Abstract
Background
Destructive bony acetabular metastases cause pain, pathological fractures, and loss of mobility. Although multiple fixation options are available, we have favored a rigid stainless steel partial pelvic cage for acetabular fixation in these patients; however, little is known about the durability of this approach.
Question/purposes
(1) How common was loss of fixation in a small series of metastatic acetabular defects treated with an acetabular cage and cemented total hip replacement? (2) What is the implant survival free from reoperation or revision at 2 and 4 years using a competing-risks survivorship estimator in patients thus treated? (3) What complications were associated with the treatment? (4) What level of postoperative mobility was achieved?
Methods
Between 2006 and 2017, we treated all acetabular metastases that needed surgical intervention, not amenable to conventional cemented THA alone with our single technique of acetabular partial pelvic cage and cemented total hip replacement. We treated 47 hips in 46 patients whose acetabular metastasis led to acetabular collapse or who were unresponsive to nonoperative measures of radiation therapy and analgesia. Routine followup occurred at 3 and 12 months; 17 of 46 patients (37%) died before 1 year, and all other patients were followed beyond 1 year. Only one patient who remains alive has not been seen in the past 5 years. Loss of fixation was determined by radiological or clinical signs of cage loosening. Survivorship free from reoperation or revision at 2 and 4 years was determined using competing-risks analysis. We did not assess patient-reported outcomes, but we did have data on the proportion of patients who were able to ambulate in the community and if so, what assistive devices they used, which we obtained by chart review.
Results
One patient experienced cage loosening identified 8 years postoperatively as a result of local disease progression and has been managed with observation. No patients underwent revision for loss of acetabular fixation. The cumulative incidence of reoperation or revision was 8% at 2 years (95% CI, 3.6–12.6) and 16% at 4 years (95% CI, 9.2–23.2). Four patients had postoperative dislocations, of which three underwent reoperation. One patient developed a postoperative deep infection and underwent reoperation. One patient died within 30 days of surgery. Only one patient did not ambulate in the community postoperatively; 23 ambulated independently, 10 with the use of a walking stick and 12 using a walker.
Conclusions
In this small series, we found this approach sufficiently durable to continue its use for patients with acetabular metastases with collapse or those not responding to nonoperative measures. However, comparison studies are needed to determine whether it is superior or inferior to other available alternatives.
Level of Evidence
Level IV, therapeutic study.
Introduction
With improvements in adjuvant treatments, patient survival is increasing, as are the functional demands in patients with metastatic carcinoma to bone. Bone is the third-most-common site of metastasis, after the lung and liver [6], and although the spine and long bone metastases predominate, acetabular metastases are not uncommon in busy orthopaedic oncology practices. They are often associated with pathological fractures and central femoral head migration that results in pain and loss of patient mobility. Surgical options for acetabular metastases are extensive and have included both biological and nonbiological reconstructions [3, 8, 11, 12, 14, 15, 18-20, 23, 26-28]. Biological fixation techniques can lead to delayed weightbearing and reduced osteointegration in the setting of a commonly radiated field [12, 17, 29]. Harrington [11] described his nonbiological technique in 1981. Extensive antegrade and retrograde modifications of this technique have been described since [3, 14, 18-20, 27]. Shortcomings of these techniques have included prosthetic dislocation (0%–20%), difficulty of pin placement, and perioperative death rates of up to 6% [20]. Alternative nonbiological methods such as custom prosthesis and pedestal cup fixation have seen high rates of infection (up to 30%), dislocation (20%) and reoperation (40%) [1, 7, 13, 16]. Many of these studies have included both primary and secondary acetabular pathology. Studies specific to acetabular metastases have been limited by small sample sizes (range, 19–81) [3, 8, 11, 14, 15, 18-20, 27, 28].
The reported shortcomings of other procedures led us to believe that immediate weightbearing, nonbiological fixation with a greater surface area, and dispersion of fixation with ischial fixation are key concepts in surgical management of acetabular metastases. These goals led us to the technique of acetabular cage fixation with cemented total hip replacement. The relative success of antiprotrusio cages and retrograde Steinman pin fixation [15, 28] has been reported in an acetabular metastases population, however, augmentation with Steinman pin fixation adds additional complexity to surgical intervention. Alternative protusio cage techniques have been used without Steinman pin augmentation [8] in small cohorts with modest results. Concern regarding acetabular cage mechanical failure in the treatment of acetabular defects [4, 5, 9, 10, 22, 25] led us to use the more rigid, greater flange surface area with ischial screw fixation, partial pelvic cage (LINK, Hamburg, Germany). To our knowledge this is the only published case series using this specific technique.
Therefore, we asked: (1) How common was loss of fixation in a small series of metastatic acetabular defects treated with an acetabular cage and cement fixation? (2) What is the implant survival free from reoperation or revision at 2 and 4 years using a competing-risks survivorship estimator in patients thus treated? (3) What complications were associated with the treatment? (4) What level of postoperative mobility was achieved?
Patients and Methods
We conducted a retrospective study of a single technique for acetabular metastasis reconstruction. There were 47 procedures performed in 46 patients treated between 2006 and 2017. All procedures were performed using the partial pelvis replacement cage by one of two surgeons (ID, SS) who work at the same two institutions, one private and one public. Institutional review board approval was obtained prior to study initiation (HREC/17/QPAH/240).
Inclusion criteria were previously mobile patients with a destructive periacetabular metastasis who experienced pain upon or were unable to bear weight. During the study period, all patients who met inclusion criteria and who were not amenable to conventional cemented THA were treated with this approach. No additional surgical techniques for acetabular metastases were used during this period by the two senior authors (ID, SS). No patients were deemed unsuitable for this technique, such that they underwent alternative methods of acetabular reconstruction.
We identified 19 patients with Harrington [11] type 2 lesions (41%) and 27 (59%) with Harrington type 3 lesions; one patient was treated for bilateral Harrington type 2 lesions. Sixteen patients (35%) had acetabular collapse or fracture with superior or medial femoral bead migration. Three patients had concomitant lesions or fracture of the proximal femur, all of these were within the head and neck region. Fourteen patients had preoperative radiotherapy (30%). The remaining patients were all referred for postoperative radiation, which was given to all but three patients.
Of the 46 acetabular metastasis patients, two had revisions of alternative fixation methods for metastatic acetabular disease. Both patients were referred after pedestal cup fixation procedures and had early fixation failures. An additional patient who underwent a total hip replacement at another institution that was subsequently failed because of unrecognized acetabular disease and progression was included in our study. For the purposes of assessing this fixation technique, we included 11 patients with multiple myeloma in this series.
We used a standard extensile posterior approach to the hip. Additional dissection to identify the ischial tuberosity for posterior column fixation was needed. We routinely released the gluteus maximus tendon and extended the hip with the knee flexed while exposing the ischial tuberosity to reduce sciatic nerve tension. Once the acetabulum was exposed and reamed, meticulous intralesional curettage was performed until cancellous bone was reached. In all patients, we used the LINK partial pelvis replacement cage (LINK, Hamburg, Germany). It is a stainless-steel cage with two cranial flanges and a single caudal flange that allows iliac and ischial fixation. The cage is 3 mm thick with the ability to use either 4.5-mm cortical or 6.5-mm cancellous screws. Once the cage was secured, with fixation into the ischium and the ilium, the acetabular defect was filled with polymethylmethacrylate cement. We consider it imperative that cement completely fills the defect. This technique was performed using a thinner revision hip nozzle on the cement gun to allow penetration through the cage holes or the adjacent bony defect. Digital pressure over the remaining defects and the use of an acetabular cement pressurizer permitted pressurization with cementation.
A cemented hooded polyethylene cup was used in all but two patients, in whom cemented constrained cups (Stryker Corp, Mahwah, NJ, USA) were used. They were inserted using a single-stage cementation technique. If a 57-mm cage or larger was inserted, we used a Contemporary Cup (Stryker Corp, Mahwah, NJ, USA) with a 32-mm head. However, if we used a 53-mm cage, a LINK cup was used to accommodate a 32-mm head. We used a 32-mm head in 38 of 47 patients. For the remaining nine patients, in which we were unable to insert a 53-mm or larger cage, we inserted a 28-mm head into a Contemporary Cup. We used a standard highly polished tapered cemented femoral stem (Stryker Corp, Mahwah, NJ, USA) in all but two patients. All patients were allowed immediate postoperative weightbearing as tolerated. Preoperative embolization was used routinely for patients with renal and thyroid primary malignancies.
Routine followup occurred at 3 and 12 months, and thereafter was individualized to the patient depending on their travelling distance, adequacy of local services, the patient’s general condition, and their treatment needs from other specialties. In addition to our own records, we obtained data from the Australian Orthopaedic Association National Joint Replacement Registry to identify any revision procedures that may have been performed elsewhere. Information from the Department of Births, Deaths and Marriages was used in conjunction with our own medical records to confirm patient survival data. Seventeen of 46 patients (37%) died before 1 year. Twenty-nine patients underwent clinical review at 1 year. No patients were lost to followup at 1 year. One patient who remains alive has not been reviewed in the past 5 years.
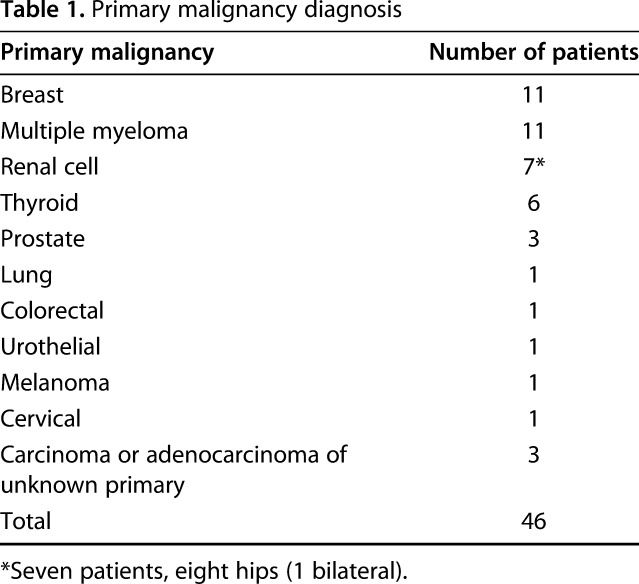
We performed 47 acetabular procedures that met treatment criteria in 46 patients, one with bilateral disease (Fig. 1). The average patient age was 65 years (range, 29–84 years). There were 26 women and 20 men. The most commonly treated primary pathologies were breast cancer and multiple myeloma (Table 1). At the time of this review, 21 patients remain alive (46%), and six patients have survived beyond 5 years. Of the 25 deceased patients, median survival was 9 months (range, 2–8 years).
Fig. 1.
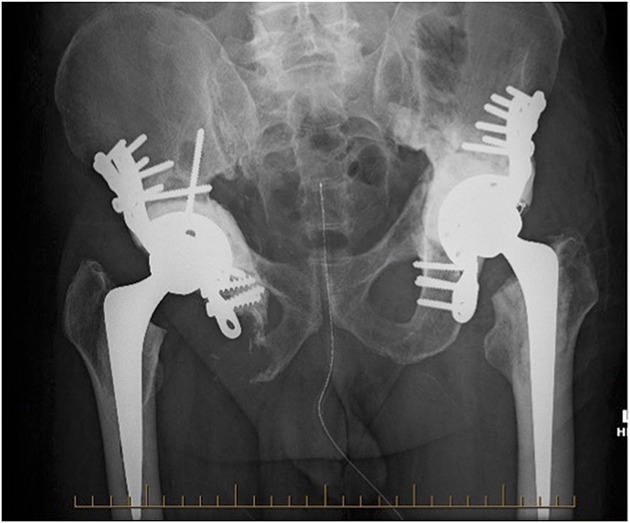
Bilateral acetabular cage fixation in a 67-year-old man with metastatic renal cell carcinoma. The radiograph was taken 12 months after right and 3 months after left acetabular reconstructions.
Table 1.
Primary malignancy diagnosis
Loss of acetabular fixation was determined by radiological or clinical signs of fixation loosening. Major surgical complications, including infection, dislocation, nerve injury, unexpected return to theater, symptomatic heterotopic ossification, massive transfusion protocol, symptomatic pulmonary embolism and perioperative death within 30 days were recorded via routine patient monitoring and followup. The patients’ mobility status at presentation and postoperative weightbearing status within 3 months was recorded (Table 2). We did not assess patient-reported outcomes, but we collected data on the proportion of patients who were able to ambulate in the community and if so, what assistive devices they used, which we obtained by chart review. Survivorship free from reoperation or revision at 2 and 4 years was determined using competing-risks analysis.
Table 2.
Pre- and postoperative weightbearing status
Results
In our series, no patient underwent revision for loss of acetabular fixation. One patient had local disease progression with radiological loss of screw fixation; however, the patient is still able to walk in the community 8 years after surgery with a single point stick and is not currently seeking revision.
Using revision or reoperation as the endpoint for survival (four events) and death as the competing event, we performed a competing risk analysis. The cumulative incidence of reoperation or revision was 8% at 2 years (95% CI, 3.6–12.6) and 16% at 4 years (95% CI, 9.2–23.2) (Fig. 2).
Fig. 2.
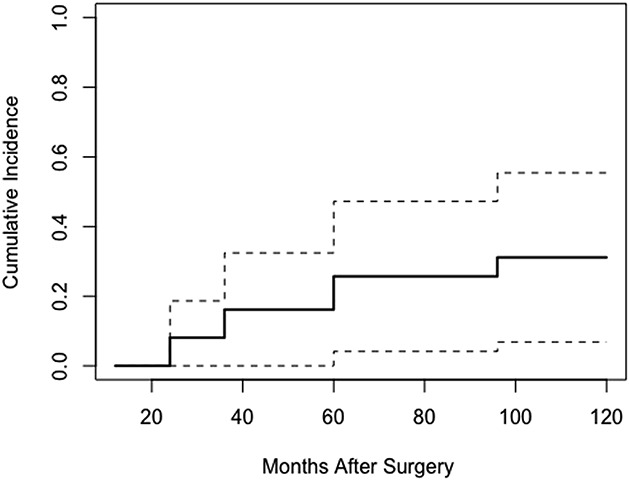
Cumulative incidence curve based on competitive risk function for death (solid line) with 95% confidence interval (dotted lines).The cumulative incidence of reoperation or revision using death as the competing risk is 8% (95% CI, 3.6–12.6) at 2 years (24 months) and 16% (95% CI, 9.2–23.%) at 4 years (48 months).
Major complications of the partial pelvic cage procedures included four dislocations, one infection, one partial sciatic nerve palsy that resolved spontaneously, one massive transfusion protocol (despite preoperative embolization), one symptomatic pulmonary embolism and one perioperative death within 30 days. No patients developed symptomatic heterotopic ossification. Of the four patients who had a dislocation (four of 46, 9%), one was successfully treated with single closed reduction, one was revised to a constrained cup alone, one underwent open reduction and reinsertion of the tapered stem, and one underwent a cement-in-cement revision and conversion to cemented constrained cup. One patient with myeloma treated with preoperative radiation developed a radiation-induced osteosarcoma diagnosed 6 years after acetabular cage fixation. After diagnosis, this patient underwent neoadjuvant chemotherapy and proceeded to hindquarter amputation 6 years after acetabular reconstruction. This patient is alive 4 years postamputation. We do not consider this a failure of the fixation method.
Of the 46 patients in this study, 45 were able to ambulate in the community postoperatively (Table 2). Twenty-three patients (50%) were able to do so without aids in the community within 3 months, 10 with the use of a walking stick (22%) and 12 using a walker (26%). The patient who died within 30 days postoperatively was the only one who did not mobilize in the community.
Discussion
Destructive bony metastases of the acetabulum are not uncommon and can cause substantial pain, pathological fractures, and loss of mobility. Multiple biological and nonbiological reconstruction techniques have been described in the evidence [3, 8, 11, 12, 14, 15, 18-20, 23, 26-28]; however, we have favored a rigid stainless steel partial pelvis cage for acetabular fixation in these patients because it allows immediate weightbearing and offers a greater surface area and dispersion of fixation with ischial fixation. Our technique in this small series has identified no revisions for loss of fixation, shown a small but substantial number of major complications, and helped restore community ambulatory function in most patients. This is a relatively safe, straightforward technique with durable fixation that can be used in diverse acetabular defects and on occasion used when other techniques would be challenging (Fig. 3A-C).
Fig. 3 A-C.
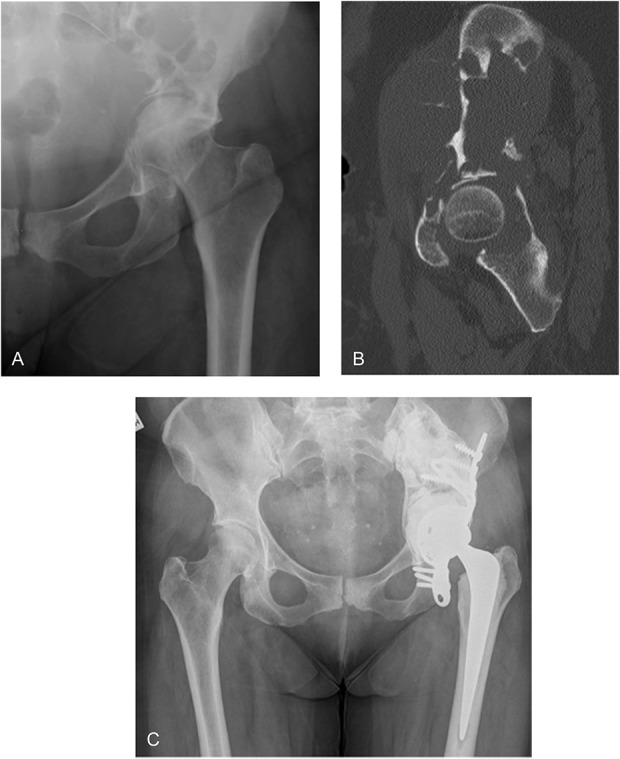
A 56-year-old woman with multiple myeloma showing large acetabular and iliac defect with protrusio. (A) The preoperative radiograph shows medial femoral head migration. (B) The sagittal CT displays a destructive iliac lesion. (C) The 4-year postoperative radiograph demonstrates stable fixation.
This study had several limitations. First, data was collected retrospectively by authors predominantly via chart review, which may subject results to assessment bias. Second, selection bias is a factor because data was collected on operatively treated acetabular metastasis patients only. We did not use strict radiographic parameters for those undergoing surgery, but rather, in discussion with our multidisciplinary team, offered the procedure to patients with pain upon or inability to bear weight who had periacetabular bone loss and a life expectancy greater than 3 months. However, among those patients, we used this approach exclusively during the study period. Further, minimum followup of 12 months and individualized followup thereafter due in part to vast geographic catchment area (range, 1–10 years) allows the possibility of transfer bias, with patient complications or symptomatic loosening being missed and the possibility of asymptomatic loosening being undetected. As the only musculoskeletal oncology service for 600 miles, we believe the risk of patients being lost to other services is reduced; however, we caution the reader to interpret our findings in light of this fact. This study was relatively small; suggesting any of a number of less-common complications might not have been detected, and our point estimates of the frequency of complications are imprecise, and would be expected to have wide confidence intervals. Lastly, there are numerous confounding variables in a metastatic carcinoma or myeloma patients that are unrelated to acetabular surgery that may affect patient outcomes and functional capacity.
In this small series, we had one radiological loss of fixation. No patient underwent revision for loss of fixation. Extensive destruction of the ischium and/or extensive proximal ilium bone loss represent a potential limitation to fixation with acetabular cages. We did not identify any patients with acetabular metastasis in our series who were deemed unsuitable for the partial pelvic cage construct, who underwent other techniques or refused surgery. We noted important design differences between the LINK partial pelvis cage and other previously reported antiprotrusio constructs [8, 15]. In comparison with its typically titanium counterparts, the stainless-steel LINK cage is thicker, more rigid, and its three long flanges enable increased surface area and dispersion of fixation, including ischial screw fixation.
Survivorship free from all-cause revision or reoperation was 91% (95% CI, 83.2–99.4) in our small series. The cumulative incidence of reoperation or revision using death as the competing event was 8% at 2 years (95% CI, 3.6–12.6) and 19% (95% CI, 9.2–23.2) at 4 years. We found no comparative studies reporting competing risk analysis in patients with acetabular metastases.
Serious complications occurred, although since this is a small group of patients widely distributed geographically, we may have missed some complications. The most frequent complication we identified was dislocation. Four of 46 patients (9%) suffered a postoperative dislocation, falling within the range reported using the modified Harrington procedures (0%–20%) [3, 8, 11, 14, 15, 18-20, 27, 28]. The causes are likely multifactorial. Head size and cup positioning were somewhat dictated by cage size and position, the patients were often frail and weak, and abductor function could be further affected by exposure of the iliac bone and potential injury to the superior gluteal nerve. Constrained liners and dual mobility cups have been used in both acetabular metastases and primary malignancy in an attempt to reduce dislocation rates with variable reported proportions of dislocation (0%–10%) [2, 23]. Results in acetabular metastases patients are encouraging [2], and there are potential benefits of higher constraint in this lower function population, but we are yet to identify strong evidence to employ a routine change of practice. We placed great emphasis on adequate ischial fixation in this technique, believing this to be one of the key factors in fixation when comparing with other cage constructs, reinforcement rings, and Harrington procedures. There have been concerns regarding the risk of sciatic nerve injury with such ischial fixation [9, 21, 24] with proportions of nerve injury up to 10%. We attribute our low proportion of sciatic nerve palsy to the following: the fact that this was a primary procedure in most patients, the care that was taken to extend the hip and flex the knee intraoperatively, and the routine release of gluteus maximus tendon.
At or within 3 months postoperatively, half of our patients could ambulate in the community independently, 72% with a single stick or less, and 98% were able to function in the community with a walker or less. These results are encouraging when compared with other papers that reported community function [11, 19, 26] using mobility assessment with a range of 58% to 84%, however, multiple fixation techniques were used in some of these papers [19, 26].
In summary, the use of a rigid stainless steel partial pelvic cage and cemented total hip replacement in the treatment of metastatic acetabular bone disease is sufficiently durable to justify its continued use. There were no revisions due to fixation failure. Early return to community function was recorded in most patients. There was a relatively small proportion of major complications, of which dislocation was the most notable. Comparison studies of the various reconstruction techniques and levels of constraint would help further guide surgeons in this challenging patient population.
Acknowledgments
We thank Dr. S. Whitehouse, a senior research fellow at Queensland University of Technology, for her assistance in statistical analysis.
Footnotes
Each author certifies that neither he, nor any member of his or her immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at both The Princess Alexandra Hospital, Woolloongabba, Queensland, Australia, and Wesley Hospital, Auchenflower, Queensland, Australia.
References
- 1.Abudu A, Grimer R, Cannon S, Carter S, Sneath R. Reconstruction of the hemipelvis after the excision of malignant tumors. J Bone Joint Surg Br. 1997;79:773-779. [DOI] [PubMed] [Google Scholar]
- 2.Bagsby DT, Wurtz LD. Effectiveness of constrained liner use during Harrington hip reconstruction in oncology patient. J Arthroplasty. 2017;32:1250-1254. [DOI] [PubMed] [Google Scholar]
- 3.Bernthal NM, Price SL, Monument MJ, Wilkinson B, Jones KB, Randall RL. Outcomes of modified Harrington reconstructions for non-primary peri-acetabular tumors: an effective and inexpensive technique. Ann Surg Oncol . 2015;22:3921-3928. [DOI] [PubMed] [Google Scholar]
- 4.Berry DJ. Antiprotrusio cages for acetabular revision. Clin Orthop Relat Res . 2004; 420: 106-112. [DOI] [PubMed] [Google Scholar]
- 5.Berry DJ, Muller ME. Revision arthroplasty using an anti-protrusio cage for massive acetabular bone deficiency. J Bone Joint Surg Br. 1992; 74: 711-715. [DOI] [PubMed] [Google Scholar]
- 6.Boyer MI. AAOS Orthopedic Comprehensive Review. Vol 1 2nd ed Rosemont: AAOS; 2014:581. [Google Scholar]
- 7.Bus MPA, Boerhout EJ, Bramer JAM, Dijkstra PDS. Clinical outcome of pedestal cup endoprosthetic reconstruction after resection of a peri-acetabular tumor. Bone Joint J. 2014;96:1706–1712. [DOI] [PubMed] [Google Scholar]
- 8.Clayer M: The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop Relat Res. 2010;468:2980-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman S, Saastamoinen H, Shasha N, Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19:436-446. [DOI] [PubMed] [Google Scholar]
- 10.Hansen E, Shearer D, Ries M. Does cemented cage improve revision THA for severe acetabular defects? Clin Orthop Relat Res . 2011;469:494-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg. 1981;63:653-664. [PubMed] [Google Scholar]
- 12.Harrington KD. The use of hemipelvic allografts or autoclaved grafts for reconstruction after wide resections of malignant tumors of the pelvis. J Bone Joint Surg. 1992;74:331. [PubMed] [Google Scholar]
- 13.Hipfl C, Stihsen C, Puchner SE, Kaider A, Dominkus M, Funovics PT, Windhager R. Pelvic reconstruction following resection of malignant bone tumours using a stemmed acetabular pedestal cup. Bone Joint J . 2017;99,841-848. [DOI] [PubMed] [Google Scholar]
- 14.Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol . 2010;101:170-174. [DOI] [PubMed] [Google Scholar]
- 15.Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg . 2012;132:405–410. [DOI] [PubMed] [Google Scholar]
- 16.Jaiswal PK, Aston WJS, Grimer RJ, Abudu A, Carter S, Blunn G, Briggs TW, Cannon S. Peri-acetabular resection and endoprosthetic reconstruction for tumors of the acetabulum. J Bone Joint Surg Br . 2008;90:1222–1227. [DOI] [PubMed] [Google Scholar]
- 17.Krieg AH, Mani M, Speth BM, Stalley PD. Extracorporeal irradiation for pelvic reconstruction in Ewing’s sarcoma. J Bone Joint Surg . 2009;91:395–400. [DOI] [PubMed] [Google Scholar]
- 18.Kunisada T, Choong PF. Major reconstruction for periacetabular metastasis: early complications and outcome following surgical treatment in 40 hips. Acta Orthop Scand. 2000;71:585-590. [DOI] [PubMed] [Google Scholar]
- 19.Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am . 2000;82:642–651. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson J, Gustafson P, Fornander E, Ornstein E. The Harrington reconstruction for advanced periacetabular metastatic destruction. Acta Orthop Scand , 2000;71:591–596. [DOI] [PubMed] [Google Scholar]
- 21.Oldenburg M, Muller RT. The frequency, prognosis and significance of nerve injuries in total hip arthroplasty. Int Orthop. 1997;21:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paprosky WG, Sporer SS, Murphy BP. Addressing severe bone deficiency: what a cage will not do. J Arthroplasty . 2007;22:111-115. [DOI] [PubMed] [Google Scholar]
- 23.Philippeau JM, Durand JM, Carret JP, Leclercq S, Waast D, Gouin F. Dual mobility design use in preventing total hip replacement dislocation following tumor resection. Orthop Traumatol Surg Res. 2010;96:2-8. [DOI] [PubMed] [Google Scholar]
- 24.Schmalzried TP, Amstutz HC, Dorey FJ. Nerve palsy associated with total hip replacement. Risk factors and prognosis. J Bone Joint Surg Am. 1991;73:1074-1080. [PubMed] [Google Scholar]
- 25.Sembrano JN, Cheng EY. Acetabular cage survival and analysis of factors related to failure. Clin Orthop Relat Res . 2008;466:1657-1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahid M, Saunders T, Jeys L, Grimer R. The outcome of surgical treatment for peri-acetabular metastases. Bone Joint J. 2014;96:132-136. [DOI] [PubMed] [Google Scholar]
- 27.Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90:84-87. [DOI] [PubMed] [Google Scholar]
- 28.Tsagozis P, Wedin R, Brosjo O, Bauer H. Reconstruction of metastatic acetabular defects using a modified Harrington procedure. Acta Orthop . 2015;86:690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wafa H, Grimer RJ, Jeys L, Abudu AT, Tillman RM, Carter SR. The use of extracorporeally irradiated autografts in pelvic reconstruction following tumour resection. Bone Joint J . 2014;96:1404–1410. [DOI] [PubMed] [Google Scholar]