Abstract
Rationale:
Arterial remodeling, a hallmark of many cardiovascular pathologies including pulmonary arterial hypertension (PAH), is regulated by TGFβ1-TGFβ receptors and the antagonistic, vasoprotective BMPR2-PPARγ axis. However, it is unclear which factors drive detrimental TGFβ1 pathways in the hypertensive pulmonary vasculature.
Objective:
We hypothesized that LDL receptor-related protein1 (LRP1) expression is decreased in PAH, leading to enhancement (disinhibition) of TGFβ1 signals, and that the PPARγ agonist pioglitazone can restore vascular homeostasis and prevent PAH resulting from LRP1 deletion in vascular smooth muscle cells (VSMC).
Methods and Results:
Targeted deletion of LRP1 in VSMC (smLRP1−/−) in mice disinhibited TGFβ1-CTGF signaling, leading to spontaneous PAH and distal pulmonary arterial muscularization as assessed by closed-chest cardiac catheterization and anti-αSMA staining. Pioglitazone inhibited the canonical TGFβ1-CTGF axis in human pulmonary artery SMC (HPASMC) and smLRP1−/− main pulmonary artery (CTGF, NOX4), and reversed PAH in smLRP1−/− mice. TGFβ1 boosted pSmad3 in PASMC from smLRP1−/− mice vs. controls. Pioglitazone-activated PPARγ binds to Smad3 in HPASMC (co-immunoprecipitation), thereby blocking its phosphorylation and overriding LRP1 deficiency. Finally, mRNA and protein expression of LRP1 was decreased in pulmonary plexiform lesions of patients with endstage idiopathic PAH (laser capture microdissection, qPCR, immunoblotting). Downregulation of LRP1 protein was also demonstrated in explanted PASMC from PAH patients and accompanied by enhanced TGFβ1-pSmad3-CTGF signaling and increased TGFβ1-induced PASMC proliferation that was prevented by pioglitazone.
Conclusions:
Here, we identify LRP1 as an integrator of TGFβ1-mediated mechanisms that regulate vascular remodeling in mice and clinical PAH, and PPARγ as a therapeutic target that controls canonical TGFβ1 pathways. Hence, pharmacological PPARγ activation represents a promising new therapy for PAH patients who lack the vasoprotective LRP1 in VSMC.
Keywords: Pulmonary arterial hypertension, vascular disease, LRP1, TGFβ, PPAR, vascular smooth muscle, pulmonary hypertension, low-density lipoprotein, peroxisome proliferator-activated receptor, TGFβ pathway aneurysm, vascular biology
Subject Terms: Basic Science Research, Cell Signaling/Signal Transduction, Pulmonary Biology, Smooth Muscle Proliferation and Differentiation, Vascular Biology
INTRODUCTION
Low-density lipoprotein receptor-related protein (LRP1) is a ubiquitously expressed cell surface receptor and a member of the LDL receptor gene family. LRP1 (or synonymous: transforming growth factor receptor 5; TGFBR5) interacts with multiple ligands, such as lipoproteins, extracellular matrix glycoproteins, cytokines, and growth factors. The latter ligands include transforming growth factor beta 1 (TGFβ1)1 and its receptor TGFBR12 as well as its downstream target, connective tissue growth factor (CTGF)3. Targeted deletion of LRP1 in vascular smooth muscle cells (VSMC) (smLRP1−/−) activates the canonical TGFβ1-pSmad2/3 pathway in the vascular wall, associated with disruption of elastic layers in the murine aorta4. A recent study suggested a critical role for LRP1 in maintaining arterial integrity, by regulating matrix deposition through CTGF5.
Pulmonary arterial hypertension (PAH) is characterized by remodeling pulmonary arterioles, leading to increased pulmonary vascular resistance, right ventricular (RV) hypertrophy, heart failure, and death. VSMC proliferation, extracellular matrix (ECM) and vascular remodeling are hallmarks of PAH. All of these features in VSMC are counteracted by the vasoprotective bone morphogenetic protein receptor 2 (BMPR2) and its downstream effector peroxisome proliferator-activated receptor gamma (PPARγ) axis6. Balanced BMP2/BMPR2 and TGFβ1/TGFBR signaling is needed for VSMC homeostasis7,8 and PPARγ links both pathways9. Recently, we unraveled PPARγ as a major gatekeeper that links vasoprotective BMP2/BMPR2 and detrimental TGFβ1/TGFBR pathways in VSMC, regulating cell proliferation and glucose metabolism9.
Others have speculated that LRP1 is a co-receptor and activator of endothelial BMPR210,11 – the receptor that is mutated or dysfunctional in many forms of clinical PAH7. Experimental evidence suggests that LRP1 associates with bone morphogenetic protein endothelial cell precursor (BMPER) and is required for BMPER endocytosis in mouse endothelial cells11. An elegant iPSC study suggests that endothelial LRP1 protects from PAH in unaffected BMPR2 mutation carriers (family members)10, probably by recycling the BMP ligand-receptor complex back to the cell surface11.
In VSMC, we showed that PPARγ activation (downstream of BMPR2) protects against PAH by inhibiting canonical and non-canonical TGFβ1 pathways9: PPARγ binds to Smad3 and Stat3, inhibiting TGFβ1-downstream signaling, glucose metabolism and SMC proliferation9. Furthermore, TGFβ1 overexpressing mice spontaneously develop PAH that can be reversed by the oral PPARγ agonist pioglitazone9.
So far, accumulating evidence indicates that LRP1 is vasoprotective, and - by physically interacting with TGFβ1, TGFBRI and possibly BMPR2 - closely involved in VSMC homeostasis. TGFβ1 overexpression induces PAH in transgenic mice9, and circulating TGFβ1 levels are heightened in human PAH12. However, LRP1 or its deficiency has not been linked to PAH yet. We hypothesized that (1) mice with targeted deletion of LRP1 in VSMC (smLRP1−/−) develop PAH in association with a disinhibited TGFβ1-CTGF axis, all of which may be reversed by PPARγ agonist treatment, and that (2) LRP1 expression is decreased in human pulmonary arterial SMC (HPASMC) of PAH patients.
METHODS
This manuscript adheres to the AHA Journals’ Transparency and Openness Promotion (TOP) Guidelines. All data and supporting materials have been provided with the published article.
Detailed methods are provided in the supplemental material.
SM22-Cre+;LRPflox/flox;LDLR−/− (smLRP−/−) with vascular TGF-β1 overexpression.
Homozygous LDL receptor (LDLR−/−) animals LRP deficient in vascular smooth muscle cells (SM22-Cre+;LRPflox/flox;LDLR−/−, referred to as smLRP−/−) have been described previously4 to develop TGFβ1 pathway enhancement. Mice were maintained on normal diet. We divided male and female smLRP1−/− (SM22-Cre+; LRP1flox/flox; LDLR−/− background) and littermate control mice (LRP1flox/flox; LDLR−/−) in 2 sets. A set of 10–12 week-old control and smLRP−/− mice (n=3–5) underwent invasive hemodynamic measurement only (RV, LV and aorta catheterization in closed chest technique). A second set of 10–12 week old control and smLRP−/− mice (n=9–11) was treated for 5 weeks either with regular chow vs. pioglitazone (20mg/kg/day) incorporated into the chow, and sacrificed at 15 to 17 weeks of age. In the second set of mice, invasive hemodynamic measurements (RV, LV, aortic catheterization) and tissue harvest were conducted after oral pioglitazone treatment for 5 weeks. All animal studies were performed according to the institutional animal care and use guidelines and the according state regulations (#2015–101088).
HPASMC isolation and culture.
Isolated HPASMC were cultured in Smooth Muscle Cell Basal Medium (CC-31–81; Lonza) supplemented with 10 % FBS and growth factors from SmGM-2 SingleQuots (CC-4149; Lonza), 100 U/ml penicillin and 0.1 mg/ml streptomycin; (15140–122; Gibco). Moreover, HPASMC were isolated from pulmonary arteries obtained from patients with PAH or those with chronic obstructive pulmonary disease (COPD), undergoing lung transplantation. Mouse PASMC were isolated from the pulmonary arteries.
Statistics.
For cell culture, each condition was tested at least in triplicates, and all experiments were repeated at least 3 times at different passages, and in cells from at least 3 different donors (unless specified differently). Values from multiple experiments are expressed as mean±SEM. Normality was tested using the Kolmogorov-Smirnov test. Statistical significance was determined for multiple comparisons using one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison (for normal distribution) or Kruskal-Wallis (for non-normal distribution) test. Student’s t-test was used for comparisons of two groups. p<0.05 was considered significant.
RESULTS
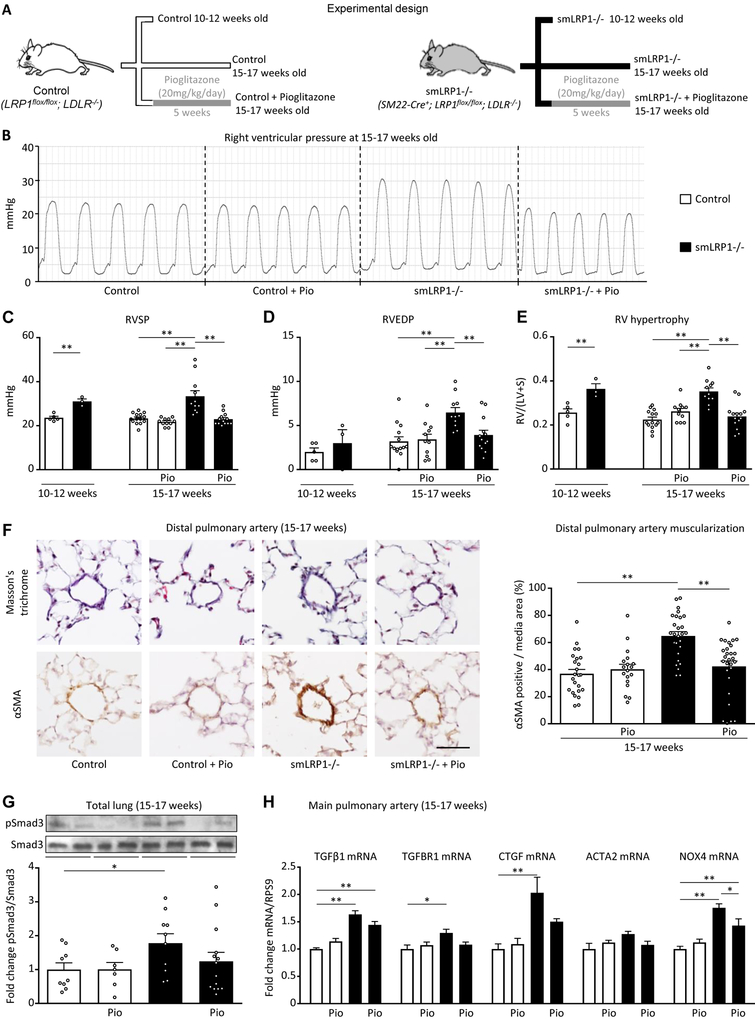
We divided male and female smLRP1−/− and littermate control mice in 2 sets, and orally treated a subset with the PPARγ agonist pioglitazone (20mg/kg/day) orally for 5 weeks (Figure 1A). Both 10–12 week-old and 15–17 week-old smLRP1−/− mice had spontaneous PAH, based on an elevated RV systolic pressure (RVSP; Table 1 and Figure 1B,C) and RV hypertrophy (increased RV/LV+septum mass ratio, Table 1 and Figure 1E). Over time, the older 15–17 week-old smLRP1−/− mice developed mild RV diastolic dysfunction, as assessed by increased RV end diastolic pressure (RVEDP; Table 1 and Figure 1D). In addition, LRP1 deletion in SMC induced pulmonary artery remodeling as judged by increased muscularization in distal pulmonary arteries (Figure 1F). Intriguingly, oral treatment with pioglitazone fully reversed PAH, RV hypertrophy, and prevented diastolic RV dysfunction, as well as pulmonary vascular remodeling. No differences were observed in aortic and left ventricular (LV) systolic and diastolic pressures (Table 1), thus excluding any relevant LV dysfunction or systemic arterial hypertension, and hence indicating pre-capillary pulmonary vascular disease. Of note, no significant differences between male and female mice were observed (data not shown).
Figure 1. The PPARγ agonist pioglitazone reverses pulmonary arterial hypertension, spontaneously developed in smLRP1−/− mice.
(A) smLRP1−/− and littermate control mice (LDLR−/− background) were divided in 2 sets: a first set untreated of 10- to 12-week-old mice (n=3–5) and a second set of 10- to 12-week-old control and smLRP1−/− mice orally treated for 5 weeks with pioglitazone (20mg/kg/day) and sacrificed at 15–17 weeks of age (n=6–13). Invasive hemodynamic measurements were performed to assess the right ventricular (B-D), pressure. (E) The right ventricle over the left ventricle+septum weight ratio (RV/(LV+S)) was also measured. (F) Masson’s trichrome staining and αSMA immunohistochemistry were performed on consecutive cuts of mouse lungs to assess the remodeling of alveolar duct pulmonary arteries (scale bar 50μm). The bar graph quantifies αSMA positive area in the media of distal pulmonary arteries. (G) In the total lung, the protein levels of pSmad3 and Smad3 (52kDa) were quantified by western blot. (H) In the main pulmonary artery, the mRNA levels of TGFβ1 targets were quantified by PCR. Values are expressed as mean±SEM; *p<0.05; **p<0.01. RVSP, right ventricular systolic pressure; RVEDP, right ventricular end diastolic pressure; LVSP, left ventricular systolic pressure; LVEDP left ventricular end diastolic pressure; SBP, systolic blood pressure.
Table 1.
Hemodynamics, heart weight, and pulmonary vascular analysis in smLRP1−/− and control mice, either untreated or treated with the PPARγ agonist pioglitazone.
| Control (C) |
Control + Pio (CP) |
smLRP1−/− (L) |
smLRP1−/− + Pio (LP) |
p | n | |
|---|---|---|---|---|---|---|
| 10- to 12-week-old | ||||||
| Body weight (g) | 27.5 ± 2.3 | 28.0 ± 2.9 | ||||
| Hemodynamics | ||||||
| HR (bpm) | 328 ± 17 | 337 ± 27 | 3 – 5 | |||
| RVSP (mmHg) | 23.6 ± 0.7 | 31.1 ± 0.8** | 3 – 5 | |||
| RVEDP (mmHg) | 2.0 ± 0.4 | 2.8 ± 1.2 | ** C vs L | 3 – 5 | ||
| 15- to 17-week-old | ||||||
| Body weight (g) | 24.4 ± 1.1 | 26.3 ± 1.4 | 27.7 ± 1.5 | 26.0 ± 0.8 | ||
| Hemodynamics | ||||||
| HR (bpm) | 291 ± 14 | 283 ± 14 | 286 ± 14 | 270 ± 11 | 9 – 11 | |
| RVSP (mmHg) | 23.0 ± 0.7 | 21.5 ± 0.5 | 33.4 ± 2.4** | 22.6 ± 0.6** | ** C vs L, ** CP vs L, ** L vs LP | 9 – 11 |
| RVEDP (mmHg) | 3.1 ± 0.5 | 3.5 ± 0.6 | 6.5 ± 0.5** | 3.4 ± 0.5** | ** C vs L, ** CP vs L, ** L vs LP | 9 – 11 |
| LVSP (mmHg) | 100.2 ± 1.9 | 98.0 ± 3.1 | 108.5 ± 3.4 | 103.8 ± 5.1 | 6 – 11 | |
| LVEDP (mmHg) | 9.8 ± 1.4 | 9.9 ± 1.5 | 13.7 ± 3.1 | 11.3 ± 1.7 | 6 – 11 | |
| SBP (mmHg) | 100.9 ± 1.7 | 98.5 ± 3.4 | 98.6 ± 3.4 | 99.9 ± 3.8 | 7 – 13 | |
| DBP (mmHg) | 67.3 ± 1.4 | 63.9 ± 2.9 | 55.9 ± 2.4** | 59.0 ± 1.2* | ** C vs L, * C vs LP | 7 – 13 |
| MAP (mmHg) | 78.5 ± 1.4 | 75.4 ± 3.1 | 70.2 ± 2.7 | 72.7 ± 2.0 | 7 – 13 | |
| Cardiac Mass | ||||||
| RV/BW (mg/g) | 0.60 ± 0.03 | 0.73 ± 0.04 | 0.93 ± 0.10** | 0.71 ± 0.07 | ** C vs L | 11 – 14 |
| RV/(LV+S) | 0.22 ± 0.01 | 0.26 ± 0.01 | 0.35 ± 0.02** | 0.24 ± 0.01** | ** C vs L, ** CP vs L, ** L vs LP | 11 – 14 |
| LV+S/BW (mg/g) | 2.70 ± 0.07 | 2.83 ± 0.14 | 2.67 ± 0.30 | 2.97 ± 0.19 | 11 – 14 |
At 10–12 week-old, smLRP1−/− and control mice received either regular chow or were orally treated with pioglitazone (20 mg/kg/day) incorporated into the chow for 5 weeks. Data were generated at the age of 10–12 and 15–17 weeks, and are shown as mean ± SEM. Statistically significant differences between groups are indicated with stars (*p<0.05; **p<0.01; student’s t-test or ANOVA, Bonferroni posthoc test). HR, heart rate; bpm, beats per minute; RVSP, right ventricle systolic pressure; RVEDP, right ventricle end diastolic pressure; LVSP, left ventricle systolic pressure; LVEDP, left ventricle end diastolic pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure, calculated as MAP = (SBP + 2 × DBP): 3; RV/BW, right ventricle / body weight; RV/(LV+S), right ventricle / (left ventricle + septum); (LV+S)/BW, (left ventricle + septum) / body weight.
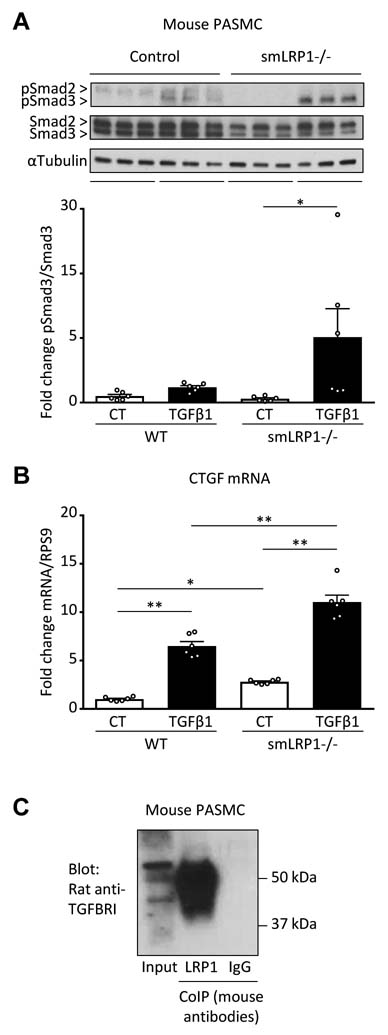
We next sought to investigate the basic molecular mechanisms of PAH development in smLRP1−/− mice. Upon TGFβ1 stimulation, CTGF recently was demonstrated to induce trans-differentiation of myoblasts into myofibroblasts - a major transformation that drives PASMC proliferation and pulmonary vascular disease13. Thus, we hypothesized that LRP1 deficiency in VSMC induces the mitogenic TGFβ1-CTGF axis that can be inhibited by PPARγ activation. Indeed, phosphorylation of Smad3 (Figure 1G) and the mRNA levels of the TGFβ1 downstream targets CTGF and NOX4 were increased in total lung lysates of smLRP1−/− mice vs. controls (Figure 1H). Consistent with our hypothesis, the heightened TGFβ1 signaling in total lung and main pulmonary arteries was inhibited by pioglitazone (Figure 1F–H). Of note, smLRP1−/− mice were shown to have increased TGFβ1-dependent nuclear pSmad2/3 accumulation in the aorta that is inhibited by the PPARγ agonist rosiglitazone4. In summary, our data, together with the published literature, support a major, protective role for LRP1 in PAH by suppressing canonical TGFβ1 pathways.
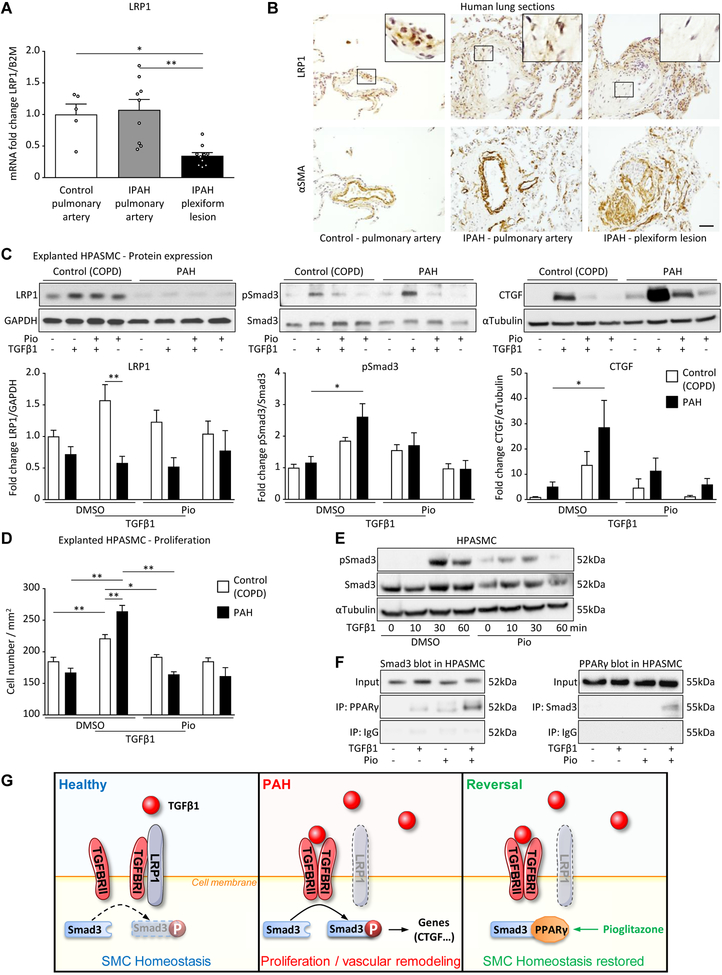
To confirm the importance of LRP1 in human PAH, we determined LRP1 expression in arteries from control subjects (downsizing donor lungs) or idiopathic PAH (IPAH) patients applying laser capture microdissection/qPCR and immunohistochemistry on lung sections. Although the basal LRP1 expression in pulmonary arteries from IPAH patients was not (mRNA) or only moderately (protein) decreased versus control, pulmonary plexiform lesions had strongly decreased LRP1 expression at both mRNA and protein level (Figure 3A,B). Next, we validated the lowered LRP1 expression in HPASMC explanted from PAH patients compared to those from patients with chronic obstructive pulmonary disease (COPD; used as control). LRP1 was moderately (not significantly) decreased in PAH-PASMC compared to control; with TGFβ1 stimulation, LRP1 protein expression was dampened by 63% (p<0.01) in PAH-PASMC vs. non-stimulated PAH-PASMC (Figure 3C). Interestingly, PAH-PASMC responded to a greater degree to TGFβ1 stimulation than control PASMC (COPD), as shown by induced (i) Smad3 phosphorylation (canonical TGFβ1 signaling), (ii) CTGF protein expression (TGFβ1 downstream target), and (iii) HPASMC proliferation (Figure 3C,D). We further confirmed the boosted TGFβ1 response (pSmad3, CTGF) in smLPR1−/− explanted PASMC compare to wildtype control (Figure 2A–B). Pioglitazone administration inhibited all the above TGFβ1-mediated effects in control and PAH PASMC (Figure 3C,D). Mechanistically, PPARγ activation by pioglitazone blocked TGFβ1-induced Smad3 phosphorylation by direct binding between Smad3 and PPARγ (Figure 3E,F). Of note, pioglitazone alone did not restore LRP1 expression in PAH-PASMC (Figure 3C). LRP1 scavenges TGFBR1 to inhibit the TGFβ1 pathway1–3 (Figure 2A–C). According to previous reports on LRP1 binding partners (see discussion) and our data, we propose that the decreased LRP1 expression in PAH-PASMC would release TGFBR1 for mitogenic ligand binding (Figure 3G), explaining the higher sensitivity and response to TGFβ1 in PAH-HPASMC vs. COPD-HPASMC (controls).
Figure 3. PASMC from PAH patients have decreased LRP1 expression and increased TGFβ1 sensitivity.
(A) LRP1 mRNA expression was analyzed by qPCR in laser microdissected pulmonary arteries (inner diameter<500mm) or plexiform lesions from IPAH patients (n=8–9) and control subjects (downsizing donor lungs, n=5). (B) Human lung serial sections were analyzed by immunohistochemistry for LRP1 and αSMA expression in peripheral pulmonary arteries or pulmonary plexiform lesions of 3 IPAH patients and 3 healthy donors (scale bar 50μm). (C-D) HPASMCs isolated from pulmonary arteries of COPD (control, n=3) or PAH patients (n=4). (C-F) The cells were seeded at 100/mm2, starved for 24hr and pre-incubated with pioglitazone (10mM) or DMSO (equivalent) for another 24hr and then stimulated with TGFβ1 (5ng/mL) for 30min (phosphorylation) or 24hr (protein expression). (C) Protein expression of LRP1 (85kDa) pSmad3 (52kDa), Smad3 (52kDa), and CTGF (38kDa) were assessed by westernblot. (D) Proliferation was assessed by crystal violet staining followed by cell count. (E) Levels of phospho-Smad3 were measured by western blot in healthy donor HPASMC (n = 6 experiments). (F) Total cell lysates from healthy donor HPASMC were subjected to co-immunoprecipitation (co-IP) and reversed Co-IP (n=3 experiments) to test Smad3-PPARγ binding. All values are expressed as mean ± SEM; *p<0.05; **p<0.01. (G) The mechanistic model incorporates the findings described in this work on LRP1 in PAH and the literature to date on LRP1 and TGFBRI interaction1–3.
Figure 2. LRP1 regulates TGFβ1 pathways in PASMC.
(A-C) PASMC were isolated from the main pulmonary artery of WT (n=2) or smLRP1−/− mice (n=2). (A) Phosphorylated and total protein expression of Smad2 (60 kDa) and Smad3 (52 kDa) expression was evaluated by western blot (n=6 experiments). (B) CTGF mRNA expression was evaluated by PCR (n=6 experiments). (C) Total cell lysates from WT mouse PASCM were subjected to coIP with anti-LRP1 or IgG (both mouse antibodies) and immunoblotted for TGFBRI (rat antibody) (n=4 experiments). All values are expressed as mean ± SEM; *p<0.05; **p<0.01.
DISCUSSION
Based on the above literature, our current and previous work4,9,14, we suggest that LRP1 deficiency in VSMC leads to induction (= disinhibition) of the canonical TGFβ1-pSmad3/CTGF pathway, thereby weakening PPARγ activity9,15,16, and thus ultimately leading to PAH in vivo (Figure 3G). We had previously linked enhanced canonical (pSmad3/CTGF) and non-canonical (pSTAT3/pFoxO1) TGFβ1/TGFBR signaling with suppression of the vasoprotective BMPR2-PPARγ axis in VSMC4,9,14. Our current study introduces a new PAH mouse model of LRP1 deficiency in VSMC in conjunction with enhanced canonical TGFβ1-pSmad3/CTGF signaling, all of which can be reversed by PPARγ agonist treatment (pioglitazone).
Of note, it is possible that LRP1 generates vasoprotection in the context of PAH via other cell types and mechanisms (beyond suppressing TGFβ1 signaling in VSMC): For example, LRP1 sustains PPARγ activity in non-SMC cell types such as endothelial cells (LRP1 metabolic, transcriptional co-activator of PPARγ)15 and pericytes (LRP1-dependent migration)17. Moreover, in macrophages, LRP1 tyrosine phosphorylation is required to activate transcription of the cholesterol transporter ABCA1 through PPARγ and LXR16. In addition, it is possible that PPARγ interacts with non-canonical TGFβ1 pathways. In SMC, we have previously described inhibitory PPARγ-Stat3 interaction9 and others have described PPARγ inhibiting NF-κB-mediated events18,19, which likely contribute to the beneficial, anti-inflammatory effects of PPARγ activation.
To the best of our knowledge, this is the first study demonstrating the vasoprotective role of LRP1 in PAH. From a clinical perspective, pioglitazone has a positive risk-benefit ratio that has been proven in recent large randomized trials (IRIS trial)20–23, and a safety profile that compares favorably with rosiglitazone20–22. Targeting PPARγ with minimal side effect is a new challenge for drug discovery research; strategies to leverage PPARγ deacetylation have been recently proposed to develop advanced PPARγ agonists21,24,25. Further preclinical and clinical studies should explore the efficacy of pioglitazone and other PPARγ agonists in advanced targeted therapy of PAH patients.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Pulmonary arterial hypertension is a proliferative cardiovascular condition regulated by TGFβ1-TGFβ receptors and the antagonistic, vasoprotective BMPR2-PPARγ axis and characterized by lipid-related insulin resistance.
LDL receptor-related protein 1 (LRP1) is vasoprotective and interacts with multiple ligands, including TGFβ1 and its receptor, and its downstream target, CTGF.
In vascular SMC, activated PPARγ inhibits canonical (pSmad3-CTGF) and non-canonical (pStat3-FoxO1) TGFβ1 pathways, glycolysis, and proliferation.
What New Information Does This Article Contribute?
First study demonstrating that LRP1 in vascular SMC protects from PAH in vivo.
Vascular expression of LRP1 is decreased in human PAH.
PPARγ activation by pioglitazone reverses PAH caused by LRP1 deficiency in SMC, inhibiting Smad3, Nox4, and CTGF and indicating that PPARγ activating agents represent a promising new therapy for PAH patients with reduced vasoprotective LRP1.
We suggest that LRP1 deficiency in vascular SMC induces (= disinhibits) a canonical TGFβ1-pSmad3-CTGF pathway, thereby weakening PPARγ activity, ultimately leading to PAH in vivo. Further preclinical studies are needed to explore whether LRP1 is vasoprotective in the context of PAH via other cell types and mechanisms beyond suppressing TGFβ1 signaling in VSMC: These non-SMC cell types may include endothelial cells (LRP1 as metabolic, transcriptional co-activator of PPARγ), pericytes (LRP1-dependent migration), and macrophages (cholesterol transport). These findings offer new important insights into the role of vascular SMC and mechanisms of vascular remodeling PAH and describe new important therapeutic avenues.
ACKNOWLEDGMENTS
We thank Dr. Jerome Terrand for assistance with in vivo experiments, and Jonas Geldner for technical assistance.
SOURCES OF FUNDING
This work was funded by the German Research Foundation (DFG grant HA4348/2–2 to GH). LC was supported by postdoctoral fellowship grants from DFG (CA 1303/1–1) and a FEBS short-term fellowship. JH was supported by grants from the NHLBI (R37 HL063762), NIA (RF AG053391), the NINDS and NIA (R01 NS093382), BrightFocus A2016396S, the Bluefield Project to Cure FTD and a Harrington Scholar Innovator Award (2019).
Nonstandard Abbreviations and Acronyms:
- PAH
pulmonary arterial hypertension
- IPAH
idiopathic PAH
- COPD
chronic obstructive pulmonary disease
- VSMC
vascular smooth muscle cells
- HPASMC
human pulmonary artery SMC
- RV
right ventricular
- LV
left ventricular
- ECM
extracellular matrix
Footnotes
DISCLOSURE
LC and JH are co-founders of Reelin Therapeutics, Inc.
REFERENCES
- 1.Huang SS, Ling T-Y, Tseng W-F, Huang Y-H, Tang F-M, Leal SM, Huang JS. Cellular growth inhibition by IGFBP-3 and TGF-beta1 requires LRP-1. FASEB J. 2003;17:2068–2081. [DOI] [PubMed] [Google Scholar]
- 2.Liu Q, Huang SS, Huang JS. Function of the type V transforming growth factor beta receptor in transforming growth factor beta-induced growth inhibition of mink lung epithelial cells. J Biol Chem. 1997;272:18891–18895. [DOI] [PubMed] [Google Scholar]
- 3.Kawata K, Kubota S, Eguchi T, Aoyama E, Moritani NH, Kondo S, Nishida T, Takigawa M. Role of LRP1 in transport of CCN2 protein in chondrocytes. J Cell Sci. 2012;125:2965–2972. [DOI] [PubMed] [Google Scholar]
- 4.Boucher P, Li W-P, Matz RL, Takayama Y, Auwerx J, Anderson RGW, Herz J. LRP1 functions as an atheroprotective integrator of TGFbeta and PDFG signals in the vascular wall: implications for Marfan syndrome. PLoS ONE. 2007;2:e448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muratoglu SC, Belgrave S, Hampton B, Migliorini M, Coksaygan T, Chen L, Mikhailenko I, Strickland DK. LRP1 protects the vasculature by regulating levels of connective tissue growth factor and HtrA1. Arterioscler Thromb Vasc Biol. 2013;33:2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansmann G, de Jesus Perez VA, Alastalo T-P, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrell NW, Bloch DB, ten Dijke P, Goumans M-JTH, Hata A, Smith J, Yu PB, Bloch KD. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol. 2016;13:106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hata A, Chen Y-G. TGF-β Signaling from Receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvier L, Chouvarine P, Legchenko E, Hoffmann N, Geldner J, Borchert P, Jonigk D, Mozes MM, Hansmann G. PPARγ Links BMP2 and TGFβ1 Pathways in Vascular Smooth Muscle Cells, Regulating Cell Proliferation and Glucose Metabolism. Cell Metab. 2017;25:1118–1134.e7. [DOI] [PubMed] [Google Scholar]
- 10.Gu M, Shao N-Y, Sa S, Li D, Termglinchan V, Ameen M, Karakikes I, Sosa G, Grubert F, Lee J, Cao A, Taylor S, Ma Y, Zhao Z, Chappell J, Hamid R, Austin ED, Gold JD, Wu JC, Snyder MP, Rabinovitch M. Patient-Specific iPSC-Derived Endothelial Cells Uncover Pathways that Protect against Pulmonary Hypertension in BMPR2 Mutation Carriers. Cell Stem Cell. 2017;20:490–504.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pi X, Schmitt CE, Xie L, Portbury AL, Wu Y, Lockyer P, Dyer LA, Moser M, Bu G, Flynn EJ, Jin S-W, Patterson C. LRP1-dependent endocytic mechanism governs the signaling output of the bmp system in endothelial cells and in angiogenesis. Circ Res. 2012;111:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selimovic N, Bergh C-H, Andersson B, Sakiniene E, Carlsten H, Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J. 2009;34:662–668. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, Sammani S, Zhou G, Raj JU, Garcia JGN, Berdyshev E, Yuan JX-J, Natarajan V, Machado RF. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190:1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvier L, Chouvarine P, Legchenko E, Hansmann G. Transforming Growth Factor β1- and Bone Morphogenetic Protein 2/PPARγ-regulated MicroRNAs in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2017;196:1227–1228. [DOI] [PubMed] [Google Scholar]
- 15.Mao H, Lockyer P, Li L, Ballantyne CM, Patterson C, Xie L, Pi X. Endothelial LRP1 regulates metabolic responses by acting as a co-activator of PPARγ. Nat Commun. 2017;8:14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xian X, Ding Y, Dieckmann M, Zhou L, Plattner F, Liu M, Parks JS, Hammer RE, Boucher P, Tsai S, Herz J. LRP1 integrates murine macrophage cholesterol homeostasis and inflammatory responses in atherosclerosis. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casey CS, Atagi Y, Yamazaki Y, Shinohara M, Tachibana M, Fu Y, Bu G, Kanekiyo T. Apolipoprotein E Inhibits Cerebrovascular Pericyte Mobility through a RhoA Protein-mediated Pathway. J Biol Chem. 2015;290:14208–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou Y, Moreau F, Chadee K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nat Commun. 2012;3:1300. [DOI] [PubMed] [Google Scholar]
- 19.Mukohda M, Lu K-T, Guo D-F, Wu J, Keen HL, Liu X, Ketsawatsomkron P, Stump M, Rahmouni K, Quelle FW, Sigmund CD. Hypertension-Causing Mutation in Peroxisome Proliferator-Activated Receptor γ Impairs Nuclear Export of Nuclear Factor-κB p65 in Vascular Smooth Muscle. Hypertension. 2017;70:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O’Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR, IRIS Trial Investigators. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young LH, Viscoli CM, Schwartz GG, Inzucchi SE, Curtis JP, Gorman MJ, Furie KL, Conwit R, Spatz ES, Lovejoy A, Abbott JD, Jacoby DL, Kolansky DM, Ling FS, Pfau SE, Kernan WN, IRIS Investigators. Heart Failure After Ischemic Stroke or Transient Ischemic Attack in Insulin-Resistant Patients Without Diabetes Mellitus Treated With Pioglitazone. Circulation. 2018;138:1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazar MA. Reversing the curse on PPARγ. J Clin Invest. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraakman MJ, Liu Q, Postigo-Fernandez J, Ji R, Kon N, Larrea D, Namwanje M, Fan L, Chan M, Area-Gomez E, Fu W, Creusot RJ, Qiang L. PPARγ deacetylation dissociates thiazolidinedione’s metabolic benefits from its adverse effects. J Clin Invest. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.