Abstract
Background:
Mycetoma is a chronic, progressive, disfiguring, and destructive disease. It caused by a variety of microorganisms including fungi and higher bacteria. It is primarily an infection of the skin and soft tissue, most frequently affecting the lower extremity and the hand and spread through fascial planes and lymphatics.
Methods:
Current medical and surgical management are still inadequate and the recurrence rate is high with severe disabilities.
Results:
This review describes some reconstructive techniques that were performed to address essential aspects with regard to mycetoma surgical management that include coverage of large skin and soft-tissue defects left after local excisions, enhancing the rate of chronic mycetoma wound healing, and preservation or restoration of functional status of the affected limbs.
Conclusion:
These applied techniques—which had acceptable preliminary outcome—have to be considered by the surgeons dealing with mycetoma to improve the functional and cosmetic outcomes and to minimize tremendous morbidities and disabilities that are associated with this neglected disease.
INTRODUCTION
Mycetoma is a chronic granulomatous inflammatory disease which is endemic in tropical and subtropic regions.1 It is caused either by actinomycetes or fungi; in vivo these cause filamentous grain containing chronic granulomata which form sinuses and reach the skin as a purulent discharge.1–3 Although rarely fatal, mortality can be secondary to abscess formation, cellulitis, osteomyelitis leading to sepsis; the latter is generally caused by superimposing bacterial infections. The foot is the most common site, but the condition can present in a variety of locations with the main complication being bony involvement. This neglected disease is important to plastic surgeons involved in Global Heath Initiatives, as its pathogenesis causes significant disfigurement and disabilities. Patients are mostly rural workers who are unable to access specialist services until such time that there are massive deformity and challenging disabilities.1 This article aims to present an overview of mycetoma and describes some reconstructive techniques that were successfully performed for various mycetoma lesions.
PATHOGENESIS
Mycetoma infection can be caused by a variety of fungi or bacteria.2,3 If secondary to fungi, it is known as eumycetoma—if bacteria it is referred to as actinomycetoma.2 The most common causative agents include the fungus Madurella mycetomatis and the bacteria Nocardia brasiliensis, Actinomadura madurae, and Streptomyces somaliensis.4 There is traumatic inoculation of the causative organism cutaneously often secondary to innocuous injury.5
RISK FACTORS
Causation is not fully understood.5 A multitude of risk factors have been described including environmental exposure to pathogenic organisms, genetic predisposition to infection, and immunosuppression.5–9 It predominantly affects adults between 20 and 40 years old, with preponderance to those with contact to the land (students, farmers, and herdsmen).5 It is agreed that the disease is not contagious between humans or from animal to human.5 It is considered as a neglected tropical disease by the World Health Organization.8
EPIDEMIOLOGY
Mycetoma is distributed in tropical and subtropical regions.4 It prevails in the mycetoma belt, which stretches from 15° South to 30° North of the Equator4 (Fig. 1) and includes Sudan, Senegal, Yemen, India, Mexico, Venezuela, and Colombia, with the highest prevalence reported in Africa, with Sudan thought to be the most endemic country in the world.1 Published prevalence rates are largely estimates and not derived from population-based studies and do not reflect the magnitude of the problem in endemic regions.4
Fig. 1.
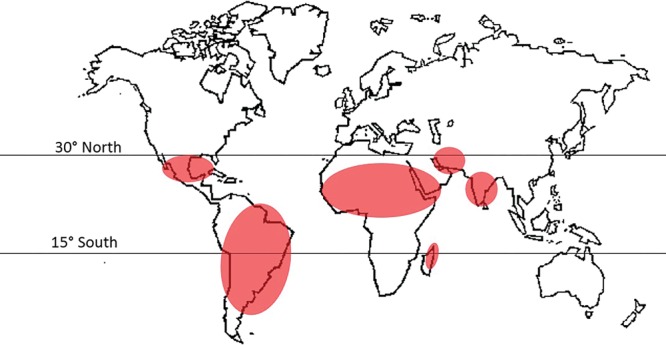
Mycetoma distribution worldwide.
CLINICAL FEATURES
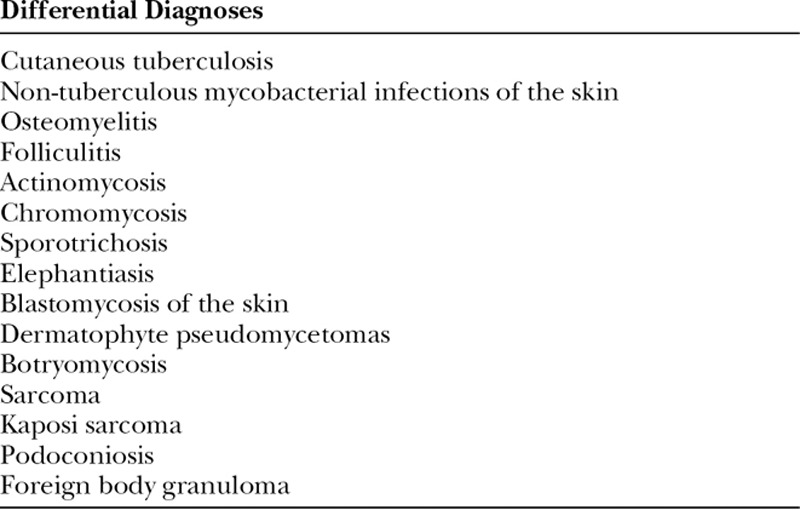
The triad of painless subcutaneous mass with multiple sinuses discharging seropurulent fluid containing mycetoma grains is pathognomonic (Fig. 2).5 During the active phase of the disease, these sinuses discharge grains of variable consistency and size whose color indicates the causative agent.5 Table 1 illustrates essential clinical and radiological features of eumycetoma and actinomycetoma. In endemic areas, mycetoma should be included as a differential diagnosis of any swelling.1,5 Differential diagnoses are listed in Table 2. The body parts that contact soil during daily activities are the most frequently affected, with the lower limb accounting for 82% of cases.1,9 The prevalence of mycetoma in limbs and the often-sizable defects create the need for significant involvement from plastic and reconstructive surgeons, as it can have dire consequences which are challenging to treat. The lack of medical and health facilities, and financial constraints compounded by poor health education in endemic areas meant that most of the studied patients presented late with advanced disease.10 Mycetoma is recognized to cause several disabilities and deformities,11–19 and it could be fatal on rare occasions (eg, cranial mycetoma).5 Rare sites include abdominal wall, chest wall, spinal cord, facial bones, mandible, paranasal sinuses, oral cavity, the eye, perineum, vulva, and testes.20–27
Fig. 2.
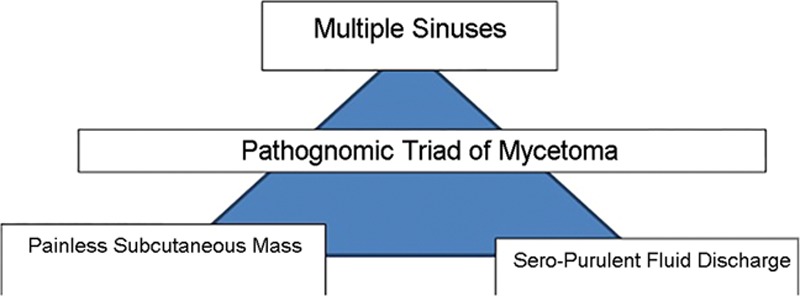
Pathognomonic triads of mycetoma.
Table 1.
Illustration of essential Clinical and Radiological features of Eumycetoma and Actinomycetoma
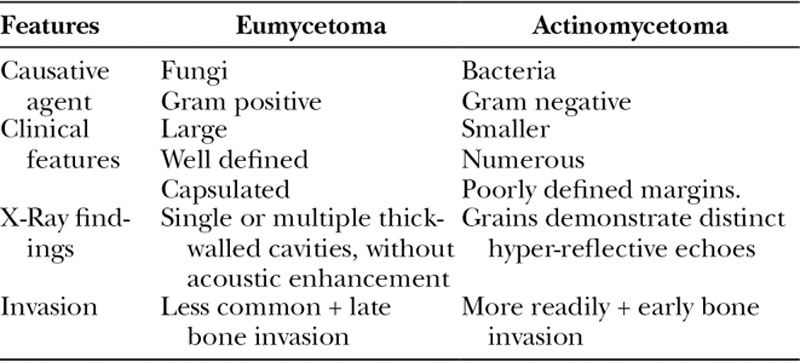
Table 2.
Differential Diagnoses of Mycetoma
Radiological Investigations
X-rays and ultrasound allow for an assessment of progression of disease. Early features show as a dense shadow or several scattered shadows similar to a granuloma28 with progression of the disease, and the cortex can be compressed by the granuloma until many punched-out cavities appear.28 Grains have specific ultrasonic appearances which allow differentiation between eumycetoma and actinomycetoma.29 Magnetic resonance imaging is used to assess the extent of bony destruction, soft-tissue involvement and might show a pathognomonic “dot-in-circle” appearance that indicates the presence of grains.30
Cytological and Histopathological Investigations
Fine needle aspiration cytology allows for confirmation of the diagnosis. It demonstrates a chronic granulomatous reaction with a purulent centre and allows different grains to be observed. Various stains (most commonly hematoxylin and eosin) can be used to identify the causative organism and host-tissue reaction.31,32 Serological and molecular tests are also proved to be valuable in diagnosis with acceptable sensitivity and specificity rates.33–35
Medical Management
The treatment and outcome depend mainly on the causative organism, the site, and extent of disease at presentation.1,5,9 Current medical treatment for mycetoma is generally inadequate; moreover, medications can be expensive with many side effects.36 Multidrug therapy is recommended to avoid drug resistance and enhance the eradication of disease.36 A multitude of azole antifungals are available for treatment of eumycetoma.36 The optimal duration of antimicrobial therapy for treatment of eumycetoma is uncertain and can involve long-term therapy in some cases going up to 12 months with bony involvement. Patients should be followed for at least 2 years after discontinuation of antimicrobial therapy before declaring a cure.36
Surgical Treatment
Local Excision
Surgical management is dependent on the size of the lesion. Small lesions (<5 cm) without bony involvement require wide local excision with concomitant antimicrobial therapy.36 Excision should always be undertaken under general anesthetic with a bloodless field using a tourniquet—a bloodless field is paramount to prevent bursting of the capsule—as bursting the capsule can lead to increased rates of recurrence.36,37 Repeated debridement is required for massive infected disease.38,39 The Mycetoma Research Center in Sudan have developed guidelines for amputation in cases of mycetoma listed in Table 3.40 Authors have utilized repeated local excisions to prevent amputation,41 but amputation can be a lifesaving procedure in selected patients and rates range from 10% to 25% in most series.39,42,43 In Sudan, a homeland of the disease, mycetoma is the third most common cause of amputation after diabetes-related complications and land-mine injuries.44 Interestingly enough, there is less phantom pain in patients with mycetoma after amputation, and this allows more successful prosthetic fitting as there is an adequate blood supply.44 The recurrence rate varies from 25% to 50%5,36 There are multifactorial causes of this high recurrence rate that include widespread disease along the fascial planes, inadequate excisions, lack of compliance with medication, and lack of health education.36,39
Table 3.
Guidelines for Amputation40

RECONSTRUCTIVE SURGERY FOR MYCETOMA
Reconstructive surgery for mycetoma was successfully performed worldwide.45–50 However, published work on reconstructive surgery for mycetoma is essentially limited in both number and case volume. Various reconstructive surgeries were applied in selected patients and had satisfactory preliminary outcomes.45–50
Imaging modalities now would enable accurate detection of the extent of the lesion.51 Deep structures and bone involvement can be identified and an adequate surgery with safe margins can be well-planned.45 A radical approach has been suggested for an adequate resection of mycetoma with applying of Enneking Principles for Oncologic Surgery.52 The rationale of this approach is to manage mycetoma lesions as malignant tumors due to the high recurrence rates after a local resection,46 although mycetoma is a benign disease with no reported malignant or premalignant potentials.5
A series of 26 patients with eumycetoma who underwent wide surgical excisions followed by reconstructive surgeries was reported from Sudan (a homeland of the disease) from 3 referral plastic surgery centers in Sudan.45 The size of lesions encountered ranged from 8 to 20 cm. The sites of lesions include the foot, knee joint, leg, hand, and gluteal region. A wide local excision was performed for large-sized lesion of >8 cm with a safe margin of 0.5–2.2 cm to eradicate the disease.45 As large skin/soft-tissue defects were left after excision, various reconstructive techniques were applied, ranging from split-thickness skin grafts (STSGs) to local or advanced flaps (eg, V-Y advancement flaps, gastrocnemius flap, dorsalis pedis flap, and lateral calcaneal artery flap). These procedures allowed closure of the skin and soft-tissue defects and hence the length of hospital stay was reduced. The patients were followed up over 9–12 months and all had good healing and return to ordinary activities but 1 patient had recurrence. According to our initial experience, we do recommend a reconstructive surgery after wide local excision (WLE) for larger-sized mycetoma lesions and when there are difficulties to close the skin primarily.
Reconstruction of a foot defect created by local excision of mycetoma lesion was reported from Israel,46 where a right foot defect following an adequate excision of mycetoma was reconstructed by a free tensor fasciae latae musculocutaneous flap with a successful tissue transfer. The function of the affected limb was preserved, and there was no recurrence on magnetic resonance imaging during follow-up.46 Authors justified their reconstructive approach by the fact that transferring a highly vascularized tissue to the mycetoma region—where the infectious process existed—after a radical resection can significantly assist in eradicating residual nests of disease by means of a better delivering of antifungal drugs to the surrounding tissues.46 This newly innovated technique can be effective to reduce recurrence rates. A similar case was reported from Kenya.47 A solitary left anteromedial leg mycetoma swelling with lytic bone invasion on leg x-rays that led into severe inability to use the limb was successively managed by local excision with reconstruction of the defect by gastrocsoleus flap and STSG in combination with antifungal treatment.46 The result was complete resolution of the swelling with no clinical and radiological signs of recurrence on follow-up. The patient became ambulant with normal weight-bearing function of his limb. It is important to note that amputation is still considered as a social stigma and has many medical and socioeconomic impacts especially in developing countries that have the greatest burden of mycetoma.39 Therefore, there is a real need to establish appropriate reconstructive options that would preserve the function of the affected limbs to avoid amputation. Nevertheless, the decision of amputation may be undeniably difficult in some cases as it can be a lifesaving procedure39 A local V-Y flap was advanced to cover a large gluteal defect following local resection of an eumycetoma in the gluteal region.49 A latissimus dorsi flap was transferred to cover a large chest wall defect following an excision of mycetoma lesion.50
It is interesting to note that STSG after application of topical negative pressure wound therapy (NPWT) to cover skin defects has been successfully described in the literature.53,54 An STSG was used for covering a skin defect after application of topical NPWT to induce the granulation tissue formation following local excision of a Madura foot.53 The lesion involved plantar arch of the right foot and was recurrent and complicated by multiple sinuses formation.53 This modality coupled with medical treatment had resulted in complete disappearance of the lesion at an 18-month follow-up.53 Although graft had developed necrosis, the presence of an adequate granulation tissue in the surgically treated area had facilitated reepithelialization of the wound.53 The final outcome of the scar was acceptable esthetically and functionally.53 A second case of employing similar technique was reported in Turkey.54 Topical NPWT was used for 2 weeks after local excision of a dorsal ankle Madura and then an STSG was applied.54 It had resulted in complete disappearance of the lesion with full graft take.54 In fact, NPWT, based on the application of subatmospheric pressure has revolutionized the management of chronic wounds complicating burn, chronic infection, exposed bone, and previous wound dehiscence.55,56 This modality facilitates healing by improving the rates of angiogenesis and endothelial proliferation, maintaining the integrity of the capillary basement membrane and reducing microbial burden within the wound.57 Therefore, it can be a good choice to induce granulation tissue formation and enhancing the rate of healing of chronic mycetoma wounds.53,54 In addition, it could enhance local inflammatory response and ensure eradication of any residual fungal elements.53 Although the value of this novel technique has not yet been fully proven,53 it merits attention by workers in the field of mycetoma to examine its efficiency based on larger-scale studies. Secondary bacterial infections represent a constant morbidity with mycetoma.5 However, the role of reconstructive surgery in reducing the incidence of these secondary infections of mycetoma wounds by covering the skin/soft-tissue defects and enhancing wound healing has not previously been discussed in the literature. This is an important relation to assess in details in future case–control studies.
Therefore, surgical management would supplement medical treatment by debridement or local excisions, therapy enhancing the response to antimicrobial therapy, and can augment functional and cosmetic outcomes by various reconstructive procedures.58 The use of latter techniques to cover large skin/soft-tissue defects following local excisions, to enhance chronic wound healing rates, and to preserve the function of disabled limbs can be rewarding as shown objectively above, hence there is a genuine need for a consensus on reconstructive approach and formulation on algorithm for reconstructive ladder for the cases that required these management options.
CONCLUSIONS
Mycetoma is a difficult-to-treat disease. A complete cure is not achieved in many patients and recurrence is not uncommon even after adequate treatment.35 Reconstructive surgery techniques have to be greatly considered by surgeons working in endemic areas. These novel surgical options can be effective in regard to many aspects of mycetoma surgical management. The benefits would include covering large skin and/or soft tissue defects following wide local excisions of massive lesions, preserving or restoring the function of disabled limbs, and enhancing the rate of chronic wound healing.
Footnotes
Published online 24 April 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Fahal AH. Mycetoma: a thorn in the flesh. Trans R Soc Trop Med Hyg. 2004;98:3. [DOI] [PubMed] [Google Scholar]
- 2.Fahal AH, Hassan MA. Mycetoma. Br J Surg. 1992;79:1138. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed AO, van Leeuwen W, Fahal A, et al. Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. Lancet Infect Dis. 2004;4:566. [DOI] [PubMed] [Google Scholar]
- 4.Van de Sande WW. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahal AH. Mycetoma: review article. Khart Med J. 2011;4:514. [Google Scholar]
- 6.Fahal AH. Mycetoma: Clinico-pathological Monograph. 2006:Khartoum, Sudan; University of Khartoum Press, 23. [Google Scholar]
- 7.Fahal AH. Serrano JA, Sandoval AH, Beaman BL. Actinomycetoma in Africa. In: Actinomicetoma. 2006:Merida, Venezuela; 456. [Google Scholar]
- 8.Van de Sande WW, Fahal AH, Goodfellow M, et al. The mycetoma knowledge gap: identification of research priorities. PLoS Negl Trop Dis. 2014;8:e2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahal AH, Suliman SH. Clinical presentation of mycetoma. Sud Med J. 1994;32:46. [Google Scholar]
- 10.Ahmed AO, Abugroun ES. Unexpected high prevalence of secondary bacterial infection in patients with mycetoma. J Clin Microbiol. 1998;36:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fahal AH, el Hag IA, Gadir AF, et al. Blood supply and vasculature of mycetoma. J Med Vet Mycol. 1997;35:101. [DOI] [PubMed] [Google Scholar]
- 12.Abd Bagi ME, Fahal AH, Sheik HE, et al. Pathological fractures in mycetoma. Trans R Soc Trop Med Hyg. 2003;97:582. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed AO, Van de Sande WW, Fahal A, et al. Management of mycetoma: major challenge in tropical mycoses with limited international recognition. Curr Opin Infect Dis. 2007;20:146. [DOI] [PubMed] [Google Scholar]
- 14.Fahal AH, Sharfy AR. Vulval mycetoma: a rare cause of bladder outlet obstruction. Trans R Soc Trop Med Hyg. 1998;92:652. [DOI] [PubMed] [Google Scholar]
- 15.Fahal AH, Suliman SH, Gadir AF, et al. Abdominal wall mycetoma: an unusual presentation. Trans R Soc Trop Med Hyg. 1994;88:78. [DOI] [PubMed] [Google Scholar]
- 16.Arbab MA, el Hag IA, Abdul Gadir AF, et al. Intraspinal mycetoma: report of two cases. Am J Trop Med Hyg. 1997;56:27. [DOI] [PubMed] [Google Scholar]
- 17.Ezaldeen EA, Ahmed RM, El Dawi N, Fahal AH. Cervical spinal cord compression: a rare and serious complication of Actinomadura pelletieri actinomycetoma. JMM Case Rep. 2015 Sep. 14;2(5). [Google Scholar]
- 18.Mohamed EW, Suleiman HS, Fadella AI, Fahal AH. Aggressive perineal and pelvic eumycetoma: an unusual and challenging problem to treat. Khart Med J. 2012;2:771. [Google Scholar]
- 19.Fahal AH, Arbab MAR, EL Hassan AM. Aggressive clinical presentation of mycetoma due to Actinomadura pelletierii. Khart Med J. 2012; 5: 699. [Google Scholar]
- 20.Gumaa SA, Mahgoub ES, el Sid MA. Mycetoma of the head and neck. Am J Trop Med Hyg. 1986;35:594. [DOI] [PubMed] [Google Scholar]
- 21.Fahal AH, Yagi HI, el Hassan AM. Mycetoma-induced palatal deficiency and pharyngeal plexus dysfunction. Trans R Soc Trop Med Hyg. 1996;90:676. [DOI] [PubMed] [Google Scholar]
- 22.Shafei H, McCormick CS, Donnelly RJ. Madura foot of the chest wall; cure after radical excision. Thorac Cardiovasc Surg. 1992;40:198. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Jain K, Parmar C, et al. Mycetoma: nonvenereal perineal lesions. Indian J Sex Transm Dis AIDS. 2010;31:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gumaa SA, Satir AA, Shehata AH, et al. Tumor of the mandible caused by Madurella mycetomil. Am J Trop Med Hyg. 1975;24:471. [DOI] [PubMed] [Google Scholar]
- 25.Clarke PR. Mycetoma of the testis. Lancet. 1953;265:1341. [DOI] [PubMed] [Google Scholar]
- 26.Klossek JM, Serrano E, Péloquin L, et al. Functional endoscopic sinus surgery and 109 mycetomas of paranasal sinuses. Laryngoscope. 1997;107:112. [DOI] [PubMed] [Google Scholar]
- 27.Gueye NN, Seck SM, Diop Y, et al. [Orbital mycetoma: a case report]. J Fr Ophtalmol. 2013;36:435. [DOI] [PubMed] [Google Scholar]
- 28.Abd El-Bagi MEB, Fahal AH. Mycetoma revisited: incidence of various radiographic signs. Saudi Med J. 2009;30:529. [PubMed] [Google Scholar]
- 29.Fahal AH, Sheikh HE, EL Lider MA, et al. Ultrasonic imaging in mycetoma. Br J Surg. 1997;78:765. [Google Scholar]
- 30.EL Shamy ME, Fahal AH, Shakir MY, Homedia MM. New MRI grading system for the diagnosis and management of mycetoma. Trans R Soc Trop Med Hyg. 2012;106:738. [DOI] [PubMed] [Google Scholar]
- 31.EL Hag IA, Fahal AH, Gasim ET. Fine needle aspiration cytology of mycetoma. Acta Cytol. 1996;40:461. [DOI] [PubMed] [Google Scholar]
- 32.Fahal AH, el Toum EA, el Hassan AM, et al. The host tissue reaction to Madurella mycetomatis: new classification. J Med Vet Mycol. 1995;33:15. [PubMed] [Google Scholar]
- 33.Gumaa SA, Mahgoub ES. Counterimmunoelectrophoresis in the diagnosis of mycetoma and its sensitivity as compared to immunodiffusion. Sabouraudia. 1975;13:309. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed AO, Mukhtar MM, Kools-Sijmons M, et al. Development of a species-specific PCR-restriction fragment length polymorphism analysis procedure for identification of Madurella mycetomatis. J Clin Microbiol. 1999;37:3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahal AH. The management of mycetoma. Post-Graduate-Caribbean. 2000;16:229. [Google Scholar]
- 36.Fahal AH. Management of mycetoma. Expert Rev Dermatol. 2010;5:87. [Google Scholar]
- 37.Fahal AH, Hassan MA, Sanhouri M. Surgical treatment of mycetoma. Sud Med J. 1994;32:98. [Google Scholar]
- 38.Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone and in combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443. [DOI] [PubMed] [Google Scholar]
- 39.Fahal AH, Shaheen S, Jones DH. The orthopaedic aspects of mycetoma. Bone Joint J. 2014;96-B:420. [DOI] [PubMed] [Google Scholar]
- 40.Fahal AH. Evidence-based guidelines for the management of mycetoma patients. 2007Khartoum, Sudan: Mycetoma Research Centre. [Google Scholar]
- 41.Bendl BJ, Mackey D, Al-Saati F, et al. Mycetoma in Saudi Arabia. J Trop Med Hyg. 1987;90:51. [PubMed] [Google Scholar]
- 42.Fahal AH., Mycetoma Williams NS, Bulstrode CJK, O’Connell PR. In: Bailey and Love’s Short Practice of Surgery. 2013:26th ed Oxford, UK: Oxford University Press; 64. [Google Scholar]
- 43.Zein HA, Fahal AH, Mahgoub el S, et al. Predictors of cure, amputation and follow-up dropout among patients with mycetoma seen at the Mycetoma Research Centre, University of Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2012;106:639. [DOI] [PubMed] [Google Scholar]
- 44.Shaheen S. Patients with lower limb amputations for mycetoma in the National Center for Prosthetics and Orthotics in the Sudan. Khart Med J. 2008;1:27. [Google Scholar]
- 45.Gismalla MDA, Abdulla GM, Ali MM, et al. Wide surgical excision and reconstruction of eumycetoma in Gezira Mycetoma Centre. Glob J Med Res. 2106; 16. [Google Scholar]
- 46.Tamir G, Adler A, Hagler J, et al. Mycetoma of the foot: surgical treatment with free flap reconstruction. Eur J Plast Surg. 1995;18:124. [Google Scholar]
- 47.Maina AM, Macharia JT. Alleviating a nomad’s anguish: successful treatment of a case of leg mycetoma-a case report. Case Rep Orthop. 2012;2012:753174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conway H, Berkeley W. Chromoblastomycosis, mycetoma form, treated by surgical excision; correction of defect by cross leg pedicled flap. AMA Arch Derm Syphilol. 1952;66:695. [DOI] [PubMed] [Google Scholar]
- 49.Ali A, Khadda S, Yadav AK, et al. Giant eumycetoma gluteal region: excision and V-Y flap advancement. Inter J of Scien Rep. 2015;1:146. [Google Scholar]
- 50.Sankale-Diouf AA, Ndiaye A, Ndiaye M. [Value of the latissimus dorsi flap in sternal reconstruction. Report of a case of mycetoma operated in Dakar]. Ann Chir Plast Esthet. 1999;44:274. [PubMed] [Google Scholar]
- 51.Sharif HS, Clark DC, Aabed MY, et al. Mycetoma: comparison of MR imaging with CT. Radiology. 1991;178:865. [DOI] [PubMed] [Google Scholar]
- 52.Enneking WF, Spanier SS, Goodman MA. Current concepts review. The surgical staging of musculoskeletal sarcoma. J Bone Joint Surg Am. 1980;62:1027. [PubMed] [Google Scholar]
- 53.Estrada-Chavez GE, Vega-Memije ME, Arenas R, et al. Eumycotic mycetoma caused by Madurella mycetomatis successfully treated with antifungals, surgery, and topical negative pressure therapy. Inter J Dermatol. 2009;48:401. [DOI] [PubMed] [Google Scholar]
- 54.Kalender AM, Baykan H, Özkan F, et al. Negative pressure wound therapy and skin graft in Madura foot treatment. Balkan Med J. 2012; 29: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cipolla J, Baillie DR, Steinberg SM, et al. Negative pressure wound therapy: unusual and innovative applications. OPUS. 2008;12:15. [Google Scholar]
- 56.Van der Velde M, Hudson DA. VADER (vacuum-assisted dermal recruitment): a new method of wound closure. Ann Plast Surg. 2005;55:660. [DOI] [PubMed] [Google Scholar]
- 57.Weed T, Ratliff C, Drake DB. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg. 2004;52:276; discussion 279. [DOI] [PubMed] [Google Scholar]
- 58.Masoodi Z, Mansoor T, Ali WM, et al. Actinomycetoma leg ulcers in north India. Wounds. 2014;26:147. [PubMed] [Google Scholar]


