Abstract
Objectives
Catathrenia (sleep groaning) is an uncommon disorder and poorly understood disorder characterized by groaning during sleep occurring in tandem with prolonged expiration. Its classification, pathogenesis and clinical relevance remain debated, at least partially due to the low number of cases in the reported literature. We report a series of 38 consecutive cases, presenting their clinical and polysomnographic characteristics, and their clinical management.
Methods
We identified 38 consecutive patients with catathrenia who had undergone full polysomnography. Catathrenia events (CEs) were examined in clusters, formulating catathrenia periods (CPs). The relationship between CPs, sleep stage distribution, EEG arousals and other poysomnographic parameters was assessed, along with the clinical presentation and management of catathrenic patients.
Results
A total of 427 CPs were identified, occurring predominantly in REM sleep (81%). 84% of the CPs were associated with an arousal preceding or coinciding with their onset and were of longer duration than those with no associated EEG arousal (57.3±56.8 vs 32.2±29.4 sec, p<0.001). Each CE had a characteristic airflow signal, with inspiration preceding a protracted expiration, followed by a brief expiration and subsequent deep inspiration. Although the majority of patients were referred on the basis of bed-partner complaints, 44.7% complained of daytime sleepiness. CPAP and sleep-consolidating pharmacotherapy lead to subjective improvement, but were limited by poor long-term adherence.
Conclusions
In the largest series of catathrenia patients reported to date, we found that this rare disorder is characterized by a distinct breathing pattern, occurring predominantly in REM sleep, with arousals almost uniformly preceding or coinciding with the onset of catathrenia periods.
Introduction
Catathrenia is an uncommon and poorly understood phenomenon, characterized by groaning and prolonged expiration during sleep. Derived from the Greek Kata (to bellow) and Threnia (to lament, and initially described by De Roeck and van Hoof in 1983(1), less than 100 cases of catathrenia have been reported in the published literature to date (1–9). Partially as a result of this, its pathogenesis and appropriate classification continues to be debated (10–12): having previously been categorised as a REM-related parasomnia (13), in the most recent iteration of the ICSD, catathrenia was re-classified as a respiratory disorder (14). Similarly, its clinical relevance – beyond the discomfiture of bed-partners – remains largely unexplored. Consequently, larger cases series are required to better characterize the disorder, and to identify any potential daytime symptoms that may be associated with it.
A typical polysomnographic description of catathrenia includes a deep inhalation followed by a protracted exhalation, which presents with a respiratory pattern of bradypnea resembling central apneas, during which moaning or groaning sounds are produced, usually lasting between 2 and 49 sec(13). The prevalence of this condition is unknown.
The first descriptions of catathrenia were during REM sleep (2), but other authors have since established that catathrenia events can continue, or even predominantly occur, in NREM(15, 16). Its exact pathogenesis remains uncertain, although authors have described a subtotal closure of the glottis during the event (17), or an association with electroencephalographic (EEG) arousals(2, 18).
Differential diagnoses of catathrenia include respiratory disorders (snoring, stridor, sleep-related laryngospasm, central sleep apnoea or others), sounds induced by seizures or parasomnias, such as sleep talking. How – or indeed if – catathrenia should be treated is unclear; the use of Continuous Positive Airway Pressure (CPAP) and sedative pharmacotherapy has been described, with rather variable results(2, 6, 9, 19, 20).
In this study we present the clinical and polysomnographic characteristics of what is to our knowledge the largest series of patients with catathrenia described to date.
Material and Methods
Patient Selection
Using an internal sleep laboratory database, we retrospectively identified patients who either had been referred for nocturnal polysomnography (NPSG) for possible catathrenia following a consultation with an experienced sleep physician, or who were unexpectedly diagnosed with catathrenia on the basis of their NPSG. All patients undergoing attended inpatient NPSG from x to xx were considered eligible for inclusion. Subjects with a clinical suspicion of catathrenia at clinic review, but who did not have NPSG confirmation of the disorder, were excluded from the analysis. Only patients undergoing full NPSG were included in the final analysis.
Sleep study methodology
Attended inpatient NPSG was performed using the standard EEG montage, with sleep stages scored according to the standard criteria of the American Academy of Sleep Medicine (AASM). Continuous recordings included electro-oculography, electrocardiography, submental and leg electromyography, pulse oximetry, nasal pressure cannulae, and respiratory inductance plethysmography with chest and abdominal belts(21). Time time-synchronized video recordings were also performed, while audio was captured by a multi-directional microphone placed above the patient.
The scoring of arousals was based on the AASM guidelines and the arousal index (AI) was reported as the number of arousals per hour of sleep. Apneas and hypopneas were scored following standard definitions to determine the apnea-hypopnea index (AHI)(21).
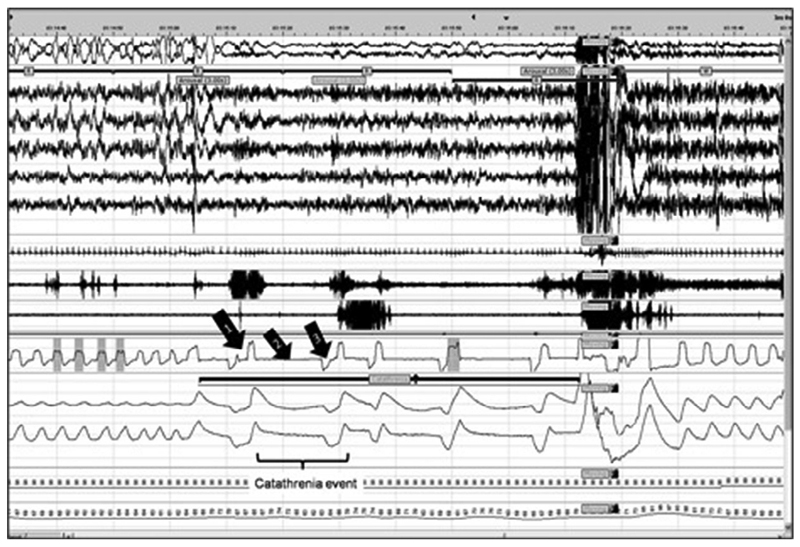
In line with previous reports(2), a catathrenia event (CE) was defined as an inspiration preceding a groaning/bradypneic event, which was followed by a brief expiration and a deep inspiration (Figure 1). These breathing events were frequently, but not uniformly, associated with groaning noises of varying amplitudes (Supplement 1). The CEs were counted and analyzed as part of clustered events, or catathrenia periods (CPs). The distribution of CPs across sleep stages, their duration, and their association with arousals were ascertained, patients’ presenting complaints were noted, and methods and outcomes of any treatment were recorded.
Figure 1. Breathing pattern during catathrenia in REM sleep and post arousal event.
Purple line indicates a catathrenia period, consisting of catathrenia events (bracket). Arrow 1: inspiration, Arrow 2: protracted expiration, Arrow 3: brief expiration. From top to bottom: electro-oculography (EOG) with rapid eye movements, electroencephalography (EEG) with C4-M1, F3-M2, F4-M1, O1-M2, O2-M1, electrocardiography (ECG), chin electromyography, left-leg tibialis electromyography, oronasal flow, chest and abdomen inductance plethysmography, pulse oximetry, pulse rate.
Statistical analysis
Statistical analysis was performed using the SPSS statistical analysis program (SPSS 21.0, IBM). Data are reported as mean ± standard deviation (SD) if not otherwise indicated. In case analysis, the role of arousals and sleep stages in catathrenia and their correlation with CP duration were analyzed using chi-square and t-test where applicable.
Results
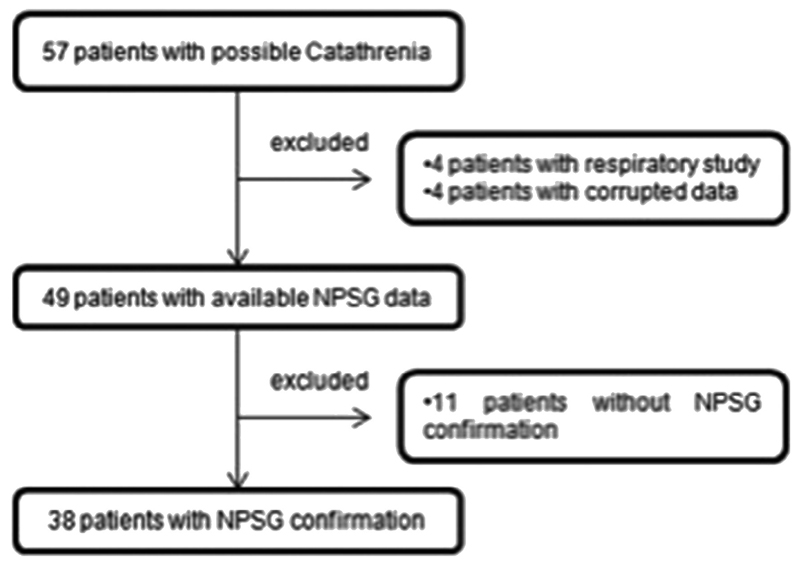
A total of 57 patients were identified with possible catathrenia, 38 of whom had polysomnographic confirmation (Figure 2). Clinical characteristics and sleep parameters of the study cohort are presented in table 1. There was a slight male predominance, with an average age at diagnosis of 33.1 ± 7.7 years. Marginal subjective sleepiness was reported. Sleep efficiency was relatively reduced with a marginally increased arousal index. 12 patients had an AHI>5 events/h (mean AHI in these patients 12.6±6.9).
Figure 2. Flow chart of the sampling methods.
Table 1. Characteristics of study cohort.
| Clinical characteristics | |
|---|---|
| Males / Females | 23/15 |
| Age (years) | 33.1 ± 7.7 |
| BMI (kg/m2) | 25.9 ± 5.3 |
| ESS | 10.1 ± 5.3 |
| Sleep parameters | |
| TST (min) | 372.7 ± 68.1 |
| SE (%) | 80.4 ± 12.5 |
| AHI (events/h) | 5.1 ± 7.5 |
| PLMI (events/h) | 10.7 ± 18.2 |
| 4% ODI (events/h) | 3.4 ± 5.8 |
| Arousal Index (events/h) | 16.6 ± 6.6 |
| NREM% of TST | 78.1 ± 7.4 |
| REM% of TST | 20.5 ± 5.8 |
| Mean SpO2% | 93.3 ± 1.6 |
Values are mean ± SD (Standard Deviation). BMI: Body Mass Index. TST: total sleep time, SE: sleep efficiency, ESS: Epworth Sleepiness Scale score, PLMI: periodic limb movement index, ODI: oxygen desaturation index, REM: rapid eye movement sleep, SpO2: peripheral oxygen saturation, NREM: non-REM sleep
Patients presented with a wide range of symptoms and complaints (Table 2); as expected, the majority of these were obtained from their bedpartners' descriptions, with moaning and breath holding described in more than half of the patients. However, a substantial proportion of patients complained of significant daytime symptoms, with almost half reporting subjective daytime sleepiness (ESS >10; 44.7%).
Table 2. Frequency of major presenting complaints among catathrenia patients.
| Symptom | Frequency |
|---|---|
| Moaning | 52.6 % |
| Breath holding | 50.0 % |
| Snoring | 18.4 % |
| Sleepiness (ESS>10) | 44.7 % |
| Humming | 5.2 % |
| Unrefreshing sleep | 7.8 % |
| Grunting | 5.2 % |
| Sleepwalking | 5.2 % |
Catathrenia periods varied significantly in frequency and duration between patients: the number of CPs identified on NPSG ranged between 1-40 events per patient, with a mean of 11.4±10.5 events/patient and a mean duration of 53.2±46.5 sec [range: 10.0-298.0 sec].
Correlations were assessed between CP duration, and the number of CPs each patient experienced, and sleep and demographic parameters as shown in Table 3. Increasing AHI and PLMI were both associated with a greater CP duration (r=0.71, p<0.001 and r=0.57, p=0.001, respectively). 81% of CPs arose from REM sleep, with an EEG arousal preceding or coinciding with the onset of 85% (363/427) of the CPs seen (Table 4). Further analysis revealed that the average duration of CPs associated with an arousal was significantly longer (57.3±56.8 sec) than those not associated with a change in sleep stage (32.2±29.4 sec) (p<0.001). The mean duration of CPs arising from REM or NREM did not differ significantly (52.2±52.5 vs 57.4±60.3, p=0.12).
Table 3. Clinical and sleep related correlations with catathrenia Period number and duration.
| Catathrenia periods (r) | CP length (r) | |
|---|---|---|
| BMI | -0.240 | 0.094 |
| TST (min) | -0.220 | -0.213 |
| NREM (%) of TST | -0.397* | 0.358* |
| REM (%) of TST | -0.245 | -0.404 |
| AHI (events/h) | -0.101 | 0.710# |
| PLMI (events/h) | -0.173 | 0.572# |
| 4% ODI (events/h) | -0.274 | 0.353* |
| SE (%) | 0.213 | -0.121 |
| Arousal index (events/h) | 0.023 | -0.234 |
| Mean SpO2% | 0.196 | -0.356 |
| ESS | 0.160 | 0.159 |
Data presented as Pearson’s or Spearman’s correlation coefficient. BMI: Body Mass Index. TST: total sleep time, NREM: non-REM sleep, REM: rapid eye movement sleep, AHI: apnea-hypopnea index, PLMI: periodic limb movement index, ODI: oxyhemoglobin desaturation index, SE: sleep efficiency, SpO2: peripheral oxygen saturation, ESS: Epworth Sleepiness Scale score.
* p<0.05 #: p<0.001
Table 4. Distribution of catathrenia periods across sleep stages and their association with preceding or coincident EEG arousal.
| NREM 1 | NREM 2 | NREM 3 | REM | Total | |
|---|---|---|---|---|---|
| CP onset not associated with an arousal, n (% of CP in each stage of sleep) | 2 (4.3) | 1 (3.5) | 0 | 61 (17.7) | 64 (15) |
| CP onset associated with arousal, n (% of CP in each stage of sleep) | 45 (95.7) | 28 (96.5) | 5 (100) | 285 (82.3) | 363 (85) |
| CP, n (% of total CPs across sleep stages) | 47 (11) | 29 (6.8) | 5 (1.2) | 346 (81) | 427 (100) |
Data presented as n (%); NREM: non-REM sleep; REM: rapid eye movement sleep; CP: catathrenia period. P<0.001 for comparison between NREM & REM.
In analyses stratified by the presence or absence of OSA (defined as an AHI ≥5 events/hr), a greater percentage of CPs associated with arousals was seen in subjects with OSA (97.1%), but no significant differences were observed in CP frequency, sleep architecture, sleep quality, or daytime symptoms. Men in overall cohort had a higher mean AHI (7.4 ± 8.9 versus 1.7 ± 2.0, p = 0.011), and PLMI (16.4 ± 22.2 versus 3.3 ± 6.8, p < 0.001) than women, but no modifying effects of gender on CP frequency or duration, or the association of CPs with arousals and REM sleep, were observed.
A number of different therapeutic approaches were adopted in this cohort. These included treatment of sleep disordered breathing with CPAP or a dental device, pharmacotherapy with hypnotic agents, and cognitive behavioural therapy for concomitant insomnia. All of these treatment modalities met with limited success, largely due to significant issues regarding patient acceptability & adherence (table 5); all patients treated with CPAP reported subjective resolution of catathrenia events, but only 3/9 continued treatment beyond 12 months. 24% (n=9) of patients did not require any treatment, after reassurance regarding the benign nature of their condition.
Table 5. Treatment approaches adopted and outcomes within this cohort.
| Subjects (n) | Effective in patients with follow up (%) | Effective in combination with other treatment options in patients with follow up | |
|---|---|---|---|
| CPAP | 9 | 3/9 (33.33) | 1 (clonazepam 1.5mg) |
| MAD | 6 | 3/6 (100) | - |
| Provent | 1 | 1/1 (100) | 1 (Mirtazapine 15mg) |
| Clonazepam | 5 | 2/3 (66.66) | 1 CPAP |
| Trazodone | 2 | 0/1 (0) | CBTi |
| Melatonin | 2 | 1/1 (100) | - |
| Zopiclone | 5 | 2/4 (50) | - |
| CBTi | 6 | 0/1 (0) | - |
| No treatment | 9 | - | - |
CPAP: continuous positive airway pressure, MAD: mandibular advancement device, CBTi: cognitive behavioral therapy for insomnia.
Discussion
The key finding in what is – to our knowledge – the largest reported series of subjects with this disorder, is the close association between catathrenia events and arousals from sleep. Over 80% of CPs in this cohort occurred in tandem with an arousal, either preceding or coinciding with the onset of the CP. Direct comparison with the published literature is not straightforward, with most studies reporting on individual episodes, rather than periods, of catathrenia, but our data are in concordance with the results of a prior smaller study, which reported nearly two thirds of CEs occurring in parallel with an arousal (18). While a number of these arousal events within our cohort were a consequence of OSA or periodic limb movements, the vast majority appeared to be spontaneous in nature. It remains unclear what role arousals may play in the pathogenesis of catathrenia, but it is well recognized that transitions from sleep to wake (and vice versa) are periods of inherent ventilatory instability(22). While this is an established pathophysiological mechanism in central sleep apnea, this area remains unexplored in catathrenia. Nonetheless, the data presented above make a reasonable argument for catathrenia to be considered a disorder of arousal, rather than a subtype of sleep disordered breathing.
We identified a clear predominance of events occurring in REM sleep, with only 19% arising from NREM. This is in agreement with the first report of this disorder, with similar observations being made in the majority of subsequent case series (1, 2, 8, 18, 19). However, this is far from a uniform finding, with a number of investigators reporting that catathrenia may occur with equal or even greater frequency in NREM sleep (15, 23). As an example of this, in a carefully characterized cohort of 7 young female subjects attending a North American sleep laboratory with a principal complaint of groaning during sleep (11), catathrenia events were identified during all sleep stages, but with a significant majority of events arising from NREM sleep. This may speak to the existence of two or more subtypes of catathrenia (12), although a clear REM predominance was seen in all of our subjects, and analyses stratified by age, gender, and the presence or absence of conventional sleep disordered breathing, failed to identify any significant variability in REM predominance within these subgroups.
A characteristic breathing pattern was observed across our cohort, with a deep inspiration followed by a prolonged expiration, usually accompanied by a groaning sound, in keeping with the current ICSD 3 description of PSG findings in catathrenia (9, 14), and facilitating its differentiation from conventional central apneic or hypopneic events. While other investigators have reported seemingly typical nocturnal vocalisations occurring in the absence of the typical breathing pattern (9), it would appear from our data that the opposite may also occur, with silent bradypnea seen in a number of our patients (online supplement 1).
Catathrenia can clearly be a significant social nuisance, with the majority of patients in our cohort, along with those in prior series, referred on the basis of their bed partner’s complaints. However, nearly half of our patients (44.7%; n=17) reported subjective daytime sleepiness, with an Epworth score >10. Again, this relationship was not modified in analyses stratified according to the presence of OSA, and may provide an additional rationale for initiating treatment of catathrenia. As noted in earlier reports (11, 12), CPAP therapy seems to be effective in controlling catathrenic events, but its utility is limited by poor patient acceptability. Sleep consolidating agents also appeared to have at least partial subjective efficacy, but dedicated prospective studies are needed to confirm this effect.
Our study has a number of important limitations. It is a retrospective study, thus constraining access to a number of variables such as age at symptom onset, descriptive information about the moaning and groaning sounds, and the outcome of any treatment initiated. A number of patients commenced CPAP primarily to treat concomitant sleep apnea, which may have influenced our results. A true general population prevalence estimate for catathrenia is not possible due to the selection bias inherent in evaluation of only patients referred for sleep consultation and NPSG.
In this study of the largest series of catathrenia patients reported to date, we found that this rare disorder is characterized by a distinct breathing pattern, occurring predominantly in REM sleep, with arousals almost uniformly preceding or coinciding with the onset of catathrenia periods. CPAP remains a viable therapeutic option, particularly for patients with concomitant OSA. The utility of sleep consolidating agents in preventing arousals and associated catathrenia warrants further evaluation.
Supplementary Material
References
- 1.De Roek J, Van Hoof E, Cluydts R. Sleep related expiratory groaning. A case report. Sleep Res. 1983;12:237. [Google Scholar]
- 2.Pevernagie DA, Boon PA, Mariman AN, Verhaeghen DB, Pauwels RA. Vocalization during episodes of prolonged expiration: a parasomnia related to REM sleep. Sleep Med. 2001;2(1):19–30. doi: 10.1016/s1389-9457(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 3.Vetrugno R, Lugaresi E, Plazzi G, Provini F, D'Angelo R, Montagna P. Catathrenia (nocturnal groaning): an abnormal respiratory pattern during sleep. Eur J Neurol. 2007;14(11):1236–43. doi: 10.1111/j.1468-1331.2007.01954.x. [DOI] [PubMed] [Google Scholar]
- 4.Vetrugno R, Provini F, Plazzi G, Vignatelli L, Lugaresi E, Montagna P. Catathrenia (nocturnal groaning): a new type of parasomnia. Neurology. 2001;56(5):681–3. doi: 10.1212/wnl.56.5.681. [DOI] [PubMed] [Google Scholar]
- 5.Steinig J, Lanz M, Krugel R, Happe S. Breath holding - A rapid eye movement (REM) sleep parasomnia (catathrenia or expiratory groaning) Sleep Med. 2008;9(4):455–6. doi: 10.1016/j.sleep.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Guilleminault C, Hagen CC, Khaja AM. Catathrenia is not expiratory snoring. Sleep. 2008;31(6):774–5. doi: 10.1093/sleep/31.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neutel D, Peralta R, Bentes C. Mystery case: catathrenia: a rare but treatable parasomnia. Neurology. 2014;82(12):e98–9. doi: 10.1212/WNL.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 8.Ramar K, Gay P. Catathrenia: getting the 'cat' out of the bag. Sleep Breath. 2008;12(4):291–4. doi: 10.1007/s11325-008-0195-5. [DOI] [PubMed] [Google Scholar]
- 9.Abbasi AA, Morgenthaler TI, Slocumb NL, Tippmann-Peikert M, Olson EJ, Ramar K. Nocturnal moaning and groaning-catathrenia or nocturnal vocalizations. Sleep Breath. 2012;16(2):367–73. doi: 10.1007/s11325-011-0503-3. [DOI] [PubMed] [Google Scholar]
- 10.Vetrugno R, Lugaresi E, Ferini-Strambi L, Montagna P. Catathrenia (nocturnal groaning): what is it? Sleep. 2008;31(3):308–9. doi: 10.1093/sleep/31.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilleminault C, Hagen CC, Khaja AM. Catathrenia: parasomnia or uncommon feature of sleep disordered breathing? Sleep. 2008;31(1):132–9. doi: 10.1093/sleep/31.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iriarte J, Campo A, Alegre M, Fernandez S, Urrestarazu E. Catathrenia: respiratory disorder or parasomnia? Sleep Med. 2015;16(7):827–30. doi: 10.1016/j.sleep.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 13.International Classification of Sleep Disorders. Diagnostic and Coding Manual. 2nd Edition. American Academy of Sleep Medicine; Westchester IL, USA: 2005. [Google Scholar]
- 14.AASM. American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. American Academy of Sleep Medicine; Darien: [Google Scholar]
- 15.Manconi M, Zucconi M, Carrot B, Ferri R, Oldani A, Ferini-Strambi L. Association between bruxism and nocturnal groaning. Mov Disord. 2008;23(5):737–9. doi: 10.1002/mds.21885. [DOI] [PubMed] [Google Scholar]
- 16.Ramar K, Olson EJ, Morgenthaler TI. Catathrenia. Sleep Med. 2008;9(4):457–9. doi: 10.1016/j.sleep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Ott SR, Hamacher J, Seifert E. Bringing light to the sirens of night: laryngoscopy in catathrenia during sleep. Eur Respir J. 2011;37(5):1288–9. doi: 10.1183/09031936.00083510. [DOI] [PubMed] [Google Scholar]
- 18.Prihodova I, Sonka K, Kemlink D, Volna J, Nevsimalova S. Arousals in nocturnal groaning. Sleep Med. 2009;10(9):1051–5. doi: 10.1016/j.sleep.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Oldani A, Manconi M, Zucconi M, Castronovo V, Ferini-Strambi L. 'Nocturnal groaning': just a sound or parasomnia? J Sleep Res. 2005;14(3):305–10. doi: 10.1111/j.1365-2869.2005.00460.x. [DOI] [PubMed] [Google Scholar]
- 20.Songu M, Yilmaz H, Yuceturk AV, Gunhan K, Ince A, Bayturan O. Effect of CPAP therapy on catathrenia and OSA: a case report and review of the literature. Sleep Breath. 2008;12(4):401–5. doi: 10.1007/s11325-008-0194-6. [DOI] [PubMed] [Google Scholar]
- 21.Richard Berry RB, Gamaldo Charlene, Harding Susan, Lloyd Robin, Marcus Carole, Vaughn Bradley. Rules, Terminology and Technical Specifications, Scoring Manual 2.0.2. American Academy of Sleep Medicine; 2013. The AASM Manual for the scoring of Sleep and Associated Events. [Google Scholar]
- 22.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: Pathophysiology and treatment. Chest. 2007;131(2):595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqui F, Walters AS, Chokroverty S. Catathrenia: a rare parasomnia which may mimic central sleep apnea on polysomnogram. Sleep Med. 2008;9(4):460–1. doi: 10.1016/j.sleep.2007.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.