Supplemental Digital Content is available in the text.
SUMMARY:
A 59-year-old woman with a history of cosmetic implants developed ipsilateral synchronous breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) and invasive ductal carcinoma in the left breast. Each tumor was subjected to next-generation sequencing, and separate analyses revealed mutually exclusive aberrations: an activating STAT3 mutation in the lymphoma and a PIK3CA in-frame deletion in the carcinoma. The patient was treated with removal of implants, capsulectomy, partial mastectomy, sentinel node biopsy, radiotherapy, and endocrine therapy with no evidence of recurrence for 1 year. This case illustrates the importance of obtaining thorough evaluation for concomitant malignancies in the breast at the time of diagnosis of BIA-ALCL. Herein, we review the current recommendations for evaluation and management of BIA-ALCL.
CASE REPORT
The patient is a 59-year-old woman who had cosmetic augmentation with Allergan 468 textured saline implants at age 41. In subsequent years, she underwent routine screening mammography according to recommended guidelines. Eighteen years after implant augmentation, she developed swelling of the left breast. Ultrasonography identified a periprosthetic fluid collection; aspiration of the seroma produced murky, yellow fluid. Cytologic analysis showed an atypical lymphocyte proliferation positive for CD3, CD30, and CD4, and negative for anaplastic lymphoma kinase (ALK), CD2, and CD5 by immunohistochemistry. Whole body positron emission tomography with computed tomography (PET-CT) showed a nonavid seroma surrounding a deflated left breast implant, but no masses or other suspicious findings (Fig. 1A). Her previous screening mammogram 6 months earlier had been negative. She underwent implant removal with capsulectomy which showed clusters of atypical pleomorphic cells with prominent nucleoli and horseshoe-shaped nuclei (hallmark cells) below the surface of the capsule with focal early invasion (Fig. 1B). The atypical cells showed positive immunohistochemical staining of CD30 (see figure, Supplemental Digital Content 1, which displays the BIA-ALCL was positive for CD30 by immunohistochemistry, http://links.lww.com/PRSGO/B33), CD3 (subset weak), CD4, T-cell intracytoplasmic antigen (TIA-1, subset), and epithelial membrane antigen (EMA), and were negative for CD2, CD5, CD7, CD8, ALK, CD15, B-cell markers, and Epstein-Barr virus (EBV)-encoded RNA in situ hybridization, consistent with ALK-negative breast implant–associated anaplastic large cell lymphoma (BIA-ALCL). The clinicopathologic stage was pT2.1
Fig. 1.
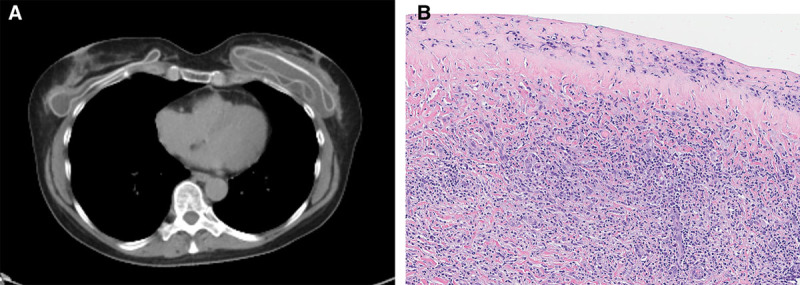
Breast implant-associated anaplastic large cell lymphoma. A, CT imaging showed seroma surrounding deflated left breast implant. B, Hematoxylin and eosin–stained section of capsulectomy specimen with BIA-ALCL underlying the surface.
Six months later she underwent screening mammography prior to the planned implant replacement and was found to have a spiculated mass in the upper outer left breast (Fig. 2A); this was not seen on the prior mammogram 12 months earlier. Core needle biopsy showed invasive ductal carcinoma (IDC), which was estrogen receptor positive, progesterone receptor positive, and HER2 negative. Diagnosed within 6 months of one another, the patient’s 2 tumors may be considered synchronous.2 With a family history of leukemia and cancer of the colon, lung, and esophagus, the patient underwent germline testing with a panel of 85 breast cancer-associated genes (Invitae, San Francisco, Calif.). No pathogenic or likely pathogenic germline aberrations were detected. She then underwent left partial mastectomy, sentinel lymph node biopsy, and bilateral oncoplastic mammoplasty. Pathology showed a 1.2-cm grade 2 IDC (Fig. 2B) with negative margins, 1 intramammary lymph node with micrometastatic carcinoma, and 2 negative sentinel nodes. The pathologic stage was pT1cN1mi(sn). Oncotype Dx testing of the IDC showed a low-risk recurrence score of 18. She was treated with hypofractionated whole breast radiotherapy with tangents to the axilla, and an aromatase inhibitor.
Fig. 2.
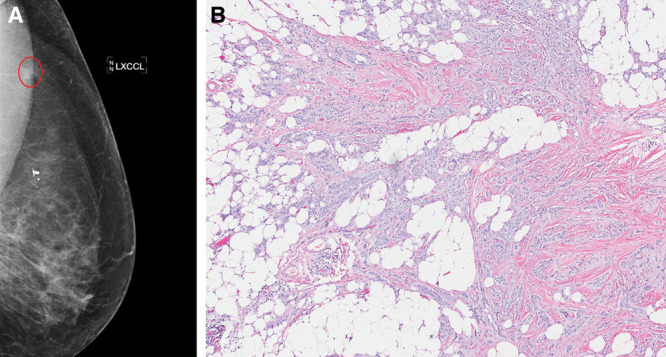
Invasive ductal carcinoma. A, Left diagnostic mammogram showing spiculated mass (circled) on craniocaudal view. B, Hematoxylin and eosin–stained section of partial mastectomy specimen with grade 2 IDC.
After obtaining informed consent, we used capture-based next-generation sequencing to more comprehensively characterize both the BIA-ALCL and the IDC. This assay (UCSF500 panel) targets the coding regions of 479 cancer-related genes, select introns from 41 genes, and the TERT promoter (see table, Supplemental Digital Content 2, which displays 479 cancer-related genes on the UCSF500 panel, http://links.lww.com/PRSGO/B34).
Sequencing libraries were prepared from genomic DNA of tumor and matched normal formalin-fixed, paraffin-embedded tissue extracted from unstained sections. Target enrichment was performed by hybrid capture using a custom oligonucleotide library. Sequencing was performed on a HiSeq 2500 (Illumina, San Diego, Calif.). In the BIA-ALCL, a pathogenic missense mutation in STAT3 (p.S614R) was identified (Fig. 3A and B). Confirmatory immunohistochemistry for phospho-STAT3 (Tyr705) highlighted the tumor cells (Fig. 3C). Copy number analysis showed chromosomal gains in 12p and 21q. Analysis of the IDC showed a likely pathogenic and activating in-frame deletion in PIK3CA (p.G106_R108del) (Fig. 4A and B). Numerous partial chromosomal gains and losses were identified; no focal amplifications or deep deletions were detected. No pathogenic or likely pathogenic germline alterations were seen in the normal tissue. No somatic variants were shared between the two malignancies (see table, Supplemental Digital Content 3, which displays all somatic variants detected in the BIA-ALCL, http://links.lww.com/PRSGO/B35; and see table, Supplemental Digital Content 4, which displays all somatic variants detected in the IDC, http://links.lww.com/PRSGO/B36).
Fig. 3.
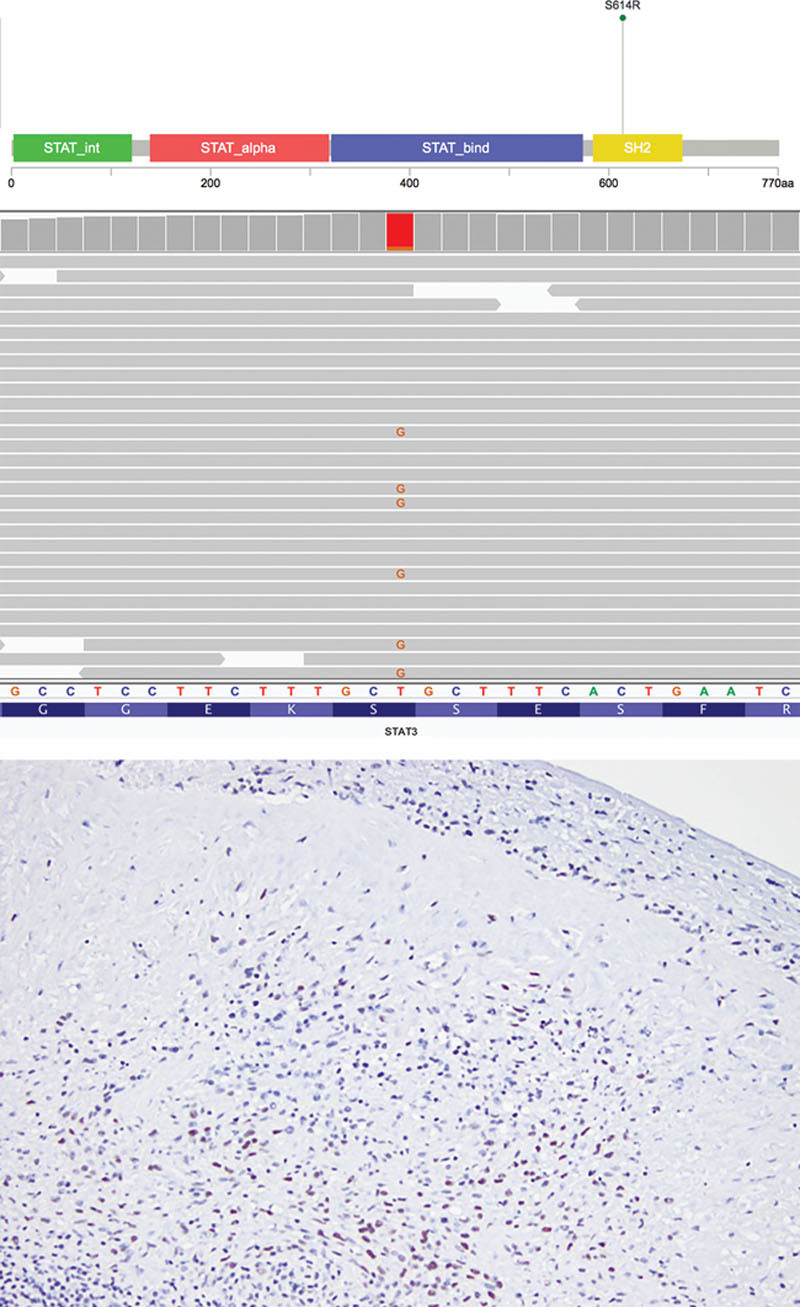
STAT3 alteration in breast implant-associated anaplastic large cell lymphoma. A, Lollipop plot and (B) Integrative Genomics Viewer depiction of STAT3 p.S614R variant in BIA-ALCL. Lollipop plot was modified from cBioPortal.3 C, The BIA-ALCL was positive for phospho-STAT3 (Tyr705) by immunohistochemistry.
The mean target sequencing coverage was 68 and 711 unique reads per target interval for the BIA-ALCL and IDC, respectively.
EVALUATION AND MANAGEMENT OF BIA-ALCL
ALCL is a rare entity, characterized by CD30 positivity and classified by clinical presentation (systemic versus cutaneous) and the presence or absence of rearrangements of ALK.4 In 1997, the first case of ALCL occurring in the periprosthetic tissue surrounding a breast implant was reported.5 Since then, numerous reports of a new clinicopathologic entity, BIA-ALCL, have been published, with approximately 500 cases reported worldwide.6,7 Although the etiology is unclear, BIA-ALCL is associated with textured implants and the presence of bacterial biofilm.8 The typical presenting symptom is a late periprosthetic seroma, but the detection of a mass, capsular contracture, axillary lymphadenopathy, B-type symptoms, and skin lesions have also been noted.6 Although the systemic form of ALK-negative ALCL typically has a poor prognosis, BIA-ALCL confined to the periprosthetic fluid appears to have favorable outcomes in most cases.7
Recent work has begun to characterize the molecular landscape of BIA-ALCL. The largest series to date showed that all cases of BIA-ALCL were negative for the alterations reported in other ALCL subtypes, namely, rearrangements in ALK, DUSP22, and TP63.9 Instead, alterations in JAK-STAT genes are relatively common (27% of cases), and the STAT3 missense variant we identified (p.S614R) has been previously reported in 3 other cases of BIA-ALCL and in the TLBR1 BIA-ALCL cell line.9–12 Located in the SH2 domain, this gain-of-function mutation results in enhanced transcriptional activity of STAT3. Also reported in systemic ALK-negative and cutaneous ALCL, STAT3 mutations are increased in malignancies associated with persistent immune stimulation.7,11,13 Although the pathogenesis of BIA-ALCL is unknown, the presence of inflammatory cytokines such as IL-6 and IL-13 suggests a component of allergy and aberrant immune response in its development.7,13
Profiling of the synchronous IDC showed no common genetic aberrations with the BIA-ALCL, suggesting different mechanisms of pathogenesis. The IDC harbored a PIK3CA alteration, which is seen in approximately 30% of breast cancers, and more commonly in estrogen receptor-positive tumors.14 Aberrant PIK3CA activation drives cellular proliferation and survival, and gain-of-function mutations are able to transform normal breast epithelial cells to carcinoma. Currently, trials of PI3K inhibitors for advanced/metastatic breast cancer are ongoing.15 Interestingly, PIK3CA-mutant breast cancer mouse models show upregulated STAT3 signaling compared to other mouse models of breast carcinogenesis, and inhibition of STAT3 sensitizes tumor cells to PI3K inhibitors.16
Awareness of the initial evaluation and management of BIA-ALCL, and the possibility of discovering concomitant malignancies are critical for plastic surgeons. Although early treatment of BIA-ALCL was variable, efforts by the Food and Drug Administration (FDA), implant manufacturers, national professional organizations, and physician scientists across many specialties have led to multidisciplinary consensus guidelines to aid in diagnosis, treatment, and tracking of this disease. In 2017, the National Comprehensive Cancer Network gathered medical, surgical, and radiation oncologists and plastic surgeons to create guidelines to better standardize the management of BIA-ALCL.
Preoperative Consultation
The initial consultation with a patient considering prosthetic breast reconstruction or augmentation should include a discussion about the risk of BIA-ALCL.17 The risk is thought to be equal between reconstructive and cosmetic patients and has variable incidence based on geographic location.18,19 Currently, the lifetime risk for developing BIA-ALCL in women with textured implants is between 1 in 4,000 and 30,000 in the United States, but may be as high as 1 in 1,000 in Australia.20 This risk is much lower than the background risk of breast cancer in women, estimated at 12.4%, or the risk of recurrence in patients who have had a mastectomy.21
Intraoperative Decision Making
In both patients undergoing augmentation mammoplasty and patients undergoing prosthetic breast reconstruction, the risk of subsequently developing BIA-ALCL appears to be similar.1 Currently, no data on implant location (submuscular or prepectoral) exist to dictate whether the plane of implant placement affects risk.22 Of the over 500 cases reported to date, only patients who have had a textured implant or expander have been confirmed to develop BIA-ALCL.23 Therefore, consideration should be paid to whether the benefits of implant texturing are worth the increased risk of developing this rare disease. As inflammation possibly from bacterial contamination at the time of surgery is thought to play a role in the development of BIA-ALCL, a 14-point intraoperative plan has been suggested which outlines various steps throughout a case that may help reduce the risk of contamination.24
Standard Postoperative Care
After routine postoperative care, there are no specific guidelines for routine monitoring to detect BIA-ALCL. Existing FDA recommendations suggest that patients with silicone implants undergo screening for silent rupture with magnetic resonance imaging of the breast at 3 years after placement, followed by every 2 years.25 Mammography should be obtained for breast cancer screening or surveillance based on prior oncologic history.26 Patients should receive education about the natural history of BIA-ALCL, typically presenting as a late-onset fluid collection, asymmetric swelling, mass, or skin changes any time after 1 year from implant placement.27,28 Although more common causes such as trauma or infection can result in these changes, and the absolute risk of ALCL in patients with these findings is low, patients should be instructed to reach out to their plastic surgeon for comprehensive evaluation.
Patients presenting for evaluation of suspected BIA-ALCL should have a thorough surgical and oncologic history obtained with the dates and specifics of procedures and treatments including what type of implant was placed. Clinical history of the changes that prompted evaluation should also be addressed. Physical exam should evaluate the chest for implant asymmetry, malposition, presence of a clinically palpable effusion or mass, skin changes, and regional adenopathy. Any abnormality should prompt subsequent imaging.29
Imaging Studies
The initial diagnostic test of choice is breast ultrasound to evaluate for periprosthetic fluid collection, mass, or regional adenopathy.26,28–30 The sensitivity of detection of a fluid collection in a patient with BIA-ALCL is 84% based on one retrospective study, and lower for detection of a mass at 46%. The specificity for effusion and mass detection were 75% and 100%, respectively.31 If an effusion is found, fluid should be aspirated and sent for evaluation. At least 20 ml of fluid should be sampled if possible, but volumes of 50–100 ml are ideal to decrease the risk of false negative or indeterminate findings.26 Fluid should be sent for culture and Gram stain, cell block cytology, immunohistochemistry, and flow cytometry.28–30 Alerting the pathologist that BIA-ALCL is a diagnostic consideration is important to ensure appropriate tests (including comprehensive flow cytometric markers) are done.
If ultrasound evaluation is inconclusive, breast magnetic resonance imaging can be considered because it is the next most sensitive imaging test for effusion.31 If a mass is found, biopsy should be performed. CT and mammography lack sufficient sensitivity and specificity to recommend their use in the workup of BIA-ALCL. However, PET should be considered in selected cases to provide information about the extent of local disease and the presence of metastatic disease once diagnosis of BIA-ALCL is made.26,29–31 Current guidelines for the workup of BIA-ALCL do not include the use of mammography in the preoperative evaluation.30 However, the diagnosis of ipsilateral IDC 6 months later in this patient suggests that thorough diagnostic breast imaging including mammography should be obtained prior to breast surgery. Although impossible to know whether mammography at the time of BIA-ALCL diagnosis would have identified the IDC, PET/CT is not a reliable replacement for thorough diagnostic workup of the breast, as the sensitivity for detection of primary breast tumors is reported to be only 68% for tumors less than 2 cm in size.32
Consultations
Patients with negative findings for lymphoma on initial workup may be appropriately managed by a plastic surgeon for management of seroma or infection. However, an indeterminate diagnosis on initial pathology warrants additional workup. This may include having the slides sent to an outside pathologist or referral to a tertiary care center, either of whom with experience in treatment of BIA-ALCL.30 A diagnosis of lymphoma should result in referral to a center with a multidisciplinary team of medical, surgical, and radiation oncologists, plastic surgeons, and hematopathologists.1,6
Surgery
Removal of the implant and total capsulectomy remains the mainstay of therapy for BIA-ALCL and is curative in a majority of cases.1,31 Consideration should be given to having a surgical oncologist present at the time of surgery, especially with extensive disease or need for lymph node surgery. Sentinel lymph node biopsy is not routinely recommended given the variable drainage patterns of the large breast implant capsule, but the removal of suspicious lymph nodes at the time of implant removal is recommended.1,26,27,30 Removal of the contralateral breast implant at the time of surgery is not mandated by guidelines, but reports of occult contralateral BIA-ALCL in patients undergoing implant removal with preoperatively diagnosed lymphoma should be discussed with patients.33 There are sparse studies evaluating the optimal timing of implant replacement following removal for BIA-ALCL. Early data suggest immediate replacement is safe and has some advantages over delayed replacement, but surgeons should choose smooth implants and treat every patient on a case-by-case basis.33
Adjuvant Therapy
For patients with BIA-ALCL confined to the capsule or implant and who complete surgical resection with no residual disease, adjuvant therapy is not currently recommended. For patients with evidence of lymphoma extending beyond the implant capsule or residual disease in the breast due to inability to completely resect the tumor, adjuvant therapy is indicated.1,29,33 Adjuvant therapy decisions should be made in a multidisciplinary setting on a case-by-case basis, but in general radiation therapy (24–36 Gy) is advocated for residual local disease in patients without a history of prior chest wall radiation, and chemotherapy is advocated for patients with positive lymph nodes or evidence of spread to distant sites. The most common chemotherapeutic agents or regimens include brentuximab vedotin, a monoclonal antibody targeting CD30 with individual case reports demonstrating efficacy in BIA-ALCL, or chemotherapy combinations recommended for peripheral T-cell lymphomas such as cyclophosphamide, doxorubicin, vincristine, and prednisone or variations thereof.26,34–36
Follow-up
After primary management, National Comprehensive Cancer Network guidelines recommend surveillance for recurrence of BIA-ALCL for a minimum of 2 years with physical examination and, if indicated, CT chest/abdomen/pelvis or whole body PET/CT every 6 months, after which patients should resume routine breast cancer screening.30 The development of recurrent clinical findings in the breast at any point should prompt referral back to their plastic surgeon, and warrants repeat diagnostic imaging.
Reporting
All cases of BIA-ALCL should be reported to the FDA through their MedWatch adverse events reporting program. In addition, the Plastic Surgery Foundation has set up a registry called Patient Registry and Outcomes For breast Implants and ALCL etiology and Epidemiology (PROFILE) that helps track and better understand the causes and treatments of BIA-ALCL.19
CONCLUSIONS
To the best of our knowledge, this is the first report of a patient with synchronous BIA-ALCL and IDC in the same breast. This case illustrates the importance of evaluating for additional malignancies in the breast when a diagnosis of BIA-ALCL is made; diagnostic mammogram at the time of workup of BIA-ALCL may be a useful adjunct to ultrasound and PET/CT to identify additional breast malignancies prior to surgery. Future studies may identify susceptibility factors and further elucidate the potential relationship between BIA-ALCL and IDC.
Fig. 4.
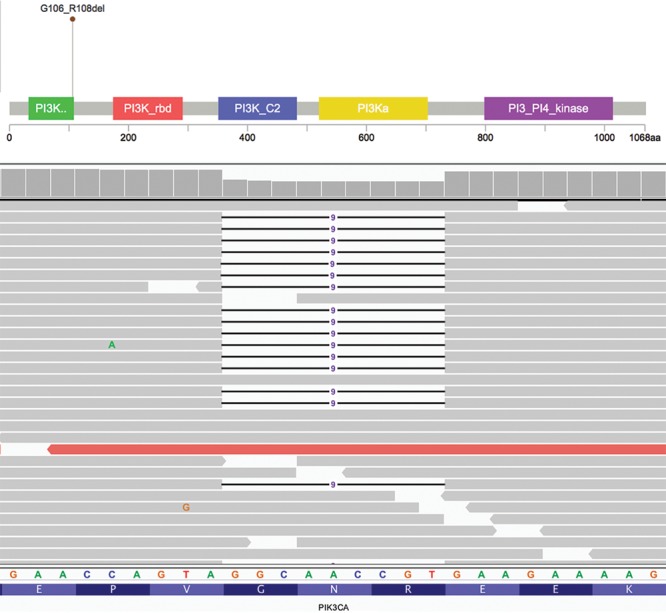
PIK3CA alteration in invasive ductal carcinoma. A, Lollipop plot and (B) Integrative Genomics Viewer depiction of PIK3CA p.G106_R108del variant in IDC. Lollipop plot was modified from cBioPortal.3
Footnotes
Published online 4 April 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Clemens MW, Medeiros LJ, Butler CE, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Testori A, Cioffi U, De Simone M, et al. Multiple primary synchronous malignant tumors. BMC Res Notes. 2015;8:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.cBioPortal for Cancer Genomics. Available at http://www.cbioportal.org. Accessed August 28, 2018.
- 4.Jewell M, Spear SL, Largent J, et al. Anaplastic large T-cell lymphoma and breast implants: a review of the literature. Plast Reconstr Surg. 2011;128:651. [DOI] [PubMed] [Google Scholar]
- 5.Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554. [DOI] [PubMed] [Google Scholar]
- 6.Leberfinger AN, Behar BJ, Williams NC, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. JAMA Surg. 2017;152:1161. [DOI] [PubMed] [Google Scholar]
- 7.Kadin ME, Morgan J, Xu H, et al. IL-13 is produced by tumor cells in breast implant-associated anaplastic large cell lymphoma: implications for pathogenesis. Hum Pathol. 2018;78:54. [DOI] [PubMed] [Google Scholar]
- 8.Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137:1659. [DOI] [PubMed] [Google Scholar]
- 9.Oishi N, Brody GS, Ketterling RP, et al. Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood. 2018;132:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blombery P, Thompson ER, Jones K, et al. Whole exome sequencing reveals activating JAK1 and STAT3 mutations in breast implant-associated anaplastic large cell lymphoma anaplastic large cell lymphoma. Haematologica. 2016;101:e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Napoli A, Jain P, Duranti E, et al. Targeted next generation sequencing of breast implant-associated anaplastic large cell lymphoma reveals mutations in JAK/STAT signalling pathway genes, TP53 and DNMT3A. Br J Haematol. 2018;180:741. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Zhang Y, Petrus MN, et al. Cytokine receptor signaling is required for the survival of ALK- anaplastic large cell lymphoma, even in the presence of JAK1/STAT3 mutations. Proc Natl Acad Sci U S A. 2017;114:3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechner MG, Megiel C, Church CH, et al. Survival signals and targets for therapy in breast implant-associated ALK–anaplastic large cell lymphoma. Clin Cancer Res. 2012;18:4549. [DOI] [PubMed] [Google Scholar]
- 14.Miller TW. Initiating breast cancer by PIK3CA mutation. Breast Cancer Res. 2012;14:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massacesi C, Di Tomaso E, Urban P, et al. PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. Onco Targets Ther. 2016;9:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merino VF, Cho S, Liang X, et al. Inhibitors of STAT3, β-catenin, and IGF-1R sensitize mouse PIK3CA-mutant breast cancer to PI3K inhibitors. Mol Oncol. 2017;11:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clemens MW, Miranda RN, Butler CE. Breast implant informed consent should include the risk of anaplastic large cell lymphoma. Plast Reconstr Surg. 2016;137:1117. [DOI] [PubMed] [Google Scholar]
- 18.Loch-Wilkinson A, Beath KJ, Knight RJW, et al. breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140:645. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Available at https://www.fda.gov/MedicalDevices/ProductsandMedical/Procedures/ImplantsandProsthetics/BreastImplants/ucm239995.htm. Accessed October 15, 2018.
- 20.Australian Government Department of Health Therapeutic Goods Administration. Breast Implants and Anaplastic Large Cell Lymphoma: Update: Additional Confirmed Cases of Anaplastic Large Cell Lymphoma. Available at https://www.tga.gov.au/alert/breast-implants-and-anaplastic-large-cell-lymphoma. Accessed October 15, 2018.
- 21.Yi M, Kronowitz SJ, Meric-Bernstam F, et al. Local, regional, and systemic recurrence rates in patients undergoing skin-sparing mastectomy compared with conventional mastectomy. Cancer. 2011;117:916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim B, Predmore ZS, Mattke S, et al. Breast implant-associated anaplastic large cell lymphoma: updated results from a structured expert consultation process. Plast Reconstr Surg Glob Open. 2015;3:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;135:695. [DOI] [PubMed] [Google Scholar]
- 24.Deva AK, Adams WP, Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132:1319. [DOI] [PubMed] [Google Scholar]
- 25.Risks of Breast Implants. Available at https://www.fda.gov/MedicalDevices/Productsand MedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm064106.htm#Rupture_Silicone_Gel-Filled. Accessed October 15, 2018.
- 26.Mehta-Shah N, Clemens MW, Horwitz SM. How I treat breast implant-associated anaplastic large cell lymphoma. Blood. 2018;132:1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santorelli A, Avvedimento S, Abbadessa A. MBN 2016 Aesthetic breast meeting BIA-ALCL consensus conference report: genetic markers for early detection of breast implant-associated anaplastic large cell lymphoma in plastic surgery procedures. Plast Reconstr Surg. 2018;142:968e. [DOI] [PubMed] [Google Scholar]
- 28.O’Neill AC, Zhong T, Hofer SOP. Implications of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) for breast cancer reconstruction: an update for surgical oncologists. Ann Surg Oncol. 2017;24:3174. [DOI] [PubMed] [Google Scholar]
- 29.Clemens MW, Horwitz SM. NCCN Consensus Guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J. 2017;37:285. [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network. T-Cell Lymphomas. Available at https://www.nccn.org/professionals/physician_gls/default.aspx#t-cell. Accessed October 15, 2018. [Google Scholar]
- 31.Adrada BE, Miranda RN, Rauch GM, et al. Breast implant-associated anaplastic large cell lymphoma: sensitivity, specificity, and findings of imaging studies in 44 patients. Breast Cancer Res Treat. 2014;147:1. [DOI] [PubMed] [Google Scholar]
- 32.Yang SK, Cho N, Moon WK. The role of PET/CT for evaluating breast cancer. Korean J Radiol. 2007;8:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shine JJ, Boghossian E, Beauchemin G, et al. Breast implant-associated anaplastic large cell lymphoma: immediate or delayed implant replacement? Aesthetic Plast Surg. 2018;42:1492. [DOI] [PubMed] [Google Scholar]
- 34.Pro B, Advani R, Brice P, et al. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130:2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson K, Alrifai T, Grant-Szymanski K, et al. Breast implant-associated anaplastic large-cell lymphoma and the role of brentuximab vedotin (SGN-35) therapy: A case report and review of the literature. Mol Clin Oncol. 2017;6:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alderuccio JP, Desai A, Yepes MM, et al. Frontline brentuximab vedotin in breast implant-associated anaplastic large-cell lymphoma. Clin Case Rep. 2018;6:634. [DOI] [PMC free article] [PubMed] [Google Scholar]


