Supplemental Digital Content is available in the text.
Key Words: small cell carcinoma, esophagus, chemotherapy, radiotherapy, chemoradiotherapy, outcome, survival
Abstract
Background and Purpose:
Small cell carcinoma of the esophagus (SCEC) is a rare subtype of esophageal cancer for which optimal treatment is unknown. We analyzed the impact of treatment factors on outcome in patients with nonmetastasized SCEC.
Methods:
Patients with a histologically confirmed SCEC without distant metastases were analyzed in a nationwide multicenter retrospective cohort. All patients received radiotherapy as part of curative treatment between January 2000 and December 2014. Details on treatment and outcome were retrieved from individual charts. Cox regression analysis was used to determine prognostic factors for survival.
Results:
Fifty-eight patients were analyzed. Median survival was 16 months (95% confidence interval, 11-21 mo). Infield recurrences occurred in 25%, distant metastases in 45%, and brain metastases in 12%. In total, 63% of patients developed a recurrence. Most recurrences (67%) occurred within 1 year. In univariable analyses an increased number of chemotherapy cycles (>3) and lower radiotherapy doses (<45 Gy) were associated with improved survival. T-stage, N-stage, treatment period, type of chemotherapy, prophylactic cranial irradiation, and age were not associated with survival. In multivariable analyses, only the number of chemotherapy cycles was associated with better survival (hazard ratio, 0.78; P=0.006).
Conclusions:
SCEC recurs frequently at distant sites after definitive chemoradiotherapy and usually within 1 year after curative treatment. With a dose of 45 to 50 Gy, infield recurrence rate was low. We found a relationship between number of received chemotherapy cycles and survival with best results obtained after at least 4 cycles of chemotherapy.
Small cell carcinoma of the esophagus (SCEC) is a rare subtype of esophageal cancer with an incidence of only 0.1% to 2.4% of all cases of esophageal cancer.1–4 It is a highly aggressive tumor, with about half of the patients presenting with metastatic disease.4,5 In localized disease, a multimodality treatment combining chemotherapy with radiotherapy and/or surgery is often used. However, even with multimodality treatment, overall survival is poor with a median survival of 8 to 21 months.4–6
Due to its rare nature, randomized studies are not available and are unlikely to be conducted. Several large retrospective series using nationwide databases show differences in survival depending on treatment modality.4,5 However, as these series lack patient specific data, they cannot draw conclusions on an optimal treatment regime.
In this retrospective nationwide study, we analyzed individual patients’ charts to determine treatment factors related to outcome in patients with nonmetastasized SCEC of the esophagus. We specifically investigated patterns of failure and the effect of type and number of chemotherapy cycles and dose of radiotherapy.
METHODS
Patients
Patients were identified through the Netherlands Cancer Registry and by the hospitals’ local data registration systems from the radiation oncology departments.7 Data on treatment and outcome were retrieved from the clinical chart and retrospectively recorded in an electronic database. All but 1 Dutch radiotherapy institute participated in the study.
We identified patients of any age who were diagnosed with a histologically confirmed SCEC of the esophagus without distant metastases at initial diagnosis. Mixed histology was accepted, but only if part of the histology was true small cell carcinoma. Neuroendocrine tumors without small cell carcinoma were thus excluded from analyses. Patients with involved supraclavicular nodes were included. All patients received treatment with curative intent including radiotherapy between January 2000 and December 2014. All data were retrieved in 2016 to ensure a minimum follow-up of at least 12 months.
The absence of distant metastases at diagnosis was confirmed by CT or PET scan. Data on pretreatment brain scans were not recorded. For esophageal radiotherapy, data regarding dose and fractionation were collected. Prophylactic cranial irradiation (PCI) was registered (yes/no). For chemotherapy, data on type of chemotherapy, number of cycles, and whether administered concurrent or sequentially were collected. For surgical treatment, date of surgery and histology after surgery were collected. For recurrences, date of recurrence, histologic confirmation (yes/no), and location in relation to radiotherapy (locoregional infield, locoregional outfield, distant, or a combination) were scored.
Brain metastases were scored both as distant metastases and separately as brain metastases. Death was reported either in the presence of disease, absence of disease, or disease status unknown.
Statistical Analyses
All data were analyzed using the statistical package IBM SPSS Statistics for Windows 24.0 (IBM Corporation, Armonk, NY). Continuous and categorical variables were summarized by descriptive statistics. Descriptive data are given as a mean (±SD) or median (range). Overall survival was calculated from the start of first treatment until the date of death or last follow-up, using the Kaplan-Meier method with the log rank test to determine significance. Data were censored at the last follow-up for patients still alive. Recurrence free rate was calculated from the start of treatment until the date of recurrence (locoregional, distant, or both), using the Kaplan-Meier method. Data were censored at date of death in the absence of disease or at date of last follow-up for patients without a recurrence.
Univariable and multivariable Cox proportional hazards models were fit to evaluate the impact of factors predictive of survival. All testing was 2-tailed with 0.05 as level of significance. Multivariable analyses were performed for factors with P<0.20 in univariate analyses.
RESULTS
Patients
Between 2000 and 2015, a total of 16,492 patient were present in the Netherlands with an esophageal carcinoma without distant metastases. Of these, 9966 patients had an adenocarcinoma and 5897 patients had a squamous cell carcinoma.7 In total, 83 patients with a small cell carcinoma were identified. Twenty-five patients (20 treated with chemotherapy only and 5 with surgery only) were excluded from the study since additional data were unavailable, rendering 58 patients available for analyses. Baseline characteristics are reported in Table 1.
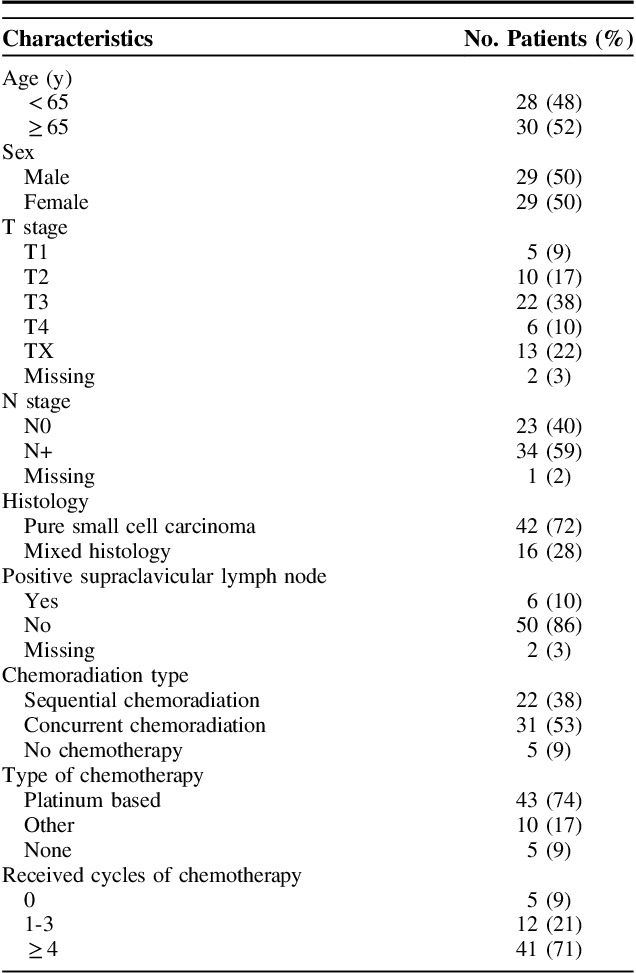
TABLE 1.
Characteristics of the Study Population (N=58)
Treatment Type
Most patients were treated with concurrent chemoradiation (31 patients, 53%), 22 patients (38%) with sequential chemoradiation and 5 patients (9%) with radiotherapy only. Four patients (7%) had a resection after neoadjuvant therapy, 1 patient received chemoradiation after endoscopic mucosal resection. Median radiotherapy dose was 45 Gy (range, 9 to 66 Gy). One patient died after complications after 9 Gy. The dose range for the remaining group was 36 to 66 Gy. The majority of patients received between 45 and 50 Gy (44 patients, 76%). Only 5 patients received >50 Gy, 3 of whom received no chemotherapy. The dose per fraction ranged between 1.8 and 3 Gy. Most patients received 1.8 or 2 Gy fractions (44 patients, 76%). Five patients received radiotherapy twice daily in 1.5 Gy fractions, with a total dose of 45 Gy. Most patients (53) received chemotherapy, of whom 43 received platinum-based therapy. Chemotherapy details are reported in Supplement 1 (Supplemental Digital Content 1, http://links.lww.com/AJCO/A256).
Survival
Median follow-up for patients alive was 43 months. Median survival for the entire cohort was 16 months [95% confidence interval (CI), 11-21 mo]. Survival at 1, 3, and 5 years was 65%, 30%, and 22%, respectively (Fig. 1). At last follow-up, 13 patients were still alive (22%). Seven patients (12%) died without evidence of disease, 32 patients (55%) died with evidence of disease and for 6 patients (10%) disease status was unknown.
FIGURE 1.
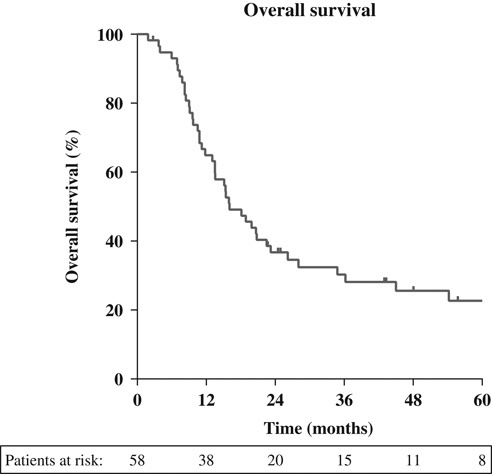
Overall survival (months).
Disease Outcome
Thirty-two of 51 patients for whom recurrence data were available, had a locoregional or distant disease recurrence (63%). For 7 patients (12%) recurrence data were unavailable. Isolated locoregional recurrence thus in the absence of systemic disease, occurred in 9 patients (18%), all of which were infield. There were no isolated regional outfield recurrences. Any infield recurrence were identified in 13 patients (25%). Distant metastases, including brain metastases, were identified in 23 patients (45%). Recurrence patterns are shown in Table 2.
TABLE 2.
Pattern of Recurrences

Median time to any recurrence was 9 months, where 69% recurred within 1 year and 91% within 1.5 years (Fig. 2). Data on development of brain metastases were available for 43 patients. Brain metastases were identified in 5 patients (12%). PCI was applied in 6 patients, of which 1 developed brain metastases. Out of the 52 patients without PCI, 4 developed brain metastases.
FIGURE 2.
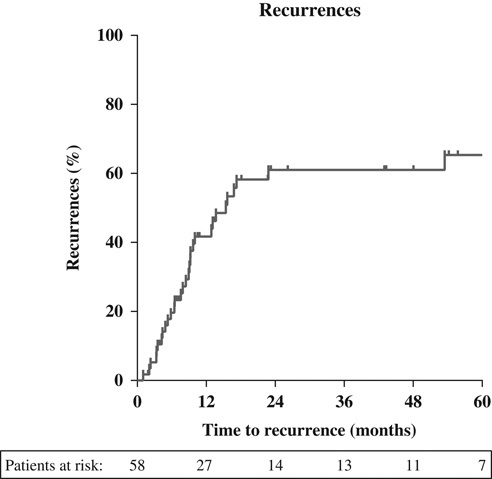
Time to recurrence (months).
The infield recurrence rate was 30% (8/27) in the group receiving ≤45 Gy, and 21% (5/24) for those receiving >45 Gy (P=ns).
Univariable Analyses
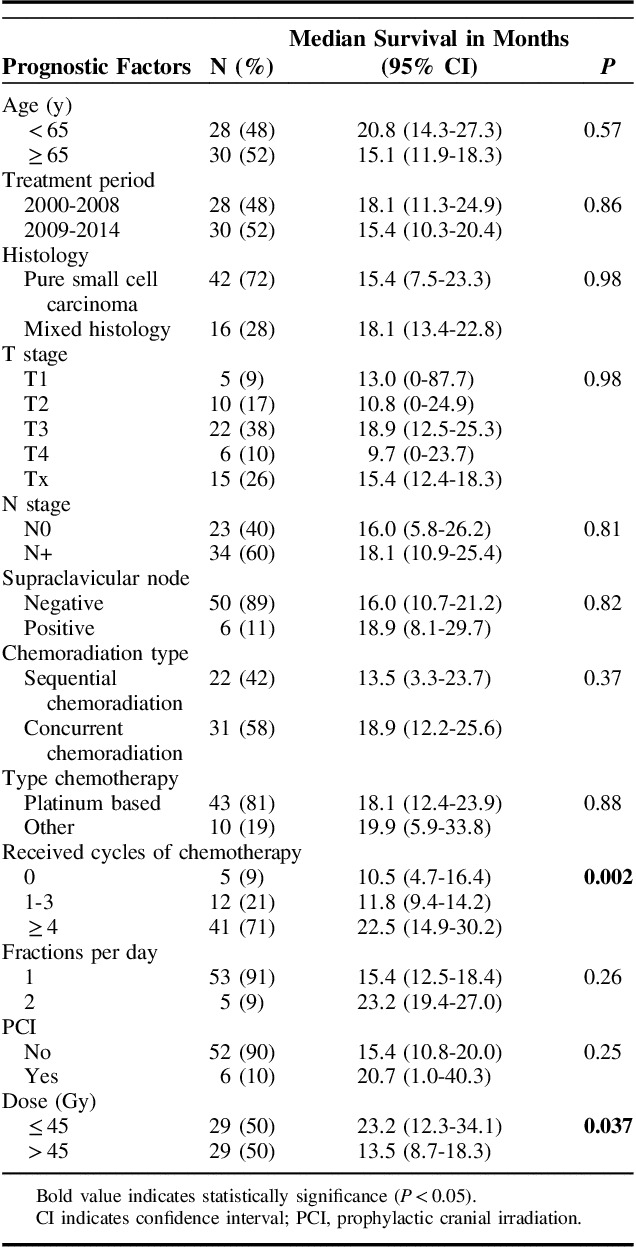
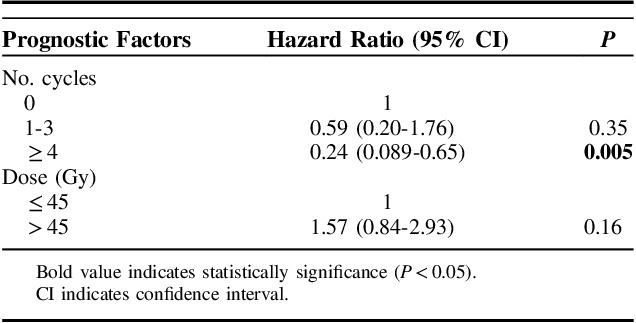
Radiotherapy dose and number of received chemotherapy cycles (grouped) were significantly associated with better survival. The T and N stage, histologic subtype, and treatment period were not associated with survival. No difference in survival was seen between concurrent or sequential chemotherapy and radiotherapy. Further details are available in Table 3.
TABLE 3.
Univariable Analyses for Overall Survival
Multivariable Analyses
Results for multivariable analyses are reported in Table 4. Only the number of chemotherapy cycles remained significant for survival. We also analyzed cycles of chemotherapy and radiotherapy dose as a continuous variable. When analyzed as a continuous variable, the hazard ratio for the number of cycles of chemotherapy was 0.78 (95% CI, 0.65-0.93; P=0.006). When radiotherapy dose was analyzed as a continuous variable, the hazard ratio was 1.03 (95% CI, 0.99-1.10; P=0.44).
TABLE 4.
Multivariable Analyses for Overall Survival
DISCUSSION
In this nationwide retrospective study we analyzed treatment outcome and recurrence patterns in patients with SCEC treated with radiotherapy with or without chemotherapy. Recurrences occur frequently and usually within 1 year after curative treatment. Most failures occurred at distant sites (37%), as found by others.8–10 The total number of infield recurrences, thus including patients with simultaneous outfield or distant failures, was 23%. Isolated infield recurrences are relatively rare, occurring in 13% of patients only, suggesting that more aggressive locoregional treatment will not have a large effect on overall survival.
The benefit from multimodality treatment over single modality in SCEC of the esophagus has been well established over the years.4,5,9,11 Kukar and colleagues analyzed the Surveillance, Epidemiology, and End Results (SEER) database, showing that both radiotherapy and surgery were associated with improved survival on multivariate analysis. However, as chemotherapy use is not registered within the SEER database, no conclusions can be drawn on the role of chemotherapy.4 Wong and colleagues performed a retrospective analyses of 583 patients with SCEC of whom 340 with nonmetastatic disease. In multivariate analysis, chemoradiation was superior in survival compared to chemotherapy alone (hazard ratio, 1.44; P=0.003).5 However, no additional data on type and number of chemotherapy cycles were available. To the best of our knowledge, our study is the first investigating these factors. We found the number of received chemotherapy cycles to be highly predictive for overall survival, where patients receiving more cycles of chemotherapy had a markedly better survival. We did not have information on the planned number of chemotherapy cycles, thus effects of preliminary termination due to toxicity could not be assessed. This could induce a potential bias, where patients in a better general condition might have received more cycles of chemotherapy. We found no significant difference in survival between platinum or non–platinum-based chemotherapy. This is in line with the results from a recent Cochrane review on outcome in small cell lung cancer.12
Reports in literature describe a wide variety of prescribed radiation doses, ranging between 40 and 70 Gy, usually in fractions of 1.8 to 2 Gy.8,13 To the best of our knowledge, this is the first study to analyze the effect of radiotherapy dose on survival. In univariable analysis, a lower radiotherapy dose was significantly associated with a better survival. In multivariable analysis however, we found no effect of radiotherapy dose on overall survival, neither when analyzing dose in groups nor when analyzing dose as a continuous variable. These results have to be interpreted with caution though. The vast majority of patients received between 45 and 50 Gy (44 patients) and only 5 patients received >50 Gy. Furthermore, 5 patients received radiotherapy twice a day, perhaps yielding a stronger radiobiological effect. As the majority of patients are clustered around such a narrow dose range any possible existing dose effect relationship is very unlikely to be found. The question whether or not a dose effect relationship exists simply cannot be answered with these data.
In small cell cancer of the lung, a dose of 60 Gy yielded superior results compared with 45 Gy in conventionally fractionated schedules once daily.14 Reducing the overall treatment time of radiotherapy by twice daily radiation has been shown to be more effective for overall survival and reduced infield recurrence (36% vs. 52%).15 A large randomized trial has recently shown that a twice daily schedule of 45 Gy to be equally effective as a once daily schedule of 66 Gy with comparable toxicity.16 In our cohort only 5 patients received radiotherapy twice daily, and only 1 patient received 66 Gy, so we cannot draw any conclusions on the possible benefit of either dose escalation or reducing overall treatment time, as has been suggested by others.8,17
Brain metastases occurred in 12% of patients. Only 6 patients received PCI, of which 1 developed brain metastases. This seems to be much lower than reported in small cell lung cancer where a meta-analysis showed an incidence of 58.6%, decreasing to 33.3% for the group treated with PCI.18 However, in other series reporting on SCEC cases, brain metastases are reported in 5% to 14% of patients mostly without PCI, which is in agreement with the presented series.10,13,19 Considering the above, we currently see no role for PCI in SCEC.
Since data were collected from medical files of nationwide radiotherapy institutions, no data are available on outcomes of surgery alone. Notwithstanding the possible benefit of esophagectomy in early stage disease,3,20–22 there has been considerable debate regarding the added value of surgery, either substituting radiotherapy or in addition to it.9,20,22 Raja and colleagues performed a meta-analysis, showing the benefit of combining either surgery or radiotherapy with chemotherapy in terms of survival. No additional benefit was found for the trimodality treatment.9 As isolated infield recurrences occurred in only 16% of patients after radiochemotherapy only in the present study, combined with a high distant recurrence rate at short term, major surgery such as an esophagectomy is unlikely to have a large clinical effect on the outcome and may not be advised as part of standard care.
Strengths and Limitations
The main strength of this study is the detailed information available from the original patient’s charts, allowing analyses of the effects of variations in treatment on both survival and failure patterns. Furthermore, it is a nationwide study representing 20 different institutions.
The main drawbacks are the small sample size, the retrospective nature and the exclusion of surgery or chemotherapy only patients. To the best of our knowledge, it is however the largest series to date containing detailed individual patient data.
CONCLUSIONS
SCEC is an aggressive disease. Recurrences occur frequently and usually within 1 year after the start of curative treatment. Most recurrences include distant metastases, emphasizing the importance of systemic treatment. Incidence of brain metastases was low, even without PCI. We found a clear relationship between number of received chemotherapy cycles and survival. In this study, the best results were obtained with regimes consisting of at least 4 cycles of chemotherapy and radiotherapy. With a radiotherapy dose of 45 to 50 Gy, infield recurrence rate was low, suggesting that more aggressive locoregional treatment will probably not affect survival.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.amjclinicaloncology.com.
ACKNOWLEDGMENTS
The authors thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the Netherlands Cancer Registry.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Beyer KL, Marshall JB, Diaz-Arias AA, et al. Primary small-cell carcinoma of the esophagus. Report of 11 cases and review of the literature. J Clin Gastroenterol. 1991;13:135–141. [DOI] [PubMed] [Google Scholar]
- 2.Brenner B, Tang LH, Klimstra DS, et al. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol. 2004;22:2730–2739. [DOI] [PubMed] [Google Scholar]
- 3.Chen W-W, Wang F, Zhang D-S, et al. Primary small cell carcinoma of the esophagus: clinicopathological study of 44 cases. BMC Cancer. 2014;14:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kukar M, Groman A, Malhotra U, et al. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol. 2013;20:4239–4244. [DOI] [PubMed] [Google Scholar]
- 5.Wong AT, Shao M, Rineer J, et al. Treatment and survival outcomes of small cell carcinoma of the esophagus: an analysis of the National Cancer Data Base. Dis Esophagus. 2017;30:1–5. [DOI] [PubMed] [Google Scholar]
- 6.Yau KK, Siu WT, Wong DCT, et al. Non-operative management of small cell carcinoma of esophagus. Dis Esophagus. 2007;20:487–490. [DOI] [PubMed] [Google Scholar]
- 7.IKNL-Integraal Kankercentrum Nederland/Comprehensive Cancer Centre, the Netherlands. Available at: www.iknl.nk. [Google Scholar]
- 8.Nemoto K, Zhao H, Goto T, et al. Radiation therapy for limited-stage small-cell esophageal cancer. Am J Clin Oncol. 2002;25:404–407. [DOI] [PubMed] [Google Scholar]
- 9.Raja S, Rice TW, Rajeswaran J, et al. Esophageal small-cell cancer: study of a rare disease. Dis Esophagus. 2013;26:690–695. [DOI] [PubMed] [Google Scholar]
- 10.Tao H, Li F, Wang J, et al. Management of treatment-naïve limited-stage small cell esophagus carcinoma. Saudi Med J. 2015;36:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casas F, Ferrer F, Farrús B, et al. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. 1997;80:1366–1372. [PubMed] [Google Scholar]
- 12.Amarasena IU, Chatterjee S, Walters JAE, et al. Platinum versus non-platinum chemotherapy regimens for small cell lung cancer. Cochrane database Syst Rev. 2015:CD006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medgyesy DC, Wolff Ra, Putnam JB, et al. Small cell carcinoma of the esophagus. Cancer. 2000;88:262–267. [PubMed] [Google Scholar]
- 14.Choi NC, Herndon JE, Rosenman J, et al. Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol. 1998;16:3528–3536. [DOI] [PubMed] [Google Scholar]
- 15.Turrisi AT, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. [DOI] [PubMed] [Google Scholar]
- 16.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng M-B, Zaorsky NG, Jiang C, et al. Radiotherapy and chemotherapy are associated with improved outcomes over surgery and chemotherapy in the management of limited-stage small cell esophageal carcinoma. Radiother Oncol. 2013;106:317–322. [DOI] [PubMed] [Google Scholar]
- 18.Aupérin A, Arriagada R, Pignon J-P, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341:476–484. [DOI] [PubMed] [Google Scholar]
- 19.Ku GY, Minsky BD, Rusch VW, et al. Small-cell carcinoma of the esophagus and gastroesophageal junction: review of the Memorial Sloan-Kettering experience. Ann Oncol. 2008;19:533–537. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Li Y, Liu X, et al. Treatment strategies and prognostic factors of limited-stage primary small cell carcinoma of the esophagus. J Thorac Oncol. 2017;12:1834–1844. [DOI] [PubMed] [Google Scholar]
- 21.Wang H-H, Zaorsky NG, Meng M-B, et al. Multimodality therapy is recommended for limited-stage combined small cell esophageal carcinoma. Onco Targets Ther. 2015;8:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W-W, Wang F-H, Chen S, et al. Detailed analysis of prognostic factors in primary esophageal small cell carcinoma. Ann Thorac Surg. 2014;97:1975–1981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.amjclinicaloncology.com.


