Abstract
While chemotherapy is the only approved non-surgical option for the majority of pancreatic cancer patients, it rarely results in a cure. The failure to respond to chemotherapy is due to the presence of an abundant dysplastic stroma that interferes in drug delivery and as a result of drug resistance. It is appropriate, therefore, to consider the stromal contribution to the resistance to chemotherapy and sidestepping this barrier with nanocarriers that improve survival outcome. In this paper, we provide a short overview of the role of the stroma in chemotherapy resistance, including the use of nanocarriers to negate this barrier. We provide a perspective and guidance towards the implementation of nanotherapeutic approaches to improve therapeutic delivery and efficacy of PDAC management.
Keywords: Pancreatic cancer, nanotherapy, engineered approach, stroma
1. Introduction
Pancreatic cancer (PDAC) is the 4th leading cause of cancer death, with ~43,090 deaths in the US in 2017 [1]. In terms of mortality trends, Cancer Facts & Figures 2018 demonstrates that the 5-year survival rate has remained unchanged from 2006 to 2015 [2]. In the US, around 56,000 new cases of PDAC will be diagnosed in 2018 [2]. Collectively, for all stages of disease, the 5-year relative survival rate is only 8%. This number includes patients with metastatic (~52% patients) and local disease (~10% patients), with a 5-year survival rate of 3% and 32%, respectively. Due to the late diagnosis and early metastasis, for the majority patients with advanced disease, chemotherapy is considered as the only approved treatment, with the standard of care involving the use of nucleoside analog gemcitabine (GEM) or a more potent but highly toxic 4-drug regimen, FOLFIRINOX (i.e. oxaliplatin, irinotecan, 5- fluorouracil, and leucovorin). Moreover, chemotherapy is also used to treat the patients who are suitable for surgery (<20%), as neoadjuvant with a hope to lower recurrence. Unfortunately, these chemo applications seldom lead to a disease cure.
Chemotherapy failure can be partly explained by the presence of a dense desmoplastic stroma serving as a physical and biological barrier for drug delivery in PDAC and an unfavorable pharmacokinetics (PK) profile [3]. It is reasonable to consider, therefore, overcoming of the stromal interference in drug delivery and chemo-resistance to improve efficacy and patient survival [3]. A popular approach to overcoming the stromal resistance is to take advantage of the ability of nanocarriers to deliver therapeutic agents to the tumor site by mechanisms that differ from the uptake and retention of classic non-encapsulated molecular drug. A recent meta-analysis on PDAC clinical trials demonstrated that nanoparticles are promising approach to increase efficacy whilst reducing toxicity of multiple cancer drugs in PDAC patients [4]. Another exciting development is the use of smart design of the nanocarriers to enable them to negotiate the stroma barrier and improve drug delivery [5]. In fact, understanding the stromal contribution to the tumor access by an enhanced permeability and retention effect (EPR) in solid tumors is of particular relevance to the study of PDAC [6–9]. The concept of “enhanced permeability” as an across-the-board explanation for nanocarrier access to solid tumor sites is over-simplified and needs to be re-interpreted [10–15]. While enlarged tumor vascular fenestrations, irregular branching and abnormal angiogenesis have been reported in different cancer scenarios (many of them are xenograft models in mice) [6], the dysplastic stroma in PDAC indicate that additional consideration needs to be given to the poorly perfused, collapsed and obstructed blood vessels in this cancer as a result of tight adherence of stromal fibroblasts or pericytes to the vascular wall. In this communication, we provide a short overview to address the inhibitory effect of the stroma on PDAC treatment, including the consideration for the use of nanocarriers to potentially engineer and past this obstacle. We also provide a perspective and guidance towards the implementation of nanotherapeutic approaches in PDAC and other stroma-rich solid tumor types.
2. The pathophysiological contribution of the PDAC stroma to disease progression
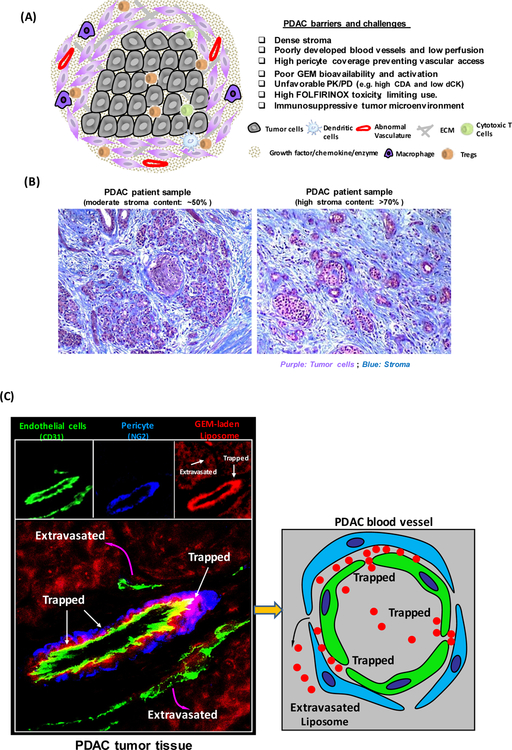
A key characteristic of PDAC is the presence of tumor stroma, which contains cellular components (e.g. fibroblasts, immune cells, stellate cells, pericytes, endothelial cells), acellular components (e.g. collagens, fibronectin, growth factors and cytokines) and biophysical components (e.g. low pH, hypoxia, high tumor interstitial pressure) (Fig. 1A) [16]. These components interact in a multiplicative fashion to promote PDAC progression and tumor metastasis [17]. Previous studies have demonstrated that during PDAC carcinogenesis normal ductal epithelial cells acquire oncogenic mutations to develop early stage lesions, a.k.a. precursor pancreatic intraepithelial neoplasm (PanIN), which further progresses through different grades (i.e. 1A, 2A, 2, and 3), ultimately form a highly invasive PDAC disease [18]. During this dynamic process, stellate cells in the stroma release pro-inflammatory cytokines and growth factors to activate immune cells, produce extracellular matrix (ECM) proteins and increase fibrotic stromal deposition [16]. PDAC cancer cells also secrete variable pro-inflammatory cytokines, such as TGF-β1, PDGF, TNFα, and IL-6 and activate stellate cells and fibroblasts, which further transform into a myofibroblast-like phenotype capable of ECM secretion [19]. ECM deposition in periacinar regions disrupt normal parenchyma, compressing tumor vasculature, and leading to hypovascularity, high interstitial fluid pressure and hypoxia, which is known to activate stellate cells and capable of perpetuating the “hypoxia-fibrosis” cycle [20]. Moreover, the fibrotic PDAC microenvironment exhibits suppressive innate and adaptive immune systems, i.e. reduced cytotoxic CD8 T cells and increased M2 macrophages, N2 neutrophils, and T-regulatory cells (Tregs) at PDAC tumor site [21]. Collectively, the dense PDAC stroma including immune suppression establishes a favorable environment for PDAC development and metastasis. However, fibrotic stroma and abnormal vasculature negatively impact drug delivery, leading to PDAC resistance to most therapeutics, including two first-generation chemo delivering nanocarriers [16].
Figure 1.
(A) Schematic to show the barriers and challenges that are responsible for failed chemotherapy in PDAC, including as a result of an abundant dysplastic stroma, which serves as a physical and biological barrier, including the immunosuppressive tumor microenvironment. (B) Trichrome staining of PDAC tissue sections. Moderate (~50%) and high (>70%) stroma content PDAC tumors were shown. Blue: stroma, e.g. collagen. Purple: Tumor cells. (C) Figure 1C demonstrates that PDAC tumor develops a dense stromal barrier, which blocks the vascular access of IV-injected red fluorescent liposomes. Higher level magnification was provided to show the localization of the liposomes in the tumor in relation to endothelial cells (CD31) and pericyte (NG2) fluorescent markers. The fluorescence microscopy image obtained from PDAC tumor site showed a region of a blood vessel where pericytes were trapping some liposomes just beyond their point of egress from the vascular fenestrations [23].
3. Why overcoming tumor stroma is important to PDAC nanotherapeutics?
The high stromal volume in PDAC (up to 70% of the total tumor volume, Fig. 1B) requires disease- specific consideration to eliminate its impact on therapy [3]. Not only is the stroma poorly vascularized, but the existing vessels are relatively less leaky due to a high pericyte coverage, which blocks the extravasation of small molecule chemo agents as well as nanoparticles to the PDAC tumor site (Fig. 1C) [22, 23]. Kataoka et al., compared size-controlled polymer micelle nanoparticle access in various solid tumor models in mice [24]. While both 30 and 100 nm nanoparticles can penetrate into a hyper- permeable colon cancer model, only the small (30 nm) micellar particle could penetrate a stroma-rich pancreatic tumor model (i.e. BxPC3) in mice to achieve an anti-tumor efficacy [24]. The study also demonstrated that the lack of egress of the 100 nm particle was due to the high pericyte coverage of the endothelial cells. The pericyte adherence to the endothelial cells could be reduced by a TGF-β inhibitor, delivered by a 100 nm particle [23]. This increases vascular access [23]. In addition to physical blockage, the stroma also contributes to chemo-resistance and an unfavorable PK and pharmacodynamic (PD) profile in vivo [3], including high level of expression of cytidine deaminase (CDA), which reduces the circulatory half-life (t1/2) of GEM to < 0.3 hour [25]. Moreover, GEM action at the tumor site also requires intracellular activation by a phosphorylation step that is catalyzed by the rate-limiting kinase deoxycytidine kinase (dCK) to generate the active metabolites, dFdCDP and dFdCTP [26]. Another important stromal contribution to tumor cell growth is through supportive cell types that promote cancer cell proliferation and metastasis by means of a number of complicated cross- talk interactions [3]. Given this background, it is important to consider overcoming the challenges of the stromal barrier to address drug delivery and unfavorable PK/PD to the cancer site, including the improvement of intratumoral distribution, bioavailability, and overcoming drug resistance.
4. State-of-the-art approaches to overcome the stromal barrier in PDAC, including the use of nanocarriers
A number of stromal treatment strategies are currently being considered to improve PDAC treatment. While it is too early to evaluate the impact of PDAC stromal treatment, the field is beginning to understand the impact of multi-stage, multi-wave and combination therapy, which influence a multitude of mechanisms such as vasculature permeability, blood vessel patency, drug activation/degradation enzymes, and/or target specific biological factors, etc. These efforts involve the use of enzymatic degradation, pharmacological suppression, tumor vasculature modification/intervention, and stromal targeting peptides, etc.
4.1. Stromal-directed agents
The first approach is the introduction of stromal-directed agents that obliterate the dense stromal microenvironment and improve drug delivery [27]. In this regard, a major advance has been the development of PEGylated hyaluronidase (PEGPH20), which is the PEGylated version of recombinant human hyaluronidase enzyme [28, 29]. In multiple solid tumor types including PDAC, PEGPH20 treatment leads to a transit degradation of hyaluronan, which is a glycosaminoglycan that is abundant in tumor stroma. A Phase 2 clinical study showed that PEGPH20 plus Abraxane® (albumin-bound paclitaxel nano-complex) and GEM led to a doubling of progression-free survival and an improvement in overall survival in patients with hyaluronan-high metastatic PDAC. This promising result is being pursued in an ongoing Phase 3 study involving ~570 patients with the purpose of comparing the anti- PDAC efficacy and safety of PEGPH20/Abraxane®/GEM versus placebo/Abraxane®/GEM. However, it is important to point out that the thromboembolic effects of PEGPH20 have resulted in a clinical hold by the FDA before the study was resumed. In addition to PEGPH20, other known anti-stromal drugs, such as hedgehog signaling inhibitors (e.g. IPI-926) [30], metalloproteinases inhibitors [31], connective tissue growth factor antagonists [16], antifibrotic agent (e.g. pirfenidone) [16], angiotensin inhibitors [32], are potentially useful for combining with nanocarriers in PDAC treatment.
4.2. Pharmacological reduction of stromal volume
The second approach is the pharmacological reduction of stromal volume, as illustrated by FDA approval for the use of Abraxane® in PDAC. Clinical data demonstrated that co-administration of this therapy promotes GEM survival outcome by 1.8 months [33, 34]. The mechanistic explanation for the stromal reduction and decreased CDA expression is the generation of oxidative stress by Abraxane®, rather than its effect as a chemotherapeutic agent [33, 34]. In cultured KPC cell lines, paclitaxel inhibited CDA expression via the generation of reactive oxygen species (ROS), an effect that is reversible by the ROS scavenger, N-acetyl-L-cysteine. However, it is important to point out that the combination of GEM/Abraxane® is premised on using conventional therapeutic doses, which may overlook the possibility that drug synergy could depend on the ratiometric combination of the drugs. In this regard, it has recently been demonstrated that the ratiometric combination of daunorubicin and cytarabine in a liposome, i.e. Vyxeos™ formulation, could provide strong drug synergy with improved outcome in acute myeloid leukemia [35]. For this reason, we developed a mesoporous silica nanocarrier that ratiometrically delivers GEM and paclitaxel by a lipid-bilayer coated nanoparticle, a.k.a. a silicasome [36]. The silicasome encapsulates GEM in the porous interior while delivering the paclitaxel from the lipid bilayer in the ratio of GEM : paclitaxel = 10 : 1 [36]. The synergy for this carrier was demonstrated by CompuSyn software. Ratiometric drug co-delivery to animals growing subcutaneous and orthotopic PANC-1 models provided more effective PDAC shrinkage than a variety of controls, including free GEM plus Abraxane® mixture. Comparable tumor shrinkage in the orthotopic model required co-administration of ~12x the amount of free Abraxane® to achieve the same outcome. This was accompanied by ~13x increased level of active GEM and ~4x decreased inactivated metabolite at tumor site [36]. Another example is the Cellax nanoparticle, a docetaxel conjugated polymeric formulation [37]. It was shown that Cellax nanoparticles were capable of depletion of cancer- associated fibroblasts and improving efficacy in patient-derived PDAC xenografts [37]. The investigators performed efficacy in PAN02 tumor xenograft model in which they showed docetaxel- Cellax nanoparticle led to 40% disease free mice at maximum tolerated dose of 170 mg DTX/kg [37]. In the breast cancer models, Cellax particles led to tumor stromal depletion and anti-metastatic effect [38]. In the mechanism study, the authors also demonstrated that Cellax adsorbed albumin was internalized by cells via an albumin and SPARC dependent fashion in the breast, prostate and lung cancer models [39].
4.3. Vascular modification
The third approach is vascular modification to improve drug delivery. This comprises a number of options, including targeting the TGF-β pathway, which is responsible for pericyte adherence to PDAC endothelial cells [40]. We and others have demonstrated that intervention in the TGF-β signaling pathway using TGF-β receptor kinase inhibitors or monoclonal antibodies can enhance vascular access and nanocarriers egress to the PDAC tumor site [40–42]. However, the use of free inhibitors or antibodies may require relatively high doses to achieve this outcome, which can be improved by the use of nanocarriers. Vascular access can also be improved by means, such as lowering of the interstitial fluid pressure [43]. In addition to the pharmacological interference, another interesting study involves the use of ultrasound microbubbles, which usually have a diameter of between 1~ 4 μm, restricting them to the vascular compartment [44]. It has been shown that disintegrating microbubbles emit acoustic forces that are capable of inducing thrombolysis, facilitating drug and gene delivery across biologic barriers [44].
4.4. Stromal targeting therapy
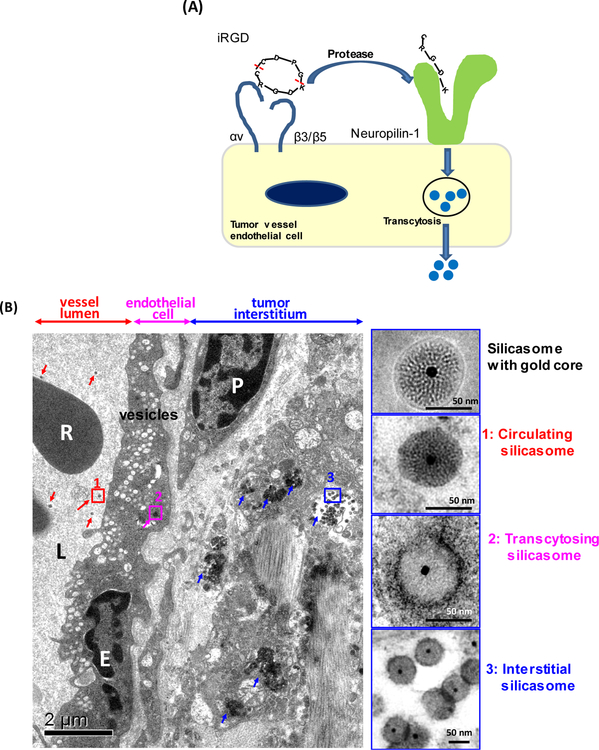
The fourth approach is to develop stromal targeting therapy. This includes the recent discovery that cyclical iRGD peptides can increase PDAC vascular access by a nutrient supply pathway [45]. The iRGD peptide initiates a transcytosis pathway, which involves the formation of cytoplasmic vesicles and vacuoles, a.k.a. a vesiculo-vascular organelle or VVO, in the endothelial cells [46]. Under physiological conditions, the transcytosis pathway is engaged in nutrient supply to the PDAC tumor growth, under the control of vascular endothelial growth factors (VEGF) [10]. These growth factors (e.g. VEGF, TGF-β, and semaphorin 3A) display a C-terminal peptide motif that binds to the neuropilin-1 (NRP-1) receptor on tumor blood vessels [47]. NRP-1 further triggers the transcytosis response which provides transport of proteins and other nutrients, but can also involve transport of drugs and nanocarriers (Fig. 2A) [47]. iRGD binds to tumor specific integrins, where it is proteolytically cleaved to release a C-terminal (CendR) motif that interacts with NRP-1 to initiate transcytosis of macromolecules and nanoparticle [45]. The peptide can be therapeutically employed to enhance the delivery of drugs, macromolecules and nanoparticles by initiating a bulk transcytosis response that can proceed independent of iRGD conjugation to the substance being transported. This pathway is likely analogous to the VVO, which has been observed for a number of years by electron microscopy in tumor vasculature [48]. Recently, we demonstrated a transcytosis-inducing iRGD peptide that can enhance the chemotherapeutic efficacy of a silica-based (silicasome) nanocarriers in PDAC [49]. The efficacy of an irinotecan-laden silicasome carrier can be significantly improved by the co-administration of an unconjugated iRGD peptide that does not require to be attached to the carrier to enhance tumor uptake, leading to enhanced killing of the primary tumor as well as metastasis inhibition in an orthotopic PDAC model. Noteworthy, we were able to visualize the transcytosis of gold-labeled silicasomes at the tumor site by TEM visualization, showing the appearance of grouped vesicles in endothelial cells, followed by particle deposition in the stroma and uptake by PDAC cells (Fig. 2B) [49]. The selection of a tumor pair with differential NRP-1 expression on the tumor vasculature demonstrated differences in carrier uptake and irinotecan delivery during iRGD treatment [49]. We propose that the NRP-1 transcytosis pathway constitutes an important component of the EPR effect for tumors with a dense stroma.
Figure 2.
Nanoparticle PDAC access was improved by iRGD co-administration via a transcytosis mediated mechanism. (A) Schematic to show the working mechanism of iRGD peptide. (B) Ultrastructural viewing of a silicasome nanocarrier transport initiated by iRGD. KPC derived orthotopic tumor bearing mice were injected with 50 mg/kg Au-core containing silicasomes with co-administrated iRGD. Tumors were harvested at 24 hours and immediately fixed for TEM analysis. The electron micrograph shows silicasomes in (i) the lumen of a tumor blood vessel (red arrows), (ii) transport in the endothelial vesicles (pink arrow), and (iii) deposition in the tumor interstitium (blue arrows). High- magnification images of regions 1 through 3 are provided in the panels on the right. E, endothelial cell; P, pericyte. Scale bar: 2 μm (left panel); 50 nm (right panels). Picture adapted from Reference [49].
5. Future discovery to develop nanotherapy for PDAC and other stroma-rich solid tumors
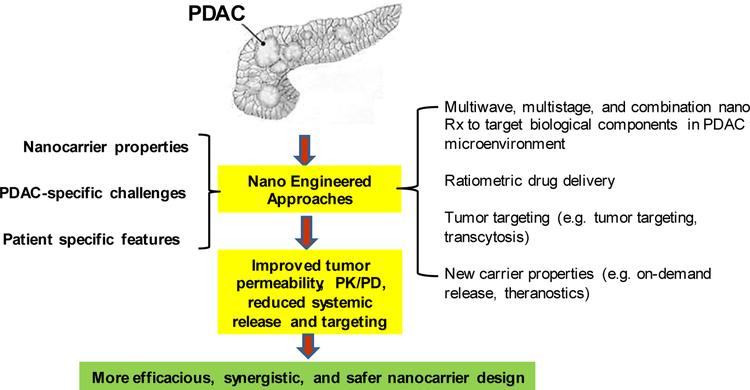
Nanotechnology has contributed in a significant way in improving chemotherapy for PDAC over the last a few years. Two nanomedicines, i.e. Abraxane® and Onivyde® (irinotecan liposome injection) were approved by FDA for PDAC treatment. We have discussed further improvement in the treatment of this disease through the use of synergistic drug combinations, tumor targeting, toxicity reduction, overcoming stroma barrier, etc. These efforts can be conceptualized by a nano-enabled “engineered approach”, which is the selective integration of the drug delivery properties with additional nanocarrier properties that address tumor-specific challenges in PDAC and other solid tumors (Fig. 3). To address heterogeneous stromal effect in PDAC and other solid tumors, one promising way to implement “engineered approach” is waves of therapy, which has been illustrated by “two-wave” approach [23]. Inspired by the ability of communication signaling in biological system, waves of therapy could also be designed by 1st wave nanoparticle to broadcast tumor location, followed by the ‘receiving’ 2nd wave nanoparticles that may carry various payload(s), thereby amplifying particle abundance in solid tumor [50]. Experimentally, it was proven that such signal could be the activation of coagulation cascade at tumor site, followed by clot-targeted nanoparticles as a 2nd wave treatment [50]. Other biological signals, such as paired nanoparticles to mimic the inflammatory cell recruitment process at disease sites, may serve as alternative options. Moreover, the waves of therapy could also include the combined use of anti-stromal agents and nanocarrier which the latter can pharmacologically impact stromal components [51–53]. However, it is also necessary to point out that the role of stroma is complicated. Classic cancer biology studies have demonstrated contrasting results, such as 1) rapid elimination of fibroblasts and fibrosis accelerated PDAC progression [54] and 2) chronic stroma depletion improves cancer drug efficacy [16]. This suggests the importance of precisely-controlled stroma manipulation, which is achievable by nanoparticles with appropriate designs for controlling PK and drug release or be responsive to internal/external stimuli (on-demand release). These options provide an opportunity to avoid the extremes, i.e. stromal depletion vs. stromal abundance, at solid tumor site.
Figure 3.
We propose an engineered approach using nanocarriers in PDAC, which can overcome stromal vascular gate or suppress the stromal abundance by the delivery of drugs that suppress pericyte coverage or decreases the stromal volume and abundance of deaminase activity. Moreover, a combination of these features could be used in synergistic designed nanocarriers. It is also a possible to include tumor targeting or the use of peptides that induce transcytosis across the stromal barrier.
Another promising approach to implement an “engineered approach” would be to design nanocarriers that can deliver drug combinations. We have mentioned that ratiometric delivery [55], exemplified by Vyxeos™ and our paclitaxel/GEM formulation [56]. The capability of tailored nanoparticle design has enabled unprecedented control in delivering a wide range of therapeutics, such as paired small molecule drugs, drug plus nucleic acid, drug plus imaging agent, etc. Use of combinatorial nanoparticles opened up many promising options toward addressing PDAC challenging, including overcoming drug resistance. As compared to traditional drug cocktail, key advantages for nano-enabled combination therapy include 1) enabling concurrent delivery of drug combinations with harmonized PK/PD profiles, 2) maintaining the synergistic drug ratio, and 3) controlling drug exposure sequence, etc. Since nanocarriers that contain multiple components are complicated formulation-wise, to secure the synergy, it is important to control batch-to-batch reproducibility during manufacture [57]. It is also important to consider the design complexity against the cost of each component and the ability to achieve GMP level manufacturing production volumes.
We also want to comment on precision nanomedicine and patient-specific response differences in engineered PDAC manotherapy. Take a GEM nano formulation for example, it requires the consideration on drug metabolic profiling and PK. For example, it would be helpful to deliver a diphosphorylated version of GEM to patients that have a relative low expression of dCK enzyme (that is a key enzyme for intracellular GEM activation). To achieve this integration of nanotherapeutics with clinical-based approaches for PDAC, it is possible to use imaging approaches for delineating GEM- responsiveness in PDAC patients (e.g., PET scanning and intratumoral drug profiling) [26]. This could constitute the basis of future translational studies that build on the development of nanocarriers that can address patient-specific disease characteristics in animals.
Last, we want to comment on the merging nano-enabled immunotherapy in PDAC. In addition to prohibiting drug access, the PDAC stroma acts as a physical and functional barrier to immune cell infiltration and anti-tumor immunity (also see Fig. 1A) [58]. While the mechanism of immunosuppression is not fully understood in PDAC, we do know that activated pancreatic stellate cells and fibroblasts impair immuno-surveillance, contributing to local immune suppression [59]. Moreover, an effective immune activation in the PDAC microenvironment has to overcome the presence of Foxp3+ Tregs (immunologically “cold”), secretion of anti-inflammatory cytokines, expression of checkpoint inhibitors [58]. The awareness of these complex tumor biology processes has allowed PDAC immunotherapy a promising approach, which includes antibodies, immune adjuvants, vaccines and cell-based treatments, etc. While just begin in PDAC, multiple nano formulations, such as polymer, liposome, micelles, and inorganic nanoparticles are under development in various cancer types including solid tumors [60–64]. Here, we want to emphasize on the impact of nanoparticle physicochemical properties on immunological therapy outcome. Take tumor-associated antigens delivery nanoparticles for example, generally speaking, dendritic cell uptake of these particles depends on size, shape, surface charge, and hydrophobicity [60]. The complexity may further increase when use these nanoparticles in vivo. For example, intracutaneously injected <200 nm polystyrene nanoparticles may drain freely to lymph nodes (LN) in B6/C57 mice, subsequently taking up by CD8α+ LN-resident DC subsets, which is favorable for cancer immunotherapy. This differs to particles that are greater than 200 nm, which appear to be taken up by circulant monocytes, then migrate to LNs [65].
There is a growing awareness that the use of selected cancer drugs in neoadjuvant therapy can enhance the recruitment of tumor-infiltrating lymphocytes, with survival benefits [66]. Recently, we demonstrated the use of nanocarriers to deliver chemo agents to mount an immune response in PDAC. One option is to use chemotherapy nanocarrier to induce immunogenic cell death (ICD), which is accompanied by the expression of calreticulin (CRT) on dying tumor cell surfaces, which provides an “eat-me” signal for dendritic cell uptake. The subsequent release of ATP and HMGB1 serves as adjuvant stimuli to the antigen presenting cells. We designed a nanocarrier for the targeted delivery of an ICD-inducing chemo agent (e.g. oxaliplatin that is a chemo component in the FOLFIRINOX regimen) to the PDAC tumor site, which can induce priming and increase the number of tumor-infiltrating lymphocytes in PDAC [58]. The same carrier was used to co-deliver with a prodrug inhibitor, which targeted metabolic immune surveillance pathway (i.e. indoleamine 2,3-dioxygenase or IDO pathway), which is overexpressed at PDAC [58]. The encapsulated co-delivery of oxaliplatin and IDO prodrug inhibitor provided a potent and synergistic activation of both the innate and cognitive immune systems at PDAC site, with survival benefit in a Kras orthotopic model [58]. This nano-enabled combination overcomes the potential guesswork associated with the classic immune checkpoint inhibitors, where only a minority of people responds because of an immunologically “cold” PDAC TME and ineffective tumor biodistribution [58]. To impact majority cancer patients with predictable benefit, it is also possible to use nano-enable approach to augment or synergize with PD-1/PD-L1-, CTL-4- and CAR-T based therapy, which are quite successful in certain cancer scenarios. Moreover, it could be quite attractive to develop image-guided nano immunotherapy with a view to predict the response, improve specificity and safety for PDAC immunotherapy.
Acknowledgements
This study was funded by the U.S. Public Health Service Grant U01CA198846.
Footnotes
Competing interests:
A.E.N. and H.M. are co-founders and equity holders in Westwood Biosciences, Inc.
References:
- [1].Cancer Facts and Figures, 2017, American Cancer Society. [Google Scholar]
- [2].Cancer Facts & Figures 2018, American Cancer Society. [Google Scholar]
- [3].Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H, The Role of Stroma in Pancreatic Cancer: Diagnostic and Therapeutic Implications, Nature Reviews Gastroenterology and Hepatology, 9 (2012) 454–467. [DOI] [PubMed] [Google Scholar]
- [4].Au M, Emeto TI, Power J, Vangaveti VN, Lai HC, Emerging Therapeutic Potential of Nanoparticles in Pancreatic Cancer: A Systematic Review of Clinical Trials, Biomedicines, 4 (2016) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ji T, Zhao Y, Ding Y, Nie G, Using Functional Nanomaterials to Target and Regulate the Tumor Microenvironment: Diagnostic and Therapeutic Applications, Advanced Materials, 25 (2013) 3508–3525. [DOI] [PubMed] [Google Scholar]
- [6].Fang J, Nakamura H, Maeda H, The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect, Advanced Drug Delivery Reviews, 63 (2011) 136–151. [DOI] [PubMed] [Google Scholar]
- [7].Bae YH, Park K, Targeted drug delivery to tumors: Myths, reality and possibility, Journal of Controlled Release, 153 (2011) 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ojha T, Pathak V, Shi Y, Hennink WE, Moonen CTW, Storm G, Kiessling F, Lammers T, Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors, Advanced Drug Delivery Reviews, 119 (2017) 44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou H, Qian W, Uckun FM, Wang L, Wang YA, Chen H, Kooby D, Yu Q, Lipowska M, Staley CA, Mao H, Yang L, IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer, ACS Nano, 9 (2015) 7976–7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nel A, Ruoslahti E, Meng H, New Insights into “Permeability” as in the Enhanced Permeability and Retention Effect of Cancer Nanotherapeutics, ACS Nano, 11 (2017) 9567–9569. [DOI] [PubMed] [Google Scholar]
- [11].Shi J, Kantoff PW, Wooster R, Farokhzad OC, Cancer nanomedicine: progress, challenges and opportunities, Nature Reviews Cancer, 17 (2016) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nakamura Y, Mochida A, Choyke PL, Kobayashi H, Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer?, Bioconjugate Chemistry, 27 (2016) 2225–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maeda H, Nakamura H, Fang J, The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo, Advanced Drug Delivery Reviews, 65 (2013) 71–79. [DOI] [PubMed] [Google Scholar]
- [14].Danhier F, To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?, Journal of Controlled Release, 244 (2016) 108–121. [DOI] [PubMed] [Google Scholar]
- [15].Wang AZ, EPR or no EPR? The billion-dollar question, Science Translational Medicine, 7 (2015) 294ec112–294ec112. [Google Scholar]
- [16].Kota J, Hancock J, Kwon J, Korc M, Pancreatic cancer: Stroma and its current and emerging targeted therapies, Cancer Letters, 391 (2017) 38–49. [DOI] [PubMed] [Google Scholar]
- [17].Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, Tuveson DA, Stromal biology and therapy in pancreatic cancer, Gut, 60 (2011) 861. [DOI] [PubMed] [Google Scholar]
- [18].Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H, The role of stroma in pancreatic cancer: diagnostic and therapeutic implications, Nature Reviews Gastroenterology &Amp; Hepatology, 9 (2012) 454. [DOI] [PubMed] [Google Scholar]
- [19].Xu Z, Pothula SP, Wilson JS, Apte MV, Pancreatic cancer and its stroma: A conspiracy theory, World Journal of Gastroenterology : WJG, 20 (2014) 11216–11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T, Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer, American Journal of Physiology-Gastrointestinal and Liver Physiology, 295 (2008) G709–G717. [DOI] [PubMed] [Google Scholar]
- [21].Thind K, Padrnos LJ, Ramanathan RK, Borad MJ, Immunotherapy in pancreatic cancer treatment: a new frontier, Therapeutic Advances in Gastroenterology, 10 (2017) 168–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dimou A, Syrigos KN, Saif MW, Overcoming the stromal barrier: technologies to optimize drug delivery in pancreatic cancer, Therapeutic Advances in Medical Oncology, 4 (2012) 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Meng H, Zhao Y, Dong J, Xue M, Lin Y-S, Ji Z, Mai WX, Zhang H, Chang CH, Brinker CJ, Zink JI, Nel AE, Two-Wave Nanotherapy To Target the Stroma and Optimize Gemcitabine Delivery To a Human Pancreatic Cancer Model in Mice, ACS Nano, 7 (2013) 10048–10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K, Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size, Nature Nanotechnology, 6 (2011) 815. [DOI] [PubMed] [Google Scholar]
- [25].Shipley LA, Brown TJ, Cornpropst JD, Hamilton M, Daniels WD, Culp HW, Metabolism and Disposition of Gemcitabine, and Oncolytic Deoxycytidine Analog, in Mice, Rats, and Dogs, Drug Metabolism and Disposition, 20 (1992) 849–855. [PubMed] [Google Scholar]
- [26].Laing RE, Walter MA, Campbell DO, Herschman HR, Satyamurthy N, Phelps ME, Czernin J, Witte ON, Radu CG, Noninvasive Prediction of Tumor Responses to Gemcitabine Using Positron Emission Tomography, Proceedings of the National Academy of Sciences of the United States of America, 106 (2009) 2847–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR, Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma, Cancer Cell, 21 (2012) 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA, Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer, Gut, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Singha NC, Nekoroski T, Zhao C, Symons R, Jiang P, Frost GI, Huang Z, Shepard HM, Tumor-Associated Hyaluronan Limits Efficacy of Monoclonal Antibody Therapy, Molecular Cancer Therapeutics, 14 (2015) 523–532. [DOI] [PubMed] [Google Scholar]
- [30].Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, DeNicola G, Feig C, Combs C, Winter SP, Ireland H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA, Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer, Science (New York, N.Y.), 324 (2009) 1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kang S.-g., Zhou G, Yang P, Liu Y, Sun B, Huynh T, Meng H, Zhao L, Xing G, Chen C, Zhao Y, Zhou R, Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C(82)(OH)(22) and its implication for de novo design of nanomedicine, Proceedings of the National Academy of Sciences of the United States of America, 109 (2012) 15431–15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Doi C, Egashira N, Kawabata A, Maurya DK, Ohta N, Uppalapati D, Ayuzawa R, Pickel L, Isayama Y, Troyer D, Takekoshi S, Tamura M, Angiotensin II type 2 receptor signaling significantly attenuates growth of murine pancreatic carcinoma grafts in syngeneic mice, BMC Cancer, 10 (2010) 67–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF, Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine, New England Journal of Medicine, 369 (2013) 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, Tuveson DA, nab-Paclitaxel Potentiates Gemcitabine Activity by Reducing Cytidine Deaminase Levels in a Mouse Model of Pancreatic Cancer, Cancer Discovery, 2 (2012) 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lancet JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE, Komrokji R, Solomon SR, Kolitz JE, Cooper M, Yeager AM, Louie AC, Feldman EJ, Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML, Blood, 123 (2014) 3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, Ji Z, Chang CH, Nel AE, Use of a Lipid-Coated Mesoporous Silica Nanoparticle Platform for Synergistic Gemcitabine and Paclitaxel Delivery to Human Pancreatic Cancer in Mice, ACS Nano, 9 (2015) 3540–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ernsting MJ, Hoang B, Lohse I, Undzys E, Cao P, Do T, Gill B, Pintilie M, Hedley D, Li S-D, Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle, Journal of controlled release : official journal of the Controlled Release Society, 206 (2015) 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD, Li S-D, Docetaxel Conjugate Nanoparticles That Target α-Smooth Muscle Actin–Expressing Stromal Cells Suppress Breast Cancer Metastasis, Cancer research, 73 (2013) 4862–4871. [DOI] [PubMed] [Google Scholar]
- [39].Hoang B, Ernsting MJ, Roy A, Murakami M, Undzys E, Li S-D, Docetaxel-Carboxymethylcellulose Nanoparticles Target Cells via a SPARC and Albumin Dependent Mechanism, Biomaterials, 59 (2015) 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].ten Dijke P, Arthur HM, Extracellular control of TGF[beta] signalling in vascular development and disease, Nat Rev Mol Cell Biol, 8 (2007) 857–869. [DOI] [PubMed] [Google Scholar]
- [41].Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, Fujii T, Komuro A, Kiyono K, Kaminishi M, Hirakawa K, Ouchi Y, Nishiyama N, Kataoka K, Miyazono K, Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-β signaling, Proceedings of the National Academy of Sciences, 104 (2007) 3460–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, Ancukiewicz M, Boucher Y, Jain RK, Xu L, TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma, Proceedings of the National Academy of Sciences, 109 (2012) 16618–16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK, Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors, Proceedings of the National Academy of Sciences, 108 (2011) 2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T, Ultrasound Microbubbles for Molecular Diagnosis, Therapy, and Theranostics, Journal of Nuclear Medicine, 53 (2012) 345–348. [DOI] [PubMed] [Google Scholar]
- [45].Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E, Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs, Science, 328 (2010) 1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin, The Journal of Experimental Medicine, 183 (1996) 1981–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E, Co-administration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs, Science (New York, N.Y.), 328 (2010) 1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF, The Vesiculo-Vacuolar Organelle (VVO): A Distinct Endothelial Cell Structure that Provides a Transcellular Pathway for Macromolecular Extravasation, Journal of Leukocyte Biology, 59 (1996) 100–115. [PubMed] [Google Scholar]
- [49].Liu X, Lin P, Perrett I, Lin J, Liao Y-P, Chang CH, Jiang J, Wu N, Donahue T, Wainberg Z, Nel AE, Meng H, Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer, The Journal of Clinical Investigation, 127 (2017) 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].von Maltzahn G, Park J-H, Lin KY, Singh N, Schwöppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN, Nanoparticles that communicate in vivo to amplify tumour targeting, Nature Materials, 10 (2011) 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Godin B, Tasciotti E, Liu X, Serda RE, Ferrari M, Multistage Nanovectors: from Concept to Novel Imaging Contrast Agents and Therapeutics, Accounts of Chemical Research, 44 (2011) 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mei L, Fu L, Shi K, Zhang Q, Liu Y, Tang J, Gao H, Zhang Z, He Q, Increased Tumor Targeted Delivery Using a Multistage Liposome System Functionalized with RGD, TAT and Cleavable PEG, International Journal of Pharmaceutics, 468 (2014) 26–38. [DOI] [PubMed] [Google Scholar]
- [53].von Maltzahn G, Park J-H, Lin KY, Singh N, Schwöppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN, Nanoparticles that Communicate In Vivo to Amplify Tumour Targeting, Nat Mater, 10 (2011) 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Özdemir Berna C., Pentcheva-Hoang T, Carstens Julienne L., Zheng X, Wu C-C, Simpson Tyler R., Laklai H, Sugimoto H, Kahlert C, Novitskiy Sergey V., De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses Harold L., Weaver Valerie M., Maitra A, Allison James P., LeBleu Valerie S., Kalluri R, Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival, Cancer Cell, 25 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mayer L, Janoff A, Optimizing Combination Chemotherapy by Controlling Drug Ratios, Mol Interv, 7 (2007) 216–223. [DOI] [PubMed] [Google Scholar]
- [56].Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, Ji Z, Chang CH, Nel AE, Use of a Lipid-Coated Mesoporous Silica Nanoparticle Platform for Synergistic Gemcitabine and Paclitaxel Delivery to Human Pancreatic Cancer in Mice, ACS Nano, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hu C-MJ, Zhang L, Nanoparticle-based combination therapy toward overcoming drug resistance in cancer, Biochemical Pharmacology, 83 (2012) 1104–1111. [DOI] [PubMed] [Google Scholar]
- [58].Lu J, Liu X, Liao Y-P, Salazar F, Sun B, Jiang W, Chang CH, Jiang J, Wang X, Wu AM, Meng H, Nel AE, Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression, Nature Communications, 8 (2017) 1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tao L, Huang G, Song H, Chen Y, Chen L, Cancer associated fibroblasts: An essential role in the tumor microenvironment, Oncology Letters, 14 (2017) 2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Conniot J, Silva JM, Fernandes JG, Silva LC, Gaspar R, Brocchini S, Florindo HF, Barata TS, Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking, Frontiers in Chemistry, 2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, Du M, Huang G, Wang C, Chen X, Porembka MR, Lea J, Frankel AE, Fu Y-X, Chen ZJ, Gao J, A STING-activating nanovaccine for cancer immunotherapy, Nature Nanotechnology, 12 (2017) 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nakamura T, Harashima H, Integration of nano drug-delivery system with cancer immunotherapy, Therapeutic Delivery, 8 (2017) 987–1000. [DOI] [PubMed] [Google Scholar]
- [63].Zhu G, Zhang F, Ni Q, Niu G, Chen X, Efficient Nanovaccine Delivery in Cancer Immunotherapy, ACS Nano, 11 (2017) 2387–2392. [DOI] [PubMed] [Google Scholar]
- [64].Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, Goldberg MS, T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity, Nature Communications, 8 (2017) 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF, Nanoparticles target distinct dendritic cell populations according to their size, European Journal of Immunology, 38 (2008) 1404–1413. [DOI] [PubMed] [Google Scholar]
- [66].Kroemer G, Galluzzi L, Kepp O, Zitvogel L, Immunogenic Cell Death in Cancer Therapy, Annual Review of Immunology, 31 (2013) 51–72. [DOI] [PubMed] [Google Scholar]