Abstract
Background
Although the use of thromboprophylaxis is well established, there is no consensus on the preferred thromboprophylaxis regimen after THA; large, population-based studies offer an opportunity to examine this problem in a robust way that can complement results from randomized trials.
Questions/purposes
Using data from a large national registry, we asked: (1) Is there any difference between low-molecular weight heparin (LMWH) and new oral anticoagulants in preventing symptomatic deep vein thrombosis (DVT) and pulmonary embolism (PE), after THA? (2) Are there any differences in safety parameters, such as bleeding, reoperations and mortality, between LMWH and new oral anticoagulants?
Methods
Between 2008 and 2012, 78,066 THAs were performed in Sweden. This study evaluated 32,663 (42%) of them, selected through the merger of several national registries. These patients underwent unilateral THA due to primary osteoarthritis. They had not experienced any venous thromboembolic events 5 years before the index operation and were not prescribed potent antithrombotic agents, of any type, in the 6 months before the index operation. Additionally, their postoperative thromboprophylaxis was confirmed in a national registry by purchase of prescribed medications. We divided the cohort into two groups: those patients who received new oral anticoagulants (5752, 18%) and those who received LMWH (26,881, 82%) as postoperative thromboprophylaxis. Our primary endpoints were the frequencies of symptomatic DVT and symptomatic PE within 3 months of surgery. Our secondary comparison was a between-group comparison of bleeding (by way of diagnostic coding), reoperation, and mortality within 3 months of surgery. Odds ratios (OR) are presented with 95% confidence intervals (CIs) as pooled results for the two groups after adjustment for duration of thromboprophylaxis (short or extended for at least 28 days), year of the index operation, Elixhauser comorbidity index, sex, age and previous treatment with platelet aggregation inhibitors.
Results
The risk of symptomatic DVT was lower in the group that received new oral anticoagulants than the group that received LMWH (0.3% versus 0.6%, OR, 0.47; 95% CI, 0.27–0.76; p = 0.026). The risk of symptomatic PE was lower in the group that received new oral anticoagulants than the group that received LMWH (0.1% versus 0.4%, OR, 0.36; 95% CI, 0.16–0.69; p = 0.005). There was no difference in the risk of bleeding (by way of diagnostic coding) (OR, 1.03; 95% CI, 0.82–1.28; p = 0.688), reoperation (OR, 1.02; 95% CI, 0.71–1.44; p = 0.860) or mortality (OR, 0.83; 95% CI, 0.31–1.88; p = 0.883) between groups.
Conclusions
New oral anticoagulants were associated with a lower risk of symptomatic DVT and symptomatic PE in this large, registry study, and we observed no differences in the risk of bleeding, reoperation, or death between the groups. Although we were able to control for a number of potential confounding variables, we cannot ascertain the indications that drove the prescription decisions in this setting, and there were important between-group differences in terms of duration of thromboprophylaxis (new oral anticoagulants generally were used for a longer period of time after surgery). Future studies, preferably large randomized trials with pragmatic inclusion criteria, to analyze symptomatic DVT, symptomatic PE and death are needed to confirm or refute our findings.
Level of Evidence
Level III, therapeutic study.
Introduction
THA is a common orthopaedic operation that carries a well-documented risk of venous thromboembolism, one of the most frequent medical complications [20]. Current studies report an incidence of symptomatic venous thromboembolism, including deep venous thrombosis (DVT) and pulmonary embolism (PE), within 90 days postoperative of between 0.7% to 1.7% in patients with postoperative thromboprophylaxis [20, 22, 27]. The use of thromboprophylaxis is established and recommended by guidelines worldwide [6, 17], but there is no consensus regarding the length of treatment and the preferred therapeutic agent.
The most commonly used thromboprophylactic medications after THA include aspirin, warfarin, fondaparinux, low-molecular weight heparins (LMWH), and new oral anticoagulants. The introduction of new oral anticoagulants represents the most recent development in thromboprophylaxis, further complicating the debate surrounding the drug of choice. New oral anticoagulants have demonstrated efficacy as thromboprophylaxis in several clinical trials [3-5, 12, 25] and meta-analyses [7, 15, 18, 26]. Compared with LMWH, they also offer reduced overall costs due to oral administration [8] and patients prefer them [29]. However, concerns have been raised regarding prolonged wound drainage [11] and an increased risk of bleeding complications [9, 19, 25].
The aim of this Swedish Hip Arthroplasty registry study was to analyze thromboprophylaxis after THA and address the following questions: (1) Is there any difference between LMWH and new oral anticoagulants in preventing symptomatic DVT and PE after THA? (2) Are there any differences in safety parameters, such as bleeding, reoperations and mortality, between LMWH and new oral anticoagulants?
Patients and Methods
Registries
The unique Swedish personal identity number facilitates linkage-studies between population and health data registries. This study is based on a previously crosslinked registry database, initiated by the Swedish Hip Arthroplasty Register [1]. All orthopaedic units report operations to the register, resulting in a completeness of more than 98% [13]. The Swedish National Patient Register, with a completeness of about 90%, registers both hospital discharges and hospital-based outpatient visits [16] and contributes with diagnoses according to the coding of the 10th revision of the International Classification of Diseases (ICD-10). All deaths are recorded by the Swedish Death Register and the Swedish Prescribed Drug Register registers all prescribed outpatient drugs in Sweden through automatic reporting by the pharmacies [28] and provided data on the studied drugs.
Patient Selection
From this merged dataset we extracted all 78,066 primary THAs performed between 2008 and 2012 (Fig. 1). This 5-year span was chosen to match the introduction of new oral anticoagulants as postoperative thromboprophylaxis and to include sufficient number of patients. We included only the first hip of patients who underwent bilateral procedures during the study period (n = 7105), which allowed for better tracking of prescriptions and complications, as well as exclusion of possible additive effect of a second operation. Subsequently, we excluded all patients with indications other then primary osteoarthritis (n = 12,186). In addition, we excluded patients who were diagnosed with any venous thromboembolism up to 5 years before their operation (n = 1121) to limit any effect of previous conditions on studied outcome.
Fig. 1.
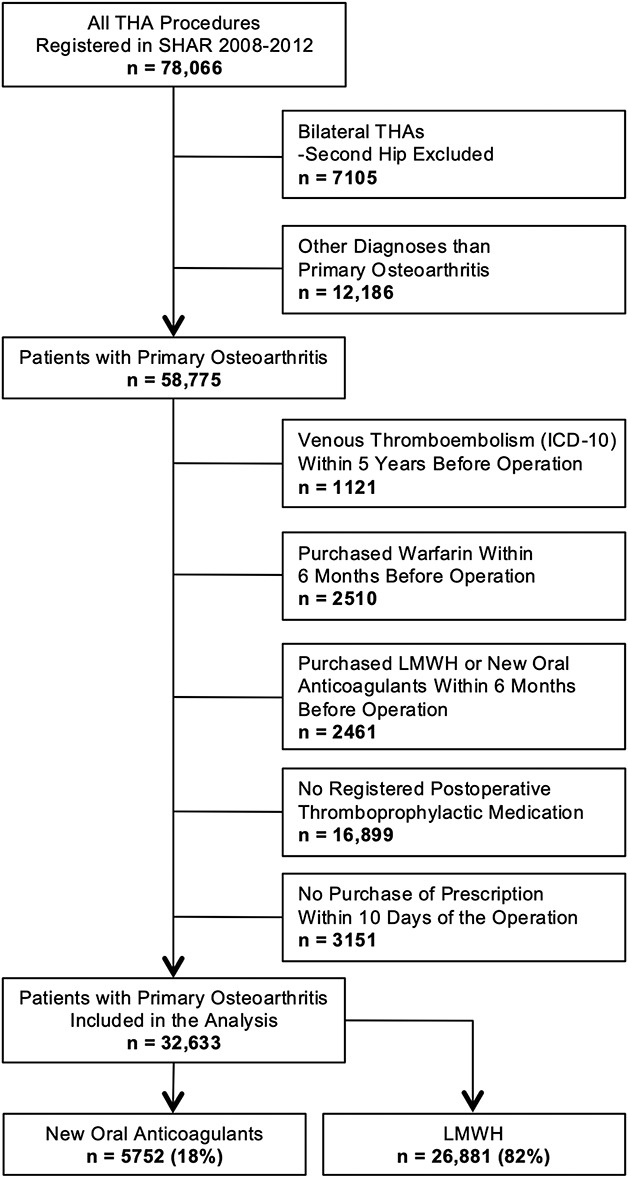
The staged patient selection is summarized by this flowchart; SHAR = Swedish Hip Arthroplasty Register; LMWH = low molecular weight heparin.
To reduce bias from ongoing or recent potent antithrombotic agents at time of surgery, we also excluded patients who had been prescribed warfarin, LMWH and new oral anticoagulants, up to 6 months before the THA (n = 4971). Nonsteroidal anti-inflammatory drugs and acetylsalicylic acid are available as over-the-counter drugs, and if not prescribed, are not registered in the Swedish Prescribed Drug Register. Therefore, this was not possible to control, and we did not include these medications in our patient selection or analysis.
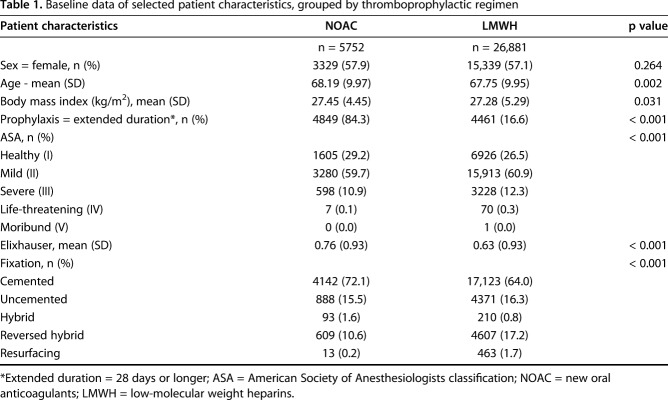
Subsequently, patients with no data on expedited postoperative thromboprophylactic treatment (n = 16,899) were excluded. This is mainly caused by a deviation in routines in Swedish hospitals. We could only quantify medication for those patients who received a prescription, because patients who were provided with medication directly by the hospital (thus without a prescription) do not appear in the Swedish Prescribed Drug Register. A subanalysis revealed that most patients purchased the prescribed thromboprophylaxis medications on the third to fifth day after the index operation. We decided that setting a cutoff of purchase within 10 postoperative days would limit the inclusion of high-risk patients by excluding those who, possibly due to comorbidities, required longer hospital care (n = 3151). Ultimately, 32,663 patients who underwent a primary THA procedure were included in this study, 5752 (18%) patients received new oral anticoagulants and 26,881 (82%) received LMWH (Table 1).
Table 1.
Baseline data of selected patient characteristics, grouped by thromboprophylactic regimen
Study Variables
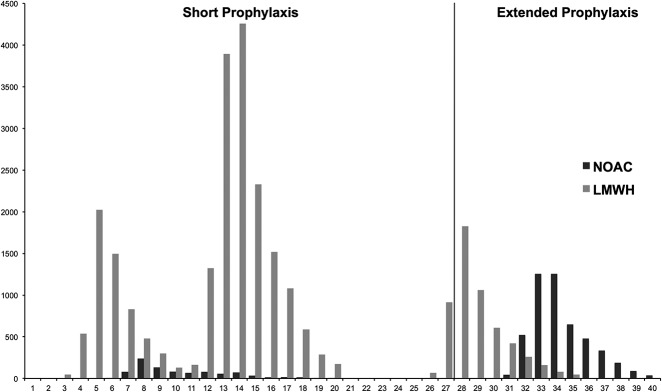
We recorded sex, age at index operation, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, Elixhauser comorbidity index [23], and type of implant fixation for each patient from the previously described cross-matched database. Additionally, we observed a variation of the length of thromboprophylaxis between hospitals. As thromboprophylaxis is started within the initial postoperative hours, the variable of duration of thromboprophylaxis was calculated as the sum of postoperative hospital stay and the amount of prescribed outpatient daily doses. If the patients were prescribed treatment for less than 28 days, we defined thromboprophylaxis as short. Consequently, we classified thromboprophylaxis of 28 days or longer as extended [17, 21]. We then divided the thromboprophylaxis regimen into two groups, LMWH and new oral anticoagulants. Most patients with new oral anticoagulants were prescribed extended thromboprophylaxis (84%), while LMWH was predominantly used as short thromboprophylaxis (83%) (Fig. 2).
Fig. 2.
The bar chart is illustrating the distribution of patients treated with new oral anticoagulants or LMWHs according to duration of thromboprophylaxis in days; LMWH = low molecular weight heparins; NOAC = new oral anticoagulants.
From the Swedish Prescribed Drug Register, we extracted the corresponding Anatomical Therapeutic Chemical (ATC) codes and included three drugs from the LMWH group: dalteparin, enoxaparin, and tinzaparin. The treatment regimen was one daily subcutaneous dose of 5000 international units (IUs) of dalteparin, 4000 IUs of enoxparin, and 4500 IUs of tinzaparin. The studied and approved new oral anticoagulants for postoperative thromboprophylaxis during our time period were a twice-daily 110-mg oral dose of dabigatran and once-daily, 10-mg oral dose of rivaroxaban (see Table, Supplemental Digital Content 1, http://links.lww.com/CORR/A159).
We did not analyze any other thromboprophylaxis because there are no treatment recommendations in Sweden that advocate simultaneous prescription of antiplatelet drugs. Patients who were prescribed these drugs preoperatively continued them postoperatively as well. Any prescriptions of platelet aggregation inhibitors 6 months before the index operation were recorded as confounders (see Table, Supplemental Digital Content 2, http://links.lww.com/CORR/A160).
The use of mechanical prophylaxis is not well established in relation to hip or knee arthroplasty in Sweden, and it is not recorded in our database.
The occurrence of all events was studied up to 90 days postoperatively. This timeline was based on previous findings of time course of thromboembolic events after joint replacement surgery [27].
Our primary outcomes of symptomatic DVT and PE, as well as the secondary outcome of bleeding were analyzed through the corresponding ICD-10 codes from the Swedish National Patient Register. Because there are no recognized standards of reporting bleeding complications, we chose a deliberately wide-ranging definition. We made no distinction between major and minor bleeding episodes, and we included every code that resulted in the inclusion of possible bleeding complication (see Table, Supplemental Digital Content 3, http://links.lww.com/CORR/A161). No national registers record decreases in hemoglobin levels or quantify administered units of blood through which severity of bleeding could be assessed. We decided not to categorize bleeding into major and minor as we had no access to individual medical records and grouping only by location would yield controversies. The secondary outcome of reoperation was extracted from the Swedish Hip Arthroplasty Register and mortality data from the Swedish Death Register.
Although the ASA class was lower (p < 0.001) in the group treated with new oral anticoagulants, the mean Elixhauser comorbidity index was higher (p < 0.001) (Table 1). Individuals with new oral anticoagulants were more frequently treated with cemented fixation. The distribution of sex, mean age, and BMI were not different between groups (Table 1).
As dabigatran and rivaroxaban were approved for postoperative thromboprophylaxis in 2008 in Europe, the new oral anticoagulants prescription in the study’s initial 2 years was low, but increased to a steady 25% share during the last 3 years of the study.
Additionally, we analyzed reoperations within 90 days, defined as any open surgical intervention related to the previous THA operation. The causes of reoperations were not analyzed, since establishing the true reason of each reoperation in a registry setting might be challenging; for example, some dislocations undergoing revision may in fact have been caused by compromised soft tissues due to infection. Similarly, it can be argued that the limit between washout due to hematoma or revision due to deep infection is fine. Consequently, we suggested that association of any synergic effect with other factors, rather than causality of medication by itself, is a better variable for safety assessment of thromboprophylaxis. Finally, we also analyzed data on mortality within 90 days from the Swedish Death Register.
Statistical Analysis
The statistical analysis was performed using R, version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria). We analyzed the data in a binary logistic regression model to determine the odds ratio (OR) with a 95% confidence interval (CI). A p value below 0.05 was considered statistically significant. Each of the observed outcomes was used as the dependent variable in the regression analysis. We calculated the OR both in a univariate and a multivariate analysis, adjusted for length of thromboprophylaxis, year of the index operation, Elixhauser comorbidity index, sex, age, and previous treatment with platelet aggregation inhibitors as confounders. A sensitivity analysis with estimation of the average treatment effect using propensity score adjustment was also performed and did not influence the conclusion of the study (see Table, Supplemental Digital Content 4, http://links.lww.com/CORR/A162).
Ethical Considerations
Ethical review approval was obtained from the Central Ethical Review Board in Gothenburg, Sweden (decision 271-14). In accordance to the Swedish Patient Data Act (2008:355) patients receive information about being registered in the Swedish Hip Arthroplasty Register and have full rights to opt-out.
Results
Symptomatic DVT and PE
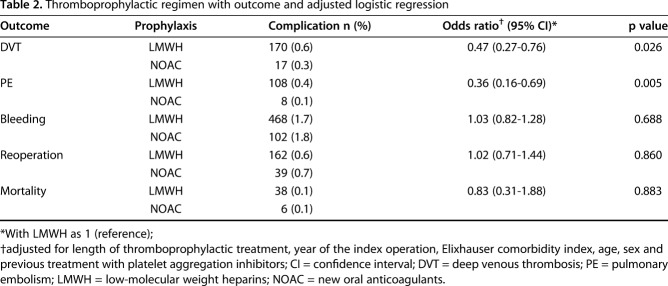
The risk of symptomatic DVT was lower in the group that received new oral anticoagulants than the group that received LMWH (0.3% versus 0.6%; OR, 0.47; 95% CI, 0.27–0.76; p = 0.026). The risk of symptomatic PE was also lower in the group that received new oral anticoagulants than the group that received LMWH (0.1% versus 0.4%; OR, 0.36; 95% CI, 0.16–0.69; p = 0.005) (Table 2).
Table 2.
Thromboprophylactic regimen with outcome and adjusted logistic regression
Bleeding, Reoperation, and Death
We found no between-group differences in terms of bleeding (OR, 1.03; 95% CI, 0.82–1.28; p = 0.688), reoperation (OR, 1.02, 95% CI, 0.71–1.44; p = 0.860) or mortality (OR, 0.83, 95% CI, 0.31–1.88; p = 0.883) (Table 2).
The limited number of patients receiving short duration thromboprophylaxis with new oral anticoagulants did not allow for reliable statistical analysis of short versus long-term prophylaxis in this group. However, adjusting for duration of thromboprophylaxis in the subset of patients treated with LMWH did not show any difference with regard to the studied outcomes of symptomatic DVT (OR, 0.74; 95% CI, 0.46–1.14; p = 0.199), symptomatic PE (OR, 1.14; 95% CI, 0.68–1.82; p = 0.593), bleeding (OR, 0.77; 95% CI, 0.54–1.05; p = 0.112), reoperation (OR, 1.24; 95% CI, 0.83–1.80; p = 0.281), or death (OR, 0.76; 95% CI, 0.26–1.78; p = 0.567).
Discussion
There is little agreement about the best choice of thromboprophylaxis for THA, with regimens and guidelines varying among countries and surgeons. In this nationwide cohort of 32,663 elective THAs, we compared two current choices, new oral anticoagulants and LMWH. Symptomatic DVT and symptomatic PE were reduced in patients receiving extended postoperative thromboprophylaxis with new oral anticoagulants compared with LMWH. We did not find any difference in bleeding, reoperations, and mortality. Although we were able to control for a number of potential confounding variables, we could not ascertain the indications that drove the prescription decisions in this setting. The advantage of new oral anticoagulants in our setting may partly be explained by easier administration, which increases patient compliance [29], but we cannot exclude the possibility that the main explanation is the pharmaceutical mechanism of action.
We recognize that our study has several limitations. The difference in duration of thromboprophylaxis between the two groups most probably reflects the absence of consensus on length of treatment. Our large study population allowed for a separate analysis of this occurrence and adjusting for the treatment length did not influence the likelihood of symptomatic DVT and PE in the LMWH group. The same analysis of effect of duration was not possible in patients with new oral anticoagulants due to insufficient numbers treated with short prophylaxis. However, the adjustment for duration in our analysis and the similar likelihood of thrombosis in the subanalysis of both LMWH subgroups supports the superiority of the new oral anticoagulants regimen.
Our registry setting cannot assess the severity of bleeding complications and we could not measure the magnitude of bleeding and its clinical importance. There are no national registers that record decreased in hemoglobin levels or quantify administered units of blood. We decided not to categorize bleeding into major and minor as we had no access to individual medical records and grouping based on ICD codes would yield uncertainties. We believe some minor bleeding (such as epistaxis) could have been missed as patients may have refrained from seeking medical assistance. Because of high completeness of the registers included, we think that the number of major bleeding episodes not reported is small. Nonetheless, despite a number of necessary exclusions, our study included a large cohort, and we have no reason to believe that the distribution of reported bleeding complications differed between the two thromboprophylaxis groups.
Although our strict inclusion criteria and analysis controlled for several confounders, we cannot rule out differences in the indications for new oral anticoagulants and LMWH. Patients at higher risk for thromboembolic events could have been treated with LMWH instead of new oral anticoagulants. Patients with recent (< 5 years) history of DVT or LE, recent (< 6 months) prescription of antithrombotic agents as well as patients who did not purchase their prophylactic drugs within the first 10 postoperative days (possibly due to a prolonged return to home) were excluded from the analysis. There may also be residual confounding in patients’ demographic and clinical characteristics. For example, patients treated with new oral anticoagulants had higher comorbidity, according to the Elixhauser comorbidity index. The higher incidence of cemented fixation among the same patient group may also partly reflect the higher comorbidity in this group, as cemented fixation is preferred for older patients, who can be expected to have a higher degree of comorbidity. However, the potential association of cement fixation and thromboembolism, beyond the immediate postoperative period, is controversial. Cemented fixation is reported as both associated with higher [24] and lower [14] incidence of DVT and PE. This association is difficult to assess in a registry setting, and would necessitate pragmatic multicenter studies. Nonetheless, the higher comorbidity identified in the new oral anticoagulants group should theoretically increase the likelihood of symptomatic DVT and PE and consequently disadvantage new oral anticoagulants [20, 21].
A structured screening for complications is not available in a single registry setting due to the frequent failure to record screening procedures and discrepancies in ICD-coding of complications. Contrary to most other publications in the field, this study focuses on real-life clinical outcomes, while several previous studies performed an active screening for venous thromboembolic events. Some asymptomatic events can, however, develop into clinically manifested or even potentially life-threatening complications. In most clinical postoperative followup routines, general screening for asymptomatic events is generally not performed. Subsequently, this may lead to asymptomatic PE developing into serious events with a potentially lethal outcome. Although we cannot confirm the cause of death, we had an overall equal incidence (1%) of mortality in both groups, regardless of the cause of death or the contribution of thromboprophylaxis to it. This study presents a pragmatic approach, reflecting true outcome with analysis of clinically presented and therefore relevant results, regardless of their severity, outcome, followup or methodology. This is also assured by the high completeness of the Swedish National Patient Register and the validity of the same register, specifically with regard to thromboembolic outcomes continuously being verified.
The incidence of symptomatic DVT and PE, as well as bleeding, reoperation and mortality may also have been decreased by our exclusion criteria. Patients with documented DVT or PE 5 years before the index operation and patients with prescribed LMWH, new oral anticoagulants, and warfarin 6 months preoperatively were excluded. We also excluded all patients who had no postoperative prescription data on thromboprophylaxis. Further exclusion of patients who did not purchase their thromboprophylaxis medications within 10 days after operation may have excluded individuals with prolonged wound drainage [10, 11] or higher comorbidity. After discussion within the Swedish Hip and Knee Association, which represents most Swedish THA surgeons, we believe that a large proportion of these excluded patients were provided a supply of thromboprophylaxis medications on discharge by the ward staff, bypassing the pharmacies and consequently their registration. From this discussion it could also be suggested that most patients received short-term prophylaxis with either new oral anticoagulants or LMWH. A separate analysis on these excluded patients was performed to determine if their removal from the study group affected our results. We found that the symptomatic DVT and PE incidence for these patients was similar to the study group (0.4 %). The distribution of the outcomes and clinical data did not show any differences between patients with missing and existing medication data. Therefore, we concluded that these exclusions should not have any impact on the conclusions drawn from in the current analysis. Based on the high number of observations and strict inclusion criteria, we think that there is a low probability for skewed distribution of possible confounders between the LMWH group and the new oral anticoagulants group.
Finally, there might have been selection bias in this nationwide observational study. The study period encompasses a relatively limited period; hence, it is unlikely that any major changes in surgical procedure or technique have been introduced. To reduce the risk of any selection bias, a sensitivity analysis using propensity score adjustment was performed and did not influence the conclusion of the study.
Several meta-analyses have reported results similar to this study’s finding of a lower incidence of DVT and PE after THA with new oral anticoagulants compared with LMWH treatment [15, 18, 19, 26]. However, these results are not directly comparable due to differences in patient selection and patient demographics. There is also a discrepancy with regard to the primary endpoints between studies. Most studies used different screening modalities to identify DVT and PE, which also detect subclinical events; this may have raised the reported incidence of DVTs and PEs and simultaneously prevented some from becoming symptomatic and detectable in clinical practice. Among recently published trials, only the large phase 4 XAMOS rivaroxaban trial had symptomatic DVT and PE as the primary endpoint and reported a similar incidence of 0.3 % in THA patients with rivaroxaban as compared with the standard of care thromboprophylaxis (OR, 0.43; 95% CI, 0.24–0.80) [25]. However, this study was a nonintervention study without any randomization or control of possible risk factors, which could have resulted in bias between treatment groups. Moreover, in this study several different thromboprophylaxis regimens were used as the comparator to the new oral anticoagulants group, which might further obscure the results.
In the current study there was no association between LMWH or new oral anticoagulants and the risk of bleeding. Any direct comparison to previous clinical trials is difficult as they often did not adhere to standardized definitions of bleeding [2]. However, analysis of bleeding is important as a safety assessment of pharmacological thromboprophylaxis. Both the RE-NOVATE I and II dabigatran trials, as well as the XAMOS trial, did not report any difference in risk of major bleeding [3, 5, 25] between new oral anticoagulants and LMWH. Some meta-analyses have reported an association of major/clinically relevant bleeding with rivaroxaban [7, 9, 19, 26], whereas others did not find any association for rivaroxaban or dabigatran [7, 9, 15, 18, 26].
In conclusion, new oral anticoagulants were associated with a lower risk of symptomatic DVT and symptomatic PE in this large registry study, and we observed no differences in the risk of bleeding, reoperation, or death between the groups. The study was performed independently of any financing or affiliation to specific companies and with no intended selection of settings, protocols or performing centers. This, together with the quality of our registers and the large cohort analyzed, reinforces the relevance and generalizability of our results.
Although we were able to control for a number of potential confounding variables, we could not ascertain the indications that drove the prescription decisions in this setting, and there were important between-group differences in terms of duration of thromboprophylaxis (new oral anticoagulants generally were used for a longer period of time after surgery). Future studies, preferably large randomized trials with pragmatic inclusion criteria that analyze symptomatic DVT, symptomatic PE and death are needed to confirm or refute our findings.
Acknowledgments
We would like to thank the Swedish orthopaedic surgeons and secretaries and, in particular, the coordinators at the Swedish Hip Arthroplasty Register for providing data and ensuring the accuracy of the data being registered in the register.
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
One of the authors certifies that he (OR) has received personal fees during the study period, in an amount of less than USD 10,000, from Zimmer Biomet (Winterthur, Switzerland) as reimbursement for presentation (Gothenburg, Sweden), outside the submitted work.
One of the authors certifies that he (MM) has received personal fees during the study period, in an amount of less than USD 10,000, from Zimmer Biomet (Winterthur, Switzerland) as reimbursement for presentation (Copenhagen, Denmark); and grants as institutional support in an amount of less than 10,000 USD, from Link (Hamburg, Germany), all outside the submitted work.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This study was performed in Sweden as a nationwide study, based on national registries with continuous data collection from all performing centers in the country. The authors in their respective centers in Gothenburg and Stockholm, Sweden performed the analysis and writing.
References
- 1.Cnudde P, Rolfson O, Nemes S, Karrholm J, Rehnberg C, Rogmark C, Timperley J, Garellick G. Linking Swedish health data registers to establish a research database and a shared decision-making tool in hip replacement. BMC Musculoskelet Disord. 2016;17:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahl OE, Quinlan DJ, Bergqvist D, Eikelboom JW. A critical appraisal of bleeding events reported in venous thromboembolism prevention trials of patients undergoing hip and knee arthroplasty. J Thromb Haemost. 2010;8:1966-1975. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W, Grp RS. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765-2775. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJ. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105:721-729. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Buller HR, Grp R-NS. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949-956. [DOI] [PubMed] [Google Scholar]
- 6.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW. Prevention of VTE in orthopedic surgery patients antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:E278S-E325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Outes A, Terleira-Fernandez AI, Suarez-Gea ML, Vargas-Castrillon E. Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta-analysis, and indirect treatment comparisons. BMJ. 2012;344:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hull RD. Treatment of pulmonary embolism: The use of low-molecular-weight heparin in the inpatient and outpatient settings. Thromb Haemost. 2008; 99: 502-510. [DOI] [PubMed] [Google Scholar]
- 9.Hur M, Park SK, Koo CH, Jung DE, Kang P, Kim WH, Kim JT, Jung CW, Bahk JH. Comparative efficacy and safety of anticoagulants for prevention of venous thromboembolism after hip and knee arthroplasty A network meta-analysis. Acta Orthop. 2017;88:634-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jameson SS, Rymaszewska M, James P, Serrano-Pedraza I, Muller SD, Hui ACW, Reed MR. Wound complications following rivaroxaban administration a multicenter comparison with low-molecular-weight heparins for thromboprophylaxis in lower limb arthroplasty. J Bone Joint Surg Am. 2012;94:1554-1558. [DOI] [PubMed] [Google Scholar]
- 11.Jensen CD, Steval A, Partington PF, Reed MR, Muller SD. Return to theatre following total hip and knee replacement, before and after the introduction of rivaroxaban a retrospective cohort study. J Bone Joint Surg Br. 2011;93:91-95. [DOI] [PubMed] [Google Scholar]
- 12.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S, Investigators R. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008; 372: 31-39. [DOI] [PubMed] [Google Scholar]
- 13.Karrholm J, Lindahl H, Malchau H, Mohaddes M, Rogmark C, Rolfson O. Swedish Hip Arthroplasty Register: Annual Report 2015. Gothenburg 2016, Sweden: Available at: https://shpr.registercentrum.se/shar-in-english/annual-reports-from-the-swedish-hip-arthroplasty-register/p/rkeyyeElz. Accessed December 1, 2018. [Google Scholar]
- 14.Kim YH, Oh SH, Kim JS. Incidence and natural history of deep-vein thrombosis after total hip arthroplasty - A prospective and randomised clinical study. J Bone Joint Surg Br. 2003;85:661-665. [PubMed] [Google Scholar]
- 15.Loke YK, Kwok CS. Dabigatran and rivaroxaban for prevention of venous thromboembolism - systematic review and adjusted indirect comparison. J Clin Pharm Ther. 2011;36:111-124. [DOI] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute for Health and Clinical Excellence: Guidance. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis and pulmonary embolism. London: 2018, United Kingdom. Available at: https://www.nice.org.uk/guidance/ng89/chapter/Recommendations#interventions-for-people-having-orthopaedic-surgery. Accessed December 1, 2018. [Google Scholar]
- 18.Neumann I, Rada G, Claro JC, Carrasco-Labra A, Thorlund K, Akl EA, Bates SM, Guyatt GH. Oral direct factor Xa inhibitors versus low-molecular-weight heparin to prevent venous thromboembolism in patients undergoing total hip or knee replacement a systematic review and meta-analysis. Ann Intern Med. 2012;156:710-719. [DOI] [PubMed] [Google Scholar]
- 19.Ning GZ, Kan SL, Chen LX, Lei SG, Feng SQ, Zhou Y. Rivaroxaban for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis with trial sequential analysis of randomized controlled trials. Sci Rep. 2016;6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen AB, Mehnert F, Sorensen HT, Emmeluth C, Overgaard S, Johnsen SP. The risk of venous thromboembolism, myocardial infarction, stroke, major bleeding and death in patients undergoing total hip and knee replacement. Bone Joint J. 2014;96:479-485. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen AB, Sorensen HT, Mehnert F, Johnsen SP, Overgaard S. Effectiveness and safety of different duration of thromboprophylaxis in 16,865 hip replacement patients - A real-word, prospective observational study. Thromb Res . 2015;135:322-328. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen AB, Sorensen HT, Mehnert F, Overgaard S, Johnsen SP. Risk Factors for venous thromboembolism in patients undergoing total hip replacement and receiving routine thromboprophylaxis. J Bone Joint Surg Am . 2010;92:2156-2164. [DOI] [PubMed] [Google Scholar]
- 23.Quan HD, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care . 2005;43:1130-1139. [DOI] [PubMed] [Google Scholar]
- 24.Schiff RL, Kahn SR, Shrier I, Strulovitch C, Hammouda W, Cohen E, Zukor D. Identifying orthopedic patients at high risk for venous thromboembolism despite thromboprophylaxis. Chest . 2005;128:3364-3371. [DOI] [PubMed] [Google Scholar]
- 25.Turpie AGG, Haas S, Kreutz R, Mantovani LG, Pattanayak CW, Holberg G, Jamal W, Schmidt A, van Eickels M, Lassen MR. A non-interventional comparison of rivaroxaban with standard of care for thromboprophylaxis after major orthopaedic surgery in 17,701 patients with propensity score adjustment. Thromb Haemost . 2014; 111:94-102. [DOI] [PubMed] [Google Scholar]
- 26.Venker BT, Ganti BR, Lin H, Lee ED, Nunley RM, Gage BF. Safety and efficacy of new anticoagulants for the prevention of venous thromboembolism after hip and knee arthroplasty: a meta-analysis. J Arthroplasty . 2017;32:645-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warwick D, Friedman RJ, Agnelli G, Gil-Garay E, Johnson K, FitzGerald G, Turibio FM. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events - Findings from the global orthopaedic registry. J Bone Joint Surg Br. 2007;89:799-807. [DOI] [PubMed] [Google Scholar]
- 28.Wettermark B, Harnmar N, MichaelFored C, Leimanis A, Olausson PO, Bergman U, Persson I, Sundstrom A, Westerholm B, Rosen M. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726-735. [DOI] [PubMed] [Google Scholar]
- 29.Wilke T. Patient preferences for an oral anticoagulant after major orthopedic surgery results of a German survey. Patient . 2009;2:39-49. [DOI] [PubMed] [Google Scholar]