Abstract
Background
There is limited information on the complications and costs of conversion THA after hemiarthroplasty for femoral neck fractures. Previous studies have found that patients undergoing conversion THA experience higher risk complications, but it has been difficult to quantify the risk because of small sample sizes and a lack of comparison groups. Therefore, we compared the complications of patients undergoing conversion THA with strictly matched patients undergoing primary and revision THA.
Questions/purposes
(1) What are the risks of complications, dislocations, reoperations, revisions and periprosthetic fractures after conversion THA compared with primary and revision THA and how has this effect changed over time? (2) What are the length of hospital stay and hospital costs for conversion THA, primary THA, and revision THA?
Methods
Using a longitudinally maintained total joint registry, we identified 389 patients who were treated with conversion THA after hemiarthroplasty for femoral neck fractures between 1985 and 2014. The conversion THA cohort was 1:2 matched on age, sex, and year of surgery to 778 patients undergoing primary THA and 778 patients undergoing revision THA. The proportion of patients having at least 5-year followup was 73% in those who underwent conversion THA, 77% in those who underwent primary THA, and 76% in those who underwent revision THA. We observed a significant calendar year effect, and therefore, compared the three groups across two separate time periods: 1985 to 1999 and 2000 to 2014. We ascertained complications, dislocations, reoperations, revisions and periprosthetic fractures from the total joint registry. Cost analysis was performed using a bottom-up, microcosting methodology for procedures between 2003 and 2014.
Results
Patients who converted to THA between 1985 and 1999 had a higher risk of complications (hazard ratio [HR], 2.3; 95% confidence interval [CI], 1.7–3.1; p < 0.001), dislocations (HR, 2.3; 95% CI, 1.3–4.2; p = 0.007), reoperations (HR, 1.7; 95% CI, 1.2–2.5, p = 0.005), and periprosthetic fractures (HR, 3.8; 95% CI, 2.2–6.6; p < 0.001) compared with primary THA. However, conversion THAs during the 1985 to 1999 time period had a lower risk of reoperations (HR, 0.7; 95% CI, 0.5–1.0; p = 0.037), revisions (HR, 0.6; 95% CI, 0.5–0.9; p = 0.014), and periprosthetic fractures (HR, 0.6; 95% CI, 0.4–0.9; p = 0.007) compared with revision THA. The risk differences across the three groups were more pronounced after 2000, particularly when comparing conversion THA patients with revision THA. Conversion THA patients had a higher risk of reoperations (HR, 1.9; 95% CI, 1.0–3.4; p = 0.041) and periprosthetic fractures (HR, 1.7; 95% CI, 1.0–2.9; p = 0.036) compared with revision THA, but there were no differences in the complication risk (HR, 1.4; 95% CI, 0.9–2.1; p = 0.120), dislocations (HR, 1.5; 95% CI, 0.7–3.2; p = 0.274), and revisions (HR, 1.4; 95% CI, 0.7–3.0; p = 0.373). Length of stay for conversion THA was longer than primary THA (4.7 versus 4.0 days; p = 0.012), but there was no difference compared with revision THA (4.7 versus 4.5 days; p = 0.484). Similarly, total inpatient costs for conversion THA were higher than primary THA (USD 22,662 versus USD 18,694; p < 0.001), but there was no difference compared with revision THA (USD 22,662 versus USD 22,071; p = 0.564).
Conclusions
Over the 30 years of the study, conversion THA has remained a higher risk procedure in terms of reoperation compared with primary THA, and over time, it also has become higher risk compared with revision THA. Surgeons should approach conversion THA as a challenging procedure, and patients undergoing this procedure should be counseled about the elevated risks. Furthermore, hospitals should seek appropriate reimbursement for these cases.
Level of Evidence
Level III, therapeutic study.
Introduction
More than 5800 hemiarthroplasties are performed each year in the United States for femoral neck fractures [2]. It has been reported that 5% to 7% of patients with hemiarthroplasties undergo revision surgery [4, 9]. Hemiarthroplasties most commonly undergo revision due to acetabular erosion and femoral component loosening that manifests as groin or thigh pain. The most common form of revision after a hemiarthroplasty is conversion to THA.
Studies that have evaluated conversion to THA for hemiarthroplasties highlight the high rates of dislocation and implant loosening [6-8, 12, 14, 15]. However, most of these studies were small, did not include a comparison cohort of primary or revision THA, and were not always hemiarthroplasties after femoral neck fractures. Figved et al. [8] specifically evaluated conversion of hemiarthroplasty after femoral neck fracture against THA using the Norwegian registry. They found that the conversion group had a greater risk of revision compared with primary THA but were at decreased risk of revision compared with revision THA. They did not compare all-cause reoperations, complications, or dislocation rates between these groups. Importantly, they did not perform a matched analysis, rather they compared their conversion cohort with all other patients in their registry. Fichman et al. [7] found that revision and dislocation rates were similar between hemiarthroplasties converted to THA and a group of first-time revision THAs, but this study was small, and the authors made no direct statistical comparisons.
Previous studies also have found that conversion THAs receive more revision-type implants than the primary THAs, and they were similar to revision THAs [3, 13, 14]. These studies did not specifically evaluate conversion after just hemiarthroplasty [3, 13, 14]. Further, few studies have evaluated the costs of conversion THA after hemiarthroplasty and compared these costs directly to primary and revision THA [5]. Thus, it would be helpful to do a formal cost analysis between matched conversion, primary, and first-time revision THAs.
Therefore, we asked: (1) What are the risks of complications, dislocations, reoperations, revisions and periprosthetic fractures after conversion THA compared with primary and revision THAs and how has this changed over time? (2) What are the length of hospital stay and costs for conversion THA, primary THA, and revision THA?
Patients and Methods
Treatment and Control Groups
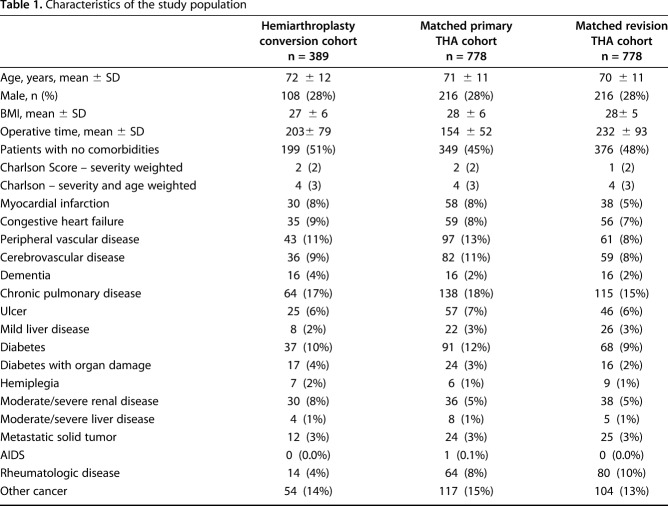
Approval was obtained from the institutional review board. Between 1985 and 2014, there were 24,030 primary THA and 10,606 revision THA procedures performed at our institution, including 389 patients who underwent conversion THA after hemiarthroplasty for femoral neck fractures. This represented 1% of all primary and revision THA procedures. We performed a detailed manual chart review to confirm all these procedures. Indications for conversion were acetabular wear (37%), femoral loosening (31%), femoral loosening and wear (25%), periprosthetic fracture (3%), infection (3%), and instability (1%). Both the femoral and acetabular components were converted in 317 of 389 (81%) patients and acetabular component-only in 72 (19%). At the time of conversion, the proportion of isolated cup implantation was six of 110 (6%) from 1985 to 1989, seven of 79 (9%) from 1990 to 1994, nine of 63 (14%) from 1995 to 1999, 15 of 57 (26%) from 2000 to 2004, 22 of 49 (45%) from 2005 to 2009, and 13 of 31 (42%) from 2010 to 2014. The decision to replace just the cup versus the stem and the cup was at the discretion of each individual surgeon. In general, the stem was also revised if it was felt to be loose. Conversion stem types were cemented (n = 218; 69%), fully porous coated (n = 37; 12%), modular fluted tapered (n = 32; 10%), and uncemented metaphyseal fit (n = 30; 9%). Cups were uncemented (n = 264; 68%) and cemented (n = 125; 32%). Femoral head size was 22 to 28 mm (n = 266; 68%), 32 mm (n = 75; 19%), and 36 to 40 mm (n = 48; 12%). Acetabular liners were standard nonelevated (n = 300; 77%), elevated or lipped (n = 87; 22%) and constrained (n = 2; 1%). Three patients had dual-mobility constructs (1%). At the time of conversion, the mean age was 72 (± 12) years, 108 (28%) patients were men, and mean BMI was 27 (± 6) kg/m2. Patients were followed until death, revision or implant removal, or until final clinical followup. Mean clinical followup of these 389 patients was 9.3 years (range, 0.01-28.4 years) (Table 1).
Table 1.
Characteristics of the study population
We matched all 389 patients undergoing conversion THA to patients undergoing primary and revision THA based on gender, age at surgery and year of surgery, resulting in 778 matched patients undergoing primary THA and 778 matched patients undergoing revision THA. There was similar followup amongst the three groups. Patients having at least 5-year followup was 73% in those undergoing conversion THA, 77% in those undergoing primary THA, and 76% in those undergoing revision THA. All 778 patients undergoing primary THA underwent THA for osteoarthritis and were matched on gender, within ± 5 years of age at surgery, and within ± 1 year of date of surgery. The mean age of the 778 patients undergoing primary THA was 71 (± 11) years, 216 (28%) patients were men, and mean BMI was 28 (± 6) kg/m2. Mean clinical followup of the 778 patients undergoing primary THA was 10 years (range, 0–31 years). Patients undergoing revision THA were similarly matched on gender, age at surgery and year of surgery. For age matching, 93% were matched within ± 5 years of age at surgery, 2% within ± 10 years of age at surgery, and the remaining 5% greater than 10 years of age at surgery. Ninety-two percent were matched within ± 1 year of date of surgery, 6% within ± 5 years of date of surgery, and an additional 2% were more than 5 years from the date of surgery. These were all first-time revisions for aseptic loosening. The mean age was 70 (± 11) years, 216 (28%) patients were men, and mean BMI was 28 (± 5) kg/m2. Mean clinical followup of the 778 patients undergoing revision THA was 9.5 years (range, 0–28 years). These were cup and stem revisions in 336 patients, cup revisions in 316, and stem revisions in 126 patients. We also retrieved comorbidities in each group using the Charlson Comorbidity Index [1]. The Charlson Comorbidity Index Score was 2 ± 2 in the conversion group, 2 ± 2 in the primary THA group, and 1 ± 2 in the revision THA group (Table 1).
Cost analyses were limited to January 2003 and December of 2014 period, and included 106 conversion THAs, 211 primary THAs for osteoarthritis, and 210 revision THAs for aseptic loosening. We applied a bottom-up, microcosting costing methodology to derive standardized costs [16]. We first obtained all line-item details of services provided during each hospital episode, such as the date, type, frequency, and billed charges of each service. We then applied a hybrid costing algorithm, separately for professional services and hospital services. Professional services were identified with either Healthcare Common Procedure Coding System (HCPCS) or Current Procedural Terminology Fourth Edition (CPT-4) codes and were assigned standardized costs by using national reimbursement amounts from the appropriate Medicare physician, clinical laboratory, and fee schedules for that year. We determined the costs of hospital services, such as room and board, radiology, physical therapy, and supplies, by multiplying the charge for each service item with the cost center-specific cost-to-charge ratios for the year in which the service was delivered. We then inflated the cost estimates to the final year of the study [16].
Statistical Analyses
Characteristics of the study population were summarized as mean (± SD) for continuous variables, or count (percentage) for categorical variables, unless otherwise noted. Temporal trends over time across the three cohorts in the risk of five outcomes, such as complications, dislocations, reoperations, revisions, and periprosthetic fractures, were analyzed using Poisson regression analyses. These Poisson models were fit using a generalized linear model framework with person-years of exposure as an offset and calendar year of THA incorporated using a smoothing spline. Confidence intervals for between-group differences in rates were generated using a bootstrap approach. In the analyses of temporal trends, we observed an interaction between calendar year and cohort effect, indicating that the differences in the risk of outcomes across the three groups differed over time. Therefore, we compared the risk of complications, dislocations, reoperations, revisions, and periprosthetic fractures across the three groups during two separate periods: 1985 to 1999 and 2000 to 2014 (Table 2). Reoperations were reported as any reoperation, including a revision. We compared time-to-event outcomes using survivorship analyses, including Kaplan-Meier analysis and Cox regression. We used survivorship analyses because they evaluate event rates while properly accounting for differential followup. To further balance the comparisons across the three groups, we adjusted the Cox regression models for the severity-weighted Charlson Index. Hazard ratios from the Cox regression models were reported with 95% confidence intervals (CIs). All analyses were conducted using SAS version 9.4M3 (SAS Institute Inc, Cary, NC, USA), and R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
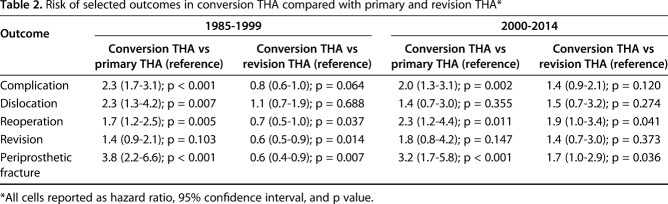
Table 2.
Risk of selected outcomes in conversion THA compared with primary and revision THA*
Results
Complications, Dislocations, Reoperation, Revisions, and Periprosthetic Fractures
Patients who converted to THA between 1985 and 1999 had a higher risk of complications (hazard ratio [HR], 2.3; 95% confidence interval [CI], 1.7–3.1; p < 0.001), dislocations (HR, 2.3; 95% CI, 1.3–4.2; p = 0.007), reoperations (HR, 1.7; 95% CI, 1.2–2.5, p = 0.005), and periprosthetic fractures (HR, 3.8; 95% CI, 2.2–6.6; p < 0.001) compared with primary THA patients (Table 2). Patients converted to THA had no difference in the risk of revisions (HR, 1.4; 95% CI, 0.9–2.1; p = 0.103) compared with primary THA. However, patients undergoing conversion THA had a lower risk of reoperations (HR, 0.7; 95% CI, 0.5–1.0; p = 0.037), revisions (HR, 0.6; 95% CI, 0.5–0.9; p = 0.014), and periprosthetic fractures (HR, 0.6; 95% CI, 0.4–0.9; p = 0.007) compared with patients undergoing revision THA. Patients converted to THA had no difference in the risk of complications (HR, 0.8; 95% CI, 0.6–1.0; p = 0.064) and dislocations (HR, 1.1; 95% CI, 0.7–1.9; p = 0.688) compared with patients undergoing revision THA (Table 2).
These risk differences were different during the 2000 to 2014 period. Patients who converted to THA between 2000 and 2014 had a higher risk of complications (HR, 2.0; 95% CI, 1.3–3.1; p = 0.002), reoperations (HR, 2.3; 95% CI, 1.2–4.4, p = 0.011), and periprosthetic fractures (HR, 3.2; 95% CI, 1.7–5.8; p < 0.001) compared with patients undergoing primary THA (Table 2). Patients converted to THA had no difference in risk of dislocations (HR, 1.4; 95% CI, 0.7–3.0; p = 0.355) and revisions (HR, 1.8; 95% CI, 0.8–4.2; p = 0.147) compared with primary THA. Patients undergoing conversion THA had a higher risk of reoperations (HR, 1.9; 95% CI, 1.0–3.4; p = 0.041) and periprosthetic fractures (HR, 1.7; 95% CI, 1.0–2.9; p = 0.036) compared with patients undergoing revision THA. Patients converted to THA had no difference in the risk of complications (HR, 1.4; 95% CI, 0.9–2.1; p = 0.120), dislocations (HR, 1.5; 95% CI, 0.7–3.2; p = 0.274), and revisions (HR, 1.4; 95% CI, 0.7–3.0; p = 0.373) compared with patients undergoing revision THA (Table 2).
In 1990, the rate of complications per 1000 person-years for patients undergoing conversion THA was 33.4 complications compared with 16.1 complications for patients undergoing primary THA (difference of -17.3 revisions per 1000 person-years; 95% CI ,-29.1 to -6.6), and 53.5 complications for patients undergoing revision THA (difference of 20.1 revisions per 1000 person-years; 95% CI, 6.4-33.8) (Fig. 1). About 15 years later in 2005, the rate of complications per 1000 person-years was 64.8 complications for patients undergoing conversion THA compared with 23.6 complications for patients undergoing primary THA (difference of -41.2 revisions per 1000 person-years; 95% CI, -65.5 to -22.3), and 37.1 complications for revision THA patients (mean difference of -27.7 revisions per 1000 person-years; 95% CI, -52.9 to -6.6) (Fig. 1).
Fig. 1.
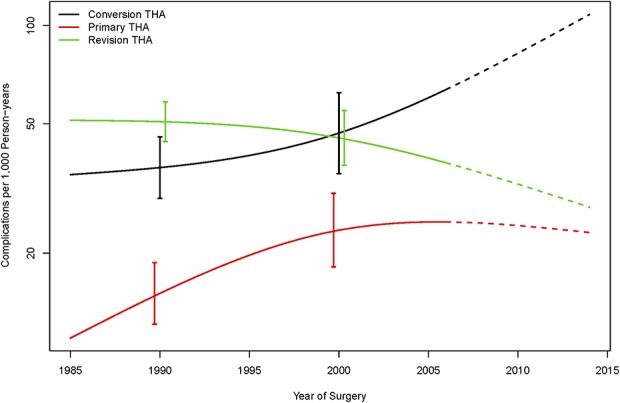
The rate of complications did not decrease over time in patients undergoing conversion THA.
The rate of revisions per 1000 person-years for patients who underwent conversion THA in 1990 was 13.1 revisions compared with 11.7 revisions for patients undergoing primary THA (corresponding to a difference of -1.4 revisions per 1000 person-years; 95% CI, -10.3 to 5.1), and 24.0 revisions for patients undergoing revision THA (corresponding to a difference of 10.9 revisions per 1000 person-years; 95% CI, 2.2–18.8). Notably, the revision rate remained relatively stable over time for patients undergoing conversion THA, but declined steadily for the patients undergoing primary THA and revision THA. By 2005, the revision rate per 1000 person-years was 15.8 for patients undergoing conversion THA, 5.6 for patients undergoing primary THA, and 8.1 for patients undergoing revision THA, corresponding to a difference of -10.2 (95% CI, -21.9 to -2.1) revisions per 1000 person-years compared with primary THA, and a difference of -7.7 (95% CI, -19.2 to 0.5) revisions per 1000 person-years in comparison to revision THA (Fig. 2).
Fig. 2.
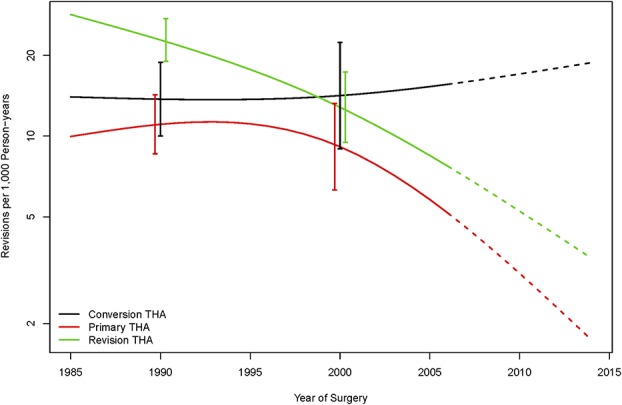
The rate of revisions did not decrease over time in patients undergoing conversion THA.
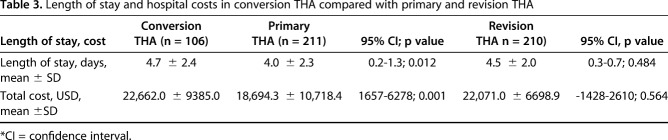
Length of Stay and Cost Analysis
Between 2003 and 2014, the length of stay was 4.7 ± 2.4 days for patients with conversion THA after hemiarthroplasty compared with 4.0 ± 2.3 days for patients with primary THA (mean difference of 0.7 days; 95% CI, 0.2–1.3; p = 0.012) and 4.5 ± 2.0 days for patients with revision THA (mean difference 0.2 days; 95% CI, -0.3 to 0.7; p = 0.484) (Table 3). The total inpatient costs was USD 22,662 ± 9385 for patients with conversion THA after hemiarthroplasty compared with USD 18,694 ± 10,718 for patients with primary THA (mean difference of USD 3968; 95% CI, 1657–6278; p < 0.001) and USD 22,071 ± 6699 for patients with revision THA (mean difference of USD 591.0; 95% CI, -1428 to 2610; p = 0.564).
Table 3.
Length of stay and hospital costs in conversion THA compared with primary and revision THA
Discussion
Patients who have had hemiarthroplasties sometimes undergo revision because of acetabular wear and femoral component loosening. Previous studies have evaluated conversion THA after hemiarthroplasty and compared the complications with primary or revision THA, but most studies were small or did not strictly match the different cohorts. To our knowledge there has never been a formal cost analysis that compared conversion THA with a matched group of both primary and revision THAs. We found that patients undergoing conversion to THA are at a higher risk for complications, reoperations, and periprosthetic fractures than patients undergoing primary THA over the 30 years of the study. We observed notable trends over time primarily due to steady improvements in revision rates after both primary and revision THA, but not necessarily in patients undergoing conversion THA. Up until 2000, patients undergoing conversion THA had a lower risk compared with patients undergoing revision THA, but this pattern reversed after 2000, and patients undergoing conversion THA experienced a higher risk of many of the adverse outcomes, especially reoperations and periprosthetic fractures, compared with patients undergoing revision THA. Furthermore, hospitals costs of patients undergoing conversion THA were higher than patients undergoing primary THA but similar to patients undergoing revision THA. Surgeons should recognize that conversion THA after hemiarthroplasty remains a high-risk procedure despite improvements in implant techniques and perioperative management. Institutions should consider these procedures as costly as revision THA and seek appropriate reimbursement.
This study had several limitations. Although we had a reasonable large cohort of 389 patients undergoing conversion THA, we were not fully powered to evaluate rare outcomes such as periprosthetic fractures, resulting in wide confidence intervals. Despite this, the long time period allowed us to examine trends over time (Fig. 1 and Fig. 2), particularly the steady decline in the risk of subsequent revisions in primary and revision THA relative to conversion THA after hemiarthroplasty. Second, selection bias is a potential concern since symptomatic patients undergoing hemiarthroplasty who did not have conversion THA were not included in the study. It is possible that inclusion of such patients could have changed the findings. Third, outcomes examined in this study were limited to clinical events and not patient-reported outcomes. For instance, pain control and physical function may have been better in one group than another. If this were the case, it may change the way we compare conversion THA relative to both primary and revision THA. Yet, by evaluating revisions, reoperations, and complications, we believe we captured all major clinical outcomes. Fourth, information bias due to incomplete identification of outcomes and/or differential followup across the three cohorts is a potential concern. However, followup was relatively complete for all patients included in this study; only 3.5% were lost to followup within 2 years. There is no evidence to assume that informative censoring is a concern, that is, loss to followup related to occurrence of outcomes of interest. Losses to followup were most likely random, and were accounted for by using survival analyses methods. Fifth, we evaluated total hospital costs but not the components that contribute to cost differences. Future studies are warranted to evaluate if the cost differences were related to length of stay and/or implant costs. Finally, we did not evaluate the type of hemiarthroplasty used at the original implantation and whether the hemiarthroplasty was implanted correctly. This could potentially affect the complexity of the subsequent conversion THA, and this may be an element to be evaluated in future studies as a potential risk factor.
Although historical practice patterns are not necessarily reflective of today’s practices, the long time period in this study allowed us to examine temporal trends over time. Over the entire 30-year period, patients undergoing conversion THA after hemiarthroplasty for femoral neck fractures were at higher risk for complications, reoperations, and periprosthetic fractures compared with primary THA. Yet, up until 2000, patients undergoing conversion THA experienced a lower risk compared with patients undergoing revision THA, but this trend changed after 2000 when their risk of adverse events was higher than patients undergoing revision THA. Two previous studies addressed the same study questions. In a cohort of 46 patients undergoing conversion THA between 2002 and 2013, Fichman et al. [7] concluded that the revision and dislocation rates were similar between hemiarthroplasties converted to THA and a group of first-time revision THAs, but with only 46 patients and a total of five complications in the conversion THA group, the numbers were too small for reliable comparisons. In a larger cohort of 595 patients undergoing conversion THA after hemiarthroplasty for femoral neck fractures performed between 1987 and 2004, Figved et al. [8] found that conversion THAs had a higher risk of revision than primary THA but lower risk compared with revision THA, similar to our findings for the same time period. Our study is unique because to our knowledge, this is the first study to cover a long time period and to directly compare the risk of complications, dislocations, reoperations, and periprosthetic fractures across strictly matched groups. In the present study, we found that patients who underwent conversion THA after hemiarthroplasty for femoral neck fractures did not experienced the same improvements in the rate of revisions as in patients who underwent primary and revision THA. The improved revision rates seen in primary and revision THA over time could be related to improved perioperative management, surgical technique, and/or implant design. The fact that hemiarthroplasty conversion remains a high-risk operation may be related to underlying patient population; many of these patients are older, and they often have poor bone quality and serious medical comorbidities [1, 10, 11]. Future studies should aim to identify risk factors in this group to better understand why their surgical outcomes have remained poor over time. Our results suggest that surgeons should prepare for conversion THA as an especially challenging procedure, and that they should educate their patients about the serious risks associated with it. Furthermore, it is noteworthy that over the course of the study, more patients having conversion THA after hemiarthroplasty had isolated cup revisions. Even without a femoral revision the rate of revision and complication in conversion THA did not decrease over time.
Patients undergoing conversion THA had longer hospital stays and incurred greater costs than primary THA, but were similar to revision THA. Previous studies [3, 8] have highlighted that conversion THA uses more hospital resources and has a complication profile more similar to revision THA, but to the authors' knowledge studies have not shown that the cost of conversion THA is similar to that of revision THA. Institutions should strive for conversion THA after hemiarthroplasty to receive reimbursement at the level of a revision procedure and not as a primary procedure. Part of the increased cost of conversion THA compared with primary THA is increased length of stay, but it is also likely related to the increased use of revision-type implants. Although the difference in length of stay was small between primary and conversion THA, this information is helpful to surgeons and institutions. In the future, this group may benefit from identifying and removing barriers to earlier discharge. Further, there should be increased research evaluating the costs of treating patients with femoral neck fracture immediately with THA versus the costs of treating patients with hemiarthroplasty who go on to conversion THA. Most patients with hemiarthroplasty will not undergo conversion THA, but an effort should be made to identify patients who may end up having conversion after hemiarthroplasty. If these patients could be identified, perhaps institutions could save resources, and patients could avoid additional operations.
In conclusion, the risk of adverse events remains elevated in conversion THA after hemiarthroplasty compared with primary THA. Despite improvements in implant techniques and perioperative management, patients undergoing conversion THA have more reoperations than patients undergoing revision THA. Surgeons should be aware of this increased risk and educate patients appropriately. Institutions should be aware of the increased hospital costs and strive to have appropriate reimbursement.
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
The institution of one or more of the authors (RJS) has received, during the study period, funding from Zimmer Biomet and Link Orthopaedics; however, no funding was relevant to the present research.
One of the authors certifies that he (RJS), or a member of his or her immediate family, has received or may receive consultancy fees and royalties, during the study period, in an amount of USD 100,001 to USD 1,000,000 from Zimmer Biomet (Warsaw, IN, USA) and USD 10,000 to USD 100,000 from Link Orthopaedics (Rockaway, NJ, USA).
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20:1633-1650. [DOI] [PubMed] [Google Scholar]
- 2.American Joint Replacement Registry. American Joint Replacement Registry Annual Report 2016, 15, 2016. Available at: http://www.ajrr.net/publications-data/annual-reports. Accessed December, 5, 2018. [Google Scholar]
- 3.Baghoolizadeh M, Schwarzkopf R. The Lawrence D. Dorr Surgical Techniques & Technologies Award: Conversion total hip arthroplasty: Is it a primary or revision hip arthroplasty. J Arthroplasty. 2016;31:16-21. [DOI] [PubMed] [Google Scholar]
- 4.Burgers PT, Van Geene AR, Van den Bekerom MP, Van Lieshout EM, Blom B, Aleem IS, Bhandari M, Poolman RW. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin G, Wright DJ, Snir N, Schwarzkopf R. Primary vs conversion total hip arthroplasty: a cost analysis. J Arthroplasty. 2016;31:362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diwanji SR, Kim SK, Seon JK, Park SJ, Yoon TR. Clinical results of conversion total hip arthroplasty after failed bipolar hemiarthroplasty. J Arthroplasty. 2008;23:1009-1015. [DOI] [PubMed] [Google Scholar]
- 7.Fichman SG, Makinen TJ, Vincent A, Lozano B, Safir O, Kuzyk PR. Complications following conversion of a hip hemiarthroplasty to a total hip arthroplasty. Int Orthop. 2015;39:2335-2339. [DOI] [PubMed] [Google Scholar]
- 8.Figved W, Dybvik E, Frihagen F, Furnes O, Madsen JE, Havelin LI, Nordsletten L. Conversion from failed hemiarthroplasty to total hip arthroplasty: a Norwegian Arthroplasty Register analysis of 595 hips with previous femoral neck fractures. Acta Orthop. 2007;78:711-718. [DOI] [PubMed] [Google Scholar]
- 9.Haidukewych GJ, Israel TA, Berry DJ. Long-term survivorship of cemented bipolar hemiarthroplasty for fracture of the femoral neck. Clin Orthop Relat Res. 2002:118-126. [DOI] [PubMed] [Google Scholar]
- 10.Ray NF, Chan JK, Thamer M, Melton LJ., 3rd Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24-35. [DOI] [PubMed] [Google Scholar]
- 11.Roche JJ, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331:1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sah AP, Estok DM., 2nd Dislocation rate after conversion from hip hemiarthroplasty to total hip arthroplasty. J Bone Joint Surg Am. 2008;90:506-516. [DOI] [PubMed] [Google Scholar]
- 13.Schwarzkopf R, Baghoolizadeh M. Conversion total hip arthroplasty: Primary or revision total hip arthroplasty. World J Orthop. 2015;6:750-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarzkopf R, Chin G, Kim K, Murphy D, Chen AF. Do conversion total hip arthroplasty yield comparable results to primary total hip arthroplasty? J Arthroplasty. 2017;32:862-871. [DOI] [PubMed] [Google Scholar]
- 15.Sierra RJ, Cabanela ME. Conversion of failed hip hemiarthroplasties after femoral neck fractures. Clin Orthop Relat Res. 2002:129-139. [DOI] [PubMed] [Google Scholar]
- 16.Visscher SL, Naessens JM, Yawn BP, Reinalda MS, Anderson SS, Borah BJ. Developing a standardized healthcare cost data warehouse. BMC Health Serv Res. 2017;17:396. [DOI] [PMC free article] [PubMed] [Google Scholar]