Abstract
This clinical practice guideline (CPG) provides clinicians with recommendations regarding chemotherapy emetogenicity classification in pediatric oncology patients. This information is critically important for the appropriate selection of antiemetic prophylaxis. Recommendations are based on a systematic review limited to pediatric patients and a framework for classification when antiemetic prophylaxis is provided. Findings of 87 publications informed the emetogenicity classification of 49 single-agent and 13 combination agent regimens. Information required for the classification of many chemotherapies commonly administered to pediatric patients is lacking. In the absence of pediatric data, consultation of methodologically sound CPGs aimed at adult oncology patients may be appropriate.
Keywords: Chemotherapy, chemotherapy-induced nausea and vomiting, emetogenicity, pediatric, vomiting, supportive care
Introduction
Chemotherapy-induced nausea and vomiting (CINV) remain clinically important treatment-related adverse effects for children and adolescents with cancer. For chemotherapy-naïve pediatric patients, the inherent emetogenicity of the chemotherapy about to be administered remains the most important determinant of CINV and, therefore, of the CINV prophylaxis recommended in modern guidelines.1–3
This clinical practice guideline (CPG) aims to provide evidence-based recommendations regarding the acute emetic potential of chemotherapy in pediatric oncology patients aged 1 month to 18 years and thus facilitate the selection of appropriate CINV prophylaxis for these patients. The scope of this CPG is limited to chemotherapy-induced vomiting (CIV) that occurs during the acute phase, defined as the 24-hour period following administration of chemotherapy, and is most applicable to chemotherapy-naïve pediatric patients. This CPG does not include anticipatory, breakthrough, delayed or refractory CIV. Nor does its scope include vomiting that occurs at end-of-life or due to other causes including radiotherapy or surgery. It is furthermore important to underscore that this CPG does not address chemotherapy-induced nausea.
The recommendations of this CPG are likely to be of most interest to physicians, pharmacists, nurse practitioners, physician assistants, nurses and others who care for pediatric oncology patients. They also may be of interest to administrators, educators, and researchers who make decisions about resource availability, provide professional education, and frame research questions about CIV and the supportive care of pediatric oncology patients. This CPG is one of a series on the subject of the prevention and management of CINV in pediatric oncology patients.1,4–6 The first of this series, a CPG on the emetogenicity of chemotherapy in children, was an adaptation of a guideline aimed at adult oncology patients.7 The CPG presented here is not a direct update of the previous publication but is a de novo CPG focused on evidence from pediatric oncology patients.
Methods
Guideline Panel
An international panel of interdisciplinary professionals was assembled to create this CPG. Members were invited to join the panel based on previous publications in the supportive care arena or who had a current research interest in CINV in children with cancer and represented a diversity of views, expertise, and practice experiences.. No panel member had a conflict of interest that precluded participation. Full details of the panel membership and conflict of interest declarations are provided in Supplementary Appendices 1 and 2. This CPG is editorially independent of its funder, the Pediatric Oncology Group of Ontario (POGO).
Evidence Identification and Review
Development of this CPG was framed around addressing the health question: What is the risk of acute CIV in children receiving a specific chemotherapy agent when given alone or in combination? With the assistance of a library scientist, literature searches of OVID MEDLINE, MEDLINE-in-process and Embase were conducted for articles indexed as of March 19, 2018. The complete search strategies are provided in Supplementary Appendix 3.
The following inclusion criteria were applied to the published articles identified by the literature search: (1) primary data reported as full papers, letters to the editor (no restriction by publication date) or conference abstracts (published in 2014 or more recently); (2) all participants were less than 25 years of age or the mean/median age was less than 16 years of age; (3) primary study in any pediatric area using chemotherapy including biologic agents; (4) describes the proportion of children who received specific chemotherapy drug(s) and who experienced vomiting during the acute phase; (5) defines acute phase as starting with the first dose of chemotherapy during a chemotherapy block and ending 24 hours after the last dose during the same block or 24 hours later regardless of the duration of the chemotherapy block or on the calendar day of the last dose of a multiple-day chemotherapy block; (6) vomiting can be attributed to chemotherapy and not due to another reason such as radiotherapy or surgery; and (7) at least three patients receiving a specific chemotherapy regimen. In addition, for multiple-day chemotherapy blocks where different chemotherapy was given on various days within the block, the proportion of children with vomiting is reported for the 24-hour period following each specific chemotherapy. There was no restriction by study design or language of publication.
Two reviewers (EPCS plus PDR, JFl or LLD) independently screened titles and abstracts and evaluated the full text of potentially relevant citations for eligibility. Discrepancies and disagreements were discussed and resolved by consensus. Agreement of inclusion between reviewers was assessed using the kappa statistic. Agreement was defined as: slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80) or almost perfect (0.81–1.00).8
Data were abstracted from the included studies and compiled into tables (EPCS or JFl), which were double-checked by a second reviewer (EPCS, PDR, or JFl). The outcome of interest was the proportion of children who experienced vomiting during the acute phase. Where the period of CIV assessment in an included study encompassed a period beyond the acute phase, only studies which reported no emesis were included. Chemotherapy agents not commercially available in Canada, the United States or the European Union or not in phase I trials (clinicaltrials.gov) were included in evidence tables, but omitted from recommendations (e.g. behenoyl-AC).
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to generate recommendations and assign the level of evidence.9
Emetogenicity Classification Framework
When a new chemotherapy is administered to pediatric patients, CIV prophylaxis is usually administered in accordance with an emetogenicity classification developed to support adult patients. Thus, in order to fully utilize the published evidence to inform chemotherapy emetogenicity in pediatric patients, we developed a framework to assign emetogenicity when antiemetic prophylaxis is administered.
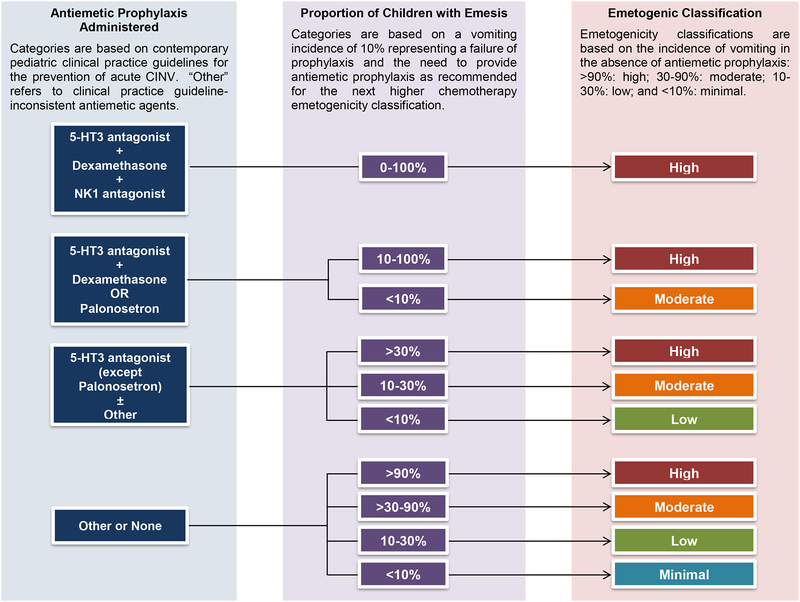
The Emetogenicity Classification Framework was developed by the CPG panel over a series of teleconferences and its face validity was evaluated by CPG panel members. The framework incorporates the traditional emetogenicity classifications that are based on the incidence of vomiting in the absence of CINV prophylaxis: >90%: high; 30–90%: moderate; 10–30%: low; and <10%: minimal.2,10 For included studies where antiemetic prophylaxis was provided, the antiemetic prophylaxis was judged to be CPG-consistent or CPG-inconsistent with respect to a specific chemotherapy emetogenicity classification. A CIV incidence of 10% or more was deemed to represent a failure of prophylaxis and the need to provide antiemetic prophylaxis as recommended for the next higher chemotherapy emetogenicity classification.11 Thus, assignment of chemotherapy emetogenicity was based on the proportion of children who experienced emesis during the acute phase and also on whether or not antiemetic prophylaxis was administered and, if administered, whether or not the antiemetic prophylaxis was CPG-consistent. Elements included in the framework for emetogenicity classification are summarized in Table 1. Figure 1 illustrates the algorithm employed to determine the emetogenicity classification of chemotherapy administered in individual studies.
TABLE 1.
Emetogenicity Classification Framework
| Element | Item |
|---|---|
| Framework Structure |
|
| |
| |
| |
| |
| |
| |
| Interpretation of Individual Included Studies |
|
| |
|
Figure 1:
Chemotherapy emetogenicity classification algorithm
Evidence Interpretation
Using the Emetogenicity Classification Framework (Table 1) and algorithm (Figure 1), the findings of each included study were interpreted to classify the emetogenicity of the chemotherapy administered. In the event of varying emetogenic classifications for a chemotherapy regimen among the included studies, study design (prospective vs. retrospective), study focus (CIV as a study aim vs. not included) and sample size were considered. Studies which were prospective, had CIV evaluation as a stated study aim and included a larger number of patients were weighted most highly. The CPG panel placed a high value on achieving complete acute CIV control since it is a major determinate of breakthrough and refractory CIV. Consistent with this value, the panel assigned a chemotherapy regimen to the higher emetogenicity classification in instances of conflicting evidence. All classifications were discussed by the CPG panel with particular attention paid to those chemotherapy regimens where classifications differed among studies. The panel did not consider cost when formulating recommendations.
Updating Plans
An update to this CPG is planned in five years or sooner in the event of the publication of important new information.
Results
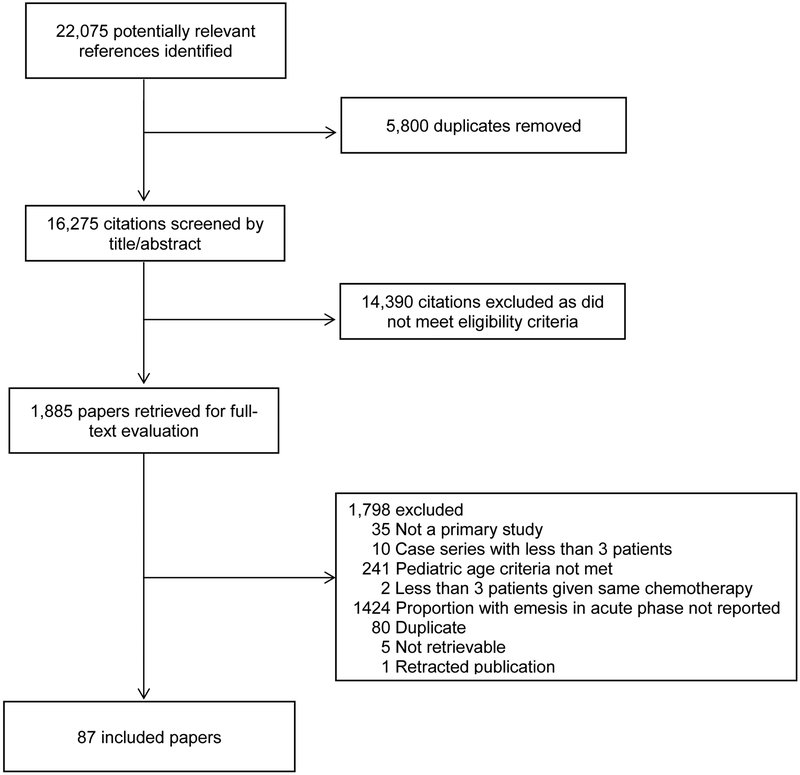
The systematic review identified 16,275 unique citations, of which 1,885 were identified as potentially relevant and retrieved for full-text evaluation. In total, 87 studies met the eligibility criteria and form the evidence base for this CPG (Figure 2). The agreement between reviewers with respect to inclusion of papers was substantial (kappa=0.66; 95% confidence interval (CI 0.58 to 0.74). The characteristics of the included studies and the evidence abstracted are presented in tables in Supplementary Appendices 4 and 5. The guideline panel’s recommendations for the classification of emetogenicity for 49 single-agent and 13 multiple-agent chemotherapy regimens described in the included studies are presented in Table 2. An expanded version of this table that includes assessments of the quality of evidence for the classification of each chemotherapy is provided in Supplementary Appendix 6.
Figure 2:
Flow diagram Depicting Study Identification, Selection and Reasons for Exclusion
TABLE 2.
Health Questions, Recommendations and Panel Remarks
Panel’s Remarks on Recommendation Interpretation:
|
| Health Questions and Recommendations: |
| 1. Which chemotherapy regimens are highlyα emetogenic? |
Single-agent regimens:
|
| 2. Which single-agent and multiple-agent chemotherapy regimens are moderatelyβ emetogenic? |
Single-agent regimens:
|
| 3. Which single-agent and multiple-agent chemotherapy regimens are of lowχ emetogenicity? |
Single-agent regimens:
|
| 4. Which single-agent and multiple-agent chemotherapy regimens are minimallyδ emetogenic? |
Single-agent regimens:
|
Frequency of emesis in the absence of prophylaxis,
> 90%;
30 to <90%;
10 to <30%;
< 10%.
Changes from pediatric evidence included in 2011 Clinical Practice Guideline:
addition;
dose revision
The chemotherapy emetogenicity classification differed between included studies for the following regimens: carboplatin IV ≥ 175mg/m2/dose, cyclophosphamide PO 2 – 3mg/kg/dose, cytarabine IV ≥ 3g/m2/day, doxorubicin IV 10 – 25mg/m2/dose, erlotinib PO 0.8 – 0.9mg/m2/day, imatinib PO ≥ 260mg/m2/day and topotecan PO 0.4 – 2.3mg/m2/day. As outlined in the methods, the guideline panel considered study design, study focus and sample size when assigning the emetogenicity when evidence from included studies conflicted. The adjudication process for these regimens is summarized in Supplementary Appendix 7.
Nine chemotherapy regimens were not included in the recommendations since they were not marketed in Canada, the USA or the European Union and not in active trials. A list of these agents is provided in Supplementary Appendix 8.
Implementation Considerations:
A chart comparing the current emetogenicity classification systems of the American Society of Clinical Oncology,2 the Multi-national Association of Supportive Care in Cancer,10 the National Comprehensive Cancer Network,12 and the previous POGO emetogenicity CPG7 as well as an alphabetical listing of the chemotherapy emetogenicity as classified in this CPG are offered in Supplementary Appendix 9 as implementation tools.
Discussion
Based on a systematic review of the published pediatric evidence, we have developed a CPG that delineates the emetogenicity of 49 single agent and 13 combination agent chemotherapy regimens when given to pediatric patients. It focuses on CIV prevention alone since few pediatric studies have evaluated nausea severity using a validated pediatric tool and the classification systems in place for adult oncology patients focus solely on CIV.
Ideally, chemotherapy emetogenicity would be determined in trials where CIV prophylaxis was not given. However, in the absence of pediatric data, pediatric patients receiving chemotherapy known to be emetogenic in adults often receive antiemetic prophylaxis based on the adult classification. As a result, descriptions of vomiting following chemotherapy administration to pediatric patients in the absence of prophylaxis are now uncommon. We therefore developed a framework and an algorithm for classifying the emetogenicity of chemotherapy agents in pediatric patients receiving CIV prophylaxis. CPG panel members found that these tools maintained face validity as the emetogenicity of each chemotherapy regimen was adjudicated. The framework and algorithm can be used to create future updates to this CPG.
The methods used to create the 2011 CPG for classification of chemotherapy emetogenicity in pediatric patients differ from this CPG. In 2011, the CPG panel opted to adapt an existing guideline developed for adult patients for use in pediatric oncology patients. Acknowledging emerging evidence that CIV risk factors including chemotherapy emetogenicity may be different in adult and pediatric patients,13–16 the 2018 CPG panel made the decision not to generalize from experience in adults and to focus on evidence in pediatric patients.
When compared to the 2011 CPG,7 the number of chemotherapy regimens with pediatric evidence to support their emetogenicity classification and included in the present CPG is increased. Nevertheless, it must be noted that evidence remains scant. For example, the classification of all but 13 regimens were based on a single study. Two changes from the 2011 CPG are notable. First, the emetogenicity of IV busulfan has been changed from moderate to high. This was based on a prospective study in 16 pediatric patients.17 Second, the emetogenicity classification of doxorubicin now varies based on dose (Table 2).
The strength of this CPG rests in its foundation in a systematic literature review and the application of a framework to assign emetogenicity. Limitations to this CPG include the quality of the included studies, many of which did not include the evaluation of CIV as a primary or secondary outcome. In addition, when faced with conflicting evidence with respect to a chemotherapy’s emetogenicity, the CPG panel transparently assigned it to the higher classification. This was consistent with the high value placed on the achievement of complete CIV control. We also assumed no CIV prophylaxis was given in papers that provided no details about prophylaxis. Both tactics may have led to a systematic bias to over-classification. This conservative approach is likely to provide more effective CIV prophylaxis in chemotherapy-naïve patients and reflects the high value the panel places on minimizing breakthrough CINV through optimal acute phase CIV control. Conversely, among studies where the period of CIV assessment may have included a period beyond the acute phase, only studies which reported no emesis were included. This may have led to a systematic bias to under-classification. Further, we were not able to distinguish between the contributions of acute and delayed CIV in multiple-day chemotherapy regimens.
The largest limitation of this CPG is the thin evidence base available to inform our health questions. There is no direct evidence to inform the CIV risk in pediatric patients for many chemotherapy agents and chemotherapy doses. For example, many chemotherapy agents that form the standard of care for the treatment of common pediatric cancers do not appear in this CPG due to lack of published evidence including: pegaspargase IV, bleomycin IV, cytarabine IT, cyclophosphamide IV < 500mg/m2/dose, and etoposide IV. These gaps present serious barriers to the provision of optimal antiemetic prophylaxis to pediatric patients. For chemotherapy that is new to pediatrics, toxicity data from early phase trials, when chemotherapy is most likely to be administered without antiemetic prophylaxis, should be reported in a way that permits emetogenicity classification using the emetogenicity classification framework. For older agents, the guideline panel encourages clinicians to conduct prospective studies to evaluate the incidence of CIV in pediatric oncology patients receiving CPG-consistent antiemetic prophylaxis. Data mining of electronic health records may also be useful in addressing many of the evidence gaps regarding chemotherapy emetogenicity.
Disparity between the proportions of adult and pediatric oncology patients who experience complete CIV control has been identified.18 An accurate assessment of a chemotherapy’s emetogenicity is fundamental to the provision of appropriate antiemetic prophylaxis and, therefore, to the achievement of optimal CIV control. This CPG offers an evidence-based classification of chemotherapy emetogenicity appropriate for pediatric oncology patients and a method for evaluating chemotherapy emetogenicity that can be used in updates of this CPG. For chemotherapy regimens that do not appear in this CPG, the panel suggests that clinicians consult high quality, adult-focused chemotherapy emetogenicity classification guidelines.
Supplementary Material
Appendix 1 Composition of the guideline panel
Appendix 2 Guideline panel conflict of interest disclosures
Appendix 3 Search strategies
Appendix 4 Characteristics of included studies
Appendix 5 Evidence tables
Appendix 6 Health questions and recommendations with quality of evidence
Appendix 7 Rationale for emetogenicity classification for agents with conflicting evidence
Appendix 8 List of chemotherapy not marketed in Canada, USA or Europe and not in clinical trials and therefore not included in recommendations.
Appendix 9 Comparison of emetogenicity classification systems
Acknowledgements:
Development of this guideline was funded by the Pediatric Oncology Group of Ontario. Dr. Thackray’s participation in this research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations:
- CINV
Chemotherapy-Induced Nausea and Vomiting
- CIV
Chemotherapy-Induced Vomiting
- CPG
Clinical Practice Guideline
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- HEC
High Emetogenic Chemotherapy
- LEC
Low Emetogenic Chemotherapy
- MEC
Moderate Emetogenic Chemotherapy
Footnotes
Conflict of Interest Statement:
The authors declare no conflicts of interest.
References:
- 1.Patel P, Robinson PD, Thackray J, et al. Guideline for the prevention of acute chemotherapy-induced nausea and vomiting in pediatric cancer patients: A focused update. Pediatr Blood Cancer. 2017;64(10). [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(28):3240–3261. [DOI] [PubMed] [Google Scholar]
- 3.Dupuis LL, Sung L, Molassiotis A, Orsey AD, Tissing W, van de Wetering M. 2016 updated MASCC/ESMO consensus recommendations: Prevention of acute chemotherapy-induced nausea and vomiting in children. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2017;25(1):323–331. [DOI] [PubMed] [Google Scholar]
- 4.Dupuis LL, Boodhan S, Holdsworth M, et al. Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer. 2013;60(7):1073–1082. [DOI] [PubMed] [Google Scholar]
- 5.Dupuis LL, Robinson PD, Boodhan S, et al. Guideline for the prevention and treatment of anticipatory nausea and vomiting due to chemotherapy in pediatric cancer patients. Pediatr Blood Cancer. 2014;61(8):1506–1512. [DOI] [PubMed] [Google Scholar]
- 6.Flank J, Robinson PD, Holdsworth M, et al. Guideline for the treatment of breakthrough and the prevention of refractory chemotherapy-induced nausea and vomiting in children with cancer. Pediatr Blood Cancer. 2016;63(7):1144–1151. [DOI] [PubMed] [Google Scholar]
- 7.Dupuis LL, Boodhan S, Sung L, et al. Guideline for the classification of the acute emetogenic potential of antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer. 2011;57(2):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 9.Brozek JL, Akl EA, Alonso-Coello P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64(5):669–677. [DOI] [PubMed] [Google Scholar]
- 10.Jordan K, Chan A, Gralla RJ, et al. 2016 Updated MASCC/ESMO consensus recommendations: Emetic risk classification and evaluation of the emetogenicity of antineoplastic agents. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2017;25(1):271–275. [DOI] [PubMed] [Google Scholar]
- 11.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(suppl_5):v232–v243. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. Antiemesis V.3.2018. http://www.nccn.org/patients. Accessed September 21, 2018.
- 13.Molassiotis A, Aapro M, Dicato M, et al. Evaluation of risk factors predicting chemotherapy-related nausea and vomiting: results from a European prospective observational study. Journal of pain and symptom management. 2014;47(5):839–848.e834. [DOI] [PubMed] [Google Scholar]
- 14.Warr DG, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of phase 3 trial of aprepitant in patients receiving adriamycin-cyclophosphamide-based chemotherapy. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2011;19(6):807–813. [DOI] [PubMed] [Google Scholar]
- 15.Hesketh PJ, Aapro M, Street JC, Carides AD. Evaluation of risk factors predictive of nausea and vomiting with current standard-of-care antiemetic treatment: analysis of two phase III trials of aprepitant in patients receiving cisplatin-based chemotherapy. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2010;18(9):1171–1177. [DOI] [PubMed] [Google Scholar]
- 16.Dupuis LL, Sung L, Tomlinson G, Pong A, Bickham K. Potential factors influencing the incidence of chemotherapy-induced vomiting (CIV) in children receiving emetogenic chemotherapy: a pooled analysis. [abstract]. Pediatr Blood Cancer. 2018;65(S2):e27455.30240102 [Google Scholar]
- 17.Flank J, Sparavalo J, Vol H, et al. The burden of chemotherapy-induced nausea and vomiting in children receiving hematopoietic stem cell transplantation conditioning: A prospective study. Bone Marrow Transplant. 2017;52(9):1294–1299. [DOI] [PubMed] [Google Scholar]
- 18.Bernhardt MB. Nausea and vomiting: Therapeutic orphans of pediatric oncology. Pediatr Blood Cancer. 2017;64:e26648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1 Composition of the guideline panel
Appendix 2 Guideline panel conflict of interest disclosures
Appendix 3 Search strategies
Appendix 4 Characteristics of included studies
Appendix 5 Evidence tables
Appendix 6 Health questions and recommendations with quality of evidence
Appendix 7 Rationale for emetogenicity classification for agents with conflicting evidence
Appendix 8 List of chemotherapy not marketed in Canada, USA or Europe and not in clinical trials and therefore not included in recommendations.
Appendix 9 Comparison of emetogenicity classification systems