Abstract
Socioeconomic disparities in childhood are associated with remarkable differences in cognitive and socio-emotional development during a time when dramatic changes are occurring in the brain. Yet, the neurobiological pathways through which socioeconomic status (SES) shapes development remain poorly understood. Behavioral evidence suggests that language, memory, social-emotional processing, and cognitive control exhibit relatively large differences across SES. Here we investigated whether volumetric differences could be observed across SES in several neural regions that support these skills. In a sample of 60 socioeconomically diverse children, highly significant SES differences in regional brain volume were observed in the hippocampus and the amygdala. In addition, SES × age interactions were observed in the left superior temporal gyrus and left inferior frontal gyrus, suggesting increasing SES differences with age in these regions. These results were not explained by differences in gender, race or IQ. Likely mechanisms include differences in the home linguistic environment and exposure to stress, which may serve as targets for intervention at a time of high neural plasticity.
Introduction
Currently, over one in five US children live below the federal poverty line (National Center for Children in Poverty, 2011). For decades, it has been recognized that socioeconomic disadvantage in childhood is associated with negative effects on cognitive and socio-emotional development (McLoyd, 1998).These effects are both statistically substantial and clinically meaningful. By the time of school entry, children from lower SES backgrounds typically score between one-half and one full standard deviation lower than other children on most academic achievement tests (Rouse, Brooks-Gunn & McLanahan, 2005). Such disparities in child development in turn have long-lasting ramifications for physical and mental health (Brooks-Gunn & Duncan, 1997). Yet, the neurobiological pathways through which socioeconomic disadvantage shapes developmental processes remain poorly understood.
Socioeconomic status (SES) is typically characterized by factors including family educational attainment, occupation, and income level (McLoyd, 1998). Studies examining the association between SES and child development have typically focused on important but generalized cognitive and academic milestones, such as child IQ, grade retention, and school graduation rates (Brooks-Gunn & Duncan, 1997). These outcome measures are likely to be at least partially accounted for by variability in the developing brain. However, until very recently, the study of SES disparities in child development operated with scarce input from neuroscience. While classic academic milestones like school graduation can tell us broadly about global effects of socioeconomic disparities on achievement, we know in fact that ‘achievement’ is the complex output of multiple cognitive systems which are supported by different brain regions and networks. Thus, although classic measures of academic achievement must at some level reflect the function of the brain, they are relatively uninformative concerning perturbations in specific cognitive and neural processes. A cognitive neuroscience approach, in contrast, reflects the fact that different neural structures and circuits support the development of distinct cognitive and socio-emotional skills, improving our efforts to provide targeted educational interventions.
In recent years, a series of studies has capitalized on this insight (Farah, Shera, Savage, Betancourt, Giannetta, Brodsky, Malmud & Hurt, 2006; Noble, McCandliss & Farah, 2007; Noble, Norman & Farah, 2005). By using behavioral tasks that selectively engage one neurocognitive system while placing a minimal burden on others, it has proven possible to investigate the degree to which socioeconomic disparities are associated with a child’s performance in one neurocognitive system relative to another. Using this approach, Noble, Farah and colleagues have demonstrated large socioeconomic disparities in early childhood language development, with more modest but consistent disparities across SES in other neurocognitive systems, such as memory and certain aspects of executive function, including cognitive control (Farah et al., 2006; Noble et al., 2007; Noble et al., 2005). However, the extent to which these neurocognitive differences - as captured by differences in behavioral performance - reflect underlying structural differences in regional brain development remains largely unknown.
Of course, SES is a general marker for a broad conglomerate of experiences and exposures. Many environmental factors have been shown to affect regionally specific brain development (McEwen & Gianaros, 2010; Rosenzweig, 2003), and thus are likely candidates in mediating the links between SES and specific neurocognitive outcomes. Further, the components of childhood SES - such as parent education and family income – may not always act in concert (Geyer, Hemström, Peter & Vågerö, 2006; Næss, Claussen, Thelle & Smith, 2005).
Brain development can be characterized as a dynamic process of progressive and regressive changes, which are influenced by both complex genetic programs and experience-dependent plasticity that varies with environmental stimulation (Rosenzweig, 2003). Maturation of the brain regions responsible for higher cognitive functioning continues throughout childhood and adolescence (Sowell, Thompson, Leonard, Welcome, Kan & Toga, 2004; Toga, Thompson & Sowell, 2006), and reductions in synaptic density along with concomitant increases in axonal myelination are thought to be the hallmarks of experience-based neural plasticity (Sowell et al., 2004; Toga et al., 2006). Recent neuroimaging research suggests that even relatively brief interventions can lead to measureable differences in brain structure in children, and that this change is directly related to improvement in cognitive skill (Keller & Just, 2009). Such work lends credence to the notion that the developing human brain is malleable, and that interventions that are targeted towards SES-related disparities in distinct cognitive functions – and the neural mechanisms that support them – may lead to better outcomes.
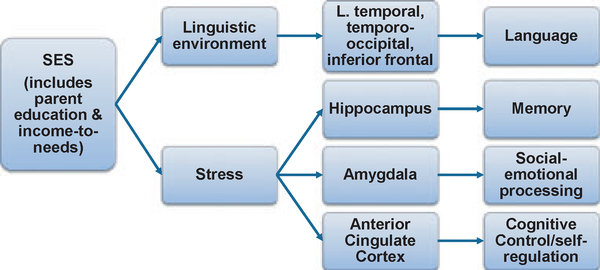
Figure 1 illustrates one theoretical model, illustrating two relatively independent pathways by which SES may operate via more proximate factors to influence cognitive and neural development.
Figure 1.
Hypothesized mechanisms by which SES operates to influence cognitive development. See text for details.
SES, linguistic exposure, and brain development
First, socioeconomic disparities in the quantity and quality of linguistic stimulation in the home have been well described (Hart & Risley, 1995; Whitehurst, 1997), with higher SES families more likely to speak to children with greater frequency and complexity (Hart & Risley, 1995); spend more hours in parent-child reading activities (Adams, 1990); and provide increased access to books (Bradley, Corwyn, Burchinal, McAdoo & Garcia Coll, 2001; Raz, 1990; Whitehurst, 1997) and other language-related resources (Bradley et al., 2001). Such differences in linguistic exposure have in turn been directly related to child language development (Bradley et al., 2001; Hart & Risley, 1995; Hoff, 2003; Noble & McCandliss, 2005; Whitehurst, 1997; Whitehurst & Lonigan, 1998). Research has also shown that differences in linguistic exposure are associated with developmental differences in language-supporting cortical regions in the left hemisphere (Conboy & Kuhl, 2007; Kuhl, 2007; Kuhl, Tsao & Liu, 2003). The language-supporting brain network in left temporal, temporo-occipital and frontal cortices is critical for the development of language skill (Dehaene-Lambertz, Hertz-Pannier, Dubois, Mériaux, Roche, Sigman & Dehaene, 2006; McCandliss & Noble, 2003; Redcay, Haist & Courchesne, 2008; Turkeltaub, Gareau, Flowers, Zeffiro & Eden, 2003; Vannest, Karunanayaka, Schmithorst, Szaflarski & Holland, 2009). Taken together, the above evidence suggests that socioeconomic disparities, which are associated with large differences in access to language-promoting resources, are likely to be associated with differences in the development of language-supporting brain regions. This proposed mechanistic pathway may therefore mediate previously described SES disparities in children’s language skills (Hoff, 2003; Noble et al., 2007; Noble et al., 2005). Further, as SES-related disparities in language-promoting experience tend to increase over time (Hart & Risley, 1995), differences in the development of language-supporting brain regions may become more pronounced in later childhood.
To date, two functional magnetic resonance imaging (fMRI) studies have reported SES differences in the function of certain language-supporting brain regions, namely the left fusiform (Noble, Wolmetz, Ochs, Farah & McCandliss, 2006) and left inferior frontal gyrus (Raizada, Richards, Meltzoff & Kuhl, 2008). However, little is known about whether these differences in brain function reflect differences at the structural level, and/or the degree to which these differences vary with age. We hypothesize that SES disparities are associated with differences in the structural development of the left temporal, temporo-occipital and frontal structures that support language development (but see Eckert, Lombardino & Leonard, 2001). Further, we predict that SES-related differences in brain structure in these regions may increase with age. As parental education level has a particularly strong influence on the home linguistic environment (Whitehurst & Lonigan, 1998), we hypothesize that this component of SES will be most important in accounting for variation in brain structure in these regions.
SES, stress, and brain development
A relatively independent literature has described SES disparities in exposure to stress, including uncertainty about material resources such as food or clothing; chaotic households; and exposure to violence (Evans, 2004). These differences in childhood exposure to stressful experiences are reflected in hormonal markers of stress, with children from lower SES backgrounds tending to show dysregulation of the stress axis and response (Evans & Kim, 2007; Lupien, King, Meaney & McEwen, 2000, 2001). Research in both animals and humans suggests that the experience of stress has important negative effects on the hippocampus (Buss, Lord, Wadiwalla, Hellhammer, Lupien, Meaney & Pruessner, 2007; McEwen & Gianaros, 2010; Tottenham & Sheridan, 2010), the amygdala (McEwen & Gianaros, 2010; Tottenham & Sheridan, 2010) and the anterior cingulate cortex (ACC) (Liston, McEwen & Casey, 2009; McEwen & Gianaros, 2010) in the medial prefrontal cortex, structures which are linked together anatomically and functionally (McEwen & Gianaros, 2010), and which are, respectively, critical for the development of memory (McEwen & Gianaros, 2010; Richmond & Nelson, 2008), socio-emotional processing (Gianaros, Horenstein, Cohen, Matthews, Brown, Flory, Critchley, Manuck & Hariri, 2007; Tottenham & Sheridan, 2010), and cognitive control/self-regulation (Gianaros et al., 2007; McEwen & Gianaros, 2010). Together these structures represent an important network for processing emotionally salient environmental stimuli (Gianaros et al., 2007). Exposure to stress may therefore operate on these structures to mediate previously described SES disparities in memory (Noble et al., 2007; Noble et al., 2005), socio-emotional processing (Hackman, Farah & Meaney, 2010; McLoyd, 1998; National Institute of Child Health Human Development Early Child Care Research Network, 2005) and cognitive control/self-regulation (Blair, Granger & Peters Razza, 2005; Mezzacappa, 2004; Noble et al., 2007; Noble et al., 2005).
One fMRI study has reported SES disparities in the function of the amygdala in human adults (Gianaros, Horenstein, Hariri, Sheu, Manuck, Matthews & Cohen, 2008), and other studies have used event-related potentials (ERP) to show SES disparities in the function of the prefrontal cortex in children (D’Angiulli, Herdman, Stapells & Hertzman, 2008; Kishiyama, Boyce, Jimenez, Perry & Knight, 2009; Stevens, Lauinger & Neville, 2009). In addition, investigators have used structural neuroimaging to show that subjectively lower social status during adulthood is associated with reduced ACC volume (Gianaros et al., 2007), and one recent study found that lower SES is associated with smaller hippocampal size in children (Hanson, Chandra, Wolfe & Pollak, 2011). We thus hypothesize that SES is associated with regional volumetric differences in the developing amygdala, hippocampus, and ACC. Further, the components of SES may differentially relate to different types of stressful childhood experience, which may in turn have regionally specific effects on neurodevelopment. For instance, parental education level may be particularly important in predicting parenting style, including warmth and nurturance (Brooks-Gunn & Markman, 2005), which may have particular importance for amygdala structure (Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly, Milner, Galvan, Davidson, Eigsti, Thomas, Freed, Booma, Gunnar, Altemus, Aronson & Casey, 2010). In contrast, lower family income may cause limited access to material resources, which may be more important for predicting hippocampal size (Hanson et al., 2011).
An integrated model
Taking these separate lines of research together, we propose to test part of the model illustrated in Figure 1, hypothesizing that SES differences are associated with differences in the structural development of brain regions that support distinct skills of language, memory, socioemotional processing, and cognitive control. Specifically, we use structural magnetic resonance imaging (MRI) in a diverse sample of children to assess the degree to which SES accounts for individual differences in the structure of the hippocampus, the amygdala, the ACC and several structures in a language-supporting network in the left temporal, temporo-occipital and frontal cortices. We will further examine the extent to which certain components of SES, namely parent education and income, have differential effects on these outcomes.
Method
Participants
Participants were typically developing, native English-speaking children and adolescents who were recruited as a part of a larger study on anatomic and functional brain development in childhood, adolescence and adulthood, which took place at the University of California, Los Angeles. For the present study, data were analyzed from a cross-sectional sample of 60 children and adolescents (31 female) who participated in the study, ranging in age from 5 to 17 years (Mean = 11.4, SD 3.1). Table 1 shows the SES and demographics of the sample, confirming that participating families were socioeconomically diverse. Parents of all participants confirmed that participants were free of neurological impairment, psychiatric disability, low birth weight, learning disability, language impairment, mental retardation, autism, and exposure to prenatal teratogens such as alcohol. Thirteen additional participants were scanned but were excluded due to excessive motion artifact or participant request to discontinue the procedure in the middle of the scan.
Table 1.
Socioeconomic status and demographics of sample
| Mean (SD; range) or N (%) | |
|---|---|
| Average parent education (years) | 15.1 (2.7; 8–21) |
| Income-to-needs ratio1 | 3.3 (1.9; 0.23–6.7) |
| Race | |
| African-American | 8 (13.3%) |
| Asian/Pacific Islander | 5 (8.3%) |
| Caucasian | 41 (68.3%) |
| Mixed race/other | 6 (10%) |
| Ethnicity | |
| Hispanic/Latino | 25 (41.7%) |
| Not Hispanic/Latino | 34 (56.7%) |
| Did not respond | 1 (1.7%) |
Total family income divided by the federal poverty level for a family of that size, in the year data were collected.
Procedures
All participants and their parents gave informed consent to participate in this study, which was approved by the Institutional Review Board of the University of California, Los Angeles.
Structured interview
Parents of participants underwent an interview during which they were asked about the highest level of education of any parents or gaurdians living in the home, the total family income during the past year, and the number of children and adults actively living in the household. Socioeconomic status variables were derived, including (1) the average number of years of education of parents or guardians living in the home, and (2) the family’s income-to-needs ratio, defined as the total family income divided by the federal poverty threshold for a family of that size, in the year the data were collected (McLoyd, 1998).
Neurocognitive data collection
As part of the larger study, a subset of participants were administered abattery of standardized cognitive measures. For the present study, some analyses include the subsample of 50 participants who completed the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) (Wechsler, 2003), a measure of full-scale IQ.
Image acquisition
Structural imaging data were obtained on a Siemens 1.5T Sonata Scanner using a 12-channel head coil. One to four sagittal T1-weighted MPRage images were collected for each participant using the following parameters: repetition time, 1900 ms; echo time, 4.38 ms; flip angle, 15; matrix size, 256 × 256; voxel size, 1 × 1 × 1 mm; acquisition time, 8 min 8s.
Image processing and analysis
Raters blind to participant age, gender and SES inspected all images. Pre-processing and definition of cortical and subcortical gray matter regions on structural images used automated brain segmentation software (FreeSurfer 4.5, http://surfer.nmr.mgh.harvard.edu) (Fischl, Salat, Busa, Albert, Dieterich, Haselgrove, van der Kouwe, Killiany, Kennedy, Klaveness, Montillo, Makris, Rosen & Dale, 2002), which has been shown to be indistinguishable in accuracy to manual labeling (Fischl et al., 2002).
Pre-processing of the data involved visually inspecting each scan for excessive motion and other artifacts, and then averaging all high-quality MPRage acquisitions for each participant to enhance signal to noise ratio (SNR). Images were then motion-corrected and brain-extracted, followed by manual inspection and editing when necessary by an expert user. Skull-stripping was processed in Brainsuite’s Brain Skull Extractor (BSE) and manually edited by an expert user. The skull-stripped image was then inserted back into the FreeSurfer processing stream. Gray/white matter boundaries were automatically delineated. A surface of connected white matter voxels was refined to generate sub-millimeter voxel resolution in the gray/white matter boundary. The gray/white matter boundary was then warped outward to approximate the pial surface with the constraints that the surface must be smooth and maintain the natural topology of the brain. All participants’ pial and white matter surfaces were inspected for errors by a trained examiner, and edited when necessary.
Total gray and white matter volumes were obtained using FreeSurfer’s ‘mri_segstats 2table’ command. Cortical gray matter volume was defined as the volume between the pial and white matter surfaces. White matter volume was calculated by subtracting the subcortical and ventricular volumes from the volume bounded by the white matter surface. Regional volumes were calculated using FreeSurfer’s automatic quantification of cortical and subcortical structures, which assigns a neuroanatomical label to each voxel in an MRI volume based on probabilistic information estimated from a manually labeled training set. The classification technique employs a non-linear registration procedure that is robust to anatomical variability, as described in detail elsewhere (Fischl et al., 2002).
Regions of interest (ROIs) were selected based on the literature to encompass regions that support skills that show SES disparities, namely language, memory, socioemotional processing and cognitive control. The development of language and reading skills in children is supported by a diverse network of regions involved in semantic, phonologic and orthographic processing, including left superior temporal gyrus (LSTG), left middle temporal gyrus (LMTG), left inferior temporal gyrus (LITG), left inferior frontal gyrus (LIFG), and left fusiform gyrus (Dehaene-Lambertz et al., 2006; Mc- Candliss & Noble, 2003; Turkeltaub et al., 2003; Vannest et al., 2009). Episodic memory skills are dependent in large part on the hippocampus (McEwen & Gianaros, 2010; Richmond & Nelson, 2008). The processing of information containing social or emotional significance relies heavily on the amygdala (McEwen & Gianaros, 2010; Tottenham & Sheridan, 2010). The anterior cingulate cortex (ACC) is heavily involved in regulating cognitive control (Gianaros et al., 2007; McEwen & Gianaros, 2010). Our analysis strategy consisted of examining the relationship between SES factors (parent education and income-to-needs ratio) and cortical volume in each ROI, adjusting for age and total cortical volume. Scan quality, gender, race and full-scale IQ were examined as possible confounders of SES effects. We further investigated the degree to which the effects of SES factors on regional brain volume might change with age, by examining SES × age interactions in each ROI.
Results
As expected, parent education and income-to-needs were highly correlated (R = 0.56; p <4.1 × 10−6). Parents of two children did not disclose income information. A regression equation was therefore constructed to predict the income-to-needs ratio from parent education using data from the 58 participants for whom both variables were available [Income-to-needs = 0.386 (parent education) – 2.48; R2 = 0.32; p <4.1 × 10−6]. This equation was then used to impute the income-to-needs ratio for the remaining two children.
Initial data analyses consisted of evaluations for any differences in these SES factors across scan quality, child age, gender and race. Each participant provided between 1 and 4 scans with usable, high-quality data (mean = 2.9, SD = 0.68). The number of high-quality scans did not vary by age (R = −0.096; p = .47) or SES (average parental education: R = 0.24; p = .063, income-to-needs: R = 0.186; p = .16). Child age was negatively correlated with parental education (R = −0.43; p < .001), but not income-to-needs ratio (R = −0.24; p < .1). There were no significant SES differences in the sample in gender (parent education: t(58) = 1.23; p = .224; income-to-needs: t(56) = 0.332; p = .741) or race (parent education: F(1, 58) = 0.156; p = .694; income-to-needs: F(1, 56) = 0.474; p < .494).
Next, multiple regressions were examined, to investigate the effects of SES on ROI volume. To correct for multiple comparisons of eight regions of interest, alpha was set at 0.00625 (e.g. 0.05/8). All models controlled for child age and total cortical volume. Although there were no differences in child gender by SES, research has suggested that gender may contribute to differences in neural volume over and above variance associated with total brain volume (Lenroot, Gogtay, Greenstein, Wells, Wallace, Clasen, Blumenthal, Lerch, Zijdenbos, Evans, Thompson & Giedd, 2007). Models were therefore examined with and without gender, and gender was retained in the models when it accounted for unique variance at the p < .05 level. This was the case in the hippocampus and the amygdala. In all other ROIs, gender did not account for unique variance, and was therefore dropped from the models for the sake of parsimony. Similarly, past work has suggested that in certain brain regions, a quadratic term for age may be more appropriate than a linear term alone (Østby, Tamnes, Fjell, Westlye, Due-Tønnessen & Walhovd, 2009). However, in no case did age2 account for significant variance in ROI volume when the linear term for age was included, and therefore the quadratic term was dropped from all models.
Table 2 shows that, when controlling for age, total cortical volume, and gender, SES was significantly associated with hippocampal volume (R2 change = 0.124; p < .001) and amygdala volume (R2 change = 0.088; p < .001). There was also a trend for an association between SES and L ITG volume (R2 change = 0.071; p < .036). No main effect of SES was found for L STG, L MTG, L IFG, left fusiform, or ACC volume, nor for total cortical volume or total white matter volume.
Table 2.
SES accounts for individual variation in amygdala and hippocampus volume
| ROI | Regression step | R2 change (sig) | Beta (sig) |
|---|---|---|---|
| Hippocampus | Step 1: | 0.457 (1.5 × 10−7 ) | |
| age, | 0.156 (0.173) | ||
| total cortical volume, | 0.380 (0.002) | ||
| gender | −0.411 (0.001) | ||
| Step 2: | 0.124 (0.001) | ||
| age, | 0.112 (0.293) | ||
| total cortical volume, | 0.423 (3.0 × 10−4 ) | ||
| gender, | −0.371 (0.001) | ||
| avg. parent education, | −0.384 (0.002) | ||
| income-to-needs | 0.392 (0.001) | ||
| Amygdala | Step 1: | 0.634 (2.9 × 10−12 ) | |
| age, | 0.313 (0.001) | ||
| total cortical volume, | 0.688 (1.1 × 10−9 ) | ||
| gender | −0.194 (0.048) | ||
| Step 2: | 0.088 (0.001) | ||
| age, | 0.235 (0.008) | ||
| total cortical volume, | 0.782 (5.5 × 10−12 ) | ||
| gender, | −0.133 (0.130) | ||
| avg. parent education, | −0.401 (1.3 × 10−4 ) | ||
| Income-to-needs | 0.209 (0.02) | ||
| Left Inferior temporal gyrus | Step 1: | 0.370 (1.9 × 10−6 ) | |
| age, | 0.017 (0.879) | ||
| total cortical volume, | 0.612 (5.5 × 10−7 ) | ||
| Step 2: | 0.071 (0.036) | ||
| age, | −0.089 (0.429) | ||
| total cortical volume, | 0.685 (5.4 × 10−8 ) | ||
| avg. parent education, | −0.351 (0.012) | ||
| income-to-needs | 0.107 (0.385) |
Note: Models of effects of SES on ROI volume. In each ROI, a regression was conducted, with the first step incorporating covariates, and the second step incorporating SES factors (parent education and the income-to-needs ratio). All models controlled for age and total cortical volume. Gender was included in models in which it accounted for unique variance at the p < .05 level. SES factors accounted for significant unique variance in the models of hippocampus and amygdala volume, when using the Bonferroni correction for multiple comparisons of eight ROIs (e.g. alpha set at 0.05/8 = 0.00625). A nonsignificant trend was also seen in left inferior temporal gyrus volume.
Although ROIs were chosen based on hypotheses concerning their specific underlying neurocognitive functions and respective relations to SES, we next explored whether these volumetric differences could be related to nonspecific ‘general intelligence’ differences across SES (though compelling arguments against this approach have been made; Dennis, Francis, Cirino, Schachar, Barnes & Fletcher, 2009). Regression analyses were re-run in the 50 subjects for whom full-scale IQ was available, in the regions showing significant or near-significant effects of SES above. After controlling for age and total cortical volume, IQ did not account for unique variance in the hippocampus, amygdala or left ITG. When SES was entered in the next step, the effects of SES on regional volumes were very similar (acknowledging reduced power in light of 10 fewer subjects in the analysis: R2 change hippocampus = 0.124, p < .008; R2 change amygdala = 0.067, p < .007; R2 change LITG = 0.078, p < .048).
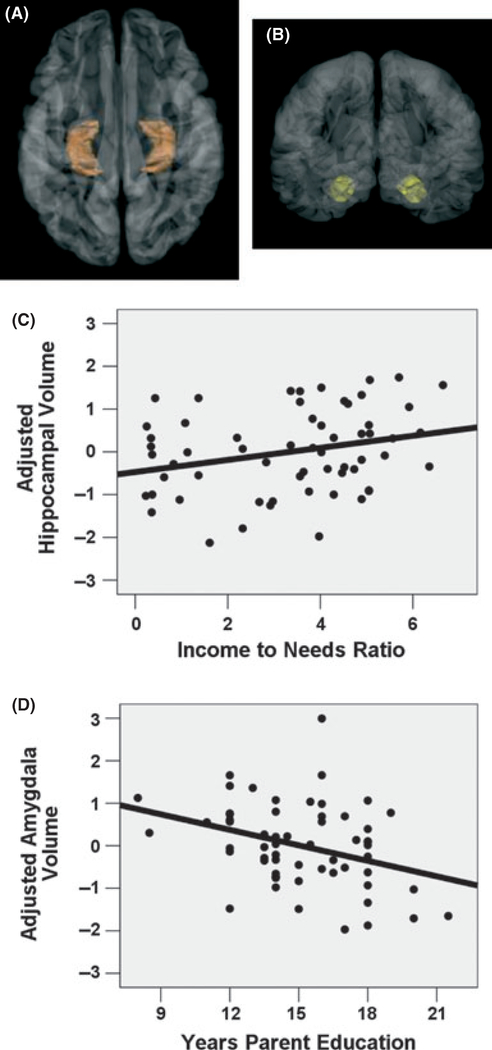
Further examination of Table 2 shows that parental education and income-to-needs ratio did not always act in the same direction in predicting ROI volume, despite the fact that these two components of SES were themselves highly correlated with each other. To assess whether this result represented ‘statistical suppression’ (Pedhazur, 1997), in which one variable suppresses irrelevant variance and improves the prediction of the other variable, these analyses were re-run examining the effects of parental education and income-to-needs separately. In the amygdala, when controlling for age, total cortical volume, and gender, parent education alone accounted for significant unique variance, with lower education levels associated with larger amygdala size (R2 change = 0.59, Beta = −0.286, p < .002; see Figure 2). In contrast, the addition of the income-to-needs ratio without including parent education in the model did not account for unique variance in amygdala size (R2 change = 0.001, Beta = 0.031; p = .724). Conversely, in the hippocampus, when adjusting for age, total cortical volume, and gender, the income-to-needs ratio alone showed a positive association with hippocampal volume (R2 change = 0.44, Beta = 0.222, p < .032; see Figure 2), whereas the inclusion of parental education without including the income-to-needs ratio did not account for unique variance in hippocampal size (R2 change = 0.02, Beta = −0.168; p = .15). Thus, parental education appears to be driving the effect of SES on amygdala volume, whereas the income-to-needs ratio appears to be driving the effect of SES on hippocampal volume.
Figure 2.
Family SES predicts child hippocampus and amygdala volumes. (A) Hippocampus ROI defined in orange. (B) Amygdala ROI defined in yellow. (C) Family income-to-needs ratio is positively correlated with child hippocampal volume, adjusted for child age, gender, and total cortical volume (Beta = 0.22; p < .032). (D) Number of years of parent education is negatively correlated with child amygdala volume, adjusted for child age, gender and total cortical volume (Beta = −0.29; p < .002). In all plots, regional volume is portrayed as the standardized residual, in standard deviations, after adjusting for covariates.
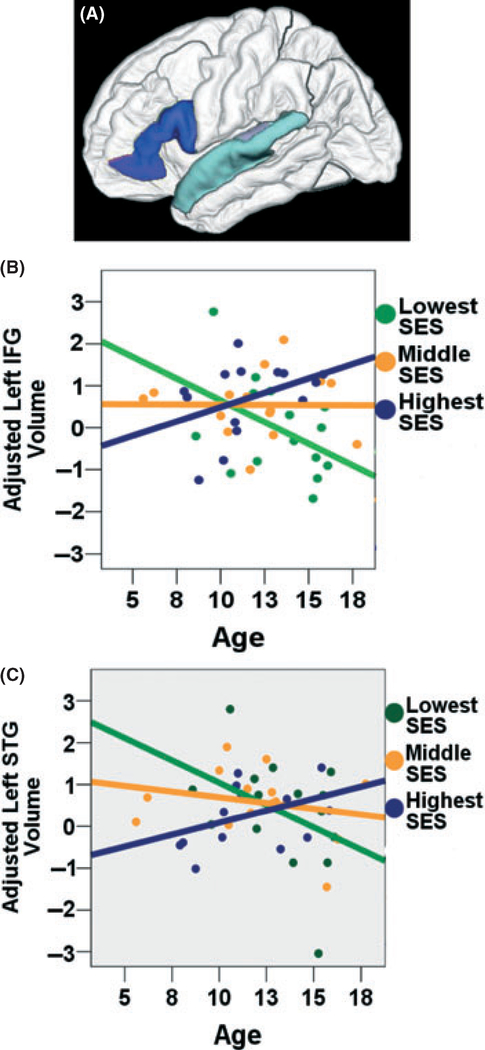
Finally, to investigate the degree to which SES effects remained constant in each ROI across age, we added terms to assess SES × age interactions in the regression models. Unfortunately, because this study represented a secondary analysis of data collected for other purposes, the sample was not originally recruited with the goal of optimizing socioeconomic diversity across the age spectrum. As stated above, there was a negative correlation between child age and parental education. Therefore, to be able to appropriately examine the data for SES × age interactions, we restricted the sample to the 44 children whose parents had an average educational level ranging from 11 to 17.5 years (mean 14.2, SD 1.7). Children in this restricted sample still ranged in age from 5 to 17 years old (mean 12.3, SD 2.9); however, there was no longer a significant correlation between age and education (r = −0.273; p < .1). Parental education × child age interactions were observed in the left superior temporal gyrus (Beta = 2.516; p < .012) and the left inferior frontal gyrus (Beta = 2.769; p < .014) as demonstrated in Table 3 and illustrated in Figure 3. In each case, positive standardized coefficients represent increasing SES disparities in volume with age. No SES × age interactions were observed in the other ROI’s, or in total cortical or total white matter volume.
Table 3.
SES × age interactions in language-related regions of interest
| ROI | Factors | Model R2 | Beta (sig) |
|---|---|---|---|
| LSTG | Age | 0.549 | −2.633 (0.010) |
| Total cortical volume | 0.734 (2.6 × 10 −8 ) | ||
| Average parent education | −1.396 (0.010) | ||
| Parent education × age | 2.516 (0.012) | ||
| LIFG | Age | 0.482 | −2.820 (0.013) |
| Total cortical volume | 0.554 (3.7 × 10 −5 ) | ||
| Average parent education | −1.217 (0.042) | ||
| Parent education × age | 2.769 (0.014) |
Note: LSTG = Left superior temporal gyrus; LIFG = Left inferior frontal gyrus. Models of effects of SES on region of interest (ROI) volume, controlling for age and total cortical volume, and including SES × age interactions. Sample is restricted to the 44 children whose parent education ranged from 11 to 17.5 years, to reduce the confound between age and parent education seen in the full sample. Parent education × child age interactions are observed in the volume of the left superior temporal gyrus and left inferior frontal gyrus, suggesting that the effects of SES in these regions are not constant at different ages. This is illustrated in Figure 3. No SES × age interactions were found in other ROIs.
Figure 3.
SES × Age interactions in left superior temporal gyrus and left inferior frontal gyrus. A priori ROIs defined in FreeSurfer were chosen that related to language processing. Among the 44 children whose parent education ranged from 11 to 17.5 years, SES × child age interactions were observed in (A) left inferior frontal gyrus (IFG; dark blue) and left superior temporal gyrus (STG; light blue). This is portrayed graphically in (B) left IFG (Beta = 2.769; p < .014), and (C) left STG (Beta = 2.516; p < .012), suggesting that SES differences in regional brain volume are not uniform at different ages. In all plots, regional volume is portrayed as the standardized residual, in standard deviations, after adjusting for total cortical volume. All analyses were performed using continuous variables for child age, parent education, and ROI volume, but are displayed with parental education represented in terciles, with 11–13.5 years of parental education in green; 13.5–15 years of parental education in orange; and 15–17.5 years of parental education in blue.
Discussion
Here we have demonstrated that socioeconomic disparities in childhood are associated with regionally specific differences in several discrete brain structures. Specifically, when controlling for age, total cortical volume, and gender, and correcting for multiple comparisons, childhood socioeconomic status factors were associated with differences in the volumes of the hippocampus and the amygdala. A non-significant trend for a difference in the volume of the left inferior temporal gyrus was also observed. In a restricted sample appropriate for testing interaction effects, SES differences in regional brain volume appear to vary with age in two language-supporting regions, the left superior temporal gyrus and left inferior frontal gyrus.
These findings are important for several reasons. First, this study focuses on children within the ‘normal range’ of experience. While the effects of extreme childhood adversity, such as trauma, abuse, or institutionalization are relatively well studied (Bremner, Randall, Vermetten, Staib, Bronen, Mazure, Capelli, McCarthy, Innis & Charney, 1997; Carrey, Butter, Persinger & Bialik, 1995; Fox, Levitt & Nelson, 2010; Tottenham & Sheridan, 2010), far less is known about the effects of socioeconomic disadvantage on specific aspects of child neurocognitive development. Given the prevalence of socioeconomic disadvantage, an understanding of the neural mechanisms by which it operates has vast potential implications for intervention and prevention.
Second, the neuroanatomic differences observed here are likely to reflect previously documented SES differences in specific neurocognitive processes, including language, memory and socio-emotional processing. No relations were found between SES and total cortical volume or total white matter volume, and all associations were consistent when controlling full-scale IQ. Specific neural and neurocognitive mechanisms may serve as more precise markers and targets for educational intervention.
Third, these findings confirmed predictions based on hypothesized environmental mediators of SES disparities in cognitive development, which are known to affect these very systems. Specifically, as reviewed above, SES disparities in children’s linguistic environments are well documented, and tend to become more pronounced with age. These environmental differences may mediate the finding of increased effects of SES with age in two language-supporting regions. In particular, we observed an SES × age interaction in the left superior temporal gyrus (an area that is largely related to the development of phonologic skill, a critical precursor to reading ability; McCandliss & Noble, 2003), as well as in the left inferior frontal gyrus (which has been implicated in the development of both phonologic and semantic processing; Dehaene-Lambertz et al., 2006; Turkeltaub et al., 2003; Vannest et al., 2009).
In a separate literature, SES disparities in the experience of stress have also been well described. As reviewed above, stress has important effects on the developing hippocampus (critical for memory) and the amygdala (supporting social-emotional processing). Thus, differences in the experience of stress may mediate our findings of SES differences in hippocampal and amygdala volumes.
The directionality of these results, and the specific components of SES involved, bears comment. In the amygdala, fewer years of parent education – but not family income – was associated with larger amygdala volumes. Studies in both animals and human children have suggested that the experience of stressful events is associated with larger amygdala size (Tottenham et al., 2010). Our findings are thus consistent with the interpretation that SES disparities in amygdala size may be mediated by differences in exposure to stress. Further, lower parent education has been associated with lower levels of parental nurturance (Brooks-Gunn & Markman, 2005), which may have particular importance for amygdala structure (Tottenham et al., 2010). Future studies are necessary, however, to directly assess the degree to which differences in parenting style explain this association.
Differences in the experience of stress have also been associated with differences in the size of the hippocampus in both animals and humans (Rao, Betancourt, Giannetta, Brodsky, Korczykowski, Avants, Gee, Wang, Hurt, Detre & Farah, 2010; Tottenham & Sheridan, 2010). Most studies of human adults suggest that higher stress is associated with smaller hippocampal volumes (Geuze, Vermetten & Bremner, 2005; Kitayama, Vaccarino, Kutner, Weiss & Bremner, 2005; Tottenham & Sheridan, 2010), although findings have been inconsistent in children (De Bellis, Hooper, Woolley & Shenk, 2010; Rao et al., 2010; Woon & Hedges, 2008). Here, we found that income-to-needs ratio – but not parental education – was positively associated with hippocampal size, similar to one previous report (Hanson et al., 2011). Perhaps stressors more directly related to income, such as limited access to material resources, have a greater influence on hippocampal development, relative to factors such as parenting style or cognitive stimulation, which may be more closely tied to parental education. Certainly, more research is needed, ideally with a sample in which socioeconomic factors, material resources, parental nurturance and regional brain volume are assessed longitudinally.
Notably, we did not find main effects of SES in language regions, when stringently adjusting for multiple comparisons, despite the fact that previous research has suggested that SES differences in neurocognitive skill are largest in the language domain (Noble et al., 2007; Noble et al., 2005). However, the absence of a main effect is uninterpretable in the presence of an interaction, and research suggests that SES differences in both linguistic stimulation and cognitive development may ‘snowball’ with age (Hart & Risley, 1995). Indeed, this is suggested by SES × age interactions found in two language-supporting regions, namely the left superior temporal gyrus and left inferior frontal gyrus.
Figure 3 shows that, at older ages, higher SES children show relatively larger volumes in these regions, compared to their lower SES peers (noting that regional volumes were measured in standard deviations, after adjusting for total cortical volume). Previous research suggests that the left superior temporal gyrus tends to show increases in gray matter density (Sowell, Peterson, Thompson, Welcome, Henkenius & Toga, 2003) and cortical thickness (Sowell et al., 2004) during childhood and adolescence. Research has also demonstrated increased cortical thickness in the left inferior frontal gyrus during this time frame (Sowell et al., 2004). One possibility is therefore that socioeconomically advantaged environments promote this process. That is, it is possible that higher SES environments are associated with relatively protracted pruning in these regions, allowing for prolonged plasticity. Prolonged pruning has been associated with increased intelligence during childhood and adolescence (Shaw, Greenstein, Lerch, Clasen, Lenroot, Gogtay, Evans, Rapoport & Giedd, 2006). Although MRI is unable to directly measure synaptic pruning or myelination, these findings may be consistent with the notion of experience-dependent plasticity (Greenough, Black & Wallace, 1987). In both the left superior temporal and left inferior frontal gyri, it is noted that volumetric disparities across SES increase with age, perhaps reflecting that experiential differences in language exposure tend to increase over time, with cumulative effects of home and school differences. Certainly, a longitudinal study would be necessary to properly investigate this possibility.
This study suffers from several limitations. By nature, it is difficult to draw strong conclusions concerning development in a cross-sectional sample. Additionally, a sample of 60 children has limited power; it is possible that a larger sample would have revealed volumetric differences related to SES in additional brain regions. Further, although results were largely consistent with our proposed model, we had no information on environmental factors, such as linguistic stimulation or exposure to stress, which would enable us to directly test the degree to which these factors mediate our findings. Finally, although the presence of these associations between SES and regional brain structure is provocative, the direction of causality is unclear. Future work will build upon these findings using a longitudinal data set in which SES, hypothesized mediators in the environment, brain structure, and specific cognitive skills are all assessed over time.
It is important to comment on the well-documented relationships between SES and race (Duncan & Magnuson, 2005). In this diverse sample of children from various socioeconomic and racial backgrounds, we did not find a relationship between SES and race, lending confidence that these factors were not confounded in our data. Importantly, self-reported race frequently does not correlate well with ancestry based on genetic analyses (Sinha, Larkin, Elston & Redline, 2006). It is thus possible that the variation in brain structure observed here is related to genotype-related variation in general anatomic architecture (similar to genetic differences in facial or other physical features), and not to meaningful differences in the function of the brain regions evaluated. Future studies would benefit from measuring admixture in genotype in the assessment of SES-related variability in brain structure, rather than self-reported race.
Finally, it must be emphasized that the neural substrates of cognition are themselves malleable phenotypes, which may be altered by experience (Rosenzweig, 2003; Sowell et al., 2004; Toga et al., 2006). Thus the fact that there are neural correlates of SES in no way connotes ‘immutability’, or rules out a plastic response to different environmental factors. Rather, it is our intent that future research will more clearly elucidate the mechanistic pathway or pathways by which different exposures and experiences which vary with SES may influence neural development. Such research will in turn produce testable hypotheses concerning modifiable targets for intervention – addressable in the home or school setting – which may be particularly effective at reducing disparities in achievement.
In conclusion, socioeconomic status varies widely among typically developing children, and has long been associated with individual differences in academic achievement. Through a cognitive neuroscience approach, we can tease apart how socioeconomic disparities in broad measures of achievement reflect developmental differences in distinct neurocognitive systems. Here we have shown that SES accounts for individual variation in the size of discrete brain regions that are critical for language, memory and socio-emotional processing. By elucidating the neural mechanisms underlying these effects, we are able to identify more precise targets for intervention, with the ultimate goal of mitigating the effects of unequal childhoods.
Supplementary Material
Acknowledgements
We would like to thank Dr Terry Jernigan for her insightful comments during the development of this manuscript. Funding for this work was supported by the National Institute of Drug Abuse Grants R01 DA017831, National Institute of Child Health and Human Development Grant R01 HD053893–01, the National Institute of Mental Health Grant R01 MH087563 awarded to ERS, and the John M. Driscoll, MD Scholars Program to KGN.
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article:
Data S1. Research Highlights.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Adams MJ (1990). Beginning to read: Thinking and learning about print. Cambridge, MA: MIT Press. [Google Scholar]
- Blair C, Granger D, & Peters Razza R (2005). Cortisol reactivity is positively related to executive function in preschool children attending Head Start. Child Development, 76 (3), 554–567. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, Burchinal M, McAdoo HP, & Garcia Coll C (2001). The home environments of children in the United States part II: Relations with behavioral development through age thirteen. Child Development, 72 (6), 1844–1867. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, & Charney DS (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse - a preliminary report. Biological Psychiatry, 41 (1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, & Duncan GJ (1997). The effects of poverty on children. Future of Children, 7 (2), 55–71. [PubMed] [Google Scholar]
- Brooks-Gunn J, & Markman LB (2005). The contribution of parenting to ethnic and racial gaps in school readiness. Future of Children, 15 (1), 139–168. [DOI] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, & Pruessner JC (2007). Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. Journal of Neuroscience, 27 (10), 2592–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey NJ, Butter HJ, Persinger MA, & Bialik RJ (1995). Physiological and cognitive correlates of child abuse. Journal of the American Academy of Child and Adolescent Psychiatry, 34, 1067–1075. [DOI] [PubMed] [Google Scholar]
- Conboy BT, & Kuhl PK (2007). Early speech perception: developing a culturally specific way of listening through social interaction In Braten S(Ed.), On being moved: From mirror neurons to empathy (pp. 175–199). Amsterdam: John Benjamins. [Google Scholar]
- D’Angiulli A, Herdman A, Stapells D, & Hertzman C (2008). Children’s event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology, 22 (3), 293–300. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Woolley DP, & Shenk CE (2010). Demographic, maltreatment, and neurobiological correlates of PTSD symptoms in children and adolescents. Journal of Pediatric Psychology, 35 (5), 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Mériaux S, Roche A, Sigman M, & Dehaene S (2006). Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proceedings of the National Academy of Sciences of the United States of America, 103 (38), 14240–14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, & Fletcher JM (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15 (03), 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, & Magnuson KA (2005). Can family socioeconomic resources account for racial and ethnic test score gaps? Future of Children, 15 (1), 35–54. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lombardino LJ, & Leonard CM (2001). Planar asymmetry tips the phonological playground and environment raises the bar. Child Development, 72 (4), 988–1002. [DOI] [PubMed] [Google Scholar]
- Evans GW (2004). The environment of childhood poverty. American Psychologist, 59 (2), 77–92. [DOI] [PubMed] [Google Scholar]
- Evans GW, & Kim P (2007). Childhood poverty and health: cumulative risk exposure and stress dysregulation. Psychological Science, 18 (11), 953–957. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, & Hurt H (2006). Childhood poverty: specific associations with neurocognitive development. Brain Research, 1, 166–174. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, & Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33 (3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fox SE, Levitt P, & Nelson CA 3rd. (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development, 81 (1), 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, & Bremner JD (2005). MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Molecular Psychiatry, 10 (2), 160–184. [DOI] [PubMed] [Google Scholar]
- Geyer S, Hemström Ö, Peter R, & Vågerö D (2006). Education, income, and occupational class cannot be used interchangeably in social epidemiology: empirical evidence against a common practice. Journal of Epidemiology and Community Health, 60 (9), 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, Critchley HD, Manuck SB, & Hariri AR (2007). Perigenual anterior cingulate morphology covaries with perceived social standing. Social Cognitive & Affective Neuroscience, 2 (3), 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, & Cohen S (2008). Potential neural embedding of parental social standing. Social Cognitive & Affective Neuroscience, 3 (2), 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Black JE, & Wallace CS (1987). Experience and brain development. Child Development, 58 (3), 539–559. [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, & Meaney MJ (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11 (9), 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, & Pollak SD (2011). Association between income and the hippocampus. PLoS ONE, 6 (5), e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, & Risley T (1995). Meaningful differences in the everyday experience of young American children. Baltimore, MD: Brookes. [Google Scholar]
- Hoff E (2003). Causes and consequences of SES-related differences in parent-to-child speech In Bornstein MH & Bradley RH (Eds.), Socioeconomic status, parenting and child development (pp. 145–160). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Keller TA, & Just MA (2009). Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron, 64 (5), 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, & Knight RT (2009). Socioeconomic disparities affect prefrontal function in children. Journal of Cognitive Neuroscience, 21 (6), 1106–1115. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, & Bremner JD (2005). Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. Journal of Affective Disorders, 88 (1), 79–86. [DOI] [PubMed] [Google Scholar]
- Kuhl PK (2007). Is speech learning ‘gated’ by the social brain? Developmental Science, 10 (1), 110–120. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Tsao F-M, & Liu H-M (2003). Foreign-language experience in infancy: effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences of the United States of America, 100 (15), 9096–9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, & Giedd JN (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage, 36 (4), 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, & Casey BJ (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America, 106 (3), 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, & McEwen BS (2000). Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry, 48 (10), 976–980. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, & McEwen BS (2001). Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development & Psychopathology, 13 (3), 653–676. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, & Noble KG (2003). The development of reading impairment: a cognitive neuroscience model. Mental Retardation & Developmental Disabilities Research Reviews, 9 (3), 196–204. [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2010). Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186 (1), 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC (1998). Socioeconomic disadvantage and child development. American Psychologist, 53 (2), 185–204. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E (2004). Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Development, 75 (5), 1373–1386. [DOI] [PubMed] [Google Scholar]
- Nsss 0, Claussen B, Thelle DS, & Smith GD (2005). Four indicators of socioeconomic position: relative ranking across causes of death. Scandinavian Journal of Public Health, 33 (3), 215–221. [DOI] [PubMed] [Google Scholar]
- National Center for Children in Poverty (2011). 50-State demographics wizard. Retrieved 9 March 2011, from http://www.nccp.org/tools/demographics/
- National Institute of Child Health Human Development Early Child Care Research Network (2005). Duration and developmental timing of poverty and children’s cognitive and social development from birth through third grade. Child Development, 76 (4), 795–810. [DOI] [PubMed] [Google Scholar]
- Noble KG, & McCandliss BD (2005). Reading development and impairment: behavioral, social, and neurobiological factors. Journal of Developmental & Behavioral Pediatrics, 26 (5), 370–378. [DOI] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, & Farah MJ (2007). Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science, 10 (4), 464–480. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, & Farah MJ (2005). Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science, 8 (1), 74–87. [DOI] [PubMed] [Google Scholar]
- Noble KG, Wolmetz ME, Ochs LG, Farah MJ, & McCandliss BD (2006). Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Developmental Science, 9 (6), 642–654. [DOI] [PubMed] [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Westlye LT, Due- Tønnessen P, & Walhovd KB (2009). Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience, 29 (38), 11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedhazur EJ (1997). Statistical control: Partial and Semipartial correlation In Pedhazur EJ, Multiple regression in behavioral research: Explanation and prediction (pp. 156–194). South Melbourne, Australia: Wadsworth Thomson Learning. [Google Scholar]
- Raizada RD, Richards TL, Meltzoff A, & Kuhl PK (2008). Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. NeuroImage, 40 (3), 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, Gee JC, Wang J, Hurt H, Detre JA, & Farah MJ (2010). Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. NeuroImage, 49 (1), 1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz IS (1990). Social background, phonological awareness and children’s reading. British Journal of Developmental Psychology, 8 (3), 209–225. [Google Scholar]
- Redcay E, Haist F, & Courchesne E (2008). Functional neuroimaging of speech perception during a pivotal period in language acquisition. Developmental Science, 11 (2), 237–252. [DOI] [PubMed] [Google Scholar]
- Richmond J, & Nelson CA (2008). Mechanisms of change: A cognitive neuroscience approach to declarative memory development In Nelson CA & Luciana M(Eds.), Handbook of developmental cognitive neuroscience (2nd edn.). Cambridge, MA: Bradford. [Google Scholar]
- Rosenzweig MR (2003). Effects of differential experience on the brain and behavior. Developmental Neuropsychology, 24 (2–3), 523–540. [DOI] [PubMed] [Google Scholar]
- Rouse C, Brooks-Gunn J, & McLanahan S (2005). Introducing the issue. Future of Children, 15 (1), 3–14. [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, & Giedd J (2006). Intellectual ability and cortical development in children and adolescents. Nature, 440 (7084), 676–679. [DOI] [PubMed] [Google Scholar]
- Sinha M, Larkin EK, Elston RC, & Redline S (2006). Self-reported race and genetic admixture [Letter to the editor]. New England Journal of Medicine, 354 (4), 421–422. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, & Toga AW (2003). Mapping cortical change across the human life span. Nature Neuroscience, 6 (3), 309–315. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, & Toga AW (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience, 24 (38), 8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Lauinger B, & Neville H (2009). Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Developmental Science, 12 (4), 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, & Sowell ER (2006). Mapping brain maturation. Trends in Neurosciences, 29 (3), 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Milner A, Galvan A, Davidson MC, Eigsti I-M, Thomas KM, Freed P, Booma ES, Gunnar M, Altemus M, Aronson J, & Casey BJ (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13 (1), 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, & Sheridan M (2010). A review of adversity, the amygdala, and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience, 3, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, & Eden GF (2003). Development of neural mechanisms for reading. Nature Neuroscience, 6 (7), 767–773. [DOI] [PubMed] [Google Scholar]
- Vannest J, Karunanayaka PR, Schmithorst VJ, Szaflarski JP, & Holland SK (2009). Language networks in children: evidence from functional MRI studies. American Journal of Roentgenology, 192 (5), 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2003). The Wechsler Intelligence Scale for Children, Fourth Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Whitehurst GJ (1997). Language processes in context: language learning in children reared in poverty In Adamson LB& Romski MA(Eds.), Research on communication and language disorders: Contribution to theories of language development (pp. 233–266). Baltimore, MD: Brookes. [Google Scholar]
- Whitehurst GJ, & Lonigan CJ (1998). Child development and emergent literacy. Child Development, 69 (3), 848–872. [PubMed] [Google Scholar]
- Woon FL, & Hedges DW (2008). Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus, 18 (8), 729–736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.