Abstract
Pancreatic adenosquamous carcinoma (PASC) is a rare, aggressive subtype of pancreatic tumor with a poor prognostic outlook compared to the much more common pancreatic adenocarcinoma. Here we present two cases of the rare PASC and analyze the radiologic findings on computed tomography (CT) and 18F- fluorodeoxyglucose positron emission tomography (FDG-PET).
Both cases involve 62-year-old women presenting with abdominal pain, nausea, and vomiting who on imaging were found to have infiltrating lobular pancreatic masses with ring enhancement on CT and peripheral hypermetabolism with central necrosis on FDG-PET. Location in the pancreas and involvement of adjacent structures differed in the two cases, resulting in varying progressive clinical manifestations.
PASC is a rare subtype of pancreatic cancer with nonspecific imaging findings. Here we presented two cases of PASC supporting previously reported imaging findings suggestive of PASC with additional FDG-PET manifestations and SUV levels, which only few reports have previously described.
Keywords: Pancreatic adenosquamous carcinoma, CT, FDG-PET
Introduction
Pancreatic cancer is the second most common gastrointestinal tract cancer after colon cancer and the fourth leading cause of cancer-related death in the United States with approximately 53,670 new cases and 43,090 deaths in 2017 [1]. More than 90% of pancreatic malignancies arise from exocrine glands with ductal adenocarcinoma accounting for almost 85% [2]. In contrast, the rare pancreatic adenosquamous carcinoma (PASC) of the pancreas accounts for 1%-4% of exocrine pancreatic malignancies [3]. First reported in 1907, PASC is defined by the presence of at least 30% malignant squamous cell carcinoma (SCC) mixed with ductal adenocarcinoma [4], [5]. Differentiation from metastatic SCC is based on the presence of glandular elements [5].
Similar to patients with adenocarcinoma, those with adenosquamous carcinoma present with abdominal pain, weight loss, anorexia, and jaundice [3], [6], [7], [8]. Treatments include surgical resection, radiation therapy, and locoregional chemotherapy. Surgical resectability is the single strongest predictor of survival in patients with PASC [9]. However, with a median survival of 7 months and long-term disease-specific 1- and 2-year survival of 30.5% and 19.7%, respectively, prognosis for patients with PASC remains much worse compared to patients with adenocarcinoma of the pancreas [10].
No specific imaging features distinguish PASC from adenocarcinoma, but several useful clues have been previously reported including an infiltrating round-lobulated mass, extensive central necrosis with ring-enhancement, location in the body or tail of the pancreas, or tumor thrombus in the portal venous system [11], [12], [13], [14]. Given its highly aggressive nature and dismal prognosis, accurate imaging diagnosis and determination of surgical resectability are of paramount importance, despite the rarity of this pancreatic carcinoma subtype.
Here, we report 2 rare cases of pancreatic adenosquamous cell carcinoma of the pancreas.
Case report #1
A 62-year-old woman presented to an outside facility with complaints of progressive left upper quadrant abdominal pain occasionally radiating to her back and across her abdomen. Additional symptoms included nausea, vomiting, and unintentional weight loss. Initial computed tomography (CT) of the abdomen and pelvis revealed a left upper quadrant mass, originally described as arising from the posterior wall of the stomach with possible ulceration. This led to endoscopic gastroduodenoscopy and subsequent biopsy revealing SCC of the stomach, a very rare tumor [15], [16]. The patient was then referred to our institution for further care.
Further work-up with diagnostic laparoscopy confirmed a mass arising from the pancreatic tail and separate from the stomach. Additionally, a suspicious firm right upper quadrant peritoneal nodule was detected incidentally and resected. CT-guided percutaneous biopsy as well as pathologic evaluation of the resected peritoneal nodule yielded SCC of the pancreas.
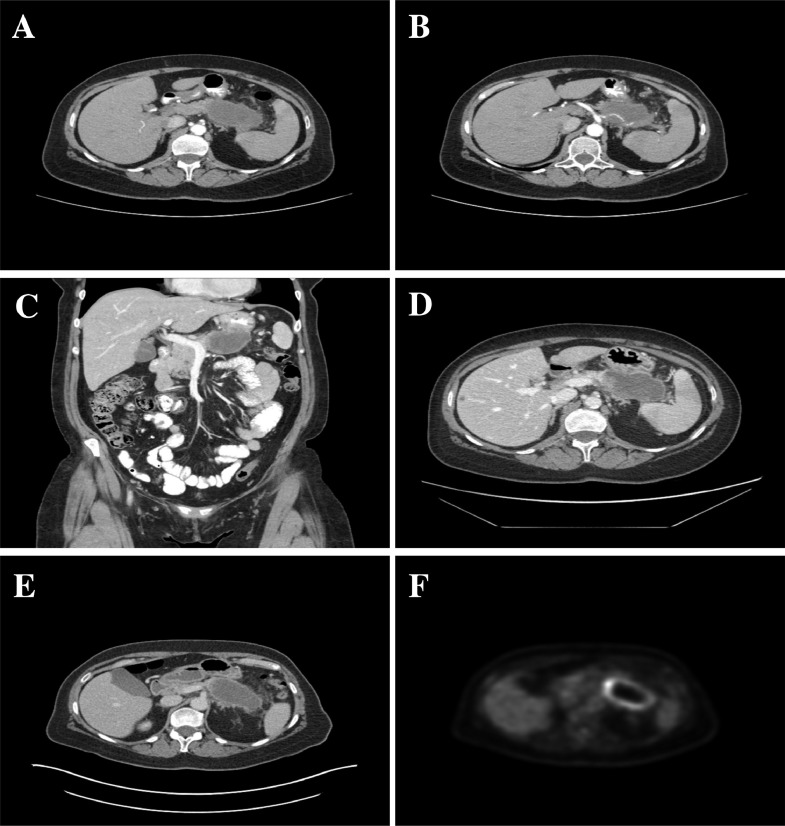
Follow-up CT of the abdomen and pelvis showed a 4.4 × 8.5 × 5.9 cm (anteroposterior × transverse × craniocaudal) centrally necrotic mass in the tail of the pancreas invading the posterior wall of the stomach, occluding the splenic vein, and encasing the splenic artery. Vascular involvement resulted in multiple splenic infarcts (Fig. 1 A-C). The 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) imaging for staging demonstrated localized disease to the pancreatic tail without additional metastases (Max standardized uptake value [SUVMAX] = 15.0 g/mL). The mass had peripheral hypermetabolism with central necrosis corresponding to the area of central necrosis on CT images (Fig. 1D and E).
Fig. 1.
Case 1: Contrast-enhanced CT of the abdomen showing a lobulated mass with peripheral ring enhancement and central hypoattenuation (A) invading the posterior gastric wall (B and C). Encasement of the splenic artery (B) and occlusion of the splenic vein (E) resulting in multiple splenic infarcts (A, B, and D). Solitary hypoattenuating metastasis in segment 5 of the liver (D). FDG-PET shows a peripheral ring of hypermetabolism and central hypometabolism corresponding to an area of necrosis (SUVMAX = 15 g/mL; F).
Laboratory studies include complete blood count, comprehensive metabolic panel, and lipase within normal limits. Serum tumor marker CA19-9 was also within normal limits. Carcinoembryonic antigen markers were not obtained. The multidisciplinary team made the decision to avoid surgery given extent of disease at the time. The patient completed 5 cycles of chemotherapy with FOLFIRINOX (folinic acid, fluorouracil, irinotecan, and oxaliplatin) followed by chemoradiation with Xeloda (capecitabine). The latter was discontinued after the discovery of a new liver lesion, which was percutaneously biopsied and pathologically proven to represent metastatic adenosquamous pancreatic cancer. This finding inferred the final diagnosis of the pancreatic mass to be adenosquamous carcinoma rather than SCC. The patient is currently continuing chemotherapy.
Case report #2
A 62-year-old woman presented to our emergency department with 3-day history of progressive poor oral intake and weight loss, malaise, nausea, vomiting, and epigastric abdominal pain. Laboratory results at presentation were in the normal range for lipase (11 U/L; normal range: ≤50). Bilirubin (1.7 mg/dL; normal range: 0.2-1.0 mg/dL) and alkaline phosphatase (390 mg/dL; normal range: 30-120 mg/dL) were elevated. Serum tumor markers revealed a normal CA 19-9 (5.0 U/mL; normal: 0-35 U/mL) but elevated carcinoembryonic antigen (11.1 ng/mL; normal: 0-5 ng/mL).
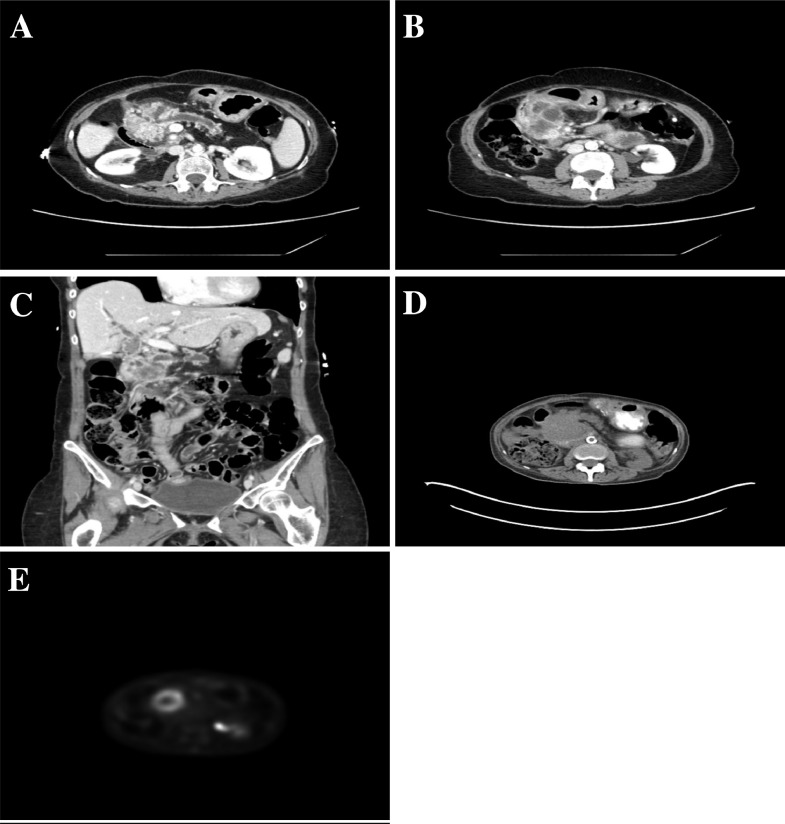
CT of the chest, abdomen, and pelvis was performed for further evaluation. A large, heterogeneous, and centrally necrotic mass was found in the head of the pancreas measuring 5.6 × 4.5 × 4.0 cm (anteroposterior × transverse × craniocaudal). This displaced the stomach anteriorly and partially encased and narrowed the superior mesenteric vein. The location resulted in marked dilation of the main pancreatic duct and biliary tree. The patient was subsequently admitted to the hospital for further management of comorbidities, mainly uncontrolled diabetes and hypertension (Fig. 2).
Fig. 2.
Case 2: Contrast-enhanced CT of the abdomen showing a lobulated, infiltrating cystic and solid mass in the head of the pancreas (A) with peripheral enhancement. Partial encasement and narrowing of the superior mesenteric vein (B). The mass is causing obstruction and marked dilation of the biliary tree (C). PET-CT (D and E) shows a peripheral ring of hypermetabolism and central hypometabolism corresponding to an area of necrosis (SUVMAX = 7.2 g/mL).
Extent of disease was evaluated with FDG-PET imaging. Tumor was localized to the pancreatic head without evidence of metastatic disease to other organs or lymph nodes (SUVMAX = 7.2 g/mL). Peripheral hypermetabolism was present with relative hypometabolism centrally corresponding to area of central necrosis on CT images.
CT-guided biopsy of the pancreatic head mass yielded a dual population of cells consistent with adenosquamous carcinoma.
The patient was not a candidate for surgical resection. Poor nutritional status precluded chemotherapy or radiation therapy. After multiple complications including several bouts of pancreatitis, cholangitis (treated with cholecystostomy tube placement), and episodes of gastrointestinal hemorrhage, the patient opted to discontinue all aggressive management and focus on comfort care. She passed away 7 months after her initial diagnosis.
Discussion
Adenosquamous carcinoma is a rare, aggressive subtype of pancreatic neoplasm characterized by at least 30% of squamous cells in a background of glandular elements [5]. As no squamous epithelium is normally present within the ductal epithelium of the pancreas, several hypotheses of the origin of PASC have been reported. To date, the leading theory is histologic transformation of pre-existing adenocarcinoma to SCC [17], [18], [19]. Other proposed mechanisms include ectopic squamous epithelium and squamous metaplasia of the glandular system or pancreatic ductal epithelium.
Although most PASCs occur in the body or tail, they can occur anywhere in the pancreas [12]. Presenting symptoms are nonspecific and indistinguishable from the more common pancreatic adenocarcinoma. Clinical presentation and progression is largely based on tumor location and involvement of adjacent structures. For example, both our patients presented with similar symptoms of abdominal pain, nausea, vomiting, and weight loss. However, as seen in case 1, location in the pancreatic tail resulted in gastric wall invasion and splenic infarcts from splenic vein and artery encasement. In contrast, case 2 dealt with location in the head of the pancreas, which resulted in obstructive jaundice and pancreatitis requiring placement of cholecystostomy drains to alleviate symptoms.
PASC carries a dismal prognosis with a median survival of 7 months and long-term disease-specific 1- and 2-year survival of 30.5% and 19.7%, respectively, much worse compared to patients with adenocarcinoma of the pancreas [10]. If the patient is a surgical candidate, resection is the single most beneficial treatment and strongest predictor for survival [9], [10]. Radiation therapy and locoregional chemotherapy have proven less effective [9]. Neither of our cases was managed surgically. Case 1 completed initial chemotherapy followed by chemoradiation, which was discontinued after the discovery of a solitary liver metastasis. The patient was restarted on chemotherapy that she is continuing to this day, 6 months after initial diagnosis. Case 2 was not a candidate for chemoradiation or radiation therapy due to poor nutritional status and additional comorbidities. She fell within the median survival rate of 7 months.
Imaging characteristics of PASC are nonspecific, but prior reports indicate a lobulated, infiltrative, predominantly cystic mass with rim-enhancement and central necrosis may be suggestive of this rare diagnosis—characteristics that are rarely seen with pancreatic adenocarcinoma [11], [12], [13], [14], [20], [21]. Additional findings such as portal vein thrombosis have also been described [12]. Both cases presented here demonstrated findings supportive of those previously reported. Both were lobulated infiltrating rim-enhancing masses with predominant cystic components representing necrosis. Findings were confirmed with FDG-PET imaging in both cases. In addition, we report SUVs for PASC that are significantly higher than those previously described for adenocarcinoma (4.7 ± 2.5 g/mL), which may aid in the differentiation of these entities [22]. Nonetheless, tissue biopsy and pathologic evaluation are ultimately needed to reach the final diagnosis. Determining staging and extent of disease for operability remains the most important role of imaging.
Conclusion
Herein, we described 2 cases of the rare adenosquamous carcinoma of the pancreas, clinical course, treatment, and imaging findings. Although definitive imaging characteristics remain largely nonspecific, our cases are supportive of previously reported imaging findings. We also report FDG-PET imaging findings and SUVs, which few have described before and may add additional information in differentiating this entity from pancreatic adenocarcinoma. Radiographic evaluation continues to play an important role in determining extent of disease to guide management. Efficient treatment strategies have yet to be established. Although surgical resection has shown improved median survival, long-term survival remains less than that for adenocarcinoma. Further accumulation of knowledge in all aspects of this entity is still needed.
Footnotes
Acknowledgments: We thank Dr. Jeffrey McClure for aiding with FDG-PET SUV levels.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2019.04.004.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1) doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald T.L., Hickner Z.J., Schmitz M., Kort E.J. Changing incidence of pancreatic neoplasms: a 16- year review of statewide tumor registry. Pancreas. 2008;37(2):134–138. doi: 10.1097/MPA.0b013e318163a329. [DOI] [PubMed] [Google Scholar]
- 3.Madura J.A., Jarman B.T., Doherty M.G., Yum M.N., Howard T.J. Adenosquamous carcinoma of the pancreas. Arch Surg. 1999;134(6):599–603. doi: 10.1001/archsurg.134.6.599. [DOI] [PubMed] [Google Scholar]
- 4.Herxheimer G. Uber heterologe cancroide. Beitr Pathol Anat. 1907;41:348–412. [Google Scholar]
- 5.Hruban R. Tumors of the pancreas. In: Silverberg S., editor. AFIP atlas of tumor pathology. 2007. pp. 177–181. Washington, DC. [Google Scholar]
- 6.Hsu J.T., Chen H.M., Wu R.C., Yeh C.N., Yeh T.S., Hwang T.L. Clinicopathologic features and outcomes following surgery for pancreatic adenosquamous carcinoma. World J Surg Oncol. 2008;6:95. doi: 10.1186/1477-7819-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okabayashi T., Hanazaki K. Surgical outcome of adenosquamous carcinoma of the pancreas. World J Gastroenterol. 2008;14(44):6765–6770. doi: 10.3748/wjg.14.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kardon D.E., Thompson L.D., Przygodzki R.M., Heffess C.S. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol. 2001;14(5):443–451. doi: 10.1038/modpathol.3880332. [DOI] [PubMed] [Google Scholar]
- 9.Katz M.H., Taylor T.H., Al-Refaie W.B. Adenosquamous versus adenocarcinoma of the pancreas: a population-based outcomes analysis. J Gastrointest Surg. 2011;15:165–174. doi: 10.1007/s11605-010-1378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd C.A., Benarroch-Gampel J., Sheffield K.M., Cooksley C.D., Riall T.S. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res. 2012;174(1):12–19. doi: 10.1016/j.jss.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imaoka H., Shimizu Y., Mizuno N., Hara K., Hijioka S., Tajika M. Clinical characteristics of adenosquamous carcinoma of the pancreas: a matched case-control study. Pancreas. 2014;43:287–290. doi: 10.3892/ol.2014.2008. [DOI] [PubMed] [Google Scholar]
- 12.Ying Q., Wang C., Wu Z., Wang M., Cheng K., Zhao X. Adenosquamous carcinoma of the pancreas: multidetector-row computed tomographic manifestations and tumor characteristics. J Comput Assist Tomogr. 2013;37(2):125–133. doi: 10.1097/RCT.0b013e31827bc452. [DOI] [PubMed] [Google Scholar]
- 13.Imaoka H., Shimizu Y., Mizuno N. Ring-enhancement pattern on contrast-enhanced CT predicts adenosquamous carcinoma of the pancreas: a matched case-control study. Pancreatology. 2014;14(3):221–226. doi: 10.1016/j.pan.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Toshima F., Inoue D., Yoshida K. Adenosquamous carcinoma of pancreas: CT and MR imaging features in eight patients, with pathologic correlations and comparison with adenocarcinoma of pancreas. Abdom Radiol. 2016;41(3):508–520. doi: 10.1007/s00261-015-0616-4. [DOI] [PubMed] [Google Scholar]
- 15.Straus R., Heschel S., Fortmann D.J. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985–995. doi: 10.1002/1097-0142(196911)24:5%3C985::AID-CNCR2820240518%3E3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Wakabayashi H., Matsutani T., Fujita I. A rare case of primary squamous cell carcinoma of the stomach and review of the 56 cases reported in Japan. J Gastric Cancer. 2014;14:58–62. doi: 10.5230/jgc.2014.14.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao S.H. Extrahepatic bile duct cancer. Report of 106 cases. Chin Med J (Engl) 1992;105(8):622–629. [PubMed] [Google Scholar]
- 18.Iemura A., Yano H., Mizoguchi A., Kojiro M. A cholangiocellular carcinoma nude mouse strain showing histologic alteration from adenocarcinoma to squamous cell carcinoma. Cancer. 1992;70(2):415–422. doi: 10.1002/1097-0142(19920715)70:2<415::aid-cncr2820700208>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Momosaki S., Yano H., Kojiro M. Establishment of a human hepatic adenosquamous carcinoma cell line (KMC-2) and its response to cytokines. Pathol Int. 1995;45(2):137–146. doi: 10.1111/j.1440-1827.1995.tb03434.x. [DOI] [PubMed] [Google Scholar]
- 20.Mergo P.J., Helmberger T.K., Buetow P.C., Helmberger R.C., Ros P.R. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics. 1997;17:281–301. doi: 10.1148/radiographics.17.2.9084072. [DOI] [PubMed] [Google Scholar]
- 21.Buetow P.C., Parrino T.V., Buck J.L., Pantongrag-Brown L., Ros P.R., Dachman A.H. Islet cell tumors of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR Am J Roentgenol. 1995;165:1175–1179. doi: 10.2214/ajr.165.5.7572498. [DOI] [PubMed] [Google Scholar]
- 22.Izuishi K., Takebayashi R., Suzuki Y. Electronic image of the month. Adenosquamous carcinoma of the pancreas. Clin Gastroenterol Hepatol. 2010;8(40):e40. doi: 10.1016/j.cgh.2009.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.