Abstract
Background
A single-center study of 144 THAs revised specifically for periprosthetic joint infection (PJI) observed that trabecular metal (TM) acetabular components had a reduced risk of rerevision for subsequent infection compared with non-TM implants. It was suggested that TM was protective against infection after revision and that TM may be useful when revising THAs for PJI. Three registry studies have subsequently assessed the effect of TM on future infection. In the National Joint Registry (NJR) for England and Wales, we earlier reported lower revision rates for infection when TM (versus non-TM) was used in primary THA, but no difference in rerevision rates for infection when TM was used for all-cause revision THAs. The latter findings in all-cause revisions were also confirmed in a study from the Swedish and Australian registries. It is possible that TM only reduces the risk of infection when it is specifically used for PJI revisions (rather than all-causes). However, to date, the registry analyses have not had large enough cohorts of such cases to assess this meaningfully.
Questions/purposes
(1) In revision THAs performed for PJI, are rerevision rates for all-cause acetabular indications lower with TM acetabular components compared with non-TM designs? (2) In revision THAs performed for PJI, are rerevision rates of any component for infection lower with TM acetabular components compared with non-TM designs?
Methods
A retrospective observational study was performed using NJR data from England and Wales, which is the world’s largest arthroplasty registry and contains details of over two million joint replacement procedures. The registry achieves high levels of patient consent (92%) and linked procedures (ability to link serial procedures performed on the same patient and hip; 94%). Furthermore, recent validation studies have demonstrated that when revision procedures have been captured within the NJR, the data completion and accuracy were excellent. Of 11,988 revisions performed for all causes, 794 were performed for PJI in which the same cementless acetabular component produced by one manufacturer was used. Acetabular components were either TM (n = 541) or non-TM (n = 253). At baseline the two groups were comparable for sex, age, body mass index, and American Society of Anesthesiologists (ASA) grade. Outcomes after revision THA (rerevision for all-cause acetabular indications and rerevision of any component for infection) were compared between the groups using Fine and Gray regression analysis, which considers the competing mortality risk. Regression models were adjusted for the propensity score, with this score summarizing many of the potential patient and surgical confounding factors (age, sex, ASA grade, surgeon grade, approach, and type of revision procedure performed).
Results
There was no difference in 5-year cumulative acetabular component survival rates between TM (96.3%; 95% confidence interval [CI], 94%-98%) and non-TM components (94.4%, 95% CI, 90%-97%; subhazard ratio, 0.78, 95% CI, 0.37-1.65; p = 0.509). There was no difference in 5-year cumulative implant survival rates free from infection between TM (94.8%; 95% CI, 92%-97%) and non-TM components (94.4%, 95% CI, 90%-97%; subhazard ratio, 0.97, 95% CI, 0.48-1.96; p = 0.942).
Conclusions
We found no evidence to support the notion that TM acetabular components used for PJI revisions reduced the subsequent risk of all-cause rerevision or the risk of rerevision for infection compared with non-TM implants from the same manufacturer. We therefore advise caution against recent claims that TM components may protect against infection.
Level of Evidence
Level III, therapeutic study.
Introduction
The burden of revision THA is increasing annually with periprosthetic joint infection (PJI) being one of the most common indications for revision surgery [1, 13]. The management of PJI is extremely challenging; the incidence is increasing, it is associated with a high risk of morbidity and mortality, and the economic burden is huge [2, 14, 17]. Trabecular metal (TM) acetabular components, made from elemental tantalum, have been widely used for revision THA over the last decade [1, 13]. A single-center study of 144 THAs revised specifically for PJI observed that TM acetabular components had a reduced risk of rerevision for subsequent infection compared with non-TM implants [17]. It was suggested that TM was protective against infection after revision and that TM may be useful when revising THAs for PJI. Three registry studies have since assessed the effect of TM on the risk of subsequent revision for infection [8, 9, 11]. In the National Joint Registry (NJR) for England and Wales, we earlier reported lower revision rates for infection when TM (versus non-TM) was used in 18,200 primary THAs although this was of questionable clinical importance [9]. Using the same registry, we also earlier reported no difference in rerevision rates for infection when TM (versus non-TM) was used for 3862 all-cause revision THAs [11]. Another study of 6843 all-cause revisions from two large registries (Sweden and Australia) similarly showed that the rerevision rate for PJI was not different when TM or non-TM components were used at revision THA [8].
It is possible that TM only reduces the risk of infection when it is specifically used for PJI revisions rather than for all-cause revisions. However, to date, the registry analyses have not had large enough cohorts of such PJI revision cases to assess this issue in a meaningful way. The study from the Swedish and Australian registries was unable to assess this issue because the authors felt that the small number of revision THAs performed for PJI (n = 272) precluded any useful analysis [8]. Our previous analysis of 247 revision THAs for PJI from the NJR showed no difference in implant survival rates for both aseptic and septic indications between TM and non-TM components; however, the analysis was similarly underpowered because of the propensity-matched analysis approach that was used [11]. Thus, we sought to use the world’s largest arthroplasty registry to evaluate the effect of TM when used in revision THAs performed for PJI.
We therefore asked the following: (1) In revision THAs performed for PJI, are rerevision rates for all-cause acetabular indications lower with TM acetabular components compared with non-TM designs? (2) In revision THAs performed for PJI, are rerevision rates of any component for infection lower with TM acetabular components compared with non-TM designs?
Materials and Methods
A retrospective observational study was performed using data from the NJR for England and Wales. The NJR was established in April 2003 to identify poorly performing implants early. The registry now contains details of over two million joint replacement procedures. Patients provide voluntary consent for their details to be recorded within the NJR. The unique patient identifiers allow linkage of primary THAs to any future procedures in which components are removed or exchanged. Details regarding patient consent, procedural linkage, and data validity in the NJR have been described [10]. The registry achieves high levels of patient consent (92%) and linked procedures (ability to link serial procedures performed on the same patient and hip; 94%) [13]. Furthermore, validation studies have demonstrated that when revision procedures have been captured within the NJR, the data completion and accuracy were excellent [15, 16]. Using unique patient identifiers, the NJR data set was linked to the Office for National Statistics database, which provides data on all-cause mortality. The study protocol was approved by the NJR Research Subcommittee (RSC2016/14).
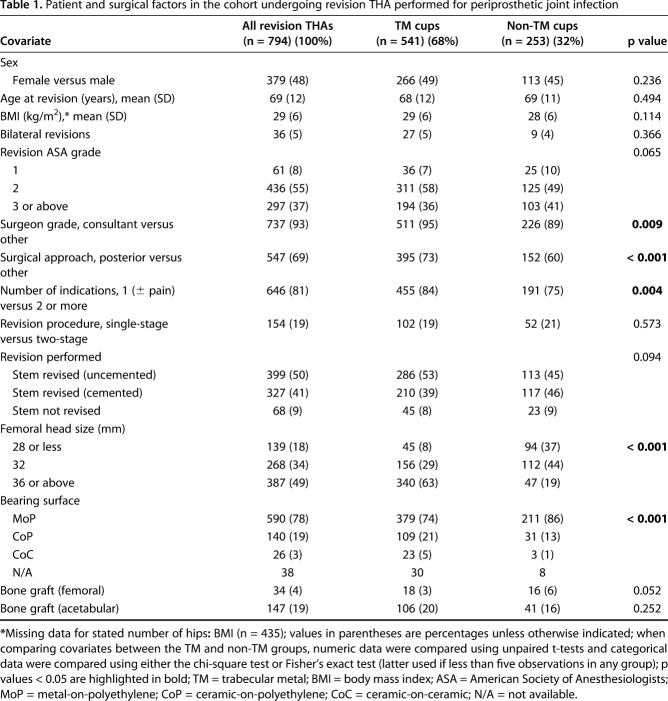
Anonymized patient data were extracted from the NJR for all revision THAs performed between April 1, 2003, and July 30, 2015, in which one of four cementless acetabular component designs produced by the same manufacturer were implanted (described subsequently). The former date represents when the NJR began collecting data, and the latter date ensured a minimum 1-year followup for assessing the study endpoints after revision THA. A total of 11,988 THAs (10,480 patients) underwent revision surgery for any cause that involved the four acetabular components studied. Only revision THAs performed for infection were subsequently included in this study, which involved 7% of the eligible cohort (794 THAs in 722 patients) (Table 1). Of these THA revisions for infection, 541 hips were TM acetabular designs and 253 hips were non-TM designs. The mean followup after revision THA was 5.3 years (range, 1.0-13.5 years), which was not different between the TM and non-TM groups.
Table 1.
Patient and surgical factors in the cohort undergoing revision THA performed for periprosthetic joint infection
The study exposure of interest was whether the revision THA acetabular component was TM or non-TM. These components were either made of porous tantalum (TM) or titanium with a fiber metal coating (non-TM). Two TM implant designs (TM Modular™ and Continuum®) and two non-TM implant designs (Trilogy® and Trilogy IT) were studied, which are all produced by one manufacturer (Zimmer Biomet, Bridgend, UK). The Trilogy and TM Modular designs are similar, and the same is true for the Trilogy IT and Continuum designs; however, the TM Modular designs are elliptical (that is, a cup labeled with a 56-mm diameter cup has a true diameter of 58 mm). The main difference between these design pairs is that only polyethylene liners can be used with the Trilogy and TM Modular designs, whereas either polyethylene or ceramic liners can be used with the Trilogy IT and Continuum designs. Details regarding the design and manufacture of these components have been described previously [11].
The two outcomes of interest after revision THA for infection were rerevision surgery of the acetabular component for all causes and rerevision surgery of any component for infection (regardless of whether the acetabular component was removed). The latter therefore included isolated femoral head and/or acetabular liner exchanges for infection. The diagnosis of infection is recorded by the revision surgeon on standardized data capture forms, which are subsequently submitted to the registry as described previously [11]. This diagnosis is based on preoperative and intraoperative findings, but does not include any laboratory analysis of samples taken during revision surgery.
The NJR also collects data on other relevant patient and surgical factors, which could be potential confounders. These include age, sex, body mass index (BMI), year of surgery, American Society of Anesthesiologists (ASA) grade, surgeon grade, surgical approach, specific revision indication, number of revision indications, and type of revision performed (single-stage or two-stage procedure, femoral stem revision or retention, revision femoral head size, bearing surface, and use of bone graft). Adjustments were made for these potential confounders in the analysis.
The patient age at revision was not different between the TM and non-TM groups (mean 68 versus 69 years; p = 0.494) (Table 1). The sex distribution was not different between the TM and non-TM groups (49% females [266 of 541] versus 45% females [113 of 253]; p = 0.236). The BMI at revision was not different between the TM and non-TM groups (mean 29 versus 28 kg/m2; p = 0.114). The frequency of bilateral THA revisions was not different between the TM and non-TM groups (5% [27 of 541] versus 4% [nine of 253]; p = 0.366). The patient ASA grade at revision was not different between the TM and non-TM groups (ASA 1: 7% [36 of 541] versus 10% [25 of 253]; ASA 2: 58% [311 of 541] versus 49% [125 of 253]; ASA 3 or above: 36% [194 of 541] versus 41% [103 of 253]; p = 0.065). The TM group more frequently had surgery performed by a consultant compared with the non-TM group (95% [511 of 541] versus 89% [226 of 253]; p = 0.009). The TM group more frequently had surgery performed using a posterior approach compared with the non-TM group (73% [395 of 541] versus 60% [152 of 253]; p < 0.001). The TM group more frequently had fewer indications for revision compared with the non-TM group (one indication ± pain [compared with two or more]: 84% [455 of 541] versus 75% [191 of 253]; p = 0.004). The frequency of single-stage revision procedures was not different between the TM and non-TM groups (19% [102 of 541] versus 21% [52 of 253]; p = 0.573). The frequency of stem revisions and fixation was not different between the TM and non-TM groups (stem revised [uncemented]: 53% [286 of 541] versus 45% [113 of 253]; stem revised [cemented]: 39% [210 of 541] versus 46% [117 of 253]; stem not revised: 8% [45 of 541] versus 9% [23 of 253]; p = 0.094). The TM group more frequently had larger femoral head sizes implanted at revision compared with the non-TM group (28 mm or less: 8% [45 of 541] versus 37% [94 of 253]; 32 mm: 29% [156 of 541] versus 44% [112 of 253]; 36 mm or above: 63% [340 of 541] versus 19% [47 of 253]; p < 0.001). The TM group more frequently had ceramic-bearing surfaces implanted at revision compared with the non-TM group (metal-on-polyethylene: 74% [379 of 541] versus 86% [211 of 253]; ceramic-on-polyethylene: 21% [109 of 541] versus 13% [31 of 253]; ceramic-on-ceramic: 5% [23 of 541] versus 1% [three of 253]; p < 0.001). The frequency of femoral bone graft used at revision was not different between the TM and non-TM groups (3% [18 of 541] versus 6% [16 of 253]; p = 0.052). The frequency of acetabular bone graft used at revision was not different between the TM and non-TM groups (20% [106 of 541] versus 16% [41 of 253]; p = 0.252).
Statistical Analysis
Cumulative implant survivorship after revision THA was determined using the Kaplan-Meier method with the two endpoints used for implant survival defined previously. Patients who did not experience an outcome event and who remained alive were censored on the study end date (July 30, 2016). The study endpoints after revision THA were compared between the TM and non-TM groups using Fine and Gray regression analysis, which considers the competing mortality risk [3]. This decision was supported by the high risk of mortality during the study, especially in the non-TM group (overall 19% [153 of 794]: TM group 13% [73 of 541] versus non-TM group 32% [80 of 253]; p < 0.001). Proportional subhazard assumptions were assessed and satisfied for all regression analyses.
Adjusted regression models for each study endpoint were assessed in which adjustment was made solely for the propensity score. There were a number of potential patient and surgical confounding factors, which could have all been adjusted for in the analysis. However, adjusting for all of these confounders individually would substantially increase the risk of overfitting the regression models, especially given the relatively small number of observed outcome events. A propensity score (ranging from 0 to 1) was generated for each revision THA using logistic regression with the methods used described in detail previously [11]. The propensity score summarizes all the potential patient and surgical confounding factors (all covariates listed in Table 1 apart from BMI as a result of this variable frequently having missing data) using one single score per revision THA.
We used Stata (Version 14.2; College Station, TX, USA) for all analyses. Probability values < 0.05 and 95% confidence intervals (CIs) were used.
Results
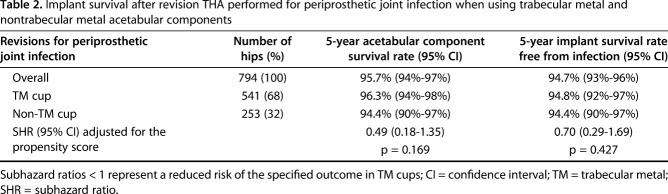
We found no difference in the all-cause risk of acetabular rerevision between the TM group and the non-TM group after THA revision for PJI. The 5-year cumulative acetabular component survivorship was 96.3% (95% CI, 94%-98%) in the TM group compared with 94.4% (95% CI, 90%-97%) in the non-TM group (subhazard ratio [SHR], 0.49; 95% CI, 0.18-1.35; p = 0.169) (Table 2). There were 28 of 794 hips (3.5%) that underwent all-cause acetabular rerevision within the study period. Mean time to all-cause acetabular rerevision was 2.1 years (range, 0.04-6.4 years). The most common reasons for these rerevisions were infection (57% [16 of 28]), aseptic loosening (32% [nine of 28]), lysis (14% [four of 28]), and dislocation/subluxation (11% [three of 28]).
Table 2.
Implant survival after revision THA performed for periprosthetic joint infection when using trabecular metal and nontrabecular metal acetabular components
We found no difference in the risk of rerevision of any component for infection between the TM group and the non-TM group after THA revision for PJI. The 5-year cumulative implant survivorship free from infection was 94.8% (95% CI, 92%-97%) in the TM group compared with 94.4% (95% CI, 90%-97%) in the non-TM group (SHR, 0.70; 95% CI, 0.29-1.69; p = 0.427) (Table 2). There were 34 of 794 hips (4.3%) that underwent rerevision for infection within the study period. Mean time to rerevision of any component for infection was 2.5 years (range, 0.05-7.2 years).
Discussion
The burden of revision THA is increasing with PJI being one of the most common indications for revision surgery [1, 13]. A single-center study of 144 THAs revised for PJI observed that TM acetabular components had a reduced risk of rerevision for subsequent infection compared with non-TM implants [17]. It was suggested that TM was protective against infection after revision and that TM may be useful when revising THAs for PJI. Three registry studies have subsequently assessed the effect of TM on future infection [8, 9, 11]. In the NJR for England and Wales, we earlier reported lower revision rates for infection when TM (versus non-TM) was used in primary THA [9], but no difference in rerevision rates for infection when TM was used for all-cause revision THAs [11]. The latter findings in all-cause revisions were also confirmed in a study from the Swedish and Australian registries [8]. It is possible that TM only reduces the risk of infection when it is specifically used for PJI revisions rather than revisions for all-causes. However, to date, the registry analyses have not had large enough cohorts of such PJI revision cases to assess this meaningfully. The present large nationwide registry study demonstrated that in revision THAs performed for PJI, the risk of all-cause acetabular component rerevision and the risk of rerevision of any component for infection were not different in patients receiving TM- and non-TM acetabular components.
This study had a number of limitations. First, our study was subject to selection bias given that TM components may have preferentially been chosen over non-TM components for PJI revisions for numerous reasons. These potential reasons include patient factors (such as age, sex, BMI, comorbidities such as diabetes, and medications such as steroids or immunosuppressants) and surgical factors (such as surgeon training and experience with each implant, case complexity, and the extent of acetabular defects). We controlled for as many of these variables as we reasonably could in the propensity score adjustment (including age, sex, ASA grade, the number of revision indications, and bone graft use). However, there were a number of factors that we could not control for, including medical and drug history, and certain aspects of case complexity. It is important to acknowledge that joint registries do not record the extent of acetabular defects encountered at revision THA nor do they have radiographic records available to retrospectively assess such defects. It has been suggested that TM components may be used in more complex revisions where larger acetabular defects are present, which may put them at an increased risk of future rerevision compared with non-TM components [8, 17]. Along with other potential patient and surgical confounders not available in the registry, the lack of acetabular defect data therefore represents an important limitation of registry analyses when assessing the outlined research questions and could influence the interpretation of our findings. Although our analysis in a large data set was as robust as possible, we would recommend future studies take these factors into account when interpreting the data. However, other registry analyses have adjusted for the use of acetabular augments, which did not change their findings [8]. Furthermore, we consider that our work adds to what is known given the very large number of revision THAs for PJI needed to satisfactorily assess our study’s key questions, which will not be available in single-center studies.
Second, registries currently do not collect data on the histopathologic and microbiologic analyses performed on tissue and fluid samples taken at revision surgery. Therefore, some of the cohort studied may have not truly had PJI after sample analysis and similarly the rerevision rates for subsequent infection may be slightly different if these sample analyses had been considered. However, this will not have influenced the acetabular rerevision rate for all causes. Third, using observational data means, we cannot infer causality. Fourth, previous NJR studies have suggested that rerevision rates may be underestimated [15, 16], although there is no reason to expect any underreporting would differ between the TM and non-TM groups. Fifth, registries do not collect data on nonrevision procedures such as closed reductions or wound washouts nor do they collect data on patient-reported outcome measures after revision surgery. It is recognized that these endpoints are important when assessing the outcomes after any surgical intervention. Sixth, it is acknowledged that the rerevision risk may have been artificially low because of the competing mortality risk, given that a number of patients died during followup, thus precluding them from undergoing rerevision. However, we have mitigated this by performing competing risk regression analyses, which accounted for the risk of mortality. Finally, our observations cannot be extrapolated to highly porous acetabular component designs produced by other manufacturers.
After revision THA performed for PJI, TM acetabular components had a risk of all-cause rerevision that was comparable to non-TM components in the present study. The perceived advantages of TM acetabular components have led to an increase in their use worldwide for both primary and revision THA [8, 9, 11]. A small study of 46 revision THAs for all causes in which TM components were used reported a 96% survival rate for the acetabular component at 11 years with excellent pain relief and good functional outcomes observed in patients with surviving implants [6]. Other authors comparing implant survival after all-cause revision THA performed with TM or non-TM acetabular components have reported better implant survival and fewer radiologic failures in the TM group [5, 17]. However, similar analyses in all-cause revision THA cohorts from national registries from Mohaddes et al. (n = 2460), Kremers et al. (n = 3448), Laaksonen et al. (n = 6843), and Matharu et al. (n = 3862) have failed to demonstrate any improvement in all-cause implant survival when using TM acetabular components compared with non-TM components [7, 8, 11, 12]. The present study is the largest analysis of revision THAs performed specifically for PJI. Our findings regarding the subsequent risk of acetabular rerevision for all causes are in concordance with previous registry data based on revision THAs performed for any indication and now therefore question the clinical benefit of TM components in revision THAs performed specifically for infection.
After revision THA performed for PJI, the risk of rerevision of any component for infection was comparable between TM and non-TM acetabular components in the present study. The notion that TM components used in THAs revised for PJI may protect against subsequent infection comes from a small single-center study involving 144 revision THAs performed for PJI [17]. After patients were followed up for a minimum of 90 days from revision (mean of 3 years), the risk of rerevision resulting from subsequent infection (defined as infection recurrence or persistence) was lower in the TM group (3%) compared with the non-TM group (18%) [17]. Potential mechanisms proposed by the authors for the reduced risk of infection observed with TM implants included the increased potential for osseointegration (which may reduce dead space for colonizing organisms), TM being more hostile to organisms with lower bacterial adhesion compared with other orthopaedic materials (possibly as a result of its three-dimensional structure) and TM enhancing local host defense systems by promoting leukocyte activation [4, 17]. However, a recent matched analysis, albeit underpowered, of revision THAs performed for PJI from the NJR (n = 247) showed no difference in implant survival rates for septic indications between TM and non-TM components [11]. The findings from the present much larger study based on an unselected national population with longer followup compared with the study by Tokarski et al. [17] (including a minimum of 1 year) also do not support the suggestion that TM components used in THAs revised for PJI are protective against subsequent infection.
This large nationwide registry study demonstrated that after revision THA performed for PJI, TM acetabular components had a risk of all-cause rerevision that was comparable with non-TM components. Contrary to the findings from a recent small study [17], we also found no evidence to support the notion that TM acetabular components used for PJI revisions reduced the subsequent risk of rerevision for infection compared with non-TM implants from the same manufacturer. At this time, we would therefore advise that clinicians exercise caution regarding previous claims that TM components may protect against subsequent infection. Future work should include testing this contention in other large patient cohorts as well as analyzing the data periodically in the medium to long term to establish whether TM components can confer any survival benefit over non-TM components at extended followup.
Acknowledgments
We thank the patients and staff of all the hospitals in England, Wales and Northern Ireland who have contributed data to the National Joint Registry. We are grateful to the Healthcare Quality Improvement Partnership (HQIP), the NJR Research Sub-committee and staff at the NJR Centre for facilitating this work. The authors have conformed to the NJR's standard protocol for data access and publication. The views expressed represent those of the authors and do not necessarily reflect those of the National Joint Registry Steering Committee or the Health Quality Improvement Partnership (HQIP) who do not vouch for how the information is presented. The Healthcare Quality Improvement Partnership (“HQIP”) and/or the National Joint Registry (“NJR”) take no responsibility for the accuracy, currency, reliability and correctness of any data used or referred to in this report, nor for the accuracy, currency, reliability and correctness of links or references to other information sources and disclaims all warranties in relation to such data, links and references to the maximum extent permitted by legislation. HQIP and NJR shall have no liability (including but not limited to liability by reason of negligence) for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the data within this report and whether caused by reason of any error, omission or misrepresentation in the report or otherwise. This report is not to be taken as advice. Third parties using or relying on the data in this report do so at their own risk and will be responsible for making their own assessment and should verify all relevant representations, statements and information with their own professional advisers.
Footnotes
One of the authors’ institutions (HGP) has received, during the study period to undertake this study, funding from Zimmer Biomet (Bridgend, UK), the manufacturer of the implants investigated in this study, in the amount of USD 10,000 to USD 100,000. One of the authors’ institutions (GSM, AJ, DWM, HGP) have been involved in the following relevant financial activities outside of this work and/or other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, this manuscript: received funding to undertake other research work from Arthritis Research UK (London, UK) in the amount of USD 100,001 to USD 1,000,000, received funding to undertake other research work from The Orthopaedics Trust (Norwich, UK) in the amount of USD 10,000 to USD 100,000, received funding to undertake other research work from The Royal College of Surgeons of England (London, UK) in the amount of USD 10,000 to USD 100,000, and received funding to undertake other research work from The Royal Orthopaedic Hospital Hip Research and Education Charitable Fund (Birmingham, UK) in the amount of USD 10,000 to USD 100,000. One of the authors (GSM) has been involved in the following relevant financial activities outside of this work and/or other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, this manuscript: paid consultant for Leigh Day (London, UK) in the amount of less than USD 10,000. One of the authors (AJ) has been involved in the following relevant financial activities outside of this work and/or other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, this manuscript: received consultancy, lecture fees, and honoraria from Servier (Neuilly-sur-Seine, France; less than USD 10,000), UK Renal Registry (Bristol, UK; less than USD 10,000), Oxford Craniofacial Unit (Oxford, UK; less than USD 10,000), IDIAP Jordi Gol (Barcelona, Spain; less than USD 10,000), and Freshfields Bruckhaus Deringer (London, UK; USD 10,000 to USD 100,000) and is a member of the Data Safety and Monitoring Board (which involved receipt of fees) from Anthera Pharmaceuticals Inc (Hayward, CA, USA; USD 10,000 to USD 100,000). One of the authors (DWM) has been involved in the following relevant financial activities outside of this work and/or other relationships or activities that readers could perceive to have influenced, or that gave the appearance of potentially influencing, this manuscript: paid consultant for Herbert Smith Freehills (Sydney, Australia) in the amount of USD 10,000 to USD 100,000. During the study period, two of the authors (DWM, HGP) have been involved in the following relevant financial activities outside of this work and/or other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, this manuscript: received research funding from Zimmer Biomet in the amount of USD 100,000 to USD 1,000,000 paid to the University of Oxford. During the study period, one of the authors (HGP) has been involved in the following relevant financial activities outside of this work and/or other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, this manuscript: paid consultant for Zimmer Biomet (Bridgend, UK) in the amount of USD 10,000 to USD 100,000 and Kennedys Law (London, UK) in the amount of USD 10,000 to USD 100,000 and received research funding from Zimmer Biomet in the amount of USD 100,000 to USD 1,000,000, UKIERI (New Delhi, India) in the amount of USD 100,000 to USD 1,000,000, and NIHR (London, UK) in the amount of USD 100,000 to USD 1,000,000 paid to the University of Leeds.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
This work was performed at the Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Nuffield Orthopaedic Centre, Oxford, UK.
References
- 1.AOANJRR. Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) Hip, Knee & Shoulder Arthroplasty Annual Report. 2018. Available at: https://aoanjrr.sahmri.com/annual-reports-2018. Accessed October 9, 2018.
- 2.Berend KR, Lombardi AV, Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res . 2013;471:510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 4.Garbuz DS, Hu Y, Kim WY, Duan K, Masri BA, Oxland TR, Burt H, Wang R, Duncan CP. Enhanced gap filling and osteoconduction associated with alendronate-calcium phosphate-coated porous tantalum. J Bone Joint Surg Am . 2008;90:1090-1100. [DOI] [PubMed] [Google Scholar]
- 5.Jafari SM, Bender B, Coyle C, Parvizi J, Sharkey PF, Hozack WJ. Do tantalum and titanium cups show similar results in revision hip arthroplasty? Clin Orthop Relat Res . 2010;468:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konan S, Duncan CP, Masri BA, Garbuz DS. Porous tantalum uncemented acetabular components in revision total hip arthroplasty: a minimum ten-year clinical, radiological and quality of life outcome study. Bone Joint J . 2016;98:767-771. [DOI] [PubMed] [Google Scholar]
- 7.Kremers HM, Howard JL, Loechler Y, Schleck CD, Harmsen WS, Berry DJ, Cabanela ME, Hanssen AD, Pagnano MW, Trousdale RT, Lewallen DG. Comparative long-term survivorship of uncemented acetabular components in revision total hip arthroplasty. J Bone Joint Surg Am . 2012;94:e82. [DOI] [PubMed] [Google Scholar]
- 8.Laaksonen I, Lorimer M, Gromov K, Rolfson O, Makela KT, Graves SE, Malchau H, Mohaddes M. Does the risk of rerevision vary between porous tantalum cups and other cementless designs after revision hip arthroplasty? Clin Orthop Relat Res . 2017;475:3015-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matharu GS, Judge A, Murray DW, Pandit HG. Trabecular metal acetabular components reduce the risk of revision following primary total hip arthroplasty: a propensity score matched study from the National Joint Registry for England and Wales. J Arthroplasty . 2018;33:447-452. [DOI] [PubMed] [Google Scholar]
- 10.Matharu GS, Judge A, Murray DW, Pandit HG. Outcomes after metal-on-metal hip revision surgery depend on the reason for failure: a propensity score-matched study. Clin Orthop Relat Res . 2018;476:245-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matharu GS, Judge A, Murray DW, Pandit HG. Trabecular metal versus non-trabecular metal acetabular components and the risk of re-revision following revision total hip arthroplasty. A propensity score-matched study from the National Joint Registry for England and Wales. J Bone Joint Surg Am . 2018;100:1132-1140. [DOI] [PubMed] [Google Scholar]
- 12.Mohaddes M, Rolfson O, Karrholm J. Short-term survival of the trabecular metal cup is similar to that of standard cups used in acetabular revision surgery. Acta Orthop . 2015;86:26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NJR. National Joint Registry (NJR) for England, Wales, Northern Ireland and the Isle of Man 15th Annual Report. 2018. Available at: http://www.njrreports.org.uk/Portals/0/PDFdownloads/NJR%2015th%20Annual%20Report%202018.pdf. Accessed October 9, 2018.
- 14.Parvizi J, Haddad FS. Periprosthetic joint infection: the last frontier. Bone Joint J . 2015;97:1157-1158. [DOI] [PubMed] [Google Scholar]
- 15.Sabah SA, Henckel J, Cook E, Whittaker R, Hothi H, Pappas Y, Blunn G, Skinner JA, Hart AJ. Validation of primary metal-on-metal hip arthroplasties on the National Joint Registry for England, Wales and Northern Ireland using data from the London Implant Retrieval Centre: a study using the NJR dataset. Bone Joint J . 2015;97:10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabah SA, Henckel J, Koutsouris S, Rajani R, Hothi H, Skinner JA, Hart AJ. Are all metal-on-metal hip revision operations contributing to the National Joint Registry implant survival curves? A study comparing the London Implant Retrieval Centre and National Joint Registry datasets. Bone Joint J . 2016;98:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokarski AT, Novack TA, Parvizi J. Is tantalum protective against infection in revision total hip arthroplasty? Bone Joint J . 2015;97:45-49. [DOI] [PubMed] [Google Scholar]