphamari district | 1,242 persons | All ages | Acute disease prevalence | The study assessed the impact of 12 rounds of MDA on clinical filariasis. A two-stage 30 cluster survey with selected villages were surveyed for Ag (ICT), episodes of ADLA and chronic LF manifestations. The study villages and the disease cases were mapped. Results showed there was no association between disease status and filarial Ag positivity. Twenty one of the 30 villages recorded at least one manifestation. Disease prevalence was 4.4 (3.4–5.7). The authors suggested the use of mobile apps for geo referencing the disease database at village level which may be helpful for scaling up MMDP activities and a home-based morbidity control protocol. However, there was no baseline information on disease in these areas to relate the change to the impact of MDA. |
| 10 | Africa | Senegal | Wilson et al, 2016112 | 14 villages in three districts | 1,131 persons | ≥5 | Ag and Ab prevalence | It assessed the possibility of adding LF tests to a standard onchocerciasis epidemiological survey to provide meaningful results for LF-onco elimination program. The status of LF in three districts was assessed using ICT and Wb123, along with the Ov16 RDT in Kedougou region of Senegal, co-endemic for both oncho and LF. A convenience sample of individuals from 14 villages from three districts were examined for both W. bancroftiand O. volvulusinfection. Prevalence of LF antigen was found as 0.5% (95%CI: 0.2–1.2%) and that of its antibody was 0.6% (0.2–1.3%). However, one of the three districts, Salemata recorded an Ag and Ab prevalence of 3.5% (1.3–7.4%) and 1.2% (0.2–4.2%). TAS required a sample of 1,500 children to assess interruption of LF transmission but in the current study the sample size was small and therefore it could not be determined if transmission was interrupted even if no ICT positives were detected. The findings of this study provided insight into the complexities that may arise if the stop MDA decision was to be taken in LF and Oncho co-endemic areas. |
| 11 | Africa | Ethiopia | Mengistu et al, 2017113 | 70 districts | Not mentioned | Not mentioned | Mf and Ag prevalence | The study described the current status of the LF elimination programme that was integrated with the onchocerciasis control program in 2009 that was implemented in 70 LF endemic districts. By 2016 it had achieved 100% geographical coverage with treatment coverages between 73 and 87% and an epidemiological coverage of 65%. Being a malarious region too, LLIN distribution and IRS also played a significant role in prevention of LF. The current status indicated that Ethiopia was poised to achieve the 2020 goal of elimination of LF if the treatment coverage was sustained and strong monitoring and evaluation were in place. |
| 12 | South East Asia | India | Vaishnav et al, 2007114 | One city (Surat) | 5,058 persons | ≥1 | Mf prevalence | This study assessed the LF situation after 6 rounds of MDA in Surat ciy. LF endemicity rate reduced from 0.24% to 0.11% (reduction 54%) and in north zone it reduced from 0.72% to 0.30% (reduction of 58%). Though, overall Mf rate had reduced due to MDA, higher rate was noticed in North zone of city where the migrant populations influx was higher. |
| 13 | South East Asia | India | Khan et al, 2015115 | Tea garden population of Dibrugarh, Assam | 634 persons | All ages | Mf, disease and vector infection prevalence | The study assessed the current filariasis situation after five rounds of MDA among tea garden workers in Assam who had recorded high Mf rates in the past. Mf, disease and entomological surveys were conducted. While Mf rate was 3.8%, disease rate was 5.7%. Vector infection (13.2%) and infectivity (3.7%) rates were high. While the distribution coverage was >80% in 2007, it reduced to 60–70% in 2013. However, there were no information on the compliance rates. The study concludes that LF elimination in the State is achievable but poor drug compliance was the main bottleneck to the elimination program. Community participation in adjunct to training of drug distributors for meeting the target of drug compliance were the essential components in the success of GPELF. |
| 14 | Africa | Tanzania | Simonsen et al, 2010116,117 | One village (Kirare) | 919 persons | ≥1 | Mf, Ag and Ab prevalence | These two studies from Tanzania, utilized the data from the surveys conducted in the Kirare village in Africa, which had completed four rounds of MDA. All individuals (only residents) in the village were examined under Mf, Ag(Og4C3) and Ab(Bm14) at the baseline and after each round of MDA upto 3rd MDA. Entomological surveys were also carried out for monitoring infection in vectors. However, children enrolled and assessed for Ag in standard Iimmediately after first MDA were followed upto 4th MDA and antigen levels were measured in them prior to each MDA. Results of these studies indicate that the prevalence of Ag and Ab post-4 MDA did not differ significantly from baseline levels. However, there were significant difference in Ag units and Ab OD values among the cohorts of individuals surveyed at both time points (Og4C3 Ag units: from 106.9 to 47.3 CFA units and Ab OD value: (from to 0.784 to 0.405). With regard to vector infection, the reduction was significant even after the first round of MDA. However the reduction in vector infectivity rates was not significant. Even the mean monthly transmission potential decreased by 87 times post 4th MDA. Ag prevalence in children of standard I immediately before the first MDA reduced significantly after 2 MDA onwards and reached 6.4% from 25.5%after the 4th MDA. |
| 15 | Africa | Tanzania | Simonsen et al, 2011 | 10 rural primary schools | 700–800 children | 6–14 | Ag prevalence | |
| 16 | Americas | Haiti | Boyd et al, 2010118 | Six villages | 455 persons | 2-4 | Mf, Ag and Ab prevalence | The authors described how even if the sentinel sites reported Mf prevalences <1%, after eight rounds of MDA, transmission could still continue to occur through a 30-cluster survey among 2-4 yo. Ag and Ab prevalences were 14.3% and 19.7%. Filarial infection was focal in nature and infection was significantly associated with non-compliance to MDA drugs. |
| 17 | Africa | Nigeria | Richards et al, 2011119 | 10 villages | 10,753 persons | ≥2 | Mf, Ag and vector infection prevalence | The study assessed the impact of MDA (7 years of monotherapy with IVM in 12 LGAs) in a LF-Oncho co-endemic area. The villages were annually monitored till 2009, for both human and vector infection since 2000 and had completed 7–10 rounds of MDA with a coverage of 72% in 2003, ≥85% in 2006 and 73% by 2009. Impact on transmission showed that by 2009 Mf prevalence decreased from 4.9% to 0.8% (86% reduction), Ag (based on ICT) from 21.6% to 7.2% (67% reduction), vector infection from 3.1 to 0.4 (86% reduction) and infectivity rate from 1.3 to 0.3% (77% reduction). Three sentinel villages had on-going transmission (based on infective L3 in mosquito) and one had a Ag prevalence of 27.3% and vector infection >2%, indicating the need for increased interventional efforts. The authors suggested that LGAs with greater endemicity (≥25% Ag prevalence at baseline) were likely to be the primary areas of risk of MDA failure. As the remaining LGAs were on the path of making decisions on stopping MDA, particularly for a co-endemic area there were two options: (i) IVM alone may have to be continued with post MDA surveillance for LF to prevent its recrudescence or (ii) after assessing the status of oncho transmission, if found interrupted stop Oncho MDA, and plan for an integrated surveillance method to carry out post-MDA surveillance for both the diseases. |
| 18 | Western Pacific | American Samoa | Mladonicky et al, 2009120 | Three villages | 579 persons | ≥5 | Ag and Vector infection prevalence | The use of Ab and xeno monitoring in LF transmission was explored in an area that had completed 6 rounds of MDA. Mf, Ag and Ab surveys were conducted in three villages covering individuals aged >4 years. The overall prevalence of Mf was <1%, Ag prevalence ranged between 3.7 and 4.6% and Ab prevalence ranged between 12.5% and 14.9% in all these villages. As there were reports of vector infection in these villages (based on an earlier study), the authors concluded that both xeno-monitoring and Ab may be useful to identify areas with potential transmission of LF. |
| 19 | Eastern Mediterranean | Egypt | Farid et al, 2007121 | Two sentinel sites | Not mentioned | All ages | Mf, Ag and Vector infection prevalence | The study was conducted in Egypt, after five rounds of MDA to appraise if MX can be used as a tool for assessing progress towards elimination of LF. The tool was tested in two sentinel villages to measure the parasite DNA rates in mosquitoes. Results showed that the parasite DNA rates reduced by 93.8% and 100% in high and low prevalence areas respectively after five rounds of MDA. These changes were consistent with decreases in Mf prevalence rates in the sites. It also provided insight regarding the minimal mosquito DNA rates necessary for sustained transmission of filariasis in Egypt. The study concluded that MX is a powerful tool for assessing the impact of MDA. |
Abbreviations: LF, Lymphatic filariasis; DEC, Diethylcarbamazine; IVM, Ivermectin; Ag, Antigeneamia; Mf, Microfilaraemia; Ab, Antibody; MDA, Mass drug administration; TAS, Transmission Assessment Survey; ICT, Immunochromatographic Test; MX, Molecular Xenomontoring.
Studies in the African,118 East Mediterranean,120 and South East Asian107,114,117 regions which used human and vector infection indicators have concluded that 4–6 rounds of MDA may not be sufficient to interrupt transmission. While the study from Nigeria118 concluded that additional interventions (like more frequent MDA treatments and insecticidal bed nets) were necessary particularly for those sentinel villages that had baseline Ag prevalence >25%, systematic non-compliance to MDA was the reason for on-going transmission in Haiti which led to two additional MDA rounds.117 The studies that had used vector infection prevalence in addition to Mf/Ag/Ab117,119,120 have suggested that MX is a powerful tool in assessing the impact of MDA and that both Ab in humans and the MX tool could be used to measure on-going transmission.
Few studies that had measured Ab prevalence alone or in addition to Mf/Ag in children105,108,115,116 indicated that measuring Ab in children is a better indicator to assess interruption of transmission as it also measures exposure to infective bites in addition to infection with adult worms. It was suggested that measuring Ab instead of Ag alone may be helpful in identifying areas with residual infections.105
Studies that used disease prevalence to measure the impact of MDA104,106,110 suggested that 10 rounds of MDA had helped in reducing the overall disease prevalence to low levels. A study from Papua New Guinea showed that after four rounds of MDA, the acute filarial morbidity reduced significantly from the pre-treatment levels and that the reduction was higher in those areas that had higher transmission indices compared to those with lower figures.106 In a community trial from India, it was found that seven rounds of MDA with DEC alone prevented incidence of new hydrocele cases and resolved the manifestation in a proportion of already affected individuals in addition to reducing the levels of Mf and transmission indices in the communities.104 Assessment of occurrence of systemic symptoms102 following a 5th round of MDA and providing free symptomatic treatment was expected to motivate people to report symptoms and seek care. However, the authors concluded that from a programmatic perspective, treatment of these systemic symptoms might not be necessary, because most of the individuals surveyed did not report these symptoms as a barrier to participating in future MDA.
Few studies have reported only the current situation of LF (Mf/Ag/Ab/disease) after six to seven rounds of MDA103,104,110,112–114 in the endemic areas and found that Mf prevalence were still above the 1% threshold. To interrupt the on-going transmission in spite of several rounds of MDA, these studies recommend the need for additional efforts by program authorities to improve the coverage of the program and better monitoring and evaluation procedures.
One study assessed the possibility of integrating antibody testing for LF and oncho, which has highlighted the complexities that may arise in making a decision on stopping MDA particularly in LF-oncho co-endemic areas of Africa.111 Another study109 observed that 7 years of monotherapy with ivermectin has not reduced Mf prevalence in a oncho-LF co-endemic area and therefore to achieve significant reduction, the authors suggested that albendazole be added to the program particularly in LF endemic areas. In another oncho-LF co-endemic area118 the study showed that while Mf and Ag rates were reduced by 83% and 67%, the mosquito infection and infectivity rates were reduced by 86% and 76% compared to the baseline. As this was a LF-oncho co-endemic area, regarding the decision of stopping MDA, it was suggested that if LF transmission was found to be interrupted, albendazole could be stopped and the MDA could continue only with ivermectin with simultaneous post-MDA surveillance for LF for resurgence. If it is found that oncho transmission also has been interrupted, an integrated surveillance can be planned for post-MDA surveillance.
Post-MDA
A total of 17 studies (eleven from South East Asia, three from Western Pacific, two from Africa and one from American regions) have reported the findings during the post-MDA monitoring phase. Of these, one additionally monitored pre-MDA period and another study monitored pre-, during- and post-MDA periods. These studies highlighted the importance of monitoring during different phases of MDA (Table 4).
Table 4.
Details of the studies where monitoring was done post-MDA
| Study no. | Region | Country | Author & year | No. of districts or villages or sites | No. of people/mosquitoes tested | Age (years) | Indicator | Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Americas | Haiti | Chu et al, 2013122 | 11 countries | 29,169 children | 6–7 | Ag and Ab prevalence | This study was carried out to assess the revised TAS protocol in its ability to make decisions to stop MDA and also to be used as a post-MDA surveillance tool. While Burkino Faso, Dominican republic, Ghana, Indonesia, Malaysia and Tanzania were in to make decision on stopping MDA, rest of the countries were already in the post_MDA scenario. The diagnostic tools used were ICT for W.bancrofti regions, and PanLF for TAS 1 or BMR1 for Brugia spp endemic areas. Sample sizes for these surveys were obtained through SSB (range: 684 in Sri Lanka to 1556 in Burkino Faso). Either school based or community based surveys were adopted. The authors suggest that TAS based on communities face several challenges than the ones based on school survey main reason being poor census, definition of EA boundaries, estimation of target age group etc., Preliminary results from separate TAS studies appear to suggest there is no statistically significant difference or change in the TAS-recommended outcome for EUs with school primary enrolment rates as low as 59% instead of the current 75% (Gass et al, 2013). The results of this study supports the reliability of this strategy but because TAS is not powered to detect change or designed to identify hotspots, post-MDA surveillance would best be complemented in the short and long term with other, complementary diagnostic tests and surveillance methods. In future, if new diagnostic tools are to be used, then the thresholds and the sample sizes may need to be modified. |
| 2 | South East Asia | India | Srivastava et al, 2014123 | 42 primary schools in Goa | 1692 children | 6–7 | Ag prevalence | The study from Goa, describes the first time implementation of the revised TAS in India. Goa, one of the historically LF endemic districts in India, started the MDA against LF in the year 2000. Four rounds of MDA with DEC alone and 5 with DEC+albendazole were completed. Post-treatment MF survey showed that Mf rates in sentinel and spot check sites were 0. In 2013 Mf surveys in additional 10 randomly selected sites also showed 0 Mf prevalence. These observations showed that the district was eligible for TAS. A sample of 1692 (based on the SSB tool for TAS by WHO), children aged 6–7 years were tested for Ag (ICT) of which only 16 were positive, less than the cut-off value of 20, indicating passing of TAS. MDA was stopped and the district is now in the post_MDA surveillance mode. The authors conclude that TAS is a scientific, practical and effective evaluation tool for decisions on stopping or continuing MDA. |
| 3 | Africa | Tanzania | Biritwuma et al, 2017124 | 98 endemic districts of Ghana | Not mentioned | All ages | Ab prevalence | The study describes the existence of persistent LF hotspots inspite of 14 rounds of MDA . The study aimed to compare the baseline Mf prevalence and anti_filariasis interventions (LLINs) among hotspots and those districts where MDA was stopped. The study population were from the 29 hotspot districts and 69 stopped-MDA districts. The data collected were the baseline Mf prevalences and data on distribution of LLINs to the population. Assessing LF status at baseline, during MDA implementation and the status in 2016 showed that by 2014 69 districts had stopped MDA and by 2016 another 12 more stopped MDA after TAS. Results show that the number of rounds of MDA required for hotspots were higher than those districts where the MDA was stopped. It was also observed that the baseline MF prevalence in these hotspot districts were 10 time higher that those districts in which MDA has been stopped. The authors indicated that these observations may have implication on programme stating the districts with high baseline MF prevalence may require more than the recommended 5–6 rounds of MDA compared those with low baseline prevalence. |
| 4 | Western Pacific | American Samoa | Shamsuzzaman et al, 2017125 | 34 districts (19 endemic and 15 low endemic) | 136,080 children | 6–7 | Ag prevalence | This paper presents TAS results, highlighting the momentous geographical reduction in risk of LF and its contribution to the global elimination target of 2020. Since 2011, a total of 59 TAS have been conducted in 26 EUs across the 19 endemic MDA districts (99,148 students tested from 1,801 schools), and 22 TAS in the 15 low endemic non-MDA districts (36,932 students tested from 663 schools). All endemic MDA districts passed TAS, except in Rangpur which required two further rounds of MDA.The distribution was geographically sparse, with only two small focal areas showing potential evidence of persistent transmission. Bangladesh is now considered to have very low or no risk of LF infection after 15 years of programmatic activities, and is on track to meet elimination targets.Other positive influencing factors for Bangladesh include good administrative development and health system infrastructure, relative political stability, strong political commitment and financial support, strong program management leadership, heightened awareness of morbidity in the endemic areas, which helped to increase drug compliance and importance of disease elimination. |
| 5 | Western Pacific | Papua New Guinea | Khieu et al, 2018126 | Four sites (two sentinel and two spot check) | Not mentioned | ≥5 | Ab prevalence | In this study, as a first step towards LF elimination, mapping exercise was carried out in all provinces and districts, based on Ag surveys during 2000–2002. Any province with even one Ag positive as endemic. Two provinces and four districts in two other provinces classified as endemic due to the focal nature of LF. MDA with DEC and albendazole was initiated in 2005 through 2009 completing five rounds with effective coverage of 65%. In sentinel sites, Mf surveys were conducted during baseline and 2nd, 3rd and 4th years and also after the final round of MDA in 2009. In spot-check sites, mf surveys were carried out during 2nd, 3rd and 4th round of MDA. It was observed that Mf prevalence became 0% from third survey onwards and in sentinel sites it was 0% in all surveys. Hence TAS was conducted following the WHO norms and it was found that the Ag prevalence in six IUs (two provinces and four districts) ranged between 0.1 and 0.7%, much below the 1% level indicating interruption of transmission. MDA was stopped in these provinces and districts. Post MDA surveillance surveys TAS 2 on grade 1 children (after 2–3 eaof stopping MDA) and TAS 3 (after 46 years of stopping MDA) were conducted in 2013 and 2015 respectively. In both, there were no Ag positive child in both TAS2 and TAS3 surveys suggesting total transmission interruption in the IUs. To ensure this, TAS 3 with an antibody survey of children in one of the historically known LF endemic district was carried out and only one child was positive substantiating the earlier finding tha that transmission has been interrupted. Having passed TAS3, as the final stage of declaring elimination of LF, in 2015 the MoH prepared the dossier documenting elimination of LF as a public health problem and submitted to WHO which was validated by the Regional Dossier Review Group of WHO Western Pacific Regional office. In 2016, WHO headquarters officially acknowledged that LF elimination was achieved in Combodia. |
| 6 | South East Asia | Nepal | Ojha et al, 2017127 | Five districts | 9495 children | 6–9 | Ag prevalence | This study in Nepal provides findings of the Pre-TAS, TAS and drug coverage surveys conducted after 6 rounds of MDA to assess if the LF transmission has been interrupted or not. The Study was conducted in 7 of the 10 endemic districts (selected purposively) prior implementing MDA. From each districts, 2 sentinel sites were selected (Based on migration, population size and LF prevalence). All sentinel and spot check sites in districts in five districts and one spot check site of another district reported <2% Ag prevalence. Remaining 4 districts reported >2% Ag in the pre-TAS surveys. None of these four districts at achieve epidemiological coverage of ≥65% MDA coverage. TAS was conducted in all these five districts and a part of the 6th district (it was split into two EUs) and all passed TAS (number of Ag positive children less the cut-off value). The results suggest that the LF transmission was interrupted in the 5th and partly in 6th district and that MDA may be stopped in these. However in the remaining four, additional rounds of MDA may be necessary. out of the 10 district. Though the MDA coverage varied between the districts (50%-84%) they still passed TAS suggesting that a MDA coverage of around 50% may be sufficient to interrupt LF transmission in urban populations. However, in sptie of high coverage, four rural districts failed to pass TAS and these had the high baseline LF prevalence. These findings corroborate with the finding of Shamsuzzaman et al, 2017 described earlier. |
| 7 | South East Asia | Myanmar | Aye et al, 2018128 | 19 districts | In 206 schools in 5 districts | >2 | Ag prevalence | This study also, like the previous one summarizes the programmatic activities right from mapping endemic districts to post-MDA surveillance of LF, the progress and impact of the those activities and highlights the first evidence that prevalence has been lowered to a level when transmission is not sustainable. Data for this study was from 15 administrative units of Myanmar, consisting of 65 districts. Mapping exercise in 19 districts and historical data showed that 45 districts were endemic for LF. Starting MDA in only two districts in 2001, it was upscaled to all 45 endemic districts by 2014 so the number of MDAs completed ranged between 2 and 12 in different districts. Seven years after the start of MDA, the first TAS wasconducted in three districts and subsequently in other districts too. TAS passed in 5 districts and MDA was stopped, but monitoring continued in these IUs for next 5 years. The results of this study highlights that Myanmar NPELF has moved forward towards elimination of LF and significant reduction in Mf prevalence and with evidence for interruption of transmission. |
| 8 | South East Asia | India | Swaminathan et al, 2012129 | Two Primary Health Centres | 35,582 persons | All ages | Ag prevalence | This study was undertaken to develop sampling strategies to decide on stopping or continuing MDA in an implementation unit. Both Mf and Ag(Og4C3) prevalence were assessed by covering all individuals in the 92 villages to see the impact of 8 rounds of MDA (upto 2007). It was found that prevalence of Mf and Ag were 0.2% and 2.3%. In these 92 villages there were 7 residual (with Mf prevalence ≥1%) and 17 transmission (atleast one Ag-positive child born during MDA period) hotspots. It was also seen that inspite of eight rounds of MDA, there was spatial clustering of infection both at household and village level. The study highlights the need for identifying factors responsible for the emergence of ‘‘transmission hotspots’’ and adoption of appropriate sampling strategies for the development of evidence-based programmatic decision-making tools. |
| 9 | South East Asia | India | Ramaiah et al, 2007130 | Ten communites | 7% of Households, adults and 20–40 children | ≥15 & 1–10 | Mf prevalence | Ten rounds of mass drug administration was done in 10 communities (5 each for DEC alone and Ivermectin alone) and 49—84% of the eligible population received treatment in different villages. Out of five villages in each treatment arm, the mf rate declined to ≤1% in four villages in the DEC arm and two villages in the ivermectin arm. No mosquitoes with infectivestage larvae were found in three of five villages in the DEC arm and two of five villages in the ivermectin arm. None of the children (n=130) were found to be positive for mf in either treatment arm. None of the 40 sampled children were found to be positive for circulating filarial antigenaemia in villages with lower endemicity in the DEC arm. The results suggest that ten rounds of DEC mass administration have the potential to interrupt transmission of infection in the majority of communities. The outcome was relatively less remarkable with ivermectin. |
| 10 | South East Asia | India | Ramaiah et al, 2013131 | Five villages | 700 persons and 10842 mosquitoes | >2 | Mf, Ag and Vector infection prevalence | Robust monitoring and evaluation of MDA is necessary to assess its impact and to stop MDA when the indicators of impact – Mf prevalence in the population or vector infection rate or antigenemia (Ag) prevalence in the children born during the MDA period – fall below the threshold level. The impact of 10 rounds of MDA (using DEC alone) on LF infection and transmission in 5 endemic communities of south India, were monitored and evaluated for 6 years after the overall Mf rate of the study communities was brought down to,1.0%, considered to be the safe and threshold level to stop MDA. Overall Mf prevalence (n=700) and vector infection rates (n=803–3520) showed a declining trend. Both Mf status in humans and infection in vectors were zero from 3rd year after stopping MDA. In only one village, community Mf rate was at 1.0% and Ag prevalence among 1–7 -year old children was 4.6% (n = 44) and vector infectivity rate during the sixth year was 0.1% (n = 852). |
| 11 | South East Asia | India | Ramaiah et al, 2002132 | 10 villages | 588 persons | individuals with ≥15 kg weight | Mf prevalence | This placebo-controlled study examined the potential of six rounds of mass treatment with DEC or IVM to eliminate Wuchereria bancroftiinfection in humans in rural areas in south India. The results indicate that DEC is as effective as or slightly better than IVM against microfilaraemia. Results from this and other recent operational studies proved that single-dose treatment with antifilarials is very effective at community level, feasible, logistically easier and cheap and hence a highly appropriate strategy to control or eliminate LF. Higher treatment coverage than that observed in this study and a few more than six cycles of treatment and more effective treatment tools/strategies may be necessary to reduce microfilaraemia to zero level in all communities, which may lead to elimination of LF. |
| 12 | South East Asia | India | Subramanian et al, 2015133 | 33 villages/wards in Tanjavur district | 20,049 mosquitoes | Not relevant | Parasite DNA rate | The monitoring and evaluation of lymphatic filariasis (LF) has largely relied on the detection of antigenemia and antibodies in human populations. Molecular xenomonitoring (MX), the detection of parasite DNA/RNA in mosquitoes, may be an effective complementary method, particularly for detecting signals in low-level prevalence areas where Culex is the primary mosquito vector. This article investigated the application of a household-based sampling method for MX in Tamil Nadu, India. Households were systematically selected using a sampling interval proportional to the number of households in the EU. Mosquito pools were collected and analyzed by real-time polymerase chain reaction (qPCR).The household-based sampling strategy for MX led to mostly reproducible results and supported the observed LF infection trends found in humans. MX has the potential to be a costeffective, non-invasive monitoring and evaluation tool with sensitive detection of infection signals in low prevalence settings |
| 13 | Western Pacific | American Samoa | Lau et al, 2016134 | 32 villages of American Samoa | 376 persons | All ages | Ag and Vector infection prevalence (MX) | The study evaluated xeno-monitoring as a surveillance tool by linking village level results of published human and vector studies. A total of 32 villages were included in the study. While a 34.4% were positive for Ag (by Og4C3), 56.3% positive for Wb123 Ab and 84.4% were positive for Bm14 Ab. Parasite DNA in vectors was detected in 15 of the 32 villages. Particularly in those villages which recorded Ag positive and Wb 123 positive persons, parasite DNA was found in 91% and 72% of the vectors. In those villages that had no positive persons (for Ag or Wb123), PCR positivity for vector infection were absent. |
| 14 | South East Asia | Bangladesh | Irish et al, 2018135 | 30 villages in two districts | 10,021 mosquitoes | Not relevant | Parasite DNA rate | In this study, MX evaluation was conducted in two areas of Bangladesh, one previously endemic district that had stopped MDA (Panchagarh), and part of a non-endemic district (Gaibandha) that borders the district where transmission was most recently recorded.The results showed that none of the mosquito pools tested were positive for W. bancrofti DNA which confirms the results of TAS conducted during 2013 and 2015. The authors suggest that MX can be used to identify missing foci of transmission with smaller geographical areas, in areas were Ag positive cases were identified in TAS |
| 15 | South East Asia | Sri Lanka | Rao et al, 2014136 | 30 health administrative units | 28,000 mosquitoes | Not relevant | Parasite DNA rate | Galle district (population 1.1 million) was divided into two EUs. These included a coastal EU with known persistent LF and an inland EU with little persistent LF. Mosquitoes were systematically sampled from ~300 trap locations in 30 randomly selected clusters (health administrative units) per EU. Approximately 28,000 Culex quinquefasciatuswere collected with gravid traps and tested for filarial DNA by qPCR. 92/625 pools (14.7%) from the coastal EU and 8/583 pools (1.4%) from the inland EU were positive for filarial DNA. Maximum likelihood estimates (MLE) for filarial DNA rates were essentially the same when the same number of mosquito pools were collected and tested from 75, 150, or 300 trap sites (range 0.61–0.78% for the coastal EU and 0.04–0.07% for the inland EU). The ability to use a smaller number of trap sites reduces the cost and time required for mosquito sampling. These results suggest there is widespread persistence of W. bancrofti infection in the coastal Galle EU 8 years after the last round of MDA in 2006, and this is consistent with other data from the district. This study has shown that MX can be used by national programs to assess and map the persistence of W. bancrofti at the level of large EUs in areas with Culex transmission. |
| 16 | Africa | Tanzania | Rebollo et al, 2015137 | Two sentinel sites (islands of Unguja and Pemba) | 3,275 children | 6–7 | Ab prevalence | The study from Tanzania was conducted in Zanzibar where five rounds of MDA with IVM and albendazole was completed and resulted in zero prevalence of MF, Ag and vector infection in sentinel and spot check villages. MDA was stopped and TAS was performed to assess if transmission was interrupted. The study was conducted in islands Pemba and Unguja of Zanzibar. Two sentinel sites from the islands of Unguja were used for monitoring and data on Mf prevalence and coverages were collected at the baseline (prior to MDA) and repeated before each round of MDA. The Mf rates in these two sites reduced from 17.8% and 17.2% to 1.0% and 0.0% respectively. Following this, TAS was conducted in the two islands and children were examined for Ag (ICT). However the number of Ag positive children was much higher than the cut-off value in Pemba but within the cut-off value for Unguja. The low prevalence of Ag in children in Unguja is attributed vector control measures like usage of LLINs and IRS. However, earlier entomological studies in Unguja immediately after stopping MDA showed that transmission was on-going in Unguja islands. The authors indicate that 5 years of MDA may not be sufficient for interrupting LF transmission. Based on this study, the government of Zanzibar decided to resume MDA with ivermectin and albendazole in both these islands in 2013.TAS will be conducted in 2015. |
| 17 | South East Asia | Sri Lanka | Rao et al, 2016138 | 19 PHIs | 17,479 children, 78,000 mosquitoes |
All ages for MF and Ag, Ab in 6–7 | Mf, Ag, Ab and Vector infection prevalence | Comprehensive surveillance was performed in 19 Public Health Inspector (PHI) areas (subdistrict health administrative units) 6 years after stopping MDA. The surveillance package included cross-sectional community surveys for microfilaremia (Mf) and circulating filarial antigenemia (CFA), school surveys for CFA and anti-filarial antibodies, and collection of Culex mosquitoes with gravid traps for detection of filarial DNA (molecular xenomonitoring, MX). Provisional target rates for interruption of LF transmission were community CFA,2%, antibody in school children,2%, and filarial DNA in mosquitoes,0.25%. Community Mf and CFA prevalence rates ranged from 0–0.9% and 0–3.4%, respectively. Infection rates were significantly higher in males and lower in people who denied prior treatment. Antibody rates in school children exceeded 2% in ten study sites; the area that had the highest community and school CFA rates also had the highest school antibody rate (6.9%). Filarial DNA rates in mosquitoes exceeded 0.25% in 10 PHI areas. |
Abbreviations: LF, Lymphatic filariasis; DEC, Diethylcarbamazine; IVM, Ivermectin; Ag - Antigeneamia; Mf, Microfilaraemia; Ab, Antibody; MDA, Mass drug administration; TAS, Transmission Assessment Survey; ICT, Immunochromatographic Test; MX, Molecular Xenomontoring.
As seen in pre- and during-MDA phases, studies in the post-MDA phase have also used Ag/Ab and vector infection indicators to monitor the LF situation. In a multi-country study (consisting of eleven countries) Chu et al.121 reported that TAS protocol is a reliable strategy (either school- or community-based) to assess interruption of transmission. Similar observation was made in India122 who reported the first successful TAS in Goa in India after nine rounds of MDA, mentioning that TAS is a scientific, practical, and effective evaluation tool for stopping MDA. The authors emphasized the importance of focal survey to identify the source of infection in communities with antigen-positive children to prevent resurgence in an area declared free from MDA.
A study from Ghana reported the existence of hotspots, despite 14 years of MDA, and attributed the persistence of hotspots to its high baseline Mf prevalence123 and reported that more than the standard five to six rounds of MDAs, say up to 14 rounds were required to interrupt transmission. Another study124 summarized the current situation of LF in Bangladesh through TAS conducted in 19 districts (which had undergone 5–12 rounds of MDA) and 15 low endemic districts (not given MDA). The results showed a significant reduction in prevalence of geographical distribution of infection across Bangladesh and attributed this to factors like good administrative development and health system infrastructure, relative political stability, strong political commitment and financial support, strong program management leadership and heightened awareness of morbidity in the endemic areas. The authors also suggested that districts that required more rounds of MDA had higher baseline Mf prevalence. Post-MDA surveillance in Cambodia125 showed that all the endemic districts had undergone five rounds of MDA with >65% coverage and had passed even TAS 3 and is currently in the final stage of declaring LF elimination. The authors have attributed this success to the commitment of the government and effective implementation of MDA, monitoring and evaluation, and surveillance activities. MDA was successfully implemented even in forested and remote endemic areas through advocacy, sensitization of various departments, active participation of provincial- and central-level program personnel in MDA activities, and financial and related support from partners and stakeholders. In Nepal126 it was shown that interruption of transmission was established in five of the ten endemic districts and in one EU of another district following successful TAS after six rounds of MDA, in-spite of not achieving the optimum coverage of 65% in three districts. In the remaining districts, MDA continued as they had a higher baseline Mf prevalence as reported in Bangladesh.124
In Myanmar127 the program initiated in one district in 2001 was up-scaled to 42 of the 45 endemic districts by 2014. This slow progress in upscaling was attributed to lack of funding, resources (eg training of basic health staff, advocacy materials), continued problems in procuring adequate quantities of DEC, as well as security-related issues. In spite of this, five districts declared interruption of transmission with two passing TAS 1 after 6–10 rounds of MDA and three districts that had passed TAS 2 after undergoing five rounds. In this study also, a predictive modeling approach showed that the decline in Mf prevalence was associated with the number of MDAs and initial Mf prevalence as observed in the above studies.
The PHC area in Thanjavur district, Tamil Nadu, India,128 reported low levels of infection (community Mf and Ag prev-0.2%,and 2.3%, and Ag prevalence in 2–8 years old children was 0.12%) after eight rounds of MDA with spatially clustered infection both at household and community level. Based on this study MDA was stopped in this area. Results of a long-term community trial that evaluated the effectiveness of ten rounds of DEC or ivermectin alone in ten villages of the Villupuram district of Tamilnadu, India129-131 concluded that ten rounds of MDA with DEC alone would be able to interrupt transmission of LF and achieve the objective of LF elimination.129 Subsequent post-MDA study130 in the same area indicated that 6 years of post-MDA monitoring and evaluation was adequate to discern the status of transmission interruption and for making an appropriate decision on certifying LF elimination.
Few studies have also evaluated the use of MX for confirming the findings of TAS and, also as a post-MDA surveillance tool. In a study from India, Ag-prevalence in children and vector infection indicators were used to assess the LF situation in one of the evaluation areas that stopped MDA (after 8 rounds).128,132 The estimated vector infection rate based on xenomonitoring corroborated with the decision based on human infection for stopping MDA in this evaluation unit, suggesting that xenomonitoring could be a potential alternative to post-MDA surveillance or validation. Further, vector infection prevalence was reported to be higher in communities which were transmission hotspots (with Ag positive children). Similar observations were made in American Samoa133 that detected parasite DNA in vector mosquitoes in the communities where Ag-positive persons were detected. In Bangladesh, one study134 reported MX evaluation for transmission interruption in two areas; one previously endemic district that had stopped MDA (Panchagarh), and another part of a non-endemic district that reported transmission. In a study from Sri Lanka, in addition to TAS, a comprehensive surveillance (a package included cross-sectional community surveys for Mf and Ag, school surveys for Ag and Ab, besides xenomonitoring) was carried out to assess the LF situation 6 years after cessation of MDA in 19 Public Health Inspector (PHI) areas of the eleven EUs in eight districts.135 The prevalence of infection in vector exceeded the provisional threshold of 0.25% in 10 of the 19 PHIs where the school- and community-based Ag- and Ab surveys reported <2% Ag and >2% Ab prevalence. Based on the results, the authors recommended use of antibody and MX testing as tools to complement TAS for post-MDA surveillance. Similarly, a study from Tanzania showed that in areas that had passed TAS, MX provided evidence of on-going transmission and this led to re-initiation of MDA for another two rounds in those areas. In a subsequent study from Sri Lanka,136 MX indicated ongoing transmission (filarial DNA prevalence of 0.63% greater than the provisional threshold of 0.25%135) in a coastal district 8 years post-MDA, although Mf prevalence (0.2%) was much lower than the threshold for stopping MDA (1%) proving xenomonitoring is more sensitive than TAS and Mf-survey.
Mapping and monitoring
Five studies reported on mapping and monitoring together (Table 5) during MDA (four studies) and post-MDA phase (one study).
Table 5.
Details of the studies that carried out both mapping and monitoring
| Study no. | Region | Country | Author& year | No. of districts or villages or sites | Indicator | No examined | Age (years) | Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | South East Asia | Haiti | Drexler et al, 2012139 | Five communes | Ag prevalence | 2639 children | 4-17 | In the study from Haiti (Drexler et al, 2017), the authors have explored if there is active transmission particularly in communities that were identified as low prevalence five communes (during mapping exercise in 2001– Ag prevalence in 6–11-year-olds). A follow-up school surveys for Ag in children were carried out in 2003. For community survey, to examined atleast 100 individuals per community, 20 households within a radius of 5075 m (in urban, peri-urban area) and 100–250 m (rural) of 5–8 index cases (Ag positive children detected in school survey) were selected. Households were selected by systematic sampling method. All individuals available in the selected household was tested for Ag(ICT) and another 200 ul of blood was collected for confirmatory test (using Og4C3). Results of the school survey showed that 2.8% of the 4–17-year-olds were Ag positive. While ICT tested positive children in all five communities. Og4C3 detected Ag positives only in three of the five communities. Analysis on distance from index cases showed that the relationship between Ag positivity and distance from index case was strongest at 20 m and it became weaker with decreasing Ag prevalence with increase in distance. |
| 2 | South East Asia | India | Upadhyayula et al, 2012140 | 120 villages from four districts (Karimnagar, Chittoor, East and West Godavari) | Mf and vector infection prevalence | 23,624 persons | All ages | The study was conducted in the state of Andhra Pradesh in India (Upadhyayula et al, 2012). This study is primarily on spatial mapping and analysis of LF over a 3-year period from 2004 to 2007, during which the MDA was implemented.Blood samples were collected from individuals and data on entomology and socio-economical aspects were collected. Mf prevalence in these districts was 31.5% (with the least being 8.9%) and the disease rate ranged between 0.5% and 2.3%. All the villages were geo-referenced with way points, and these were used to create maps on filariasis at village level. While both east and west Godavari districts were hyper endemic, Karim Nagar and Chittoor fell under medium endemicity. The spatial mapping showed various levels of infection even after implementation of MDA |
| 3 | Africa | Tanzania | Simonsen et al, 2014141 | Seven districts of Tanga region | Mf and Ag prevalence | 2753 persons for Ag 555 persons for Mf 1700 mosquitoes |
All ages | The study describes the LF control started in rural areas of Tanga region of Tanzania in 2004 completing 8 rounds currently. Levels of transmission and human infection decreased initially, and became less pronounced in subsequent years. Even after 6 MDAs transmission was on-going at a reduced level. Seven other districts in the Tanga region (total 8 districts) were included for monitoring in 2013. Spot check community and school based were conducted in Tanga district. Four hamlets of Kirare village were for the community study and standard I pupils (6–7-year-olds) were examined for Ag (ICT) every year. In other districts, two villages were purposively selected from each district. A total of 200 6–7-year-olds were screened for Ag during 2013. Those positive for Ag only were bled for Mf smears. Vector surveillance was done using light traps in 50 selected households of Kirare village. Mapping was done by using the geo-coordinates of the sites and kriging was used to estimate prevalence in regions that were not surveyed. Results from Tanga district surveys indicate that both Mf and Ag prevalences reduced compared to baseline levels. Prevalence of chronic filarial manifestations (hydrocele/elephantiasis) was less than half of that in 2004. Dissection of msoquitoes post MDA 7 and eight rounds showed no filarial infections. Ag prevalence in 6-7yo since the start of MDA had a declining trend and after the 8th round, the prevalence was 2.3% (91% reduction compared to pre-MDA level of 25%). The authors conclude that though there is a positive downward trend in LF transmission and human infection, LF is still widespread in many parts of the Tanga region even after eight rounds of MDA, particularly in coastal areas. Lower infection rates in inland districts suggests that MDA could be stepped down after rigorous assessment so that resources could be diverted to upscale control activities in coastal districts. |
| 4 | Western Pacific | American Samoa | Lau et al, 2014142 | Three islands | Ag and Ab prevalence | 555 persons | All ages | In this study (Lau et al, 2014), the success story of MDA for 5 years (2000–2006) following which the MDA was stopped (based on TAS) and the post MDA surveillance thereafter is described in detail. The LF antigen (Og4C3) and antibody (Wb123 and Bm14) tests were performed on the adult serum samples (n=807) from a serum bank where the samples were collected for a study on leptospirosis, 4 years after the last effective round of MDA. Information on the residence of the participant whose serum was used was used to look into geographical clustering of serologically positive cases. Results showed that which Ag prevalence was 0.75%, antibody prevalences were 8.1% for Wb123 an 17.9% for Bm14. Antigen and antibody prevalences were inversely associated with number of years of living in American Samoa. While antigen prevalence showed spatial variation, antibody prevalence did not show any spatial pattern. Higher prevalence of antigen in adults shows that residual infection is still there in the communities. Further, migrants (from neighbour LF endemic areas) showed higher antigen prevalences. Authors suggest that TAS may not be able to detect transmission hotspots. In the discussion the authors suggest that the risk of LF and drivers of transmission are likely to be heterogeneous within any evaluation units, and could be influenced by many factors such as climatic conditions, population density, urban versus rural areas, MDA coverage, and vector species and density. Therefore the average prevalence in an evaluation unit could mask focal areas of high prevalence (hotspots) if they are surrounded by large areas of low prevalence. Hotspots are more likely to be missed if they are small, in evaluation areas with greater spatial heterogeneity, and when prevalence is very low such as in the post-MDA surveillance phase. |
| 5 | Western Pacific | Papua New Guinea | Graves et al, 2013143 | 324 survey sites between 1980 and 2011 in 80 districts | Mf and Ag prevalence | 37,425 persons for MF 43,264 persons for ICT 16,221 persons for Og4c3 |
All ages | This study is a review in which the LF elimination programme in Papua New Guinea at district level since 1980 to 2011, ie pre and during MDA. Data from a total of 312 district level surveys were utilized for this study. Data on Mf and Ag(ICT and Og4C3) were collected. Results indicate that, on combining these data, the estimates for Mf, ICT and Og4C3 when crudely averaged over districtswere 18.5% for Mf, 10.1% for ICT and 45.5% for Og4C3. Comparison in terms of diagnostic methods showed that Mf prevalence was always lower than ICT prevalence in most surveys. Only one survey reported higher Mf prevalence than that of Ag prevalence (using Og4C3). With respect to time period over every 10 years, it was seen that there was a decline in Mf and Og4C3 prevalences. However no obvious decline was observed in Ag prevalence based on ICT. Results indicated that there was large variation in LF prevalence in the country which may be related to the diverse altitude, geographical and ecological factors and also to the mosquito vectors in different regions of the country. Further, the heterogeneity could be due to the previous malaria control activities and recent distribution of insecticide mosquito nets and to some extent the MDA for LF in limited sites. It was also shown that the number of LF endemic districts differed with GPELF criteria specified earlier and the modified forms. While the earlier criteria classified 60 endemic districts, the modified gave only 36 endemic districts. The alternative criteria defining endemic districts as that one with ≥1% Ag prevalence classified only 34 as endemic districts. On the whole, based on these criteria, about 20 districts do not require MDA as per GPELF criteria 1 and 2. At the outset this review highlights the gaps in data and the knowledge on the remaining unknown districts. |
Abbreviations: LF, Lymphatic filariasis; DEC, Diethylcarbamazine; IVM, Ivermectin; Ag, Antigeneamia; Mf, Microfilaraemia; Ab, Antibody; MDA, Mass drug administration; TAS, Transmission Assessment Survey; ICT, Immunochromatographic Test; MX, Molecular Xenomontoring.
In Haiti, of the five districts that were mapped as low endemic based on Ag survey prior to MDA,137 transmission was on-going during MDA in three districts and a possibility of spatial dependency up to a distance of 20 m from an index case (an Ag-positive child). Spatial mapping of Mf prevalence (overall 31.5%, least 8.5%) after three rounds of MDA in four endemic districts of Andhra Pradesh in India showed spatial heterogeneity with non-interruption of transmission.138
A study in Tanzania139 showed that even after six rounds of MDA, transmission was on-going but at a lower level. However, the Ag prevalence in 6–7-year-olds had started showing a declining trend. One study,140 using samples from a serum bank assessed the prevalence of LF Ag and Ab, 4 years after stopping MDA. They reported that while Ag prevalence was very low (0.8%), Ab prevalence (8.1%) was high as expected. It was seen that while Ag prevalence showed spatial variation, Ab prevalence did not. Another study141 from Papua New Guinea that reviewed the district level distribution of MDA during 1980–2011, reported that targeting of districts by level of prevalence will strengthen the control program, facilitate monitoring of the disease trend and increase the likelihood of reaching the target of LF elimination by 2020.
Discussion
This review on mapping and monitoring of LF is based on 84 studies from 32 countries representing all WHO regions except the European region over two decades between 1997 and 2018. Most African countries followed the mapping protocol for delimitation in addition to historical data. Countries from other regions used WHO mapping strategy and other methods like the Key informant technique,70 physical examination by health workers for chronic symptoms of filariasis, disease prevalence and Mf prevalence for mapping LF. The review has shown how the WHO mapping strategy that initially used convenience sampling design evolved to become a more robust 30-cluster-based design in line with the confirmatory mapping tool94,95 to delimit areas requiring MDA. This confirmatory mapping tool tested Ag among older children aged 9–14 years in 30 randomly selected schools and was proved to be more effective in low-prevalence settings. Though this tool is similar to the LF-TAS, the sample size requirement is only one-fourth of that of TAS and covers older children and is currently termed as “Mini-TAS.” In the current scenario, where most LF endemic countries have completed at least five rounds of MDA, it is proposed to use this for re-mapping in uncertain and naive areas bordering the endemic districts or the unsurveyed areas that have the risk of LF transmission to help the country program to bring in or exclude those areas from the MDA net. However, the challenge will be to choose the appropriate test (Ag/Ab surveys in humans or MX for vector infection) for re-mapping to identify new transmission hotspots if any, and prevent resurgence during post-MDA surveillance.
This review has highlighted how mapping helped in implementing the elimination program only in the areas known to be endemic for LF thereby preventing MDA in areas where the LF was non-endemic. Mf and Ag surveys were used in prioritizing areas requiring MDA and these data were subsequently used to visualize the extent of LF distribution. Accounting for the spatial component in these data with appropriate spatial models, LF prevalence was predicted up to a distance of 25–50 km around the surveyed locations to provide additional information on risk of LF to the program authorities to act upon.60,90,93,96–98 Such data also provided scientific evidence on the clustering nature of LF around a transmission/residual foci which again could be used to extend MDA or other supplementary interventions in those neighboring areas. In addition, mapping data collected over time from different countries were also useful for modeling the trends of LF prevalence accounting for the effect of climate change on parasite transmission.91,92
The mapping initiative for LF also paved the way to integrate mapping of all NTDs (LF, podoconiosis, schistosomiasis, loasis and STH) which was proven to be cost effective and feasible while covering a large geographical area.64,88,89,100,142 However, it could be logistically intensive and methodologically difficult due to the difference in the tests used and the target groups to be covered. As this is one of the important activities under the WHO road map143 for implementing interventions against NTDs, the challenges for the endemic countries would be in terms of strong in-country leadership, man power and resources to conduct the surveys and application to capture the data to make it cost effective and feasible.
One of the reasons for the success of this large scale elimination program is attributed to the strong in-built component of monitoring and evaluation during- and post-MDA. The review has shown that impact of MDA is generally measured in terms of Mf and Ag prevalence in humans. However, in recent times Ab prevalence in humans and MX for assessing parasite DNA in vectors are being used for the purpose in view of their high sensitivity in detecting exposure/transmission. It was observed that the WHO recommended strategy of 5–6 rounds of MDA was not sufficient in interrupting transmission103,107,109,111,114,118,119,123 suggesting the requirement of additional interventions like frequent MDA treatments, distribution of insecticide treated nets and other localized vector control measures.118 Additional efforts warranting improved MDA coverage and adequate monitoring by the implementing authorities were recommended. Non-interruption of transmission and persistence of hotspots in spite of several rounds of MDA (up to 12 rounds) were found to be significantly associated with high baseline prevalence of Mf or Ag118,123,124 and systematic non-compliance to MDA.117 These imply that the number of rounds required to reach the threshold of <1% Mf prevalence (or 2% Ag prevalence) cannot be generalized for all endemic areas. The success stories in Bangladesh124 and Cambodia125 indicated that in addition to the adequate MDA coverage, the reasons for success of the elimination program in a country is driven by 1) the commitment of the government and effective implementation of MDA even in remote areas; 2i) active participation of provincial- and central-level program personnel; 3) adequate financial and related support from stakeholders and partners; and 4) a rigorous monitoring and evaluation plan and post-MDA surveillance activities. The impact of MDA on LF disease, both chronic and acute forms were promising with not only reduction in the disease prevalence following 5–8 rounds of MDA but also in preventing new cases of hydrocele, thereby indirectly achieving the MMDP goals too.104,106
In LF-oncho co-endemic areas, impact assessment surveys during MDA showed that several rounds of MDA with monotherapy of Ivermectin alone, 100, 107, 113 could not interrupt LF transmission and inclusion of albendazole in the subsequent rounds helped in reducing the Mf and Ag prevalence in humans and vector infection and infectivity rates significantly, respectively.109,111 However, the decision on stopping MDA in such areas would imply stopping albendazole but continuing with ivermectin alone along with post-MDA surveillance to ensure there is no resurgence of the disease.
TAS, the decision-making tool for stopping or continuing MDA was adjudged to be a robust, scientific and reliable tool based on the results from 12 countries,121 including India,122 based on a single EU. Detection of filaria parasite DNA in vectors in areas with Ag-positive children, showed that the results of TAS and MX corroborate with each other, when the decision to continue MDA is to be made. However in a few countries, it was also shown that assessing the impact of MDA on transmission interruption was more accurate if Ag tests in TAS are complemented with Ab tests during post-MDA surveillance as they provide additional evidence of on-going transmission/exposure in the community, particularly in children.105,115,116,132,134 In some instances, even when both Ag (TAS) and Ab tests indicated interruption of transmission, vector infection prevalence through MX was found to be well above the provisional threshold level of 0.5% (indicating on-going transmission) suggesting that TAS may not be a sensitive tool in assessing complete interruption of transmission,133,135,136,142 particularly during post-MDA surveillance. In view of this, it was recommended MX/Ab surveys may be used as a complementary tool for TAS to assess transmission, particularly during post-MDA surveillance and validation phases.133,135,136,142 These observations suggest that though TAS is a powerful tool while making decisions on stopping MDA or not, it may or may not be very sensitive particularly during the post-MDA surveillance and validation phases when the transmission levels are very low. Therefore MX, a sensitive tool in detecting on-going transmission may also be done alongside so that there is added support to the results by TAS. However, implementing MX under programmatic mode will be a major challenge as the expertise and the facilities for running the molecular assays need to be made available at all implementation unit levels.
Currently, in order to eliminate LF in hard core areas with persisting transmission, WHO has recommended the use of triple drug regimen (IDA, Ivermectin + Diethylcarbamazine + Albendazole) for MDA towards accelerated elimination of LF by 2020.6 While the monitoring and impact evaluation methods are in place for the existing LF elimination strategy of MDA with two drug regimens (DA, DEC + Albendazole),4 the challenge will be to develop an appropriate monitoring and evaluation (M & E) strategy, as this strategy is expected to interrupt transmission with 2–3 rounds of MDA in the endemic areas. In addition to the appropriate indicators sensitive enough to measure the human infection levels and or vector infection/infectivity levels, the target population need to be examined and the diagnostic to be used in humans for assessing transmission interruption needs to be identified.
Conclusions
The review has highlighted the necessity of mapping for LF before a massive intervention program is to be implemented in large geographical area as it helps in identifying only those areas that require the intervention, thereby saving resources and man-power. If the area is endemic for other NTDs as well, an integrated mapping approach would be cost effective as well as feasible. For example in India, if an area is prone for dengue, chikungunya and LF, serological or MX surveys with appropriate test kits/devices with good sampling designs can be used to carry out integrated mapping of these diseases at the same time sharing the man-power and other resources.144 However, to include this as a part of a national program, a substantial investment has to be made in providing the infrastructure and training to personnel to carry out serological and MX surveys for the NTDs prevalent in the area. The review compared the performance of both serological (Ag/Ab) and molecular tools in assessing the status of LF transmission and highlighted that MX could play a major role in the context of M & E for LF. As most endemic countries under GPELF are progressing towards post-MDA surveillance, validation and elimination phases, M & E component needs to be revised/strengthened with appropriate tools either Ag complemented with Ab or MX for verifying the absence of transmission, assessing the risk of resurgence, identifying hotspots, particularly in the context of introduction of IDA as MDA for accelerated elimination.
With this holistic approach, national programs should take advantage of the existing serological tools and MX and incorporate them wherever necessary in their integrated vector management program to control, monitor and eliminate all vector borne diseases. And wherever possible both mapping of diseases and monitoring of interventions should adopt an integrated approach, to assess the current situation with minimum resources.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Michael E, Bundy DAP. Global mapping of lymphatic filariasis. Parasitol Today. 1997;13(12):472–476. [DOI] [PubMed] [Google Scholar]
- 2.WHA. WHA50.29 Elimination of lymphatic filariasis as a public health problem. Hbk Res.. 1997. Vol. III (3rd ed.), 1.16.3.3; 7.1.3 [Google Scholar]
- 3.WHO. Global programme to elimination lymphatic filariasis. Annual Report on Lymphatic filariasis. 2002; WHO/CDS/CPE/CEE/2002.28. [Google Scholar]
- 4.WHO. Transmission assessment surveys in the global programme to eliminate lymphatic filariasis: WHO position statement. Wkly Epidemiol Rec. 2012;87(48):478–482. [PubMed] [Google Scholar]
- 5.WHO. Operational Guidelines for Rapid Mapping of Bancroftian Filariasis in Africa. WHO/CDS/CPE/CEE/2000.9 [press release] Geneva: World Health Organization; 2000. [Google Scholar]
- 6.WHO. Global programme to eliminate lymphatic filariasis: progress report, 2016. Wkly Epidemiol Rec. 2017;46:589–608. [Google Scholar]
- 7.Babu BV. A rapid method to assess the coverage of the mass drug administration of diethylcarbamazine in the program to eliminate lymphatic filariasis in India. Southeast Asian J Trop Med Public Health. 2005;36(1):44–45. [PubMed] [Google Scholar]
- 8.Bhumiratana A, Pechgit P, Koyadun S, Siriaut C, Yongyuth P. Imported bancroftian filariasis: diethylcarbamazine response and benzimidazole susceptibility of Wuchereria bancrofti in dynamic cross-border migrant population targeted by the National Program to Eliminate Lymphatic Filariasis in South Thailand. Acta Trop. 2010;113(2):121–128. doi: 10.1016/j.actatropica.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 9.Boakye DA, Baidoo HA, Glah E, Brown C, Appawu M, Wilson MD. Monitoring lymphatic filariasis interventions: adult mosquito sampling, and improved PCR-based pool screening method for Wuchereria bancrofti infection in Anopheles mosquitoes. Filaria J. 2007;6:13. doi: 10.1186/1475-2883-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cano J, Rebollo MP, Golding N, et al. The global distribution and transmission limits of lymphatic filariasis: past and present. Parasit Vectors. 2014;7:466. doi: 10.1186/1756-3305-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carme B. Mapping of lymphatic filariasis: “to be (in English) or not to be”. Med Trop (Mars). 2010;70(5–6):425–427. [PubMed] [Google Scholar]
- 12.Carme B. Rapid assessment procedure for loiasis and mapping lymphatic filariasis: two perfect illustrations of “to be in English or not to be”. PLoS Negl Trop Dis. 2012;6(12):e1863. doi: 10.1371/journal.pntd.0001863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesnais CB, Vlaminck J, Kunyu-Shako B, et al. Measurement of circulating filarial antigen levels in human blood with a point-of-care test strip and a portable spectrodensitometer. Am J Trop Med Hyg. 2016;94(6):1324–1329. doi: 10.4269/ajtmh.15-0916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rochars MB, Direny AN, Roberts JM, et al. Community-wide reduction in prevalence and intensity of intestinal helminths as a collateral benefit of lymphatic filariasis elimination programs. Am J Trop Med Hyg. 2004;71(4):466–470. [PubMed] [Google Scholar]
- 15.Dunyo SK, Appawu M, Nkrumah FK, Baffoe-Wilmot A, Pedersen EM, Simonsen PE. Lymphatic filariasis on the coast of Ghana. Trans R Soc Trop Med Hyg. 1996;90(6):634–638. [DOI] [PubMed] [Google Scholar]
- 16.Eigege A, Richards FO Jr., Blaney DD, et al. Rapid assessment for lymphatic filariasis in central Nigeria: a comparison of the immunochromatographic card test and hydrocele rates in an area of high endemicity. Am J Trop Med Hyg. 2003;68(6):643–646. [PubMed] [Google Scholar]
- 17.El Setouhy M, Ramzy RM. Lymphatic filariasis in the Eastern Mediterranean Region: current status and prospects for elimination. East Mediterr Health J. 2003;9(4):534–541. [PubMed] [Google Scholar]
- 18.Fontes G, Rocha EM, Brito AC, Antunes CM. Lymphatic filariasis in Brazilian urban area (Maceio, Alagoas). Mem Inst Oswaldo Cruz. 1998;93(6):705–710. [DOI] [PubMed] [Google Scholar]
- 19.Freeman AR, Lammie PJ, Houston R, et al. A community-based trial for the control of lymphatic filariasis and iodine deficiency using salt fortified with diethylcarbamazine and iodine. Am J Trop Med Hyg. 2001;65(6):865–871. [DOI] [PubMed] [Google Scholar]
- 20.Gbakima AA, Appawu MA, Dadzie S, et al. Lymphatic filariasis in Ghana: establishing the potential for an urban cycle of transmission. Trop Med Int Health. 2005;10(4):387–392. doi: 10.1111/j.1365-3156.2005.01389.x [DOI] [PubMed] [Google Scholar]
- 21.Gunawardena S, Gunawardena NK, Kahathuduwa G, et al. Integrated school-based surveillance for soil-transmitted helminth infections and lymphatic filariasis in Gampaha district, Sri Lanka. Am J Trop Med Hyg. 2014;90(4):661–666. doi: 10.4269/ajtmh.13-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gyapong JO, Adjei S, Gyapong M, Asamoah G. Rapid community diagnosis of lymphatic filariasis. Acta Trop. 1996;61(1):65–74. [DOI] [PubMed] [Google Scholar]
- 23.Gyapong JO, Adjei S, Sackey SO. Descriptive epidemiology of lymphatic filariasis in Ghana. Trans R Soc Trop Med Hyg. 1996;90(1):26–30. [DOI] [PubMed] [Google Scholar]
- 24.Gyapong JO, Owusu IO, da-Costa Vroom FB, Mensah EO, Gyapong M. Elimination of lymphatic filariasis: current perspectives on mass drug administration. Res Rep Trop Med. 2018;9:25–33. doi: 10.2147/RRTM.S125204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gyapong JO, Webber RH, Morris J, Bennett S. Prevalence of hydrocele as a rapid diagnostic index for lymphatic filariasis. Trans R Soc Trop Med Hyg. 1998;92(1):40–43. [DOI] [PubMed] [Google Scholar]
- 26.Harb M, Faris R, Gad AM, Hafez ON, Ramzy R, Buck AA. The resurgence of lymphatic filariasis in the Nile delta. Bull World Health Organ. 1993;71(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- 27.Harris JR, Wiegand RE. Detecting infection hotspots: modeling the surveillance challenge for elimination of lymphatic filariasis. PLoS Negl Trop Dis. 2017;11(5):e0005610. doi: 10.1371/journal.pntd.0005610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodges MH, Smith SJ, Fussum D, et al. High coverage of mass drug administration for lymphatic filariasis in rural and non-rural settings in the Western Area, Sierra Leone. Parasit Vectors. 2010;3:120. doi: 10.1186/1756-3305-3-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodges MH, Sonnie M, Turay H, Conteh A, MacCarthy F, Sesay S. Maintaining effective mass drug administration for lymphatic filariasis through in-process monitoring in Sierra Leone. Parasit Vectors. 2010;5:232. doi: 10.1186/1756-3305-5-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houston R. Salt fortified with diethylcarbamazine (DEC) as an effective intervention for lymphatic filariasis, with lessons learned from salt iodization programmes. Parasitology. 2000;121(Suppl):S161–S173. [DOI] [PubMed] [Google Scholar]
- 31.Koudou BG, de Souza DK, Biritwum NK, et al. Elimination of lymphatic filariasis in west African urban areas: is implementation of mass drug administration necessary? Lancet Infect Dis. 2018;18(6):e214–e20. doi: 10.1016/S1473-3099(18)30069-0 [DOI] [PubMed] [Google Scholar]
- 32.Leang R, Socheat D, Bin B, Bunkea T, Odermatt P. Assessment of disease and infection of lymphatic filariasis in Northeastern Cambodia. Trop Med Int Health. 2004;9(10):1115–1120. doi: 10.1111/j.1365-3156.2004.01311.x [DOI] [PubMed] [Google Scholar]
- 33.Lenhart A, Eigege A, Kal A, et al. Contributions of different mosquito species to the transmission of lymphatic filariasis in central Nigeria: implications for monitoring infection by PCR in mosquito pools. Filaria J. 2007;6:14. doi: 10.1186/1475-2883-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsay SW, Thomas CJ. Mapping and estimating the population at risk from lymphatic filariasis in Africa. Trans R Soc Trop Med Hyg. 2000;94(1):37–45. [DOI] [PubMed] [Google Scholar]
- 35.Mandal NN, Bal MS, Das MK, Achary KG, Kar SK. Lymphatic filariasis in children: age dependent prevalence in an area of India endemic for Wuchereria bancrofti infection. Trop Biomed. 2010;27(1):41–46. [PubMed] [Google Scholar]
- 36.Manhenje I, Galán-Puchades MT, Fuentes MV. Socio-environmental variables and transmission risk of lymphatic filariasis in central and northern Mozambique. Geospat Health. 2013;7(2):391–398. doi: 10.4081/gh.2013.96 [DOI] [PubMed] [Google Scholar]
- 37.Melrose W, Rahmah N. Use of Brugia Rapid dipstick and ICT test to map distribution of lymphatic filariasis in the Democratic Republic of Timor-Leste. Southeast Asian J Trop Med Public Health. 2006;37(1):22–25. [PubMed] [Google Scholar]
- 38.Meyrowitsch DW, Nguyen DT, Hoang TH, Nguyen TD, Michael E. A review of the present status of lymphatic filariasis in Vietnam. Acta Trop. 1998;70(3):335–347. [DOI] [PubMed] [Google Scholar]
- 39.Modi A, Gamit S, Jesalpura BS, Kurien G, Kosambiya JK. Reaching endpoints for lymphatic filariasis elimination- results from mass drug administration and nocturnal blood surveys, South Gujarat, India. PLoS Negl Trop Dis. 2017;11(4):e0005476. doi: 10.1371/journal.pntd.0005476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohammad A, Hussain AKS, Swain S, Kadam S, Sanghamitra P. Mass drug administration for lymphatic filariasis elimination in a coastal state of India: a study on barriers to coverage and compliance. Infect Dis Poverty. 2014;3: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammed KA, Molyneux DH, Albonico M, Rio F. Progress towards eliminating lymphatic filariasis in Zanzibar: a model programme. Trends Parasitol. 2006;22(7):340–344. doi: 10.1016/j.pt.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 42.Molyneux DH. Filaria control and elimination: diagnostic, monitoring and surveillance needs. Trans R Soc Trop Med Hyg. 2009;103(4):338–341. doi: 10.1016/j.trstmh.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 43.Msyamboza K, Ngwira B, Banda R, Mkwanda S, Brabin B. Sentinel surveillance of lymphatic filariasis, schistosomiasis soil transmitted helminths and malaria in rural southern Malawi. Malawi Med J. 2010;22(1):12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joseph N, Subba SH, Jain A, Unnikrishnan B, Nagaraj K, Kotian SM. Awareness of health personnel about lymphatic filariasis and mass drug administration in Karnataka state of South India. Australas Med J. 2011;4(2):87–93. doi: 10.4066/AMJ.2011.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odermatt P, Leang R, Bin B, Bunkea T, Socheat D. Prevention of lymphatic filariasis with insecticide-treated bednets in Cambodia. Ann Trop Med Parasitol. 2008;102(2):135–142. doi: 10.1179/136485908X252313 [DOI] [PubMed] [Google Scholar]
- 46.Pani SP, Srividya A, Krishnamoorthy K, Das PK, Dhanda V. Rapid assessment procedures (RAP) for lymphatic filariasis. Natl Med J India. 1997;10(1):19–22. [PubMed] [Google Scholar]
- 47.Pelletreau S, Nyaku M, Dembele M, et al. The field-testing of a novel integrated mapping protocol for neglected tropical diseases. PLoS Negl Trop Dis. 2011;5(11):e1380. doi: 10.1371/journal.pntd.0001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pichon G, Merlin M, Fagneaux G, Riviere F, Laigret J. [Studies of the numerical distribution of microfilariae in foci of lymphatic filariasis (author‘s transl)]. Tropenmed Parasitol. 1980;31(2):165–180. [PubMed] [Google Scholar]
- 49.Adhikari RK, Sherchand JB, Mishra SR, Ranabhat K, Wagle RR. Awareness and coverage of mass drug administration for elimination of lymphatic filariasis: a community based cross sectional study in Nepal. J Community Health. 2015;40(1):30–40. doi: 10.1007/s10900-014-9891-1 [DOI] [PubMed] [Google Scholar]
- 50.Rebollo MP, Bockarie MJ. Shrinking the lymphatic filariasis map: update on diagnostic tools for mapping and transmission monitoring. Parasitology. 2014;141(14):1912–1917. doi: 10.1017/S0031182014001231 [DOI] [PubMed] [Google Scholar]
- 51.Schneider MC, Aguilera XP, Barbosa da Silva Junior J, et al. Elimination of neglected diseases in latin america and the Caribbean: a mapping of selected diseases. PLoS Negl Trop Dis. 2011;5(2):e964. doi: 10.1371/journal.pntd.0001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh S, Raina VK, Bora D, Dhariwal AC, Lal S. Lymphatic filariasis in Bilaspur district, Chhattisgarh. J Commun Dis. 2005;37(2):125–130. [PubMed] [Google Scholar]
- 53.Srividya A, Michael E, Palaniyandi M, Pani SP, Das PK. A geostatistical analysis of the geographic distribution of lymphatic filariasis prevalence in southern India. Am J Trop Med Hyg. 2002;67(5):480–489. [DOI] [PubMed] [Google Scholar]
- 54.Steel C, Golden A, Kubofcik J, et al. Rapid Wuchereria bancrofti-specific antigen Wb123-based IgG4 immunoassays as tools for surveillance following mass drug administration programs on lymphatic filariasis. Clin Vaccine Immunol. 2013;20(8):1155–1161. doi: 10.1128/CVI.00252-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sukhvir S, Bora D, Dhariwal AC, Pawan D, Shiv L. Epidemiological, clinical and entomological observations on lymphatic filariasis in urban Puri, Orissa. J Commun Dis. 2008;40(2):161–165. [PubMed] [Google Scholar]
- 56.Terranella A, Eigiege A, Gontor I, et al. Urban lymphatic filariasis in central Nigeria. Ann Trop Med Parasitol. 2006;100(2):163–172. doi: 10.1179/136485906X86266 [DOI] [PubMed] [Google Scholar]
- 57.Triteeraprapab S, Karnjanopas K, Porksakorn C, Sai-Ngam A, Yentakam S, Loymak S. Lymphatic filariasis caused by Brugia malayi in an endemic area of Narathiwat Province, southern of Thailand. J Med Assoc Thai. 2001;84(Suppl 1):S182–8. [PubMed] [Google Scholar]
- 58.Wynd S, Carron J, Selve B, Leggat PA, Melrose W, Durrheim DN. Qualitative analysis of the impact of a lymphatic filariasis elimination programme using mass drug administration on Misima Island, Papua New Guinea. Filaria J. 2007;6:1. doi: 10.1186/1475-2883-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yousrya M, Abdel-Hamid MIS, Kenawy M. Geographical distribution and relative abundance of culicine mosquitoes in relation to transmission of lymphatic filariasis in El Menoufia Governorate, Egypt. J Egypt Soc Parasitol. 2011;41(1):109–118. [PubMed] [Google Scholar]
- 60.Gyapong JO, Kyelem D, Kleinschmidt I, et al. The use of spatial analysis in mapping the distribution of bancroftian filariasis in four West African countries. Ann Trop Med Parasitol. 2002;96(7):695–705. doi: 10.1179/000349802125001735 [DOI] [PubMed] [Google Scholar]
- 61.Sherchand JB, Obsomer V, Thakur GD, Hommel M. Mapping of lymphatic filariasis in Nepal. Filaria J. 2003;2(1):7. doi: 10.1186/1475-2883-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ngwira BM, Tambala P, Perez AM, Bowie C, Molyneux DH. The geographical distribution of lymphatic filariasis infection in Malawi. Filaria J. 2007;6:12. doi: 10.1186/1475-2883-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruberanziza E, Mupfasoni D, Karibushi B, et al. Mapping of lymphatic filariasis in Rwanda. J Lymphoedema. 2009;4(1):20–23. [Google Scholar]
- 64.Sturrock HJ, Picon D, Sabasio A, et al. Integrated mapping of neglected tropical diseases: epidemiological findings and control implications for northern Bahr-el-Ghazal State, Southern Sudan. PLoS Negl Trop Dis. 2009;3(10):e537. doi: 10.1371/journal.pntd.0000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iboh CI, Okon OE, Opara KN, Asor JE, Etim SE. Lymphatic filariasis among the Yakurr people of Cross River State, Nigeria. Parasit Vectors. 2012;5:203. doi: 10.1186/1756-3305-5-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shawa ST, Mwase ET, Pedersen EM, Simonsen PE. Lymphatic filariasis in Luangwa District, South-East Zambia. Parasit Vectors. 2013;6(1):299. doi: 10.1186/1756-3305-6-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mwase ET, Stensgaard AS, Nsakashalo-Senkwe M, et al. Mapping the geographical distribution of lymphatic filariasis in Zambia. PLoS Negl Trop Dis. 2014;8(2):e2714. doi: 10.1371/journal.pntd.0002714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rebollo MP, Sime H, Assefa A, et al. Shrinking the lymphatic filariasis map of Ethiopia: reassessing the population at risk through nationwide mapping. PLoS Negl Trop Dis. 2015;9(11):e0004172. doi: 10.1371/journal.pntd.0004172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sabesan S, Palaniyandi M, Das PK, Michael E. Mapping of lymphatic filariasis in India. Ann Trop Med Parasitol. 2000;94(6):591–606. [DOI] [PubMed] [Google Scholar]
- 70.Awolola TS, Manafa OU, Idowu ET, Adedoyin JA, Adeney AK. Epidemiological mapping of lymphatic filariasis in southern Nigeria. Preliminary sury of Akinyele local govenment area. Afr J Clin Exp Microbiol. 2004;5(3):231–234. [Google Scholar]
- 71.Chhotray GP, Ranjit MR, Khuntia HK, Acharya AS. Precontrol observations on lymphatic filariasis & geo-helminthiases in two coastal districts of rural Orrisa. Indian J Med Res. 2005;122(5):388–394. [PubMed] [Google Scholar]
- 72.Das VN, Siddiqui NA, Kumar N, et al. A pilot study on the status of lymphatic filariasis in a rural community of Bihar. J Commun Dis. 2006;38(2):169–175. [PubMed] [Google Scholar]
- 73.Singh S, Bora D, Dhariwal AC, Singh R, Lal S. Lymphatic filariasis in rural areas of Patna District, Bihar. A challenge ahead. J Commun Dis. 2006;38(2):160–163. [PubMed] [Google Scholar]
- 74.Bonfim C, Aguiar-Santos AM, Pedroza D Jr., et al. Social deprivation index and lymphatic filariasis: a tool for mapping urban areas at risk in northeastern Brazil. Int Health. 2009;1(1):78–84. doi: 10.1016/j.inhe.2009.06.007 [DOI] [PubMed] [Google Scholar]
- 75.Bonfim C, Netto MJ, Pedroza D, Portugal JL, Medeiros Z. A socioenvironmental composite index as a tool for identifying urban areas at risk of lymphatic filariasis. Trop Med Int Health. 2009;14(8):877–884. doi: 10.1111/j.1365-3156.2009.02317.x [DOI] [PubMed] [Google Scholar]
- 76.Kelly-Hope LA, Thomas BC, Bockarie MJ, Molyneux DH. Lymphatic filariasis in the Democratic Republic of Congo; micro-stratification overlap mapping (MOM) as a prerequisite for control and surveillance. Parasit Vectors. 2011;4:178. doi: 10.1186/1756-3305-4-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brandao E, Bonfim C, Cabral D, et al. Mapping of Wuchereria bancrofti infection in children and adolescents in an endemic area of Brazil. Acta Trop. 2011;120(1–2):151–154. doi: 10.1016/j.actatropica.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 78.Brandao E, Bonfim C, Alves A, et al. Lymphatic filariasis among children and adolescents: spatial identification via socio-environmental indicators to define priority areas for elimination. Int Health. 2015;7(5):324–331. doi: 10.1093/inthealth/ihv053 [DOI] [PubMed] [Google Scholar]
- 79.Koroma JB, Bangura MM, Hodges MH, Bah MS, Zhang Y, Bockarie MJ. Lymphatic filariasis mapping by immunochromatographic test cards and baseline microfilaria survey prior to mass drug administration in Sierra Leone. Parasit Vectors. 2012;5:10. doi: 10.1186/1756-3305-5-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shiferaw W, Kebede T, Graves PM, et al. Lymphatic filariasis in western Ethiopia with special emphasis on prevalence of Wuchereria bancrofti antigenaemia in and around onchocerciasis endemic areas. Trans R Soc Trop Med Hyg. 2012;106(2):117–127. doi: 10.1016/j.trstmh.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 81.Okorie PN, Ademowo GO, Saka Y, et al. Lymphatic filariasis in Nigeria; micro-stratification overlap mapping (MOM) as a prerequisite for cost-effective resource utilization in control and surveillance. PLoS Negl Trop Dis. 2013;7(9):e2416. doi: 10.1371/journal.pntd.0002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nana-Djeunga HC, Tchatchueng-Mbougua JB, Bopda J, et al. Mapping of bancroftian filariasis in cameroon: prospects for elimination. PLoS Negl Trop Dis. 2015;9(9):e0004001. doi: 10.1371/journal.pntd.0004001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ngwira BM, Jabu CH, Kanyongoloka H, et al. Lymphatic filariasis in the Karonga district of northern Malawi: a prevalence survey. Ann Trop Med Parasitol. 2002;96(2):137–144. doi: 10.1179/0003498302125000411 [DOI] [PubMed] [Google Scholar]
- 84.Onapa AW, Simonsen PE, Baehr I, Pedersen EM. Rapid assessment of the geographical distribution of lymphatic filariasis in Uganda, by screening of schoolchildren for circulating filarial antigens. Ann Trop Med Parasitol. 2005;99(2):141–153. doi: 10.1179/136485905X19829 [DOI] [PubMed] [Google Scholar]
- 85.Hassan AN, Dister S, Beck L. Spatial analysis of lymphatic filariasis distribution in the Nile Delta in relation to some environmental variables using geographic information system technology. J Egypt Soc Parasitol. 1998;28(1):119–131. [PubMed] [Google Scholar]
- 86.Gyapong JO, Remme JH. The use of grid sampling methodology for rapid assessment of the distribution of bancroftian filariasis. Trans R Soc Trop Med Hyg. 2001;95(6):681–686. [DOI] [PubMed] [Google Scholar]
- 87.Medeiros Z, Bonfim C, Brandao E, et al. Using kernel density estimates to investigate lymphatic filariasis in northeast Brazil. Pathog Glob Health. 2012;106(2):113–117. doi: 10.1179/2047773212Y.0000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sime H, Deribe K, Assefa A, et al. Integrated mapping of lymphatic filariasis and podoconiosis: lessons learnt from Ethiopia. Parasit Vectors. 2014;7:397. doi: 10.1186/1756-3305-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Finn TP, Stewart BT, Reid HL, et al. Integrated rapid mapping of neglected tropical diseases in three States of South Sudan: survey findings and treatment needs. PLoS One. 2012;7(12):e52789. doi: 10.1371/journal.pone.0052789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stensgaard AS, Vounatsou P, Onapa AW, et al. Bayesian geostatistical modelling of malaria and lymphatic filariasis infections in Uganda: predictors of risk and geographical patterns of co-endemicity. Malar J. 2011;10:298. doi: 10.1186/1475-2875-10-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slater H, Michael E. Predicting the current and future potential distributions of lymphatic filariasis in Africa using maximum entropy ecological niche modelling. PLoS One. 2012;7(2):e32202. doi: 10.1371/journal.pone.0032202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Slater H, Michael E. Mapping, bayesian geostatistical analysis and spatial prediction of lymphatic filariasis prevalence in Africa. PLoS One. 2013;8(8):e71574. doi: 10.1371/journal.pone.0071574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moraga P, Cano J, Baggaley RF, et al. Modelling the distribution and transmission intensity of lymphatic filariasis in sub-Saharan Africa prior to scaling up interventions: integrated use of geostatistical and mathematical modelling. Parasit Vectors. 2015;8:560. doi: 10.1186/s13071-015-1166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gass KM, Sime H, Mwingira UJ, et al. The rationale and cost-effectiveness of a confirmatory mapping tool for lymphatic filariasis: examples from Ethiopia and Tanzania. PLoS Negl Trop Dis. 2017;11(10):e0005944. doi: 10.1371/journal.pntd.0005944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sime H, Gass KM, Mekasha S, et al. Results of a confirmatory mapping tool for Lymphatic filariasis endemicity classification in areas where transmission was uncertain in Ethiopia. PLoS Negl Trop Dis. 2018;12(3):e0006325. doi: 10.1371/journal.pntd.0006325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sabesan S, Raju KH, Subramanian S, Srivastava PK, Jambulingam P. Lymphatic filariasis transmission risk map of India, based on a geo-environmental risk model. Vector Borne Zoonotic Dis. 2013;13(9):657–665. doi: 10.1089/vbz.2012.1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dhimal M, Gautam I, Kress A, Muller R, Kuch U. Spatio-temporal distribution of dengue and lymphatic filariasis vectors along an altitudinal transect in Central Nepal. PLoS Negl Trop Dis. 2014;8(7):e3035. doi: 10.1371/journal.pntd.0003035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chand G, Kaushal LS, Choudhari NK, Singh N. Mapping is a prerequisite for elimination of filariasis and effective targeting of filarial ‘hot spots‘. Pathog Glob Health. 2016;110(4–5):157–163. doi: 10.1080/20477724.2016.1205302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wijegunawardana ND, Gunawardene YI, Manamperi A, Senarathne H, Abeyewickreme W. Geographic information system (GIS) mapping of lymphatic filariasis endemic areas of Gampaha District, Sri Lanka based on epidemiological and entomological screening. Southeast Asian J Trop Med Public Health. 2012;43(3):557–566. [PubMed] [Google Scholar]
- 100.Stanton MC, Mkwanda S, Mzilahowa T, Bockarie MJ, Kelly-Hope LA. Quantifying filariasis and malaria control activities in relation to lymphatic filariasis elimination: a multiple intervention score map (MISM) for Malawi. Trop Med Int Health. 2014;19(2):224–235. doi: 10.1111/tmi.12266 [DOI] [PubMed] [Google Scholar]
- 101.Smith EL, Mkwanda SZ, Martindale S, Kelly-Hope LA, Stanton MC. Lymphatic filariasis morbidity mapping: a comprehensive examination of lymphoedema burden in Chikwawa district, Malawi. Trans R Soc Trop Med Hyg. 2014;108(12):751–758. doi: 10.1093/trstmh/tru150 [DOI] [PubMed] [Google Scholar]
- 102.Hochberg N, Michel MC, Lammie PJ, et al. Symptoms reported after mass drug administration for lymphatic filariasis in Leogane, Haiti. Am J Trop Med Hyg. 2006;75(5):928–932. [PubMed] [Google Scholar]
- 103.Mukhopadhyay AK, Patnaik SK, Babu PS. Status of lymphatic filariasis in parts of east Godavari district of Andhra Pradesh, India. J Vector Borne Dis. 2007;44(1):72–74. [PubMed] [Google Scholar]
- 104.Yuvaraj J, Pani SP, Vanamail P, Ramaiah KD, Das PK. Impact of seven rounds of mass administration of diethylcarbamazine and ivermectin on prevalence of chronic lymphatic filariasis in south India. Trop Med Int Health. 2008;13(5):737–742. doi: 10.1111/j.1365-3156.2008.02044.x [DOI] [PubMed] [Google Scholar]
- 105.Joseph H, Maiava F, Naseri T, Silva U, Lammie P, Melrose W. Epidemiological assessment of continuing transmission of lymphatic filariasis in Samoa. Ann Trop Med Parasitol. 2011;105(8):567–578. doi: 10.1179/2047773211Y.0000000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tisch DJ, Alexander ND, Kiniboro B, et al. Reduction in acute filariasis morbidity during a mass drug administration trial to eliminate lymphatic filariasis in Papua New Guinea. PLoS Negl Trop Dis. 2011;5(7):e1241. doi: 10.1371/journal.pntd.0001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shriram AN, Krishnamoorthy K, Sivan A, Saha BP, Kumaraswami V, Vijayachari P. Impact of MDA and the prospects of elimination of the lone focus of diurnally sub periodic lymphatic filariasis in Nicobar Islands, India. Acta Trop. 2014;133:93–97. doi: 10.1016/j.actatropica.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 108.Dewi RM, Tuti S, Ganefa S, et al. Brugia Rapid antibody responses in communities of Indonesia in relation to the results of ‘transmission assessment surveys‘ (TAS) for the lymphatic filariasis elimination program. Parasit Vectors. 2015;8:499. doi: 10.1186/s13071-015-1093-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Endeshaw T, Taye A, Tadesse Z, et al. Presence of Wuchereria bancrofti microfilaremia despite 7 years of annual ivermectin monotherapy mass drug administration for onchocerciasis control: a study in north-west Ethiopia. Pathog Glob Health. 2015;109(7):344–351. doi: 10.1080/20477724.2015.1103501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hafiz I, Graves P, Haq R, Flora MS, Kelly-Hope LA. Clinical case estimates of lymphatic filariasis in an endemic district of Bangladesh after a decade of mass drug administration. Trans R Soc Trop Med Hyg. 2015;109(11):700–709. doi: 10.1093/trstmh/trv084 [DOI] [PubMed] [Google Scholar]
- 111.Wilson NO, Badara Ly A, Cama VA, et al. Evaluation of lymphatic filariasis and onchocerciasis in three Senegalese Districts treated for onchocerciasis with ivermectin. PLoS Negl Trop Dis. 2016;10(12):e0005198. doi: 10.1371/journal.pntd.0005198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mengistu B, Deribe K, Kebede F, et al. The national programme to eliminate lymphatic filariasis from Ethiopia. Ethiop Med J. 2017;55(Suppl 1):45–54. [PMC free article] [PubMed] [Google Scholar]
- 113.Vaishnav KG, Desai HS, Srivastava PK, et al. Impact of mass drug administration on elimination of lymphatic filariasis in Surat city, India. J Commun Dis. 2012;44(4):251–259. [PubMed] [Google Scholar]
- 114.Khan AM, Dutta P, Sarmah CK, et al. Prevalence of lymphatic filariasis in a tea garden worker population of Dibrugarh (Assam), India after six rounds of mass drug administration. J Vector Borne Dis. 2015;52(4):314–320. [PubMed] [Google Scholar]
- 115.Simonsen PE, Pedersen EM, Rwegoshora RT, Malecela MN, Derua YA, Magesa SM. Lymphatic filariasis control in Tanzania: effect of repeated mass drug administration with ivermectin and albendazole on infection and transmission. PLoS Negl Trop Dis. 2010;4(6):e696. doi: 10.1371/journal.pntd.0000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simonsen PE, Magesa SM, Derua YA, Rwegoshora RT, Malecela MN, Pedersen EM. Monitoring lymphatic filariasis control in Tanzania: effect of repeated mass drug administration on circulating filarial antigen prevalence in young schoolchildren. Int Health. 2011;3(3):182–187. doi: 10.1016/j.inhe.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 117.Boyd A, Won KY, McClintock SK, et al. A community-based study of factors associated with continuing transmission of lymphatic filariasis in Leogane, Haiti. PLoS Negl Trop Dis. 2010;4(3):e640. doi: 10.1371/journal.pntd.0000640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Richards FO, Eigege A, Miri ES, et al. Epidemiological and entomological evaluations after six years or more of mass drug administration for lymphatic filariasis elimination in Nigeria. PLoS Negl Trop Dis. 2011;5(10):e1346. doi: 10.1371/journal.pntd.0001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mladonicky JM, King JD, Liang JL, et al. Assessing transmission of lymphatic filariasis using parasitologic, serologic, and entomologic tools after mass drug administration in American Samoa. Am J Trop Med Hyg. 2009;80(5):769–773. [PubMed] [Google Scholar]
- 120.Farid HA, Morsy ZS, Helmy H, Ramzy RM, El Setouhy M, Weil GJ. A critical appraisal of molecular xenomonitoring as a tool for assessing progress toward elimination of lymphatic filariasis. Am J Trop Med Hyg. 2007;77(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- 121.Chu BK, Deming M, Biritwum NK, et al. Transmission assessment surveys (TAS) to define endpoints for lymphatic filariasis mass drug administration: a multicenter evaluation. PLoS Negl Trop Dis. 2013;7(12):e2584. doi: 10.1371/journal.pntd.0002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Srivastava PKK, Sachin G, Sunita P, et al. Elimination of lymphatic filariasis in Goa: first successful transmission assessment survey in India. J Commun Dis. 2014;46(2):7–16. [Google Scholar]
- 123.Biritwum NK, Garshong B, Alomatu B, de Souza DK, Gyapong M, Kyelem D. Improving drug delivery strategies for lymphatic filariasis elimination in urban areas in Ghana. PLoS Negl Trop Dis. 2017;11(5):e0005619. doi: 10.1371/journal.pntd.0005619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shamsuzzaman AK, Haq R, Karim MJ, et al. The significant scale up and success of transmission assessment surveys ‘TAS‘ for endgame surveillance of lymphatic filariasis in Bangladesh: one step closer to the elimination goal of 2020. PLoS Negl Trop Dis. 2017;11(1):e0005340. doi: 10.1371/journal.pntd.0005340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khieu V, Or V, Tep C, et al. How elimination of lymphatic filariasis as a public health problem in the Kingdom of Cambodia was achieved. Infect Dis Poverty. 2018;7(1):15. doi: 10.1186/s40249-018-0394-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ojha CR, Joshi B, Kc KP, et al. Impact of mass drug administration for elimination of lymphatic filariasis in Nepal. PLoS Negl Trop Dis. 2017;11(7):e0005788. doi: 10.1371/journal.pntd.0005788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aye NN, Lin Z, Lon KN, et al. Mapping and modelling the impact of mass drug adminstration on filariasis prevalence in Myanmar. Infect Dis Poverty. 2018;7(1):56. doi: 10.1186/s40249-018-0420-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Swaminathan S, Perumal V, Adinarayanan S, Kaliannagounder K, Rengachari R, Purushothaman J. Epidemiological assessment of eight rounds of mass drug administration for lymphatic filariasis in India: implications for monitoring and evaluation. PLoS Negl Trop Dis. 2012;6(11):e1926. doi: 10.1371/journal.pntd.0001926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ramaiah KD, Das PK, Vanamail P, Pani SP. Impact of 10 years of diethylcarbamazine and ivermectin mass administration on infection and transmission of lymphatic filariasis. Trans R Soc Trop Med Hyg. 2007;101(6):555–563. doi: 10.1016/j.trstmh.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 130.Ramaiah KD, Vanamail P. Surveillance of lymphatic filariasis after stopping ten years of mass drug administration in rural communities in south India. Trans R Soc Trop Med Hyg. 2013;107(5):293–300. doi: 10.1093/trstmh/trt011 [DOI] [PubMed] [Google Scholar]
- 131.Ramaiah KD, Vanamail P, Pani SP, Yuvaraj J, Das PK. The effect of six rounds of single dose mass treatment with diethylcarbamazine or ivermectin on Wuchereria bancrofti infection and its implications for lymphatic filariasis elimination. Trop Med Int Health. 2002;7(9):767–774. [DOI] [PubMed] [Google Scholar]
- 132.Subramanian S, Jambulingam P, Chu BK, et al. Application of a household-based molecular xenomonitoring strategy to evaluate the lymphatic filariasis elimination program in Tamil Nadu, India. PLoS Negl Trop Dis. 2017;11(4):e0005519. doi: 10.1371/journal.pntd.0005519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lau CL, Won KY, Lammie PJ, Graves PM. Lymphatic filariasis elimination in American Samoa: evaluation of Molecular Xenomonitoring as a surveillance tool in the Endgame. PLoS Negl Trop Dis. 2016;10(11):e0005108. doi: 10.1371/journal.pntd.0005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Irish SR, Al-Amin HM, Paulin HN, et al. Molecular xenomonitoring for Wuchereria bancrofti in Culex quinquefasciatus in two districts in Bangladesh supports transmission assessment survey findings. PLoS Negl Trop Dis. 2018;12(7):e0006574. doi: 10.1371/journal.pntd.0006574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rao RU, Nagodavithana KC, Samarasekera SD, et al. A comprehensive assessment of lymphatic filariasis in Sri Lanka six years after cessation of mass drug administration. PLoS Negl Trop Dis. 2014;8(11):e3281. doi: 10.1371/journal.pntd.0003281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rao RU, Samarasekera SD, Nagodavithana KC, et al. Programmatic use of Molecular Xenomonitoring at the level of evaluation units to assess persistence of lymphatic filariasis in Sri Lanka. PLoS Negl Trop Dis. 2016;10(5):e0004722. doi: 10.1371/journal.pntd.0004722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Drexler N, Washington CH, Lovegrove M, et al. Secondary mapping of lymphatic filariasis in Haiti-definition of transmission foci in low-prevalence settings. PLoS Negl Trop Dis. 2012;6(10):e1807. doi: 10.1371/journal.pntd.0001807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Upadhyayula SM, Mutheneni SR, Kumaraswamy S, Kadiri MR, Pabbisetty SK, Yellepeddi VS. Filaria monitoring visualization system: a geographical information system-based application to manage lymphatic filariasis in Andhra Pradesh, India. Vector Borne Zoonotic Dis. 2012;12(5):418–427. doi: 10.1089/vbz.2011.0713 [DOI] [PubMed] [Google Scholar]
- 139.Simonsen PE, Derua YA, Magesa SM, et al. Lymphatic filariasis control in Tanga Region, Tanzania: status after eight rounds of mass drug administration. Parasit Vectors. 2014;7:507. doi: 10.1186/1756-3305-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lau CL, Won KY, Becker L, et al. Seroprevalence and spatial epidemiology of lymphatic filariasis in American Samoa after successful mass drug administration. PLoS Negl Trop Dis. 2014;8(11):e3297. doi: 10.1371/journal.pntd.0003297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Graves PM, Makita L, Susapu M, et al. Lymphatic filariasis in Papua New Guinea: distribution at district level and impact of mass drug administration, 1980 to 2011. Parasit Vectors. 2013;6:7. doi: 10.1186/1756-3305-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rebollo MP, Mohammed KA, Thomas B, et al. Cessation of mass drug administration for lymphatic filariasis in Zanzibar in 2006: was transmission interrupted? PLoS Negl Trop Dis. 2015;9(3):e0003669. doi: 10.1371/journal.pntd.0003669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.WHO. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases a Roadmap for Implementation. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 144.Ramesh A, Cameron M, Spence K, et al. Development of an urban molecular xenomonitoring system for lymphatic filariasis in the Recife Metropolitan Region, Brazil. PLoS Negl Trop Dis. 2018;12(10):e0006816. doi: 10.1371/journal.pntd.0006816 [DOI] [PMC free article] [PubMed] [Google Scholar]