Abstract
Skeletal homeostasis is closely effectuated by the regulation of bone formation and bone resorption. Osteoclasts are multinuclear giant cells responsible for bone resorption. Overactivated osteoclasts and excessive bone resorption result in various lytic bone diseases, such as osteoporosis, osteoarthritis, periprosthetic infection, and inflammatory aseptic loosening of orthopedic implants. In consideration of the severe side effects caused by the currently available drugs, exploitation of novel drugs has gradually attracted attention. Because of its anti-inflammatory, antioxidant, and antitumor capacities, diallyl disulfide (DADS), a major oil-soluble organosulfur ingredient compound derived from garlic, has been widely researched. However, the effects of DADS on osteoclasts and lytic bone diseases are still unknown. In this study, we investigated the effects of DADS on receptor activator of NF-κB ligand (RANKL)- and LPS-mediated osteoclastogenesis, LPS-stimulated proinflammatory cytokines related to osteoclasts, and LPS-induced inflammatory osteolysis. The results showed that DADS significantly inhibited RANKL-mediated osteoclast formation, fusion, and bone resorption in a dose-dependent manner via inhibiting the NF-κB and signal transducer and activator of transcription 3 signaling and restraining the interaction of NF-κB p65 with nuclear factor of activated T cells cytoplasmic 1. Furthermore, DADS also markedly suppressed LPS-induced osteoclastogenesis and reduced the production of proinflammatory cytokines with LPS stimulation to indirectly mediate osteoclast formation. Consistent with the in vitro results, DADS prevented the LPS-induced severe bone loss by blocking the osteoclastogenesis. All of the results indicate that DADS may be a potential and exploitable drug used for preventing and impeding osteolytic lesions.—Yang, J., Tang, R., Yi, J., Chen, Y., Li, X., Yu, T., Fei, J. Diallyl disulfide alleviates inflammatory osteolysis by suppressing osteoclastogenesis via NF-κB–NFATc1 signal pathway.
Keywords: natural products, osteoclast, bone resorption
Skeletal homeostasis integrity is preserved by maintaining a delicately balanced remodeling process, which is tightly regulated via osteoclastic bone resorption and osteoblastic bone formation (1, 2). With characteristics of excessive bone mass loss, inflammatory bone erosion, which is primarily caused by microbial products and inflammatory cytokines stimulating osteoclasts and enhancing the bone absorptive capacity, constantly occurs after infection and chronic inflammation in the orthopedics field and confuses the orthopedist (3).
Osteoclasts, multinucleated giant cells derived from the monocyte/macrophage hematopoietic lineage, are formed through multiple steps, including cell-to-cell contact, fusion, differentiation, and maturation (4, 5). M-CSF and receptor activator of NF-κB ligand (RANKL) are recognized to be critical cytokines for the differentiation and maturation of osteoclasts (6). Combination of RANKL with its receptor, receptor activator of NF-κB (RANK), recruits TNF receptor–associated factor (TRAF) 6 (7) to correspondingly activate downstream signaling cascades such as NF-κB and MAPKs, including p38, JNK, and ERK, resulting in the sequential activation of nuclear factor of activated T cells cytoplasmic 1 (NFATc1) and c-Fos, known as master regulators of osteoclast differentiation and maturation (8). Activation of these signaling effectors mentioned above up-regulates the expression of osteoclastic genes such as tartrate-resistant acid phosphatase (TRAP), matrix metalloproteinase 9 (MMP9), calcitonin receptor (CTR), and cathepsin K (CTSK), which eventually leads to the development of multinucleated bone-resorbing osteoclasts (8). NF-kB, a crucial transcription factor in RANKL-induced osteoclastogenesis and a heterodimer comprising p50 and p65 subunits, controls the expression of numerous genes involved in cell proliferation, apoptosis, and inflammation (9). NF-κB is inactive in the cytoplasm owing to its combination with the endogenic specific inhibitor protein IκB in unstimulated cells. Stimulation of RANKL leads to the activation of the kinase of IκB and IκB-α phosphorylation (10). Subsequently, the dissociative p65 subunit gets translocated to the nucleus and then initiates the target gene’s transcriptions (for instance, activating the NFATc1 promoter to encourage NFATc1 expression) (11).
LPS, an important component of gram-negative bacteria (12), is known as a potent inducer of inflammation and causes inflammatory bone loss through syntheses of proinflammatory cytokines such as IL-1β, IL-6, and TNF-a (13, 14), which have been identified to promote osteoclastogenesis and ultimately lead to the destructive bone loss primarily via the NF-κB and MAPKs signal pathway (15–17). Locally subcutaneous injections of LPS also had been shown to significantly increase the number of osteoclasts and the eroded surface area in mouse skull (18, 19). Another transcription factor of great importance involved in the induction of proinflammatory cytokines is signal transducer and activator of transcription (STAT). Among the STAT families, the importance of STAT3 has been demonstrated in bone physiology (for instance, in RANKL-mediated osteoclastogenesis) (20, 21). Moreover, STAT3 is involved in LPS-induced expression of iNOS, which is partly dependent on Ser727 phosphorylation (22). The previous study also demonstrated that knockdown of STAT3 resulted in a significant reduction in IL-1β, IL-6, and NO production with LPS stimulation, followed by decreasing osteoclast formation (23). NO, a signaling molecule playing various vital roles in biologic processes, enhances osteoclastogenesis by mediating cell fusion and increasing actin remodeling in mononuclear preosteoclasts, thereby mediating fusion and formation of multinucleated osteoclasts (24).
Drug discoveries of natural products and their derivatives are of great significance for the clinical therapeutics in the world. Diallyl disulfide (DADS), a major oil-soluble organosulfur ingredient compound derived from garlic (25), which has been considered a flavoring agent and a well-known herb for years (26), has various pharmacological properties and chemopreventive effects, such as having anti-inflammatory, antioxidant, and antitumor capacities (27–30). DADS has been demonstrated to exhibit a significant reduction in NO production via inhibiting the expression of iNOS as well as suppressing proinflammatory cytokines, including IL-1β, IL-6, and TNF-a in RAW264.7 cells and other cell types stimulated with LPS (27, 31, 32). Recent studies also demonstrated that DADS restored and protected the IL-1β–induced impaired chondrogenesis via the NF-kB signal pathway (33, 34). Interestingly, allicin, another compound derived from garlic, had been demonstrated to exhibit inhibitory effects on osteoclastogenesis via suppression of M-CSF and RANKL production by osteoblasts in vitro coculture (35). However, the effects of DADS on osteoclasts and lytic bone diseases are still unknown.
In the present study, we aimed to clarify that DADS suppressed RANKL-induced osteoclast formation and function via inhibiting NF-κB and STAT3 signaling and restraining the interaction of NF-κB p65 with NFATc1, and we also demonstrated that DADS inhibited LPS-induced osteoclastogenesis and reduced the production of proinflammatory cytokines with LPS stimulation. Furthermore, the data in vivo obtained from micro computed tomography (micro-CT) and histology analysis further confirmed that DADS significantly prevented the LPS-induced severe bone loss, which indicated that DADS could be valuable in the treatment and prevention of osteolytic bone diseases.
MATERIALS AND METHODS
Materials and reagents
DADS (purity >98%), TRAP Stain Kit, and LPS were purchased from MilliporeSigma (Burlington, MA, USA). DMEM, α minimal essential medium (α-MEM), and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Recombinant mouse M-CSF and recombinant mouse RANKL were obtained from R&D Systems (Minneapolis, MN, USA). Actin Cytoskeleton and Focal Adhesion (FAK) Staining Kit was purchased from Merck (Darmstadt, Germany). Osteo Assay Stripwell Plates for bone resorption were purchased from Corning (Corning, NY, USA). Antibodies against NFATc1 and c-Fos were purchased from Abcam (Cambridge, MA, USA). Specific antibodies against phosphorylated (p)-NFκB p65, NF-κB p65, p–IκB-α, IκB-α, p-STAT3 Ser727, p-STAT3 Tyr705, STAT3, and β-actin were obtained from Bioworld Technology (St. Louis Park, MN, USA). Mouse IL-1β, IL-6, and TNF-α ELISA Kit were obtained from Bioss (Beijing, China). Pierce Classic Magnetic Immunoprecipitation (IP)/Coimmunoprecipitation (Co-IP) Kit was purchased from Thermo Fisher Scientific.
Cell culture
RAW264.7 cells (mouse macrophage cells) were obtained from the American Type Culture Collection (Rockville, MD, USA) and cultured in DMEM Complete Medium supplemented with 1% penicillin-streptomycin (Thermo Fisher Scientific) and 10% FBS at 37°C in a 5% CO2 condition. Bone marrow macrophages (BMMs) were obtained from 6-wk-old C57/BL6 mice. The whole procedure was performed according to the guidelines of the animal care and use committee of Third Military Medical University. Briefly, the cells were isolated from the femur and tibia bone marrow and cultured for 3 d in α-MEM supplemented with 10% FBS and 25 ng/ml M-CSF. The attached cells were considered BMMs.
Cytotoxicity assay
Stock solution of DADS was prepared in DMSO, and serial dilutions were made in DMEM or α-MEM prior to application. RAW264.7 cells (2 × 103 cells/well) and BMMs (5 × 103 per well) were seeded into 96-well plates in triplicates and cultured overnight at 37°C in a 5% CO2 condition. The following day, cells were incubated with varying concentrations of DADS for 24 or 72 h, respectively. Cell viabilities were assessed by Cell Counting Kit-8 (CCK8; Solarbio, Beijing, China) reagent according to the manufacturer’s procedures. In a nutshell, the medium was removed, and the fresh medium containing 1/10 (v/v) CCK-8 reagent was added to each well and incubated for an additional 2 h. The absorbency of cells was measured using a 96-well plate reader at 450 nm on the instrument (Synergy H4; BioTek Instruments, Winooski, VT, USA).
Osteoclastogenesis assay
The formation of osteoclasts from RAW264.7 cells and BMMs was performed as previously described by Dou et al. (36). After having been seeded into 96-well plates and cultured overnight, RAW264.7 cells (3 × 103 cells/well) and BMMs (5 × 103 per well) were respectively incubated in DMEM and α-MEM containing 10% FBS, RANKL (50 ng/ml), and M-CSF (50 ng/ml) with or without DADS for 72 or 120 h to generate multinucleated osteoclasts. For TRAP stain, cells were fixed with 4% paraformaldehyde for 15 min after being washed twice with PBS and then stained with TRAP staining solution on the basis of the manufacturer’s directions. TRAP-positive multinucleated cells containing 3 or more nuclei were identified as osteoclasts and counted under the optical microscope (DMI 6000B; Leica Microsystems, Wetzlar, Germany). In addition, RAW264.7 cells were seeded into 96-well plates at a density of 3 × 103 cells per well and pretreated with PBS or RANKL (50 ng/ml) and M-CSF (50 ng/ml) for 24 h. Successively, the medium was replaced with fresh medium containing PBS or LPS (100 ng/ml) alone or together with DADS for an additional 72 h for generating multinucleated osteoclasts. After the culture, cells were stained for TRAP staining. TRAP-positive multinucleated cells containing 3 or more nuclei were counted. The purpose was to investigate the effects of DADS on osteoclastogenesis under LPS-induced inflammatory conditions.
Resorption pit assay
RAW264.7 cells were incubated in 96-well Osteo Assay Surface Plates (Corning) at a density of 1 × 104 cells per well and induced with RANKL (50 ng/ml) and M-CSF (50 ng/ml) with or without DADS for 5 d. To analyze the surface for pit formation, cells were eliminated with sodium hypochlorite for 5 min at room temperature and then washed twice with distilled water. Individual pits or multiple pit clusters were observed using a microscope at ×40 magnification. The absorption area was analyzed by ImageJ (National Institutes of Health, Bethesda, MD, USA).
FAK staining
RAW 264.7 cells (3 × 103 cells/well) were treated with RANKL (50 ng/ml) and M-CSF (50 ng/ml) alone or together with DADS for 72 h to generate multinucleated cells. For FAK staining, cells were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.1% Triton X-100 for 5 min at room temperature. After being blocked with the blocking buffer for 30 min, cells were stained for vinculin and F-actin with anti-vinculin antibody (1:300) and tetramethylrhodamine-conjugated phalloidin (1:500) revealed with Alexa Fluor 488–conjugated secondary antibody. Finally, nuclei counterstaining was performed by DAPI (1: 1000) for 5 min. Fluorescence microscopy was conducted to observe the cells.
NO analysis
NO detection was performed as previously described by Park et al. (37) via Griess reagent. RAW264.7 cells were seeded into 48-well plates at a density of 3 × 104 cells per well and pretreated with (50 ng/ml) and M-CSF (50 ng/ml) for 24 h. In succession, the medium was replaced with fresh medium containing LPS (100 ng/ml) with or without DADS for an additional 12, 24, or 48 h, respectively. The supernatant from the cultured cells was centrifuged to remove cell debris and transferred to 96-well plates. The supernatant was then reacted using Nitric Oxide (NO) Detection Kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. The absorbency of the supernatant was measured using a 96-well plate reader at 450 nm. Sodium nitrite was used as a standard curve.
RNA isolation and real-time quantitative PCR
RAW 264.7 cells were induced with RANKL (50 ng/ml) and M-CSF (50 ng/ml) in the presence or absence of DADS for 24 or 72 h, respectively. In addition, RAW264.7 cells were pretreated with RANKL and M-CSF for 24 h and successively stimulated with LPS (100 ng/ml) with or without DADS for an additional 72 h. Total RNA was isolated with Trizol reagent (Thermo Fisher Scientific). Genomic DNA was removed at 42°C for 2 min. The cDNA was synthesized from 1 µg total RNA using reverse transcriptase with oligo-deoxythymine (dT) primers at 37°C for 15 min followed by 85°C for 5 s according to the manufacturer’s instructions (Takara Bio, Kyoto, Japan); then, real-time quantitative PCR (qPCR) was performed to investigate the expression of the indicated genes using Sybr Premix Ex Taq II (Takara Bio) in a PCR detection system (Bio-Rad, Hercules, CA, USA). Real-time qPCR was performed with the following thermocycling conditions: preincubation at 95°C for 30 s followed by 40 cycles of amplification at 95°C for 5 s and 60°C for 1 min and then cooling at 65°C for 5 s. Primer sequences are as shown in Table 1, and glyceraldehyde 3-phosphate dehydrogenase was used as an internal control.
TABLE 1.
Primer sequences for qPCR
| Primer sequence, 5′–3′ |
||
|---|---|---|
| Gene | Forward | Reverse |
| TRAP | CACTCCCACCCTGAGATTTGT | CATCGTCTGCACGGTTCTG |
| MMP9 | CTGGACAGCCAGACACTAAAG | CTCGCGGCAAGTCTTCAGAG |
| CTSK | GAAGAAGACTCACCAGAAGCAG | TCCAGGTTATGGGCAGAGATT |
| CTR | CGCATCCGCTTGAATGTG | TC TGTCTTTCCCCAGGAAATGA |
| c-Fos | CGGGTTTCAACGCCGACTA | TTGGCACTAGAGACGGACAGA |
| NFATc1 | CCCGTCACATTCTGGTCCAT | CAAGTAACCGTGTAGCTGCACAA |
| DC-STAMP | CTAGCTGGCTGGACTTCATCC | TCATGCTGTCTAGGAGACCTC |
| OC-STAMP | GGGCTACTGGCATTGCTCTTAGT | CCAGAACCTTATATGAGGCGTCA |
| TNF-α | AGGCGGTGCTTGTTCCTCA | AGGCGAGAAGATGATCTGACTGCC |
| IL-1β | CTCAACTGTGAAATGCCACC | TGTCCTCATCCTGGAAGGT |
| IL-6 | TGGGAAATCGTGGAAATGAGA | ACTCTGGCTTTGTCTTTCTTGT |
| iNOS | GCAGAATGTGACCATCATGG | ACAACCTTGGTGTTGAAGGC |
| GAPDH | AAATGGTGAAGGTCGGTGTG | TGAAGGGGTCGTTGATGG |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Western blotting analysis
RAW 264.7 cells were induced with RANKL and M-CSF to generate multinucleated osteoclasts. Additionally, RAW264.7 cells were treated with vehicle or DADS (80 μg/ml) for 2 h followed by stimulation with the indicated concentration of RANKL for 0, 15, 30, or 60 min. Cells were lysed in a lysis buffer containing 10 mM Tris, pH 7.2, 150 mM NaCl, 5 mM EDTA, 0.1% SDS, 1% Triton X-100, and 1% deoxycholic acid supplemented with protease and phosphatase inhibitor cocktail. All of the protein concentrations were assessed and subsequently adjusted to 40 μg/μl using the bicinchoninic acid protein assay reagent (Beyotime Biotechnology). For Western blots, 40 μg of protein samples were subjected to SDS-PAGE followed by transfer onto PVDF membranes. After blocking in 5% skim milk for 2 h, they were incubated with rabbit primary antibodies at the following dilutions overnight at 4°C: NFATc1, 1:1000; c-Fos, 1:1000; NF-κB p65, 1:1000; p–NF-κB p65, 1:1000; IκB-α, 1:1000; p–IκB-α, 1:1000; STAT3, 1:1000; p-STAT3 Ser727, 1:1000; p-STAT3 Tyr705, 1:1000; β-actin, 1:1000. Next, they were incubated with secondary antibodies (1:2000) at room temperature for 1 h. Finally, protein bands were visualized by exposing in ChemiDoc XRS+ Imaging System (Bio-Rad) and analyzed by ImageJ software. β-Actin served as an internal control.
Co-IP
RAW 264.7 cells were induced with RANKL alone or together with DADS (80 μg/ml) for 48 h. After having been washed twice with PBS, cells were lysed in a lysis buffer at 4°C for 30 min followed by centrifugation at 12,000 g for 10 min to remove cell debris. The cell lysate was incubated with anti–NF-κB p65 (1:100) overnight at 4°C to form the immune complex. The following day, IP of NF-κB p65 was performed using Protein A/G Magnetic Beads (Thermo Fisher Scientific) according to the manufacturer’s protocol. Briefly, the antigen-antibody complex was bound to Protein A/G Magnetic Beads for 1 h at room temperature. After that, the beads were collected with a magnetic stand and washed twice with IP lysis and wash buffers and once with purified water. Next, the antigen-antibody complex was eluted for 10 min with elution buffer and then analyzed by using Western blotting.
LPS-induced calvarial osteolysis mice model
A mouse calvarial osteolysis model was established as previously described in refs. 18 and 19. Twenty-four healthy 6-wk-old C57/BL6 female mice were obtained from the animal center of Third Military Medical University. The whole procedure was performed according to the guidelines of the animal care and use committee of Third Military Medical University. The mice were randomly allocated to 4 groups: sham (injection with PBS), LPS (LPS treatment with 5 mg/kg), low-dose DADS (LPS treatment and injection with 20mg/kg DADS), and high-dose DADS (LPS treatment and injection with 40 mg/kg DADS). The detailed methods of subcutaneous injections were described in in refs. 38 and 39. All animals were anesthetized by an intraperitoneal injection of 4% chloral hydrate (5 μl/g) to reduce the level of suffering. The heads of the anesthetized mice were shaved to receive subperiosteal injections of PBS, LPS, or LPS + DADS. The total volume of the injections was 100 μl each time. After weighing the mice, the injections were administered with the 1-ml syringe at a point on the sagittal midline suture of the calvarium located between the ears and eyes. Next, a skin mound was formed, and then the needle was slowly withdrawn to prevent the liquid from overflowing. Subsequently, the injections were administered every alternate day over a 14-d period. At the end of the experiment, the mice were arranged for cranial micro-CT (Quantum FX CT; PerkinElmer, Waltham, MA, USA) and blood was taken by excising the eyeball for ELISA experiments under deep anesthesia. After that, the mice were sacrificed and the calvaria was separated for histologic analysis. Successively, the isolated cranial samples were fixed with 4% paraformaldehyde, decalcified with 10% EDTA, and made into 5-μm–thick sections for hematoxylin and eosin (H&E) staining and TRAP staining according to the previous method. The data obtained were used to describe the inflammatory response and osteoclast formation in vivo.
Serum IL-1β, IL-6, and TNF-α levels were detected using a mouse IL-1β, IL-6, and TNF-α ELISA kit according to the manufacturer’s protocol. The serums of mice were obtained from whole blood after standing for 30 min at room temperature and centrifugation at 3000 g for 10 min at 4°C. The absorbency of each standard and sample was measured at 450 nm. Standard concentration gradient was used as a standard curve.
Statistics
All data were presented as the mean ± sd from at least 3 independent experiments. Statistical differences were performed using 1-way ANOVA and SPSS 19.0 software (IBM, Armonk, NY, USA). Values of P < 0.05 and P < 0.01 were considered to be statistically significant.
RESULTS
Toxicity evaluation of DADS
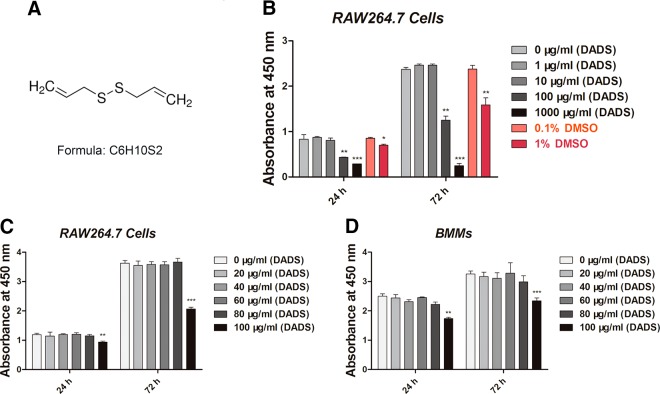
RAW264.7 cells were treated with various concentrations of DADS (0, 1, 10, and 100 μg/ml containing 0.1% DMSO and 1000 μg/ml containing 1% DMSO) for 24 or 72 h, respectively, followed by CCK8 assays. The results revealed that the DADS (Fig. 1A) dose at 1000 μg/ml containing 1% DMSO (24 h) and 1% DMSO (72 h) both strongly inhibited cell proliferation. The DADS dose at 100 μg/ml containing 0.1% DMSO (24 h) also inhibited cell proliferation, whereas 0.1% DMSO (72 h) did not (Fig. 1B). The same results were obtained from the following CCK8 assays. All the results revealed that the concentrations of DADS <100 μg/ml had little effect on cell proliferation and were selected for further study (Fig. 1C, D).
Figure 1.
DADS toxicity evaluation on RAW264.7 cells and BMMs. A) Chemical structure of DADS. B) CCK8 analysis of cell viability of RAW264.7 cells treated with different concentrations of DADS (0, 1, 10, and 100 μg/ml containing 0.1% DMSO and 1000 μg/ml containing 1% DMSO, 0.1% DMSO, and 1% DMSO) for 24 or 72 h. C, D) CCK8 analysis of cell viability of RAW264.7 cells or BMMs treated with DADS (0, 20, 40, 60, 80, and 100 μg/ml) for 24 or 72 h. Data in the figures represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.01 (based on 1-way ANOVA).
DADS inhibits RANKL-mediated osteoclast formation
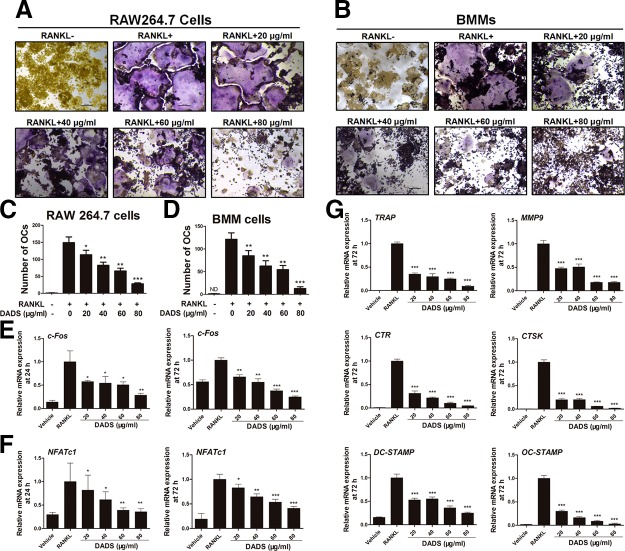
According to the results of the CCK8 assay, the dosages of DADS at 20, 40, 60, and 80 μg/ml were selected for further study. RAW264.7 cells and BMMs were induced with RANKL (50 ng/ml) and M-CSF (50 ng/ml) alone or together with DADS to generate multinucleated osteoclasts. To examine the effects of DADS on RANKL-induced osteoclastogenesis, TRAP stain was performed (Fig. 2A, B). TRAP-positive osteoclasts (nuclei ≥ 3) in each well (96-well plate) were quantificationally evaluated (Fig. 2C, D). From the results, we preliminarily noticed that DADS restrains RANKL-induced osteoclastogenesis. Moreover, we further examined the effects of DADS on the expression of various osteoclast marker genes related to osteoclastogenesis, such as TRAP, CTSK, CTR, MMP9, NFATc1, and c-Fos. The results from real-time quantitative PCR (qPCR) indicated that the expression levels of c-Fos and NFATc1 at 24 and 72 h were significantly down-regulated by DADS treatments (Fig. 2E, F), as did the other marker genes, including TRAP, CTSK, CTR, and MMP9 (Fig. 2G), which were consistent with the TRAP staining. All the results revealed that DADS inhibited RANKL-induced osteoclast differentiation in a dose-dependent manner.
Figure 2.
DADS inhibits RANKL-induced osteoclastogenesis in a dose-dependent way. A, B) Representative images of RAW264.7 cells or BMMs stained for TRAP (red) treated with RANKL (50 ng/ml) and M-CSF (50 ng/ml) for 3 and 5 d, respectively. In total, 6 different groups were set according to the vehicle and the indicated concentrations of DADS (0, 20, 40, 60, and 80 μg/ml). Scale bars, 200 μm. C, D) Quantification of osteoclasts (nuclei ≥3) in each well (96-well plate). E–G) RAW264.7 cells were incubated with RANKL (50 ng/ml) and M-CSF (50 ng/ml) with or without varying doses of DADS for 24 and 72 h, respectively. The mRNA expression of osteoclasts marker genes was analyzed by real-time qPCR (n = 3). OC, osteoclast. Data represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.01 (based on 1-way ANOVA).
DADS regulates RANKL-induced osteoclast fusion
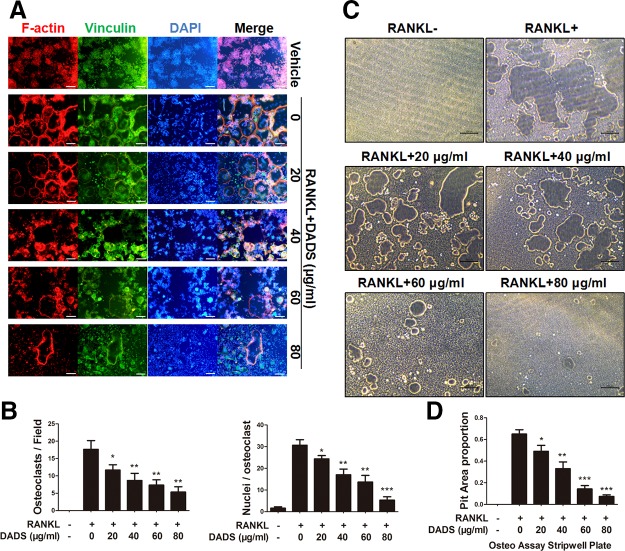
To test the effects of DADS on osteoclast fusion, RAW264.7 cells were incubated with RANKL and M-CSF treatment with or without varying doses of DADS to generate multinucleated osteoclasts. FAK stain was performed to assess the number of osteoclasts and average nuclei of the multinucleated cells (Fig. 3A). Quantitative analysis showed that the number of osteoclasts and average nuclei of osteoclasts was significantly decreased by DADS treatments in a dose-dependent way (Fig. 3B). Furthermore, dendritic cell–specific transmembrane protein (DC-STAMP) and osteoclast stimulatory transmembrane protein (OC-STAMP), 2 of the osteoclastogenic genes, are essential for osteoclast fusion. The results from real-time qPCR revealed that the expression levels of these fusion genes were obviously restrained by DADS (Fig. 2G), aligned with FAK staining results. Altogether, DADS had inhibitory effects on RANKL-induced osteoclast fusion similar to the osteoclastogenesis.
Figure 3.
DADS suppresses RANKL-induced osteoclast fusion and bone resorption activity. A) Representative images of FAK staining of RAW264.7 cells treated with RANKL and M-CSF alone or together with the indicated concentrations of DADS treatment. F-actin using tetramethylrhodamine-conjugated phalloidin (red), focal contacts using anti-vinculin mAb, and nuclear counterstaining using DAPI (blue). Scale bars, 200 μm. B) Quantitative analysis of osteoclasts (nuclei ≥3) and average osteoclast nuclei number in each field. C) Representative images of RAW264.7 cells cultured in an Osteo Assay Stripwell Plate (96-well) incubated with RANKL and M-CSF for 5 d with or without DADS. Scale bars, 200 μm. D) Quantification of RANKL-induced osteoclastic bone resorption. Data represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.01 (based on 1-way ANOVA).
DADS suppresses the bone resorption activity of mature osteoclasts
Because DADS had a dose-dependent inhibitory effect on osteoclastogenesis and also inhibited cell-cell fusion of osteoclasts. Pit formation assays were performed to explore whether DADS possessed similar effects on osteoclastic bone resorption. RAW264.7 cells were treated with RANKL (50 ng/ml) and M-CSF (50 ng/ml) alone or together with indicated concentrations of DADS for 5 d in 96-well plates (Corning). Bone resorption areas were quantificationally analyzed by ImageJ to assess bone resorption activity of osteoclasts, and the result illustrated that DADS markedly decreased the pit areas of great representativeness in a dose-dependent manner (Fig. 3C, D). Consequently, all of the results suggested that DADS could strongly attenuate osteoclastic bone resorption activity.
DADS negatively regulates LPS-induced osteoclastogenesis
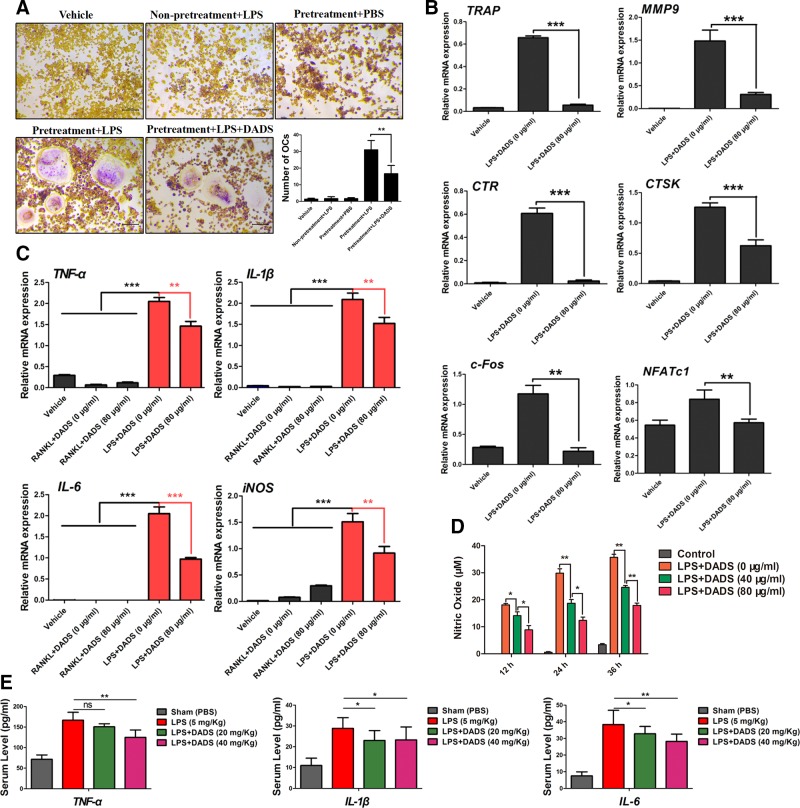
The generation of osteoclasts with LPS induction was established as previously described in refs. 40 and 41. RAW264.7 cells were pretreated with RANKL (50 ng/ml) and M-CSF (50 ng/ml) for 24 h and were successively incubated with LPS (100 ng/ml) for an additional 72 h to generate multinucleated osteoclasts. TRAP stain was performed to assess the ability of LPS-induced osteoclastogenesis. The results from TRAP staining revealed that LPS did not result in the formation of TRAP-positive multinucleated cells in the absence of pretreatment with RANKL. On the contrary, pretreatment with RANKL prior to LPS stimulation successfully generated TRAP-positive osteoclasts. Also, the results showed that the inhibitory action on LPS-evoked osteoclastogenesis was significantly mediated by the indicated dosages of DADS treatment (Fig. 4A). Furthermore, the effects of DADS on the expression of various osteoclast marker genes were examined using real-time qPCR. The results showed that DADS significantly down-regulated the expression of these marker genes, consistent with the TRAP staining (Fig. 4B), suggesting that DADS could have inhibitory effects on osteoclastogenesis under LPS-induced bone inflammatory conditions.
Figure 4.
DADS restrains LPS-induced osteoclastogenesis and reduces the proinflammatory cytokines. A) Representative images of RAW264.7 cells stained for TRAP (red) pretreated with RANKL and M-CSF for 24 h and successively cultured with LPS (100 ng/ml) for an additional 72 h and quantification of osteoclasts (OCs) (nuclei ≥3) in each well. Scale bars, 200 μm. B) RAW264.7 cells were pretreated with RANKL and M-CSF for 24 h and successively induced with LPS (100 ng/ml) alone or together with DADS for an additional 72 h. Osteoclast marker gene expression was analyzed by real-time qPCR (n = 3). C) The mRNA expression of proinflammatory genes including TNF-α, IL-1β, IL-6, and NO was assessed by real-time qPCR in the process of LPS-induced osteoclastogenesis (n = 3) compared with the RANKL-induced osteoclastogenesis. D) NO analysis in the process of LPS-induced osteoclastogenesis using the NO detection kit. E) Serum level of TNF-α, IL-1β, and IL-6 in the LPS-induced calvarial osteolysis mouse model were analyzed using an ELISA kit. Data represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.01 (based on 1-way ANOVA).
DADS decreases proinflammatory cytokines with LPS stimulation to mediate osteoclastogenesis
Owing to the inhibitory effects of DADS on LPS-induced osteoclastogenesis, the expression of proinflammatory cytokines with LPS stimulation was investigated, including TNF-α, IL-1β, IL-6 and NO, which had been identified to promote osteoclastogenesis and ultimately lead to the destructive bone loss. First, real-time qPCR was performed to assess the expression of the genes mentioned above in mRNA level. The results revealed that DADS significantly inhibited the expression of these genes with LPS stimulation (Fig. 4C). In addition, we tested the NO production of cell culture supernatant in the process of LPS-induced osteoclastogenesis, and the results showed that DADS evidently reduced the NO production (Fig. 4D). In the end, we quantitatively analyzed the serum level of TNF-α, IL-1β, and IL-6 in vivo via mouse ELISA Kit. The results indicated that DADS markedly restrained the production of these proinflammatory cytokines (Fig. 4E). In summary, DADS could negatively regulate LPS-induced osteoclastogenesis indirectly through reduction of the indicated proinflammatory cytokines with LPS stimulation.
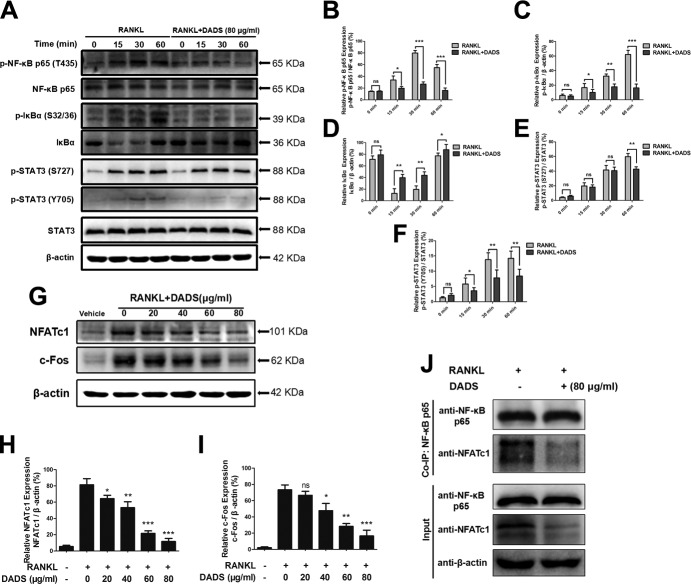
DADS inhibits NF-κB and STAT3 signaling during osteoclastogenesis
Because NF-κB and STAT3 had been implicated in osteoclastogenesis, we investigated the effects of DADS on RANKL-mediated NF-κB and STAT3 signal pathway during osteoclastogenesis by Western blots. To determine these, RAW264.7 cells were treated with vehicle or DADS (80 μg/ml) for 2 h followed by stimulation with the indicated concentration of RANKL for 0, 15, 30, or 60 min. The results showed that the RANKL-stimulated activation of NF-κB was inhibited in the presence of DADS (Fig. 5A). DADS at this specified concentration significantly suppressed the phosphorylation of NF-κB p65 at 30 and 60 min compared with control groups (Fig. 5B). Similarly, the phosphorylation and degradation of IκB-α were obviously inhibited by DADS (Fig. 5C, D), which made the p65 subunit disconnected and translocated to the nucleus for initiating the target gene transcription. Furthermore, we determined the impact of DADS on the RANKL-activated STAT3 signaling level. It was greatly demonstrated that DADS markedly attenuated the Ser727 and Tyr705 phosphorylation of STAT3 (Fig. 5E, F), which was in complete contrast to control groups. Taken together, our data indicate that DADS inhibited RANKL-induced osteoclast formation and function by regulating the activities of NF-κB and STAT3 signal pathway.
Figure 5.
DADS inhibits NF-ĸB and STAT3 signaling and suppresses RANKL-induced osteoclastogenesis via NF-ĸB–NFATc1 signal pathway. A) RAW264.7 cells were treated with vehicle or DADS (80 μg/ml) for 2 h followed by stimulation with RANKL for 0, 15, 30, or 60 min. The cell lysates were analyzed using Western blotting for p-NFκB p65, NFκB p65, p–IκB-α, IκB-α, STAT3, and p-STAT3. B) p-NFκB p65 relative to total NFκB p65. C) p–IκB-α relative to β-actin. D) IκB-α relative to β-actin. E) p-STAT3 Ser727 relative to total STAT3. F) p-STAT3 Tyr705 relative to total STAT3. G) RAW264.7 cells were incubated with RANKL and M-CSF with or without DADS for 72 h. The expression of NFATc1 and c-FOS was analyzed using Western blot. H) Quantification of NFATc1 expression relative to β-actin. I) Quantification of c-Fos expression relative to β-actin. J) Co-IP was performed to evaluate the protein interaction of NFκB p65 and NFATc1. β-Actin was used as a loading control. Quantifications were performed using ImageJ. Data represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.01 (based on 1-way ANOVA).
DADS suppresses RANKL-induced osteoclastogenesis via NF-ĸB–NFATc1 signal pathway
On account of the master regulator functions of NFATc1 and c-Fos on osteoclast differentiation and maturation, the protein expression levels of NFATc1 and c-Fos were estimated by Western blots (Fig. 5G). For this purpose, RAW 264.7 cells were induced with RANKL and M-CSF with or without various concentrations of DADS for 72 h. The results revealed that DADS significantly down-regulated the protein expression levels of NFATc1 and c-Fos in a dose-dependent way (Fig. 5H, I). Furthermore, Co-IP was performed to evaluate the protein interaction of NF-κB p65 and NFATc1, and the results showed that the combination of NF-κB p65 with NFATc1 was obviously inhibited by the indicated concentration of DADS treatment (Fig. 5J). In summary, our data confirmed that DADS could suppress RANKL-induced osteoclastogenesis via NF-ĸB–NFATc1 signal pathway.
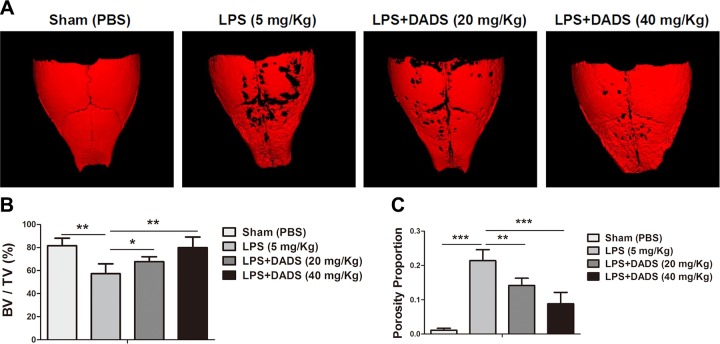
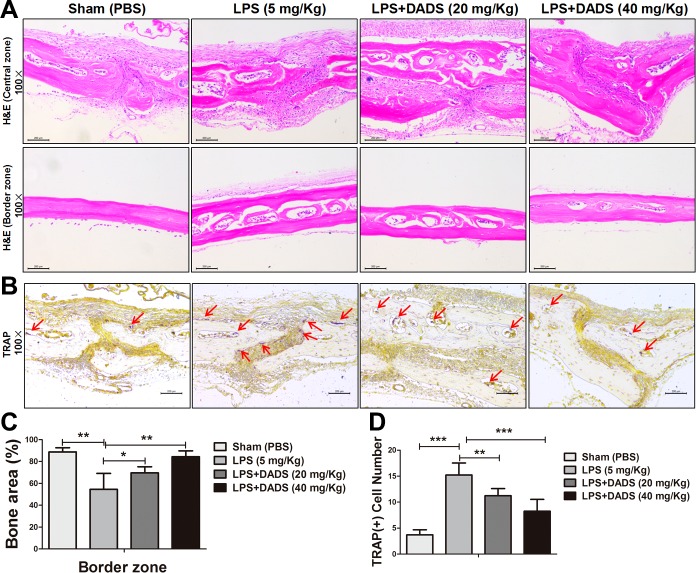
DADS prevents inflammatory osteolysis in vivo
After evaluating the inhibitory effects of DADS on osteoclastogenesis, bone resorption activity, and production of proinflammatory cytokines in vitro, we further determined whether the potential protective effects could be observed in a mouse model of LPS-induced calvarial osteolysis. The mouse was administered local subcutaneous injections of LPS (5 mg/kg, 14-d period) to the sagittal suture of the calvarium in the absence or presence of DADS. The bone mass of calvarium was quantitatively analyzed by micro-CT and 3-dimensional reconstruction images (Fig. 6A). The results showed that treatments with DADS (low-dose group and high-dose group) significantly prevented the bone volume/total volume from reducing and decreased the percentage of porosity after LPS induction (Fig. 6B, C). Furthermore, the histologic analysis with H&E and TRAP staining also confirmed that DADS protected against LPS-induced bone erosion (Fig. 7A, B). DADS treatments obviously impeded the loss of bone area, consistent with the imaging bone parameters (Fig. 7C). Similarly, the number of TRAP-positive multinucleated osteoclasts (red arrows) observed in the LPS-induced group relative to the DADS groups was markedly reduced. (Fig. 7D). Taken together, our data demonstrate that DADS may serve as a potential and effective antiresorptive agent in the treatment of LPS-induced osteolysis.
Figure 6.
DADS protects against LPS-induced osteolysis in murine calvaria. A) Micro-CT scanning and 3-dimensional reconstruction of cranium from sham group (PBS), control group (LPS; 5 mg/kg body weight), LPS with low-dose DADS (20 mg/kg), and LPS with high-dose DADS (40 mg/kg). B) Quantification of percentage of bone volume/total volume (BV/TV). C) Quantitative analysis of porosity proportion in murine calvaria. Data represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.01 (based on 1-way ANOVA).
Figure 7.
Histologic and histomorphometric analysis of the influence of DADS on LPS-induced bone loss in vivo. A) Representative images of cranial sections stained with H&E from each group. Scale bars, 200 μm. B) Representative images of cranial sections stained with TRAP. Red arrows indicate TRAP-positive cells. Scale bars, 200 μm. C) Quantification of percentage of bone area in the border zone of the cranial H&E staining by using ImageJ software. D) Quantitative analysis of TRAP (+) cell number. Data represent means ± sd. *P < 0.05, **P < 0.01, ***P < 0.01 (based on 1-way ANOVA).
DISCUSSION
Human bone remodeling, undergoing a continuous cycle throughout life, is closely effectuated by the regulation of bone-forming osteoblasts and bone resorption osteoclasts (1, 2). Overactivated osteoclasts and excessive bone resorption in the process of remodeling ultimately result in various lytic bone diseases, such as osteoporosis, osteoarthritis, periprosthetic infection, and inflammatory aseptic loosening of orthopedic implants (42–45). As is generally known, bisphosphonate is commonly used for the treatment of osteolytic bone diseases; however; it can also result in some confusing side effects for orthopedists (46). Natural products derived from plants have attracted attention to the treatment of excessive bone erosion–related diseases via restraining the formation and function of osteoclasts.
As a major oil-soluble organosulfur ingredient compound of garlic, DADS has previously demonstrated anti-inflammatory, antioxidative, and antitumor activities (29–31). Accordingly, we supposed that DADS might also be an efficacious drug candidate to treat the osteolytic-related diseases. The current study is the first to investigate the impacts of DADS on RANKL and LPS-induced osteoclastogenesis, bone resorption activity of osteoclasts, and protection of bone erosion–related diseases. We demonstrated that DADS down-regulated RANKL-induced osteoclastogenesis via suppressing the number of osteoclasts. Furthermore, the resorption activity of mature osteoclasts was obviously inhibited by DADS, suggesting that DADS not only regulated the formation and differentiation of osteoclasts but also affected their function. Research of the mechanism revealed that DADS repressed RANKL-induced osteoclastogenesis by reducing the phosphorylation of NF-κB p65 and STAT3, inhibiting phosphorylation and degradation of IκB-α and restraining the interaction of NF-κB p65 with NFATc1. Meanwhile, it has been confirmed that DADS also negatively regulated osteoclastogenesis with LPS stimulation and decreased the proinflammatory cytokines, including IL-1β, IL-6, TNF-α, and NO, which are known to promote and enhance osteoclastogenesis and ultimately cause excessive bone loss, revealing that DADS restrains LPS-induced osteoclastogenesis through both direct and indirect ways. Additionally, the in vivo data from micro-CT and histologic analysis similarly demonstrated the protective effects of DADS against LPS-induced osteolysis. All of the results indicate that DADS may be a potential and exploitable drug significantly used for preventing and impeding osteolytic lesions.
Osteoclasts, with characteristics of the multinucleated giant cells, originate from monocyte/macrophage progenitor cells or hematopoietic stem cells, which experience self-renewal and differentiation into different hematopoietic cell types (5, 47). RANKL and M-CSF are indispensable and exclusive cytokines for osteoclastogenesis. RANKL and RANK initiate TRAF6 signaling, resulting in the activation of NF-κB essential modulator and MAPKs including p38, JNK, and ERK and eventually activates NFATc1 and c-Fos, responsible for the expression of osteoclastic specific genes including TRAP, MMP9, CTR, and CTSK, leading to the proliferation, differentiation and maturation of osteoclasts (6–8). Our results showed that DADS down-regulated the expression of the osteoclast marker genes mentioned above as well as NFATc1 and c-Fos during osteoclast formation and maturation in a dose-dependent manner. DC-STAMP and OC-STAMP, two of the osteoclastogenic genes, are essential for cellular fusion of the osteoclast progenitors (45). OC-STAMP−/− cells, as well as DC-STAMP−/− cells, develop into TRAP+ mononuclear cells by RANKL stimulation (48, 49). Our research revealed that DADS repressed RANKL-induced osteoclast fusion, consistent with the inhibition of DC-STAMP and OC-STAMP at the mRNA level. As one of the key transcription factors, NF-κB composed of dimeric transcription molecules is inactive in the cytosol with a steady state and subsequently translocates into the nucleus upon activation, which is dependent on the classic or the alternative pathways (10, 50). In the classic pathway, the IKK complex containing IKK-α, IKK-β, and IKK-γ phosphorylates IκBs, which are the inactivators of NF-κB. The phosphorylation of the IκB molecules leads to their ubiquitination and subsequent degradation, freeing NF-κB. Activated NF-κB dimers induce the NFATc1 promoter to encourage the expression of NFATc1, which was known as the master regulator of osteoclast differentiation and maturation. In other words, NF-kB p65 is the upstream signal of NFATc1. The activation and translocation of NF-kB p65 to the nucleus were the main reasons for NFATc1 expression. Accordingly, it has been shown to be essential and crucial for osteoclastogenesis (11, 51). In this study, we discovered that DADS reduced the degradation of IκB-α and the phosphorylation of NF-κB p65. Additionally, the results from Co-IP indicated that the combination of NF-κB p65 with NFATc1 was obviously inhibited by the indicated concentration of DADS treatment. In other words, DADS suppressed the activation and translocation of NF-kB p65 to the nucleus and ultimately decreased NFATc1 expression. Furthermore, STAT3 signaling, an indispensable key molecule for chronic inflammation, is also involved in regulating osteoclastogenesis (22, 23). The recent study showed that peroxiredoxin II negatively regulates LPS-induced osteoclast formation via suppressing STAT3 signaling (21). Our study demonstrated that DADS can also decrease the phosphorylation of STAT3 in RANKL-induced osteoclastogenesis, collectively indicating that DADS could restrain the expression of c-Fos and NFATc1 during osteoclast formation by dulling the activation of NF-κB and STAT3 signal pathway.
Microbial products and inflammatory cytokines have a hand in osteoclastogenesis in different ways. LPS is a potent endotoxin derived from gram-negative bacteria and a notorious pathogenic molecule involved in the development of osteolytic bone loss, leading to significant morbidity and substantial healthcare expenditure (12–14). LPS signaling through TLR activates various signaling pathways, including NF-κB, MAPK, and STAT3 pathways (52). Enhanced osteoclastogenesis has been demonstrated only in preosteoclasts that were treated with RANKL prior to LPS stimulation, suggesting that LPS promotes the formation and maturation of osteoclasts, but not in the early progenitors (53, 54). Additionally, LPS is recognized to stimulate the production of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and NO from macrophages or other cells in the inflammatory site, which are able to directly enhance osteoclast differentiation and ultimately lead to the destructive bone loss. TNF-α, a potent inducer of osteoclast formation and bone resorption, acts as a pivotal part in bone metabolism and inflammatory bone diseases. TNF-α can induce RANK expression in osteoclast precursors as well as M-CSF and RANKL expression in stromal cells, osteoblasts, and activated T cells (55–57) and directly or indirectly accelerate the formation of TRAP+ multinucleated osteoclasts by activating TRAF2/5–NF-κB signaling (58, 59). Similarly, IL-1β is also a powerful stimulator of osteoclastogenesis and bone resorption by inducing RANKL expression and directly stimulating osteoclast precursor differentiation via MAPKs and NF-κB signal pathway (16, 60, 61). In short, IL-1β is deemed to be one of the strongly osteoclastogenic cytokines. The proinflammatory cytokine IL-6, which transduces signals through the IL-6 receptor (IL-6R) and combines with the glycoprotein 130 subunit is positively involved in osteoclastogenesis via NF-κB and JAK-STAT3 signal pathway, resulting in the expression of osteoclast marker genes (62, 63). Besides, IL-6 affirmatively participates in osteoclast formation through the induction of RANKL expression in osteoblasts and stromal cells (64). NO is generated from oxygen and l-arginine by NOS, which includes 3 isoforms: a neuronal form (nNOS or NOS1), an endothelial form (eNOS or NOS3), and an inducible form (iNOS or NOS2). Among these, iNOS is responsible for the generation of NO, which promotes cell fusion of osteoclast precursor and increases actin remodeling in mononuclear preosteoclasts, thereby eventually regulating the formation of multinucleated osteoclasts (24, 65, 66). Our study showed that DADS directly or indirectly inhibited LPS-induced osteoclastogenesis.
In summary, for the first time, our study demonstrated that DADS could suppress RANKL-induced osteoclast formation and function by suppression of NF-κB and STAT3 signal pathways. DADS could also inhibit LPS-induced osteoclastogenesis and decrease the proinflammatory cytokines related to osteoclasts. Furthermore, DADS protects against LPS-induced osteolysis in vivo, consistent with its effects in vitro. Therefore, DADS has potential therapeutic effects against osteoclast-related osteolytic diseases such as osteoarthritis, periprosthetic infection, and inflammatory osteolysis.
ACKNOWLEDGMENTS
This work was funded by grants from Medical Research Funding of People’s Liberation Army (PLA) of China (AWS14C003), Special Funds for Social Undertaking and Livelihood Security Projects of Chongqing (CSTC2016SHMSZX130068), and Youth Dvelopment Program of Medical Technology of PLA (16QNP103). The authors declare no conflicts of interest.
Glossary
- α-MEM
α minimal essential medium
- BMM
bone marrow macrophage
- CCK8
Cell Counting Kit-8
- Co-IP
coimmunoprecipitation
- CTR
calcitonin receptor
- CTSK
cathepsin K
- DADS
diallyl disulfide
- DC-STAMP
dendritic cell–specific transmembrane protein
- FAK
actin cytoskeleton and focal adhesion
- FBS
fetal bovine serum
- H&E
hematoxylin and eosin
- IP
immunoprecipitation
- micro-CT
micro computed tomography
- MMP9
matrix metalloproteinase 9
- NFATc1
nuclear factor of activated T cells cytoplasmic 1
- OC-STAMP
osteoclast stimulatory transmembrane protein
- RANK
receptor activator of NF-κB
- RANKL
RANK ligand
- STAT
signal transducer and activator of transcription
- TRAF
TNF receptor–associated factor
- TRAP
tartrate-resistant acid phosphatase
- qPCR
quantitative PCR
AUTHOR CONTRIBUTIONS
J. Yang, R. Tang, and J. Yi performed Western blot, real-time quantitative PCR, and staining; J. Yang and Y. Chen performed the animal studies; X. Li and T. Yu performed the bone marrow macrophage isolations; J. Yang and J. Yi wrote the paper; J. Yang and J. Fei designed this study; R. Tang and J. Yi analyzed the data; and R. Tang and J. Fei performed the manuscript proofreading.
REFERENCES
- 1.Lerner U. H. (2000) Osteoclast formation and resorption. Matrix Biol. 19, 107–120 10.1016/S0945-053X(00)00052-4 [DOI] [PubMed] [Google Scholar]
- 2.Xiong J., Onal M., Jilka R. L., Weinstein R. S., Manolagas S. C., O’Brien C. A. (2011) Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 10.1038/nm.2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenzo J., Horowitz M., Choi Y. (2008) Osteoimmunology: interactions of the bone and immune system. Endocr. Rev. 29, 403–440 10.1210/er.2007-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teitelbaum S. L. (2000) Bone resorption by osteoclasts. Science 289, 1504–1508 10.1126/science.289.5484.1504 [DOI] [PubMed] [Google Scholar]
- 5.Quinn J. M., Gillespie M. T. (2005) Modulation of osteoclast formation. Biochem. Biophys. Res. Commun. 328, 739–745 10.1016/j.bbrc.2004.11.076 [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto T., Suda T. (2003) Differentiation and function of osteoclasts. Keio J. Med. 52, 1–7 10.2302/kjm.52.1 [DOI] [PubMed] [Google Scholar]
- 7.Boyle W. J., Simonet W. S., Lacey D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 10.1038/nature01658 [DOI] [PubMed] [Google Scholar]
- 8.Quan G. H., Wang H., Cao J., Zhang Y., Wu D., Peng Q., Liu N., Sun W. C. (2015) Calycosin suppresses RANKL-mediated osteoclastogenesis through inhibition of MAPKs and NF-κB. Int. J. Mol. Sci. 16, 29496–29507 10.3390/ijms161226179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharti A. C., Takada Y., Aggarwal B. B. (2004) Curcumin (diferuloylmethane) inhibits receptor activator of NF-κ B ligand-induced NF-κ B activation in osteoclast precursors and suppresses osteoclastogenesis. J. Immunol. 172, 5940–5947 10.4049/jimmunol.172.10.5940 [DOI] [PubMed] [Google Scholar]
- 10.Karin M., Yamamoto Y., Wang Q. M. (2004) The IKK NF-κ B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 3, 17–26 10.1038/nrd1279 [DOI] [PubMed] [Google Scholar]
- 11.Tomomura M., Suzuki R., Shirataki Y., Sakagami H., Tamura N., Tomomura A. (2015) Rhinacanthin C inhibits osteoclast differentiation and bone resorption: roles of TRAF6/TAK1/MAPKs/NF-κB/NFATc1 signaling. PLoS One 10, e0130174 10.1371/journal.pone.0130174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raetz C. R., Whitfield C. (2002) Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700 10.1146/annurev.biochem.71.110601.135414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam S., Hassan F., Tumurkhuu G., Dagvadorj J., Koide N., Naiki Y., Mori I., Yoshida T., Yokochi T. (2007) Bacterial lipopolysaccharide induces osteoclast formation in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 360, 346–351 10.1016/j.bbrc.2007.06.023 [DOI] [PubMed] [Google Scholar]
- 14.Smith B. J., Lerner M. R., Bu S. Y., Lucas E. A., Hanas J. S., Lightfoot S. A., Postier R. G., Bronze M. S., Brackett D. J. (2006) Systemic bone loss and induction of coronary vessel disease in a rat model of chronic inflammation. Bone 38, 378–386 10.1016/j.bone.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 15.Azuma Y., Kaji K., Katogi R., Takeshita S., Kudo A. (2000) Tumor necrosis factor-α induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 275, 4858–4864 10.1074/jbc.275.7.4858 [DOI] [PubMed] [Google Scholar]
- 16.Wei S., Kitaura H., Zhou P., Ross F. P., Teitelbaum S. L. (2005) IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Invest. 115, 282–290 10.1172/JCI200523394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng W., Liu H., Luo T., Liu D., Du J., Sun J., Wang W., Han X., Yang K., Guo J., Amizuka N., Li M. (2017) Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-κB, ERK and JNK signaling pathways. Sci. Rep. 7, 41411 10.1038/srep41411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei C. M., Liu Q., Song F. M., Lin X. X., Su Y. J., Xu J., Huang L., Zong S. H., Zhao J. M. (2018) Artesunate inhibits RANKL-induced osteoclastogenesis and bone resorption in vitro and prevents LPS-induced bone loss in vivo. J. Cell. Physiol. 233, 476–485 10.1002/jcp.25907 [DOI] [PubMed] [Google Scholar]
- 19.Song F., Wei C., Zhou L., Qin A., Yang M., Tickner J., Huang Y., Zhao J., Xu J. (2018) Luteoloside prevents lipopolysaccharide-induced osteolysis and suppresses RANKL-induced osteoclastogenesis through attenuating RANKL signaling cascades. J. Cell. Physiol. 233, 1723–1735 10.1002/jcp.26084 [DOI] [PubMed] [Google Scholar]
- 20.Walton K. J., Duncan J. M., Deschamps P., Shaughnessy S. G. (2002) Heparin acts synergistically with interleukin-11 to induce STAT3 activation and in vitro osteoclast formation. Blood 100, 2530–2536 10.1182/blood.V100.7.2530 [DOI] [PubMed] [Google Scholar]
- 21.Song D., Cao Z., Tickner J., Qiu H., Wang C., Chen K., Wang Z., Guo C., Dong S., Xu J. (2018) Poria cocos polysaccharide attenuates RANKL-induced osteoclastogenesis by suppressing NFATc1 activity and phosphorylation of ERK and STAT3. Arch. Biochem. Biophys. 647, 76–83 10.1016/j.abb.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 22.Schuringa J. J., Schepers H., Vellenga E., Kruijer W. (2001) Ser727-dependent transcriptional activation by association of p300 with STAT3 upon IL-6 stimulation. FEBS Lett. 495, 71–76 10.1016/S0014-5793(01)02354-7 [DOI] [PubMed] [Google Scholar]
- 23.Park H., Noh A. L., Kang J. H., Sim J. S., Lee D. S., Yim M. (2015) Peroxiredoxin II negatively regulates lipopolysaccharide-induced osteoclast formation and bone loss via JNK and STAT3. Antioxid. Redox Signal. 22, 63–77 10.1089/ars.2013.5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilforoushan D., Gramoun A., Glogauer M., Manolson M. F. (2009) Nitric oxide enhances osteoclastogenesis possibly by mediating cell fusion. Nitric Oxide 21, 27–36 10.1016/j.niox.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 25.Kimbaris A. C., Siatis N. G., Daferera D. J., Tarantilis P. A., Pappas C. S., Polissiou M. G. (2006) Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason. Sonochem. 13, 54–60 10.1016/j.ultsonch.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 26.Chi M. S., Koh E. T., Stewart T. J. (1982) Effects of garlic on lipid metabolism in rats fed cholesterol or lard. J. Nutr. 112, 241–248 10.1093/jn/112.2.241 [DOI] [PubMed] [Google Scholar]
- 27.Shin I. S., Hong J., Jeon C. M., Shin N. R., Kwon O. K., Kim H. S., Kim J. C., Oh S. R., Ahn K. S. (2013) Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food Chem. Toxicol. 62, 506–513 10.1016/j.fct.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 28.Lee I. C., Kim S. H., Baek H. S., Moon C., Kang S. S., Kim S. H., Kim Y. B., Shin I. S., Kim J. C. (2014) The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem. Toxicol. 63, 174–185 10.1016/j.fct.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 29.Saud S. M., Li W., Gray Z., Matter M. S., Colburn N. H., Young M. R., Kim Y. S. (2016). Diallyl disulfide (DADS), a constituent of garlic, inactivates NF-κB and prevents colitis-induced colorectal cancer by inhibiting GSK-3β. Cancer Prev. Res. (Phila) 9, 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Druesne N., Pagniez A., Mayeur C., Thomas M., Cherbuy C., Duée P. H., Martel P., Chaumontet C. (2004) Diallyl disulfide (DADS) increases histone acetylation and p21(waf1/cip1) expression in human colon tumor cell lines. Carcinogenesis 25, 1227–1236 10.1093/carcin/bgh123 [DOI] [PubMed] [Google Scholar]
- 31.Park H. Y., Kim N. D., Kim G. Y., Hwang H. J., Kim B. W., Kim W. J., Choi Y. H. (2012) Inhibitory effects of diallyl disulfide on the production of inflammatory mediators and cytokines in lipopolysaccharide-activated BV2 microglia. Toxicol. Appl. Pharmacol. 262, 177–184 10.1016/j.taap.2012.04.034 [DOI] [PubMed] [Google Scholar]
- 32.Feng C., Luo Y., Nian Y., Liu D., Yin X., Wu J., Di J., Zhang R., Zhang J. (2017) Diallyl disulfide suppresses the inflammation and apoptosis resistance induced by DCA through ROS and the NF-κB signaling pathway in human barrett’s epithelial cells. Inflammation 40, 818–831 10.1007/s10753-017-0526-4 [DOI] [PubMed] [Google Scholar]
- 33.Bahrampour Juybari K., Kamarul T., Najafi M., Jafari D., Sharifi A. M. (2018) Restoring the IL-1β/NF-κB-induced impaired chondrogenesis by diallyl disulfide in human adipose-derived mesenchymal stem cells via attenuation of reactive oxygen species and elevation of antioxidant enzymes. Cell Tissue Res. 373, 407–419 [DOI] [PubMed] [Google Scholar]
- 34.Hosseinzadeh A., Jafari D., Kamarul T., Bagheri A., Sharifi A. M. (2017) Evaluating the protective effects and mechanisms of diallyl disulfide on interlukin-1β-induced oxidative stress and mitochondrial apoptotic signaling pathways in cultured chondrocytes. J. Cell. Biochem. 118, 1879–1888 10.1002/jcb.25907 [DOI] [PubMed] [Google Scholar]
- 35.Guan X., Zhou Y., Chen H., Wang C., Wang H. (2018) Antibacterial, anti-inflammatory, and anti-osteoclastogenesis roles of allicin in periodontitis. Int. J. Clin. Exp. Med. 11, 6721–6730 [Google Scholar]
- 36.Dou C., Li J., Kang F., Cao Z., Yang X., Jiang H., Yang B., Xiang J., Xu J., Dong S. (2016) Dual effect of cyanidin on RANKL‐induced differentiation and fusion of osteoclasts. J. Cell. Physiol. 231, 558–567 10.1002/jcp.24916 [DOI] [PubMed] [Google Scholar]
- 37.Park E. J., Kim S. A., Choi Y. M., Kwon H. K., Shim W., Lee G., Choi S. (2011) Capric acid inhibits NO production and STAT3 activation during LPS-induced osteoclastogenesis. PLoS One 6, e27739 10.1371/journal.pone.0027739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiang C. Y., Kyritsis G., Graves D. T., Amar S. (1999) Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect. Immun. 67, 4231–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang R., Yi J., Yang J., Chen Y., Luo W., Dong S., Fei J. (2019) Interleukin‐37 inhibits osteoclastogenesis and alleviates inflammatory bone destruction. J. Cell. Physiol. 234, 7645–7658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teramachi J., Inagaki Y., Shinohara H., Okamura H., Yang D., Ochiai K., Baba R., Morimoto H., Nagata T., Haneji T. (2017) PKR regulates LPS-induced osteoclast formation and bone destruction in vitro and in vivo. Oral Dis. 23, 181–188 10.1111/odi.12592 [DOI] [PubMed] [Google Scholar]
- 41.Zou W., Bar-Shavit Z. (2002) Dual modulation of osteoclast differentiation by lipopolysaccharide. J. Bone Miner. Res. 17, 1211–1218 10.1359/jbmr.2002.17.7.1211 [DOI] [PubMed] [Google Scholar]
- 42.Kassem M., Brixen K., Blum W. F., Mosekilde L., Eriksen E. F. (1994) Normal osteoclastic and osteoblastic responses to exogenous growth hormone in patients with postmenopausal spinal osteoporosis. J. Bone Miner. Res. 9, 1365–1370 10.1002/jbmr.5650090907 [DOI] [PubMed] [Google Scholar]
- 43.Geurts J., Patel A., Hirschmann M. T., Pagenstert G. I., Müller-Gerbl M., Valderrabano V., Hügle T. (2016) Elevated marrow inflammatory cells and osteoclasts in subchondral osteosclerosis in human knee osteoarthritis. J. Orthop. Res. 34, 262–269 10.1002/jor.23009 [DOI] [PubMed] [Google Scholar]
- 44.Sabokbar A., Kudo O., Athanasou N. A. (2003) Two distinct cellular mechanisms of osteoclast formation and bone resorption in periprosthetic osteolysis. J. Orthop. Res. 21, 73–80 10.1016/S0736-0266(02)00106-7 [DOI] [PubMed] [Google Scholar]
- 45.Neale S. D., Sabokbar A., Howie D. W., Murray D. W., Athanasou N. A. (1999) Macrophage colony-stimulating factor and interleukin-6 release by periprosthetic cells stimulates osteoclast formation and bone resorption. J. Orthop. Res. 17, 686–694 10.1002/jor.1100170510 [DOI] [PubMed] [Google Scholar]
- 46.Heymann D., Fortun Y., Rédini F., Padrines M. (2005) Osteolytic bone diseases: physiological analogues of bone resorption effectors as alternative therapeutic tools. Drug Discov. Today 10, 242–247 10.1016/S1359-6446(04)03265-9 [DOI] [PubMed] [Google Scholar]
- 47.Seita J., Weissman I. L. (2010) Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 640–653 10.1002/wsbm.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yagi M., Miyamoto T., Sawatani Y., Iwamoto K., Hosogane N., Fujita N., Morita K., Ninomiya K., Suzuki T., Miyamoto K., Oike Y., Takeya M., Toyama Y., Suda T. (2005) DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 202, 345–351 10.1084/jem.20050645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyamoto H., Suzuki T., Miyauchi Y., Iwasaki R., Kobayashi T., Sato Y., Miyamoto K., Hoshi H., Hashimoto K., Yoshida S., Hao W., Mori T., Kanagawa H., Katsuyama E., Fujie A., Morioka H., Matsumoto M., Chiba K., Takeya M., Toyama Y., Miyamoto T. (2012) Osteoclast stimulatory transmembrane protein and dendritic cell–specific transmembrane protein cooperatively modulate cell–cell fusion to form osteoclasts and foreign body giant cells. J. Bone Miner. Res. 27, 1289–1297 10.1002/jbmr.1575 [DOI] [PubMed] [Google Scholar]
- 50.Ghosh S., Karin M. (2002) Missing pieces in the NF-kappaB puzzle. Cell 109, S81–S96 [DOI] [PubMed] [Google Scholar]
- 51.Yamashita T., Yao Z., Li F., Zhang Q., Badell I. R., Schwarz E. M., Takeshita S., Wagner E. F., Noda M., Matsuo K., Xing L., Boyce B. F. (2007) NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 282, 18245–18253 10.1074/jbc.M610701200 [DOI] [PubMed] [Google Scholar]
- 52.Lu Y. C., Yeh W. C., Ohashi P. S. (2008) LPS/TLR4 signal transduction pathway. Cytokine 42, 145–151 10.1016/j.cyto.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 53.Takami M., Kim N., Rho J., Choi Y. (2002) Stimulation by toll-like receptors inhibits osteoclast differentiation. J. Immunol. 169, 1516–1523 10.4049/jimmunol.169.3.1516 [DOI] [PubMed] [Google Scholar]
- 54.Sul O. J., Park H. J., Son H. J., Choi H. S. (2017) Lipopolysaccharide (LPS)-induced autophagy is responsible for enhanced osteoclastogenesis. Mol. Cells 40, 880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Komine M., Kukita A., Kukita T., Ogata Y., Hotokebuchi T., Kohashi O. (2001) Tumor necrosis factor-α cooperates with receptor activator of nuclear factor kappaB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone 28, 474–483 10.1016/S8756-3282(01)00420-3 [DOI] [PubMed] [Google Scholar]
- 56.Kitaura H., Kimura K., Ishida M., Kohara H., Yoshimatsu M., Takano-Yamamoto T. (2013) Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin. Dev. Immunol. 2013, 181849 10.1155/2013/181849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitaura H., Zhou P., Kim H. J., Novack D. V., Ross F. P., Teitelbaum S. L. (2005) M-CSF mediates TNF-induced inflammatory osteolysis. J. Clin. Invest. 115, 3418–3427 10.1172/JCI26132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanazawa K., Azuma Y., Nakano H., Kudo A. (2003) TRAF5 functions in both RANKL- and TNFalpha-induced osteoclastogenesis. J. Bone Miner. Res. 18, 443–450 10.1359/jbmr.2003.18.3.443 [DOI] [PubMed] [Google Scholar]
- 59.Kanazawa K., Kudo A. (2005) TRAF2 is essential for TNF-α-induced osteoclastogenesis. J. Bone Miner. Res. 20, 840–847 10.1359/JBMR.041225 [DOI] [PubMed] [Google Scholar]
- 60.Ruscitti P., Cipriani P., Carubbi F., Liakouli V., Zazzeroni F., Di Benedetto P., Berardicurti O., Alesse E., Giacomelli R. (2015) The role of IL-1β in the bone loss during rheumatic diseases. Mediators Inflamm. 2015, 782382 10.1155/2015/782382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jules J., Zhang P., Ashley J. W., Wei S., Shi Z., Liu J., Michalek S. M., Feng X. (2012) Molecular basis of requirement of receptor activator of nuclear factor κB signaling for interleukin 1-mediated osteoclastogenesis. J. Biol. Chem. 287, 15728–15738 10.1074/jbc.M111.296228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rose-John S. (2018) Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 10, a028415 10.1101/cshperspect.a028415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blanchard F., Duplomb L., Baud’huin M., Brounais B. (2009) The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 20, 19–28 10.1016/j.cytogfr.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 64.Yoshitake F., Itoh S., Narita H., Ishihara K., Ebisu S. (2008) Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J. Biol. Chem. 283, 11535–11540 10.1074/jbc.M607999200 [DOI] [PubMed] [Google Scholar]
- 65.Alderton W. K., Cooper C. E., Knowles R. G. (2001) Nitric oxide synthases: structure, function and inhibition. Biochem. J. 357, 593–615 10.1042/bj3570593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H. (2010) Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 23, 75–93 10.1016/j.niox.2010.04.007 [DOI] [PubMed] [Google Scholar]