Abstract
Objective
To assess the separate and combined associations of maternal pre-pregnancy BMI and gestational weight gain with the risks of pregnancy complications and their population impact.
Design
Individual participant data meta-analysis of 39 cohorts.
Setting
Europe, North America and Oceania.
Population
265,270 births.
Methods
Information on maternal pre-pregnancy BMI, gestational weight gain, and pregnancy complications was obtained. Multilevel binary logistic regression models were used.
Main outcome measures
Gestational hypertension, pre-eclampsia, gestational diabetes, preterm birth, small and large size for gestational age at birth.
Results
Higher maternal pre-pregnancy BMI and gestational weight gain were, across their full ranges, associated with higher risks of gestational hypertensive disorders, gestational diabetes and large size for gestational age at birth. Preterm birth risk was higher at lower and higher BMI and weight gain. Compared to normal weight mothers with medium gestational weight gain, obese mothers with high gestational weight gain had the highest risk of any pregnancy complication (Odds Ratio 2.51 (95% Confidence Interval 2.31, 2.74)). We estimated that 23.9% of any pregnancy complication was attributable to maternal overweight/obesity and 31.6% of large size for gestational age infants was attributable to excessive gestational weight gain.
Conclusions
Maternal pre-pregnancy BMI and gestational weight gain are, across their full ranges, associated with the risks of pregnancy complications. Obese mothers with high gestational weight gain are at the highest risk of pregnancy complications. Promoting a healthy pre-pregnancy BMI and gestational weight gain may reduce the burden of pregnancy complications and ultimately the risk of maternal and neonatal morbidity.
Keywords: weight gain, body mass index, pregnancy complications, preterm birth, birth weight
Tweetable abstract
Promoting a healthy body mass index and gestational weight gain might reduce the population burden of pregnancy complications.
INTRODUCTION
Obesity among women of reproductive age is increasing in prevalence worldwide.1 A meta-analysis of published data of 38 cohorts reported that not only maternal obesity but also modest increases in maternal body mass index (BMI) were associated with an increased risk of fetal and infant death. For women with a BMI of 30 kg/m2, absolute risks per 10,000 pregnancies were 102 and 43 fetal and infant deaths, respectively.2 Maternal overweight and obesity are also associated with increased risks of more common pregnancy complications, such as gestational hypertensive disorders, gestational diabetes, preterm birth and large size for gestational age at birth.3–5 Next to maternal pre-pregnancy BMI, excessive gestational weight gain, defined by the US Institute of Medicine (IOM) criteria, is associated with increased risks of pregnancy complications.6–9 However, most previous studies have lacked power to robustly assess whether differences in risk are also present for modest changes in maternal pre-pregnancy BMI and gestational weight gain and by severity of obesity. Although the associations of maternal obesity and excessive weight gain with pregnancy complications have been extensively studied, less is known on the population disease burden attributable to these conditions.10–12 Gaining insight into the population attributable risks will allow the development of future population preventive strategies designed to reduce the risks of common pregnancy complications. Furthermore, a meta-analysis of individual participant data (IPD) on this topic, in contrast to the previously performed meta-analyses of published results,4, 9 allows more powerful and flexible analyses, better harmonization of the data, consistent adjustment for potential confounders and leads to a reduced risk of publication bias.
Therefore, we conducted a meta-analysis of IPD among 265,270 singleton births from 39 American, European and Oceania pregnancy and birth cohorts to assess the associations of maternal pre-pregnancy BMI and gestational weight gain with the risks of gestational hypertension, pre-eclampsia, gestational diabetes, preterm birth, and small and large size for gestational age at birth and to assess their population impact.
METHODS
Inclusion criteria and participating cohorts
We used data from an existing international collaboration on maternal obesity and childhood outcomes. Pregnancy and birth cohort studies were eligible if they included mothers with singleton live-born children born from 1989 onwards, had information available on maternal pre- or early-pregnancy BMI and at least one offspring measurement (birth weight or childhood BMI) and were approved by their local institutional review boards. We invited 50 cohorts from Europe, North America and Oceania selected from existing collaborations on childhood health (EarlyNutrition Project, CHICOS Project, www.birthcohorts.net assessed until July 2014), of which 39 agreed to participate, providing data of 277,042 singleton births. Of those, information on maternal pre- or early-pregnancy BMI and at least one pregnancy complication was available for 265,270 singleton births (flowchart in Figure S1). Anonymized datasets were stored on a single central secured data server with access for the main analysts (SS, EV). A description of the eligibility criteria and the references of the study design and profile papers of each included cohort are given in Table S1. Participants were not involved in the development of the study.
Maternal anthropometrics
Maternal anthropometrics were measured, derived from clinical records or self-reported (cohort-specific information in Table S2). Maternal BMI before pregnancy, available in 96% of the study population, was used in the analyses. For participants without information on pre-pregnancy BMI, BMI obtained before 20 weeks of gestation was used. Maternal BMI was categorized into underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obesity grade 1 (30.0–34.9 kg/m2), obesity grade 2 (35.0–39.9 kg/m2), and obesity grade 3 (≥40.0 kg/m2),13 and into 11 groups with a range of 2.5 kg/m2 each. Information on total gestational weight gain, defined as the difference between the latest weight before delivery and pre-pregnancy weight, was provided by the cohorts and was classified as inadequate, adequate, or excessive weight gain in relation to maternal pre-pregnancy BMI according to the IOM guidelines.14 We calculated weight gain until 20 weeks of gestation as the difference between a weight obtained until median 15.4 weeks (95% range 10.0, 19.3) and pre-pregnancy weight. We calculated maternal pre-pregnancy BMI specific weight gain for gestational age z-scores based on reference charts created using data from this collaboration (Appendix S1).15 These z-scores were categorized into 6 categories (<−2.0 standard deviation (SD), −2.0 to −1.1 SD, −1.0 to −0.1 SD, 0 to 0.9 SD, 1.0 to 1.9 SD and ≥2.0 SD) and into low (≤−1.1 SD), medium (−1.0 to 0.9 SD) and high (≥1.0 SD) weight gain.
Pregnancy complications
Information on gestational hypertension, pre-eclampsia, gestational diabetes, gestational age at birth and birth weight was measured, derived from clinical records or reported (cohort-specific information in Table S2). Preterm birth was defined as <37 weeks of gestation.16 We created sex- and gestational age-adjusted birth weight SD scores based on a North-European reference chart.17 Small and large size for gestational age at birth were defined per cohort as sex- and gestational age-adjusted birth weight below the 10th percentile and above the 90th percentile, respectively. Any pregnancy complication was defined as at least one of the pregnancy complications. A core outcome set was not used in this study.
Covariates
Information on covariates was assessed by questionnaires and provided by the cohorts as categorical covariates: educational level (low, medium, high), parity (nulliparous, multiparous), smoking habits during pregnancy (yes, no), and child’s sex. Maternal age was categorized based on data availability as <25.0, 25.0-29.9, 30.0-34.9, and ≥35.0 years. As part of the analysis plan, covariates were selected based on the graphical criteria for confounding and data availability in the cohorts.18 Maternal ethnicity was not included due to the fact that most cohorts were largely Caucasian and a high percentage of missings in ethnic specific information. Cohort-specific information is given in Table S3.
Statistical analysis
We conducted one-stage IPD meta-analysis by analyzing individual level data from all cohorts simultaneously in a multilevel model. Our model followed a two-level hierarchical structure with participants (level 1) nested within cohorts (level 2).19 We used generalized linear mixed models with a binomial distribution and logit link. We defined the models assuming a random intercept at cohort level to allow variation in the baseline risk for each cohort. We used these models to examine the separate and combined associations of maternal pre-pregnancy BMI and gestational weight gain, in clinical categories and across the full range, with the risks of pregnancy complications. We only examined the associations of gestational weight gain clinical categories with the risks of small and large size for gestational age at birth due to the possibility of reverse causality for the other outcomes. The associations of excessive weight gain with gestational hypertensive disorders might be partly explained by pathologic fluid retention as part of the disease. Women diagnosed with gestational diabetes might try to improve their diet and restrict their total weight gain. Preterm birth shortens the gestation and thus women are less likely to gain excessive gestational weight. The proportion of pregnancy complications at a population-level attributable to each maternal pre-pregnancy BMI and gestational weight gain clinical category was estimated by calculation of population attributable risk fractions. For this, we used the adjusted Odds Ratio (OR) and the prevalence of the exposure category in the population.20 To study the effects of weight gain across the full range on gestational hypertension, pre-eclampsia and gestational diabetes, we used weight gain z-scores until 20 weeks of gestation to avoid reverse causality. For the models using maternal pre-pregnancy BMI and gestational weight gain z-scores continuously, the inclusion of quadratic terms did not improve the fit. We did not observe statistical interactions between both maternal BMI and gestational weight gain with child’s sex. All models were adjusted for maternal age, educational level, parity, and smoking habits during pregnancy. Models for birth complications were additionally adjusted for child’s sex. Models for weight gain across the full range were also adjusted for maternal pre-pregnancy BMI. As sensitivity analyses, we conducted two-stage IPD meta-analyses and tested for heterogeneity between the cohorts estimates.19, 21 We used missing values in covariates as an additional group to prevent exclusion of non-complete cases. We performed statistical analyses using the Statistical Package of Social Sciences version 21.0 for Windows (SPSS Inc, Chicago, IL, USA) and Review Manager (RevMan) version 5.3 of the Cochrane Collaboration (The Nordic Cochrane Centre, Copenhagen, Denmark).
RESULTS
Participants’ characteristics
Table S4 shows cohort-specific information on maternal anthropometrics and pregnancy complications. Overall, the median maternal pre/early-pregnancy BMI and total gestational weight gain were 22.7 kg/m2 (95% range: 18.1-34.7 kg/m2), and 14.0 kg (95% range: 3.9-27.0 kg), respectively.
Maternal pre-pregnancy BMI and risks of pregnancy complications
Table 1 shows that, as compared to normal weight mothers, underweight, overweight and obesity grades 1 to 3 mothers had higher risks of any pregnancy complication (all p-values<0.05). The highest risk of any pregnancy complication was observed for obesity grade 3 mothers (OR 2.99 (95% Confidence Interval (CI) 2.68, 3.34)). Mothers with obesity grade 3 had also the highest risks of gestational hypertension (OR 5.40 (95% CI 4.47, 6.51)), pre-eclampsia (OR 6.50 (95% CI 5.48, 7.73)), gestational diabetes (OR 7.59 (95% CI 6.14, 9.38)), preterm birth (OR 1.52 (95% CI 1.24, 1.87)) and large size for gestational age at birth (OR 3.06 (95% CI 2.69, 3.49)). We estimated that 23.9% of any pregnancy complication, and specifically, 35.6% of gestational hypertension, 34.6% of pre-eclampsia, 42.8% of gestational diabetes, 3.9% of preterm birth and 20.6% of large size for gestational age at birth were attributable to maternal overweight and obesity (Table 1).
Table 1.
Maternal pre-pregnancy body mass index and gestational weight gain clinical categories and the risks of pregnancy complicationsa
| Pregnancy complications Odds Ratio (95% Confidence Interval) and Population Attributable Risk Fractions (PAR), % |
|||||||
|---|---|---|---|---|---|---|---|
| Any pregnancy complication | Gestational hypertension | Pre-eclampsia | Gestational diabetes | Preterm birth | Small size for gestational age | Large size for gestational age | |
| Pre-pregnancy body mass index | |||||||
|
Underweight (<18.5 kg/m2) |
1.08 (1.03, 1.13)* | 0.63 (0.55, 0.73)** | 0.67 (0.57, 0.78)** | 0.66 (0.53, 0.82)** | 1.20 (1.10, 1.31)** | 1.67 (1.58, 1.76)** | 0.45 (0.41, 0.50)** |
| PAR 2.3b | NA | NA | NA | PAR 0.8 | PAR 2.7 | NA | |
| ncases/total=3079/9586 | ncases/total=216/9416 | ncases/total=168/9368 | ncases/total=85/10449 | ncases/total=599/10455 | ncases/total=1900/10382 | ncases/total=383/8865 | |
|
Normal weight (18.5-24.9 kg/m2) |
Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| ncases/total=46774/159983 | ncases/total=5066/155612 | ncases/total=4100/154646 | ncases/total=1857/168117 | ncases/total=7852/172123 | ncases/total=18185/159778 | ncases/total=14674/156267 | |
|
Overweight (25.0-29.9 kg/m2) |
1.35 (1.32, 1.38)** | 2.04 (1.94, 2.15)** | 1.96 (1.86, 2.07)** | 2.22 (2.06, 2.40)** | 1.06 (1.01, 1.11)* | 0.79 (0.76, 0.82)** | 1.61 (1.56, 1.66)** |
| PAR 11.4c | PAR 17.1 | PAR 16.0 | PAR 19.4 | PAR 1.2 | NA | PAR 10.8 | |
| ncases/total=16817/47825 | ncases/total=2531/45509 | ncases/total=2202/45180 | ncases/total=1156/49203 | ncases/total=2433/50852 | ncases/total=4074/44476 | ncases/total=6837/47239 | |
|
Obesity (≥30.0 kg/m2) |
2.02 (1.96, 2.08)** | 3.68 (3.46, 3.91)** | 3.70 (3.48, 3.93)** | 4.59 (4.22, 4.99)** | 1.33 (1.25, 1.41)** | 0.79 (0.75, 0.83)** | 2.28 (2.19, 2.37)** |
| PAR 12.5c | PAR 18.5 | PAR 18.6 | PAR 23.4 | PAR 2.7 | NA | PAR 9.8 | |
| ncases/total=9330/20834 | ncases/total=1687/18863 | ncases/total=1621/18797 | ncases/total=1020/21148 | ncases/total=1322/21992 | ncases/total=1694/18134 | ncases/total=3929/20369 | |
|
Obesity grade 1 (30.0-34.9 kg/m2) |
1.87 (1.80, 1.93)** | 3.31 (3.08, 3.55)** | 3.20 (2.98, 3.44)** | 3.97 (3.61, 4.37)** | 1.30 (1.21, 1.39)** | 0.78 (0.73, 0.83)** | 2.15 (2.05, 2.25)** |
| PAR 8.2c | PAR 12.5 | PAR 12.0 | PAR 15.5 | PAR 1.8 | NA | PAR 6.6 | |
| ncases/total=6505/15181 | ncases/total=1136/13900 | ncases/total=1047/13811 | ncases/total=636/15405 | ncases/total=936/16006 | ncases/total=1235/13363 | ncases/total=2725/14853 | |
|
Obesity grade 2 (35.0-39.9 kg/m2) |
2.36 (2.21, 2.51)** | 4.66 (4.17, 5.20)** | 4.81 (4.31, 5.37)** | 5.85 (5.09, 6.73)** | 1.38 (1.22, 1.57)** | 0.79 (0.71, 0.89)** | 2.56 (2.37, 2.77)** |
| PAR 3.7c | PAR 6.1 | PAR 6.3 | PAR 7.9 | PAR 0.7 | NA | PAR 2.7 | |
| ncases/total=2091/4308 | ncases/total=412/3812 | ncases/total=410/3810 | ncases/total=271/4386 | ncases/total=287/4557 | ncases/total=345/3662 | ncases/total=888/4205 | |
|
Obesity grade 3 (≥40.0 kg/m2) |
2.99 (2.68, 3.34)** | 5.40 (4.47, 6.51)** | 6.50 (5.48, 7.73)** | 7.59 (6.14, 9.38)** | 1.52 (1.24, 1.87)** | 0.86 (0.70, 1.04) | 3.06 (2.69, 3.49)** |
| PAR 1.7c | PAR 2.4 | PAR 2.9 | PAR 3.5 | PAR 0.3 | NA | PAR 1.1 | |
| ncases/total=734/1345 | ncases/total=139/1151 | ncases/total=164/1176 | ncases/total=113/1357 | ncases/total=99/1429 | ncases/total=114/1109 | ncases/total=316/1311 | |
| Gestational weight gain | |||||||
| Inadequate weight gain | 1.57 (1.51, 1.63)** | 0.65 (0.62, 0.68)** | |||||
| PAR 11.0 | NA | ||||||
| ncases/total=6512/40322 | ncases/total=2150/35960 | ||||||
| Adequate weight gain | Reference | Reference | |||||
| ncases/total=7406/66330 | ncases/total=5592/64516 | ||||||
| Excessive weight gain | 0.62 (0.60, 0.65)** | 2.11 (2.04, 2.18)** | |||||
| NA | PAR 31.6 | ||||||
| ncases/total=5632/70709 | ncases/total=11994/77071 | ||||||
ncases/total represent the number of cases for each pregnancy complication in each clinical category/the population in each clinical category. Values are odds ratios (95% confidence intervals) from multilevel binary logistic regression models that reflect the risk of pregnancy complications per pre-pregnancy body mass index and gestational weight gain clinical category compared with the reference group (normal weight and adequate weight gain). Mothers diagnosed with pre-eclampsia were excluded from the models for gestational hypertension. The reference group for the analyses on pre-eclampsia comprises the mothers without both pre-eclampsia and gestational hypertension. The reference group for the analyses on small and large size for gestational age at birth is appropriate size for gestational age at birth. Models are adjusted for maternal age, educational level, parity, and smoking habits during pregnancy. Models for birth complications are additionally adjusted for child’s sex. *P-value<0.05; **P-value<0.001. PAR is the population attributable risk fraction in percentage that reflect the proportion of pregnancy complications at a population-level attributable to each maternal pre-pregnancy body mass index and gestational weight gain clinical category. NA, not applicable.
PAR calculated based on preterm birth and small size for gestational age at birth.
PAR calculated based on gestational hypertension, pre-eclampsia, gestational diabetes, preterm birth and large size for gestational age at birth.
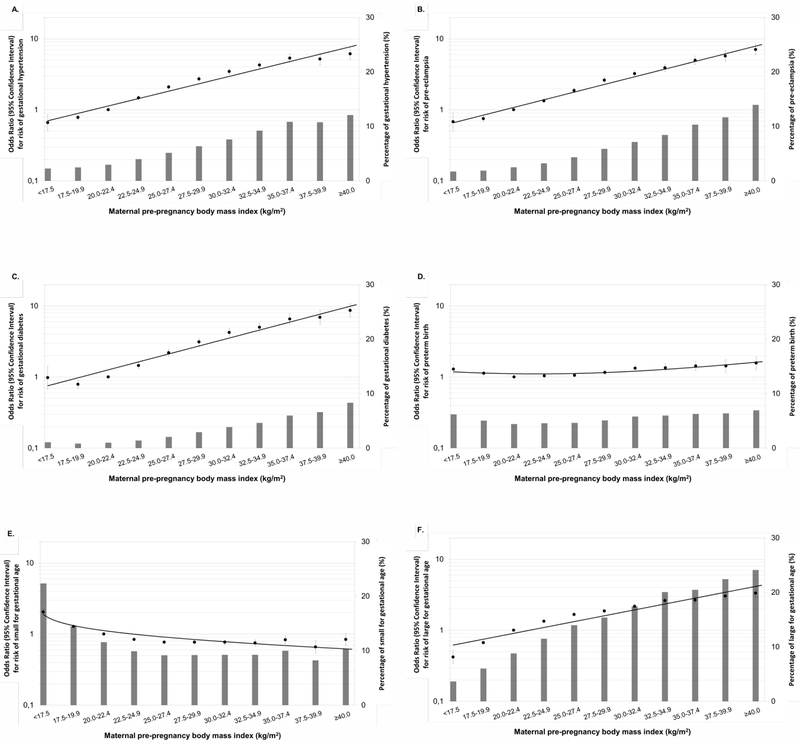
Figure 1 shows that higher maternal pre-pregnancy BMI was across the full range associated with higher risks of gestational hypertensive disorders, gestational diabetes and large size for gestational age at birth and with a lower risk of small size for gestational age at birth (p-values<0.05). Both lower and higher maternal pre-pregnancy BMI were associated with a higher risk of preterm birth (p-values<0.05). Similar results were observed in the unadjusted models (Table S5 and Figure S2). The risks of pregnancy complications per kg/m2 are given in the footnotes of Figure 1 and Figure S2. Similar results were observed in two-stage IPD meta-analysis (Figure S3).
Figure 1.
Maternal pre-pregnancy body mass index and the risks of pregnancy complicationsa
aValues are odds ratios (95% confidence intervals) on a log scale from multilevel binary logistic regression models that reflect the risk of pregnancy complications per pre-pregnancy body mass index group compared with the reference group (largest group, 20.0-22.4 kg/m2). The bars represent the percentage of each pregnancy complication per body mass index group. Mothers diagnosed with pre-eclampsia were excluded from the models for gestational hypertension. The reference group for the analyses on pre-eclampsia comprises the mothers without both pre-eclampsia and gestational hypertension. The reference group for the analyses on small and large size for gestational age at birth is appropriate size for gestational age at birth. Models are adjusted for maternal age, educational level, parity, and smoking habits during pregnancy. Models for birth complications are additionally adjusted for child’s sex. Excel’s trendline function was used to fit the curve to the data. The risks of pregnancy complications per kg/m2 were: gestational hypertension OR 1.11 (95% CI: 1.11, 1.12), pre-eclampsia OR 1.11 (95% CI: 1.11, 1.12), gestational diabetes OR 1.12 (95% CI: 1.12, 1.13), preterm birth OR 1.02 (95% CI: 1.01, 1.02), small size for gestational age at birth OR 0.96 (95% CI: 0.95, 0.96), and large size for gestational age at birth: OR 1.08 (95% CI 1.08, 1.08).
Gestational weight gain and risks of pregnancy complications
Table 1 shows that, as compared to mothers with adequate gestational weight gain, mothers with excessive gestational weight gain had a lower risk of small size for gestational age at birth (OR 0.62 (95% CI 0.60, 0.65)) and a higher risk of large size for gestational age at birth (OR 2.11 (95% CI 2.04, 2.18)). We estimated that 11.0% of small size for gestational age at birth and 31.6% of large size for gestational age at birth were attributable to inadequate and excessive gestational weight gain, respectively.
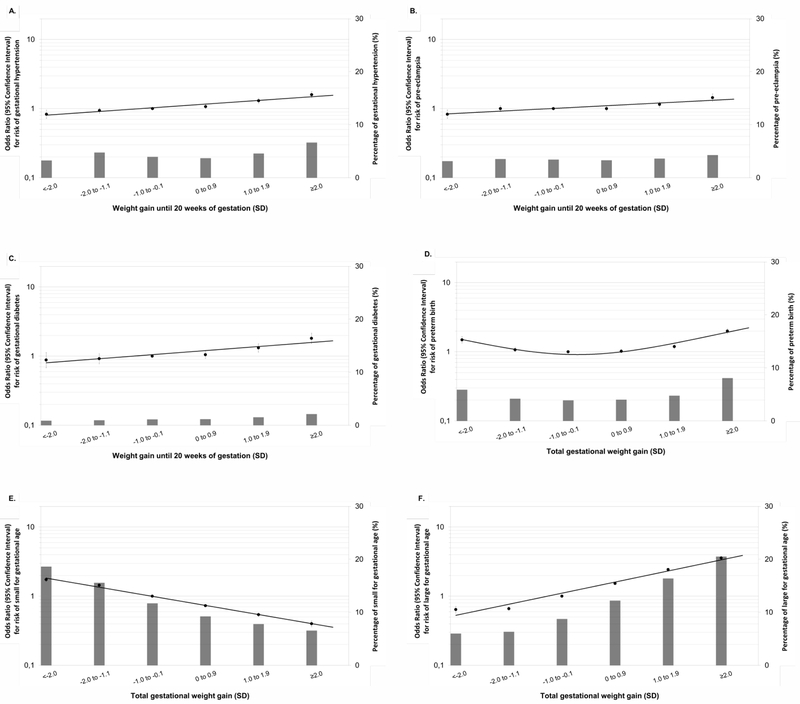
Figure 2 shows that higher weight gain z-scores until 20 weeks of gestation were associated with higher risks of gestational hypertension, pre-eclampsia and gestational diabetes. Both lower and higher total gestational weight gain z-scores were associated with a higher risk of preterm birth (p-values<0.05). Higher total gestational weight gain z-scores were, across the full range, associated with a lower risk of small size for gestational age at birth and a higher risk of large size for gestational age at birth (p-values<0.05). Similar results were observed in the unadjusted models (Table S5 and Figure S4). The risks of pregnancy complications per SD increase in gestational weight gain are given in the footnotes of Figure 2 and Figure S4. Similar results were observed in two-stage IPD meta-analysis (Figure S5).
Figure 2.
Gestational weight gain and the risks of pregnancy complicationsa
aValues are odds ratios (95% confidence intervals) on a log scale from multilevel binary logistic regression models that reflect the risk of pregnancy complications per gestational weight gain group compared with the reference group (largest group, −1.0 to −0.1 SD). The bars represent the percentage of each pregnancy complication per gestational weight gain group. Mothers diagnosed with pre-eclampsia were excluded from the models for gestational hypertension. The reference group for the analyses on pre-eclampsia comprises the mothers without both pre-eclampsia and gestational hypertension. The reference group for the analyses on small and large size for gestational age at birth is appropriate size for gestational age at birth. Models are adjusted for maternal age, educational level, parity, smoking habits during pregnancy and maternal pre-pregnancy body mass index. Models for birth complications are additionally adjusted for child’s sex. Excel’s trendline function was used to fit the curve to the data. The risks of pregnancy complications per SD increase in gestational weight gain were: gestational hypertension OR 1.12 (95% CI: 1.09, 1.14), pre-eclampsia OR 1.07 (95% CI: 1.05, 1.10), gestational diabetes OR 1.14 (95% CI: 1.10, 1.18), preterm birth OR 1.09 (95% CI: 1.07, 1.11), small size for gestational age at birth OR 0.73 (95% CI: 0.72, 0.74), and large size for gestational age at birth OR 1.53 (95% CI 1.51, 1.55).
Maternal pre-pregnancy BMI and gestational weight gain and risks of pregnancy complications
Table 2 shows that, as compared to normal weight mothers with medium gestational weight gain, overweight and obese mothers had higher risks of any pregnancy complication, independent of their gestational weight gain (p-values<0.05). The highest risk of any pregnancy complication was observed for obese mothers with high weight gain (OR 2.51 (95% CI 2.31, 2.74)). Low and high gestational weight gain were also, among normal weight mothers, associated with a higher risk of any pregnancy complication (p-values<0.05). Obese mothers with high gestational weight gain had the highest risks of gestational hypertension (OR 4.52 (95% CI 3.86, 5.31)), pre-eclampsia (OR 4.58 (95% CI 3.90, 5.37)), gestational diabetes (OR 7.84 (95% CI 6.38, 9.62)), preterm birth (OR 2.14 (95% CI 1.86, 2.46)) and large size for gestational age at birth (OR 4.77 (95% CI 4.35, 5.22)). Underweight mothers with low gestational weight gain had the highest risk of small size for gestational age at birth (OR 3.12 (95% CI 2.75, 3.54)). Similar results were observed in the unadjusted models (Table S6).
Table 2.
Maternal pre-pregnancy body mass index and gestational weight gain categories and the risks of pregnancy complicationsa
| Pregnancy complications Odds Ratio (95% Confidence Interval) |
|||||||
|---|---|---|---|---|---|---|---|
| Any pregnancy complication | Gestational hypertension | Pre-eclampsia | Gestational diabetes | Preterm birth | Small size for gestational age | Large size for gestational age | |
| Underweight | |||||||
| Low weight gain (≤−1.1 SD) |
1.09 (0.94, 1.26) | 0.56 (0.39, 0.79)* | 0.45 (0.26, 0.78)* | 1.39 (0.77, 2.49) | 1.82 (1.47, 2.27)** | 3.12 (2.75, 3.54)** | 0.23 (0.15, 0.35)** |
| ncases/total=310/960 | ncases/total=33/1002 | ncases/total=13/982 | ncases/total=12/1077 | ncases/total=92/1304 | ncases/total=360/1332 | ncases/total=21/993 | |
| Medium weight gain (−1.0 to 0.9 SD) |
1.04 (0.96, 1.12) | 0.65 (0.51, 0.81)** | 0.68 (0.53, 0.86)* | 0.55 (0.34, 0.90)* | 1.24 (1.08, 1.42)* | 1.76 (1.63, 1.90)** | 0.45 (0.38, 0.53)** |
| ncases/total=1139/4022 | ncases/total=81/3999 | ncases/total=68/3986 | ncases/total=17/4302 | ncases/total=231/4890 | ncases/total=889/4916 | ncases/total=164/4191 | |
| High weight gain (≥1.0 SD) |
1.13 (0.98, 1.30) | 1.07 (0.76, 1.50) | 1.22 (0.82, 1.79) | 0.56 (0.23, 1.36) | 1.23 (0.97, 1.57) | 0.79 (0.67, 0.95)* | 0.98 (0.79, 1.22) |
| ncases/total=330/1021 | ncases/total=37/974 | ncases/total=27/964 | ncases/total=5/1152 | ncases/total=72/1396 | ncases/total=145/1324 | ncases/total=88/1267 | |
| Normal weight | |||||||
| Low weight gain (≤−1.1 SD) |
1.04 (1.01, 1.08)* | 0.98 (0.90, 1.07) | 1.02 (0.92, 1.13) | 0.90 (0.73, 1.09) | 1.17 (1.09, 1.26)** | 1.81 (1.73, 1.89)** | 0.52 (0.49, 0.56)** |
| ncases/total=5702/19877 | ncases/total=853/19649 | ncases/total=539/19335 | ncases/total=136/20792 | ncases/total=946/21290 | ncases/total=3647/20991 | ncases/total=885/18229 | |
| Medium weight gain (−1.0 to 0.9 SD) |
Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| ncases/total=17957/68457 | ncases/total=1918/66938 | ncases/total=1606/66626 | ncases/total=497/70805 | ncases/total=3196/84958 | ncases/total=8584/79555 | ncases/total=6592/77563 | |
| High weight gain (≥1.0 SD) |
1.10 (1.06, 1.14)** | 1.39 (1.28, 1.52)** | 1.24 (1.12, 1.37)** | 1.34 (1.14, 1.58)** | 1.34 (1.25, 1.43)** | 0.57 (0.54, 0.61)** | 2.26 (2.17, 2.37)** |
| ncases/total=5910/20051 | ncases/total=817/19247 | ncases/total=530/18960 | ncases/total=218/20991 | ncases/total=1321/25135 | ncases/total=1607/21532 | ncases/total=3674/23599 | |
| Overweight | |||||||
| Low weight gain (≤−1.1 SD) |
1.23 (1.16, 1.32)** | 1.46 (1.25, 1.71)** | 1.86 (1.61, 2.15)** | 1.91 (1.46, 2.50)** | 1.15 (1.01, 1.30)* | 1.23 (1.14, 1.33)** | 0.92 (0.84, 1.01) |
| ncases/total=1541/5219 | ncases/total=185/5024 | ncases/total=217/5056 | ncases/total=62/5333 | ncases/total=278/6510 | ncases/total=759/6117 | ncases/total=496/5854 | |
| Medium weight gain (−1.0 to 0.9 SD) |
1.38 (1.33, 1.43)** | 2.10 (1.94, 2.27)** | 2.10 (1.93, 2.28)** | 2.40 (2.09, 2.75)** | 1.07 (1.00, 1.15) | 0.77 (0.73, 0.81)** | 1.77 (1.69, 1.85)** |
| ncases/total=7096/21817 | ncases/total=1118/20804 | ncases/total=986/20672 | ncases/total=368/22326 | ncases/total=1025/25596 | ncases/total=1930/22445 | ncases/total=3390/23905 | |
| High weight gain (≥1.0 SD) |
1.63 (1.54, 1.73)** | 2.71 (2.41, 3.06)** | 2.54 (2.23, 2.90)** | 3.49 (2.89, 4.22)** | 1.49 (1.34, 1.66)** | 0.51 (0.46, 0.57)** | 3.46 (3.24, 3.69)** |
| ncases/total=2089/5613 | ncases/total=361/5237 | ncases/total=282/5158 | ncases/total=153/5767 | ncases/total=395/6911 | ncases/total=368/5460 | ncases/total=1423/6515 | |
| Obesity | |||||||
| Low weight gain (≤−1.1 SD) |
1.70 (1.56, 1.85)** | 3.06 (2.57, 3.66)** | 3.52 (3.00, 4.14)** | 4.44 (3.41, 5.77)** | 1.36 (1.15, 1.62)** | 0.99 (0.87, 1.12) | 1.45 (1.29, 1.63)** |
| ncases/total=916/2534 | ncases/total=148/2344 | ncases/total=182/2378 | ncases/total=68/2577 | ncases/total=148/2957 | ncases/total=279/2639 | ncases/total=337/2697 | |
| Medium weight gain (−1.0 to 0.9 SD) |
2.06 (1.96, 2.16)** | 3.88 (3.53, 4.26)** | 4.01 (3.64, 4.40)** | 5.09 (4.40, 5.89)** | 1.32 (1.20, 1.46)** | 0.80 (0.74, 0.86)** | 2.57 (2.43, 2.72)** |
| ncases/total=3818/9080 | ncases/total=724/8208 | ncases/total=695/8179 | ncases/total=344/9220 | ncases/total=534/10807 | ncases/total=810/8924 | ncases/total=1928/10042 | |
| High weight gain (≥1.0 SD) |
2.51 (2.31, 2.74)** | 4.52 (3.86, 5.31)** | 4.58 (3.90, 5.37)** | 7.84 (6.38, 9.62)** | 2.14 (1.86, 2.46)** | 0.60 (0.51, 0.70)** | 4.77 (4.35, 5.22)** |
| ncases/total=1098/2323 | ncases/total=202/2074 | ncases/total=194/2066 | ncases/total=134/2374 | ncases/total=230/2820 | ncases/total=165/2085 | ncases/total=732/2652 | |
ncases/total represent the number of cases for each pregnancy complication in each group/the population in each group. Values are odds ratios (95% confidence intervals) from multilevel binary logistic regression models that reflect the risk of pregnancy complications per combined pre-pregnancy body mass index and gestational weight gain categories compared with the reference group (normal weight and medium weight gain). For any pregnancy complication, gestational hypertension, pre-eclampsia and gestational diabetes, weight gain z-scores until 20 weeks of gestation were used and for preterm birth, and small and large size for gestational age at birth, total gestational weight gain z-scores were used. Mothers diagnosed with pre-eclampsia were excluded from the models for gestational hypertension. The reference group for the analyses on pre-eclampsia comprises the mothers without both pre-eclampsia and gestational hypertension. The reference group for the analyses on small and large size for gestational age at birth is appropriate size for gestational age at birth. Models are adjusted for maternal age, educational level, parity, and smoking habits during pregnancy. Models for birth complications are additionally adjusted for child’s sex.
P-value<0.05;
P-value<0.001.
Significant interaction terms were present (p-values<0.05) for preterm birth, and small and large size for gestational age at birth.
DISCUSSION
Main findings
In this IPD meta-analysis, higher maternal pre-pregnancy BMI and gestational weight gain were, across the full range, associated with higher risks of gestational hypertensive disorders, gestational diabetes and large size for gestational age at birth. Preterm birth risk was higher at both BMI and weight gain extremes. Obese mothers with high gestational weight gain had the highest risk of any pregnancy complication. We estimated that up to 24% of any pregnancy complication could be attributed to maternal overweight and obesity, whereas up to 32% of large size for gestational age infants could be attributed to excessive gestational weight gain. However, the estimated population attributable risks should be carefully interpreted since the causality of the observed associations remains unknown.
Strengths and limitations
We performed a large meta-analysis of IPD from many cohorts. As part of an international collaboration between pregnancy and birth cohort studies, we invited all cohorts from Europe, North America, and Oceania that we were able to identify from existing large international collaborations on childhood health and that met the inclusion criteria. Therefore, we believe this meta-analysis covers a large proportion of individual participant data available on this topic. However, we cannot disregard the possibility of data missing from other cohorts, especially recent cohorts, that were not included. We did not rely on published data, limiting any potential publication bias and enabling a consistent definition of exposures, confounders and outcomes. The large sample size enabled us to study the risks of pregnancy complications in relatively rare conditions, such as severe obesity. We did not consider additional levels, such as country and continent, in our multilevel modelling due to the high computational complexity required for this approach and the likely minimal influence of this on the findings. We performed two-stage meta-analyses as sensitivity analyses, which gave similar results and showed moderate-to-high heterogeneity between the cohorts estimates. Missing values of covariates were used as an additional group. This approach, although commonly used in large IPD meta-analyses due to the constraints in applying more advanced imputation strategies, might lead to bias.22 However, in the current study, bias is unlikely considering the small percentage of missings and the similar findings between unadjusted and adjusted models. We relied on weights obtained partly by self-report, which might be a source of error. However, a large systematic review showed that reporting error did not bias associations between pregnancy-related weight and birth outcomes.23 We used maternal pre-pregnancy BMI specific weight gain for gestational age z-scores, which classify weight gain independently of gestational age.15 This approach allows assessing the unbiased associations between gestational weight gain and pregnancy outcomes that are highly correlated with gestational age at birth. This method is needed because the absolute value related to the z-score changes across pregnancy. However, the use of z-scores might complicate the clinical interpretation of the observed associations. Some cohorts relied on self-reporting to obtain information on gestational hypertensive and diabetic disorders. If misclassification of women occurred, our associations might be attenuated. As in any observational study, residual confounding by unmeasured lifestyle related variables may be an issue.
Interpretation
Maternal obesity is a major public health concern.24 A meta-analysis of published cohort studies showed that maternal obesity is associated with a higher risk of fetal and infant death.2 Maternal obesity is also associated with increased risks of more common pregnancy complications, such as gestational hypertensive disorders, gestational diabetes, preterm birth and large size for gestational age at birth,3–5 which are important risk factors for both maternal and neonatal morbidity and mortality.25–28 In line with these previous studies, we observed that maternal pre-pregnancy overweight and obesity are related to increased risks of any of these pregnancy complications. Mothers with obesity grade 3 showed the highest risks. Importantly, we estimated that over 40% of gestational hypertensive and diabetic disorders could be attributed to maternal overweight and obesity. Smaller but yet considerable risk fractions attributable to maternal overweight/obesity were observed for preterm birth (3.9%) and large size for gestational age at birth (20.6%). Overall, 23.9% of any pregnancy complication was estimated to be attributable to maternal pre-pregnancy overweight/obesity, which underlines their major public health implications and the possibility to substantially reduce pregnancy complications by optimizing maternal BMI.
The associations of maternal BMI with pregnancy complications were also present across the full range. Even modest increases of maternal pre-pregnancy BMI were associated with higher risks of gestational hypertensive disorders, gestational diabetes and large size for gestational age at birth. The association of maternal pre-pregnancy BMI with the risk of preterm birth tended to be U-shaped. Thus, our findings suggest that mothers do not necessarily need to become overweight or obese to be at risk of pregnancy complications, since higher risks of pregnancy complications were already observed for an increase in BMI within the healthy range.
Next to pre-pregnancy BMI, excessive gestational weight gain may affect the risks of pregnancy complications.6–9 We observed gradually higher risks of gestational hypertension, pre-eclampsia and gestational diabetes over the full range of weight gain. Similar to the association of maternal BMI, the association of total gestational weight gain z-scores with preterm birth tended to be U-shaped. We also observed that not only excessive weight gain but also higher weight gain across the full range was associated with a higher risk of large size for gestational age at birth. At the population level, 31.6% of large size for gestational age infants could be attributed to excessive weight gain. Altogether, these findings suggest that gradual increases in gestational weight gain, and not only excessive weight gain, are associated with higher risks of pregnancy complications. We also assessed the combined effects of pre-pregnancy BMI and gestational weight gain on pregnancy complications. Previous studies showed that mothers with both high BMI and gestational weight gain had the highest risk of large size for gestational age. The risk of preterm birth was increased at both extremes.29–33 In line with these previous studies, we observed that obese mothers with high weight gain were at the highest risk of any pregnancy complication. Importantly, we also observed that overweight and obese mothers are at risk of these complications, regardless how much weight they gain during pregnancy. These findings show the importance of promoting a healthy weight status before and during pregnancy.
The mechanisms underlying the associations of maternal adiposity and pregnancy complications are not fully understood yet, but might include insulin resistance, endothelial dysfunction, oxidative stress, lipotoxicity, inflammation, and infection.3, 4, 34 The associations of maternal adiposity with large size for gestational age infants might be explained by fetal over-nutrition, since an increased placental transfer of nutrients to the fetus might lead to an increased synthesis of insulin and insulin-like growth factors, both of which are growth-promoting hormones.35 The causal role of glucose is also suggested in a large Mendelian randomization study.36 Gestational weight gain reflects fat storage during pregnancy, but also reflects fetus growth, amniotic fluid, placenta, uterine and mammary tissue expansion, increased blood volume, and extracellular fluid.37 These factors may all have different roles in the associations with pregnancy complications. From the current observational data, we cannot drive conclusions on the mechanisms underlying the observed associations.
We observed that a high percentage of pregnancy complications is attributable to suboptimal maternal BMI and gestational weight gain, which suggests the potential for prevention of pregnancy complications by optimizing these maternal measures. Thus far, randomized trials focused on lifestyle interventions to improve gestational weight gain and subsequent pregnancy complications are disappointing. An IPD meta-analysis from randomized trials focused on lifestyle interventions in pregnancy showed a reduction in gestational weight gain but no effects on gestational hypertensive and diabetic disorders, preterm birth and size for gestational age.38 Strategies to improve body mass index before pregnancy rather than during pregnancy may be more effective in prevention of pregnancy complications.
CONCLUSION
Maternal pre-pregnancy BMI and gestational weight gain are, across the full range, associated with the risks of pregnancy complications. Obese mothers with high gestational weight gain are at the highest risk of pregnancy complications. Up to 30% of any pregnancy complication is estimated to be attributable to overweight/obesity or excessive gestational weight gain. Our findings provide evidence for advocating a healthy BMI in women who are planning to become pregnant and an adequate weight gain during pregnancy to reduce the burden of obstetric and neonatal morbidity.
Supplementary Material
Acknowledgements
Funding
Cohort-specific information is given in the Appendix S2.
DISCLOSURE OF INTERESTS
Keith M. Godfrey has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. Debbie A. Lawlor has received support from Roche Diagnostics and Medtronic in relation to biomarker research that is not related to the research presented in this paper. Andrea von Berg has received reimbursement for speaking at symposia sponsored by Nestlé and Mead Johnson, who partly financially supported the 15 yrs follow-up examination of the GINIplus study. The rest of the authors reported no conflicts of interest.
Footnotes
DETAILS OF ETHICS APPROVAL
Cohorts were approved by their local institutional review boards and consent to participate was obtained from participants.
REFERENCES
- 1.Poston L, Caleyachetty R, Cnattingius S, Corvalan C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol 2016;4:1025–36. [DOI] [PubMed] [Google Scholar]
- 2.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA 2014;311:1536–46. [DOI] [PubMed] [Google Scholar]
- 3.Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikstrom AK, et al. Maternal obesity and risk of preterm delivery. JAMA 2013;309:2362–70. [DOI] [PubMed] [Google Scholar]
- 4.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015;16:621–38. [DOI] [PubMed] [Google Scholar]
- 5.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 2006;368:1164–70. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar LM, Hutcheon JA, Parisi SM, Pugh SJ, Abrams B. Comparison of gestational weight gain z-scores and traditional weight gain measures in relation to perinatal outcomes. Paediatr Perinat Epidemiol 2015;29:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013;21:1046–55. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet 2010;376:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017;317:2207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacInnis N, Woolcott CG, McDonald S, Kuhle S. Population Attributable Risk Fractions of Maternal Overweight and Obesity for Adverse Perinatal Outcomes. Sci Rep 2016;6:22895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oteng-Ntim E, Kopeika J, Seed P, Wandiembe S, Doyle P. Impact of obesity on pregnancy outcome in different ethnic groups: calculating population attributable fractions. PLoS One 2013;8:e53749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z, Phung H, Freebairn L, Sexton R, Raulli A, Kelly P. Contribution of maternal overweight and obesity to the occurrence of adverse pregnancy outcomes. Aust N Z J Obstet Gynaecol 2018. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization Regional Office for Europe. Body mass index - BMI. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed August 19, 2015.
- 14.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 15.Santos S, Eekhout I, Voerman E, Gaillard R, Barros H, Charles MA, et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med 2018;16:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker J, McGuire W. Epidemiology of preterm birth. BMJ 2004;329:675–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niklasson A, Ericson A, Fryer JG, Karlberg J, Lawrence C, Karlberg P. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977–1981). Acta Paediatr Scand 1991;80:756–62. [DOI] [PubMed] [Google Scholar]
- 18.Santos S, Zugna D, Pizzi C, Richiardi L. Sources of confounding in life course epidemiology. J Dev Orig Health Dis 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 19.Debray TP, Moons KG, Abo-Zaid GM, Koffijberg H, Riley RD. Individual participant data meta-analysis for a binary outcome: one-stage or two-stage? PLoS One 2013;8:e60650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flegal KM, Graubard BI, Williamson DF. Methods of calculating deaths attributable to obesity. Am J Epidemiol 2004;160:331–8. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groenwold RH, White IR, Donders AR, Carpenter JR, Altman DG, Moons KG. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ 2012;184:1265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obes Rev 2017;18:350–69. [DOI] [PubMed] [Google Scholar]
- 24.Haslam DW, James WP. Obesity. Lancet 2005;366:1197–209. [DOI] [PubMed] [Google Scholar]
- 25.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–9. [DOI] [PubMed] [Google Scholar]
- 26.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet 2005;365:891–900. [DOI] [PubMed] [Google Scholar]
- 28.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol 2013;25:124–32. [DOI] [PubMed] [Google Scholar]
- 29.Ay L, Kruithof CJ, Bakker R, Steegers EA, Witteman JC, Moll HA, et al. Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. The Generation R Study. BJOG 2009;116:953–63. [DOI] [PubMed] [Google Scholar]
- 30.Nohr EA, Vaeth M, Baker JL, Sørensen TIA, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 2008;87:1750–9. [DOI] [PubMed] [Google Scholar]
- 31.Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol 2014;123:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietz PM, Callaghan WM, Cogswell ME, Morrow B, Ferre C, Schieve LA. Combined effects of prepregnancy body mass index and weight gain during pregnancy on the risk of preterm delivery. Epidemiology 2006;17:170–7. [DOI] [PubMed] [Google Scholar]
- 33.Nohr EA, Bech BH, Vaeth M, Rasmussen KM, Henriksen TB, Olsen J. Obesity, gestational weight gain and preterm birth: a study within the Danish National Birth Cohort. Paediatr Perinat Epidemiol 2007;21:5–14. [DOI] [PubMed] [Google Scholar]
- 34.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017;356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition--an old hypothesis with new importance? Int J Epidemiol 2013;42:7–29. [DOI] [PubMed] [Google Scholar]
- 36.Tyrrell J, Richmond RC, Palmer TM, Feenstra B, Rangarajan J, Metrustry S, et al. Genetic Evidence for Causal Relationships Between Maternal Obesity-Related Traits and Birth Weight. JAMA 2016;315:1129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol 1976;19:489–513. [DOI] [PubMed] [Google Scholar]
- 38.International Weight Management in Pregnancy Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ 2017;358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.