Opinion statement
Targeted therapies and more recently immune checkpoint inhibitors (ICI) have transformed the treatment landscape of advanced NSCLC. Clinical trials investigating immune checkpoint inhibitors (ICI) have usually excluded patients with oncogenic drivers, so that the outcome of these agents in this population is poorly known. In patients with oncogenic addiction, targeted therapy remains clearly the best option, and the place of immunotherapy in this population has not been clearly defined yet.
Based on available data, we suggest that (i) immunotherapy single agent should be proposed only after exhaustion of more validated treatments, (ii) combinations of immunotherapy with targeted therapies are of interest provided that we can manage toxicity and find the best sequence, (iii) a combination of immunotherapy with chemotherapy may be appealing in patients pretreated with targeted agents. The best way to opt in for the best strategy will depend upon the identification of adequate biomarkers. New basic and clinical research is awaited in this field.
Keywords: Immunotherapy, Oncogenic addiction, Targeted therapy, EGFR, ALK, Non-small cell lung cancer
Introduction
Lung cancer is the main cause of cancer-related mortality worldwide. Its management underwent significant transformation over the past 10–15 years leading to two new therapeutic strategies: targeted therapies and immunotherapy. Genotype-directed treatments targeting oncogenic addictions (EGFR, BRAF, and HER2 mutations, or ALK and ROS1 rearrangements) demonstrated high response rates and prolonged PFS.
Immunotherapy has been also recently developed in non-small cell lung cancer (NSCLC). The positive results of clinical trials assessing PD-1 and PD-L1 inhibitors in both metastatic and locally advanced stages revolutionized the treatment landscape of NSCLC [1–3]. In all the studies considering these agents however, only a minority of patients (15–20%) derived a durable benefit and a new pattern of hyperprogressive patients has been identified, suggesting an urgent need for new biomarkers of both response and resistance.
The place taken by ICI in patients with oncogenic driver is actually debated, since they have been usually excluded from phase 3 immunotherapy trials. However, despite a usual dramatic and durable activity of targeted therapies, resistance inexorably develops [1]. Treatment options upon exhaustion of targeted therapies are limited, underscoring the need to explore the potential interest of ICI in these populations.
Another challenge is to further understand the biological drivers of inflammation and immune escape in NSCLC with oncogenic addiction and to identify subgroups deriving benefits.
We aim herein to review the main data available regarding the outcome of patients harboring an oncogenic addiction and treated with ICI either alone, in combination with chemotherapy or concomitantly to targeted therapy.
What is the immunogenicity of lung cancer with oncogenic addiction?
The evolution of tumors bearing a molecular alteration usually depends on a single dominant mechanism following the principle of oncogenic addiction, which has been described as the dependence of tumor cells upon the specific activity of an activated oncogene [4]. A single mutation or translocation is supposed to confer a survival advantage to the respective cells and it is usually isolated, explaining the low tumor mutation burden observed in these tumors [5], leading to less inflamed tumor microenvironments with death of tumor-infiltrating CD8+ lymphocytes, explaining the low response rate to PD-1 inhibitors observed amongst EGFR- and ALK-driven NSCLC [6••].
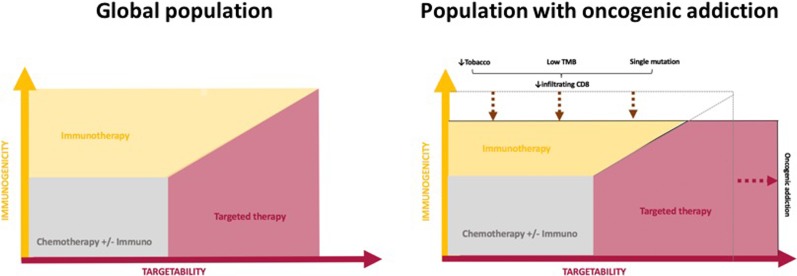
EGFR, BRAFv600E mutations, ALK, ROS, or RET rearrangements but also MET exon 14 mutations are usually found in non-smokers, in contrast with KRAS and BRAF non V600E which are more likely found in smokers [7]. It is clearly established that tobacco exposure is associated with higher tumor mutation burden (TMB) and correlates with higher responsiveness to ICI, while non-smokers will less likely respond to these agents due to excluded, non-inflamed microenvironments [6••, 8–10]. Figure 1 and Figure 2 summarize the sensitivity to ICI and targeted therapy regarding each oncogenic addiction.
Fig. 1.
Immunogenicity and sensitivity to targeted agents in situation of oncogenic addiction.
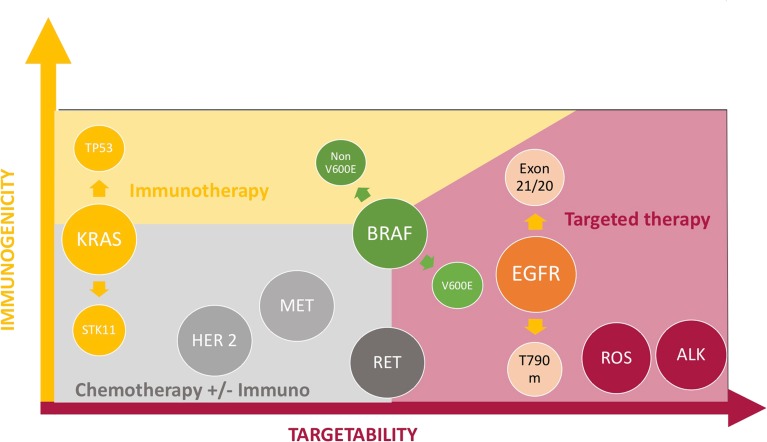
Fig. 2.
Likelihood of sensitivity to ICI and/or genotype-directed agents in each oncogenic addiction setting.
Anti-tumor activity of ICI as monotherapy according to each type of mutation
KRAS
KRAS mutations are the most frequent molecular alterations encountered in advanced NSCLC and are correlated with both poor prognosis and poor outcomes under chemotherapy [11–13]. Moreover, all studies involving therapies targeting KRAS failed to demonstrate any clinical benefit up to now [14, 15] due notably to the complexity of its downstream signaling pathways.
ICI however clearly represent an attractive alternative in KRAS-mutated patients, since correlations between KRAS mutations and sustained responses to checkpoint inhibitors have systematically been reported in trials [1, 8] . In a recent work, Miao et al. reported that clonal driver alterations in KRAS were associated with complete or partial response to ICI [16]. This relationship is likely due to a strong epidemiologic association with tobacco that generates a high mutation burden [17, 18].
In our retrospective IMMUNOTARGET cohort, we reported an interesting 3.2 months of median PFS in the overall KRAS population but failed to detect a significant impact of the different KRAS mutations subtypes on PFS or OS [19••]. PD-L1 expression in this population was associated with better outcomes.
More interestingly, some molecular alterations have been shown to have a direct impact, independently on their epidemiologic relationship with tobacco, on the tumor microenvironment. In particularly, STK11 mutations, inactivated in 20 to 30% of adenocarcinomas, and often associated with KRAS mutations, are associated with non-inflamed tumor microenvironment in a murine model. STK11 inactivation leads to the an increased infiltrate of protumoral neutrophils, a decrease of tumor-infiltrating cells and reduced PD1 and PD-L1 expressions in the tumor microenvironment, both in a KRAS-driven mouse model of lung adenocarcinoma and in cell lines [15]. Furthermore, tumors mutated for both KRAS and TP53 (KP) have a higher mutation load than tumors co-mutated for KRAS/STK11 (KL), and KP tumors are characterized by a stronger inflammatory response [20, 21]. In agreement with these preclinical signals, recent data showed that TP53 and STK11 may also accurately stratify patients between responders (57% ORR for KRAS-P53 tumors) and non-responders (0% ORR in KRAS-STK11 tumors) in the Checkmate 057 trial [22•].
EGFR
Regarding EGFR, contradictory results have been reported in preclinical studies [23] However, clinical studies have systematically reported disappointing results. Gainor et al. identified a very low response rate (3.6%) in patients harboring EGFR mutations treated by immunotherapy and showed an association with non-inflamed tumor microenvironment [6••]. A recent meta-analysis involving three randomized trials of immunotherapy in pretreated patients (CheckMate 057, Keynote 010, and POPLAR) reported that ICI did not improve overall survival compared with docetaxel in EGFR-mutated patients (HR 1.11; 95% CI 0.80–1.53, p = 0.54, interaction p = 0.005) [24•]. In a recent phase II study of pembrolizumab in PD-L1 positive, TKI-naïve EGFR-mutated patients presented no response even in tumors with high (≥ 50%) PD-L1 expression highlighting that frontline ICI is inappropriate in EGFR patients [25]. A phase 2, open-label, single-arm trial (ATLANTIC) studied the efficacy of durvalumab, a PD-L1 inhibitor, in pretreated NSCLC including 111 patients with EGFR or ALK alteration [26•]. Patients with EGFR or ALK alteration had previously received standard treatment with tyrosine kinase inhibitors. Amongst the 111 oncogene addicted patients, 77 presented PD-L1 expression in at least 25% of tumor cells. The aimed response rate was 12.2% in oncogene addicted patients with PD-L1 expression > 25% of tumor cells, while in patients with < 25% PD-L1 expression, the objective response rate was only 3.6%. Progression-free survival was 1.9 months and was not influenced by PD-L1 expression.
Several explanations to these poor outcomes have been identified, such as an association of EGFR mutation with low TMB and a lack of T cell infiltration [6••, 27] while PD-L1 expression is variable [26•, 27•, 28•].
Our IMMUNOTARGET cohort support these findings, with however some differences between sub groups. PFS was 1.4 months for the T790M attached subgroup; 1.8 months for exon 19 deletions; 2.5 for exon 21 mutations, and 2.8 for other mutations (p < 0.0001) [21]. In contrast, Negrao et al presented appealing data with exon 20 EGFR-mutated tumors compared with classic EGFR-mutations and demonstrated higher OR and disease control rates at 6 and 12 months respectively 13% and 4% versus 6% and 0% for classic EGFR group) but also higher PFS (2.9 vs 1.9 months HR 0.45, p = 0.002) and OS (HR 0.2, p < 0.001), compared with common EGFR mutations [29]. Further studies are requested to confirm these differences in outcomes between EGFR mutations subtypes and to adjust these outcomes to tobacco use (more frequent amongst exon 20 mutated patients) and ideally TMB.
ALK, ROS, and other translocations
Few studies included ALK patients and all reported very poor outcome under ICI. However, the number of patients was usually very low, precluding definitive conclusions [1, 6••, 8, 26•].
Other translocations, such as RET or ROS1, have been less studied. Poor results were found in the IMMUNOTARGET cohort amongst the concerned population. ALK, ROS1, and RET were analyzed together in a “rearrangement” subgroup, showing a 4.9% ORR (2/30) [21].
ICI outcomes in RET-rearranged patients have been studied elsewhere. In another retrospective cohort, it was found that RET rearrangements were associated with low TMB and poor response to immunotherapy compared with unselected patients [30•]. Altogether, even if data for ALK /ROS /RET translocations are preliminary and concern a low number of patients, we do not recommend ICI as single agents in patients with ALK/ROS1/RET rearranged NSCLC. Further studies are needed in order to see if these patients will benefit from combination therapies.
BRAF mutation
The efficacy of immune checkpoint inhibitors in BRAF-mutated NSCLC has also been scarcely studied. Indeed, none of the large clinical trials evaluating anti-PD1/anti-PD-L1 agents in advanced NSCLC patients reported results in this specific subgroup. In a retrospective study of 39 BRAF mutant patients, Dudnik et al. showed interesting results of immunotherapy with median PFS of 3.7 and 4.1 months and response rates of 25 and 33% in patients with V600E and no V600E mutations, respectively. Of note, no significant difference was found between V600E and non-V600E patients in this study [31•].
The IMMUNOTARGET database, however, allowed us to collect data from 35 BRAF-mutated patients who received immunotherapy, of which 17 (48.5%) were V600E. In this cohort, BRAF mutations were associated with slightly better results compared with EGFR mutations: ORR was 24% and median PFS of 3.1 months. In BRAF patients, PFS was influenced by smoking, with smokers having better PFS than non-smokers. It therefore seems that immunotherapy should be considered in BRAF-mutated patients, especially if they are smokers. Moreover, non-V600E mutations tended to be associated with better response rates and PFS than V600E mutations, likely due its epidemiologic association with tobacco use compared with V600E patients [19••].
MET alteration
In a recent study investigating ICI outcomes in MET exon 14-altered lung cancers, ORR was 17% (4/24) and median PFS was 1.9 months (95% CI 1.7–2.7). The authors concluded that responses to ICI may be achieved, but overall clinical efficacy remains modest, suggesting that targeted therapies and chemotherapy must be also favored in this population [32]. The IMMUNOTARGET MET exon 14 cohort proved better outcomes with a median PFS of 4.7 months [20]. This result is supported by another recent limited series (n = 8) from Dudnik et al. in which the median PFS was 4.0 months (95% CI, 2.4–NR). However, the limited number of patients analyzed does not allow us to draw definitive conclusions.
How to obtain better results?
Except for KRAS patients, response rates to immunotherapy used as a single agent in all oncogene addiction situations were globally shown to be impaired compared with wild-type patients [19••, 24•, 33, 34]. We forecast two main strategies to optimize the integration of immunotherapy in this population: the first one is to identify more reliable biomarkers. The second one is to use them combined with other drugs.
Biomarkers
PD-L1
Currently, PD-L1 expression remains the most reliable predictive biomarker to guide PD-1 inhibitors treatment. A recent meta-analysis has clearly demonstrated its interest (OR 2.51), even using a cut-off of 1% (OR 2.17) [35]. The value of this biomarker has been clearly proved in most of second-line trials but also in the frontline setting, with pembrolizumab showing its superiority over platinum doublet chemotherapy in terms of progression-free but also overall survival in patients whose tumor strongly expressed PD-L1 (≥ 50%), establishing it as a new standard of care [3]. In the IMMUNOTARGET study, PD-L1 expression was significantly associated not only with better outcomes in both EGFR and KRAS subgroups but also in the whole population [22•].
PD-L1 expression is however challenging to interpret in cases of oncogenic addictions. PD-L1 expression may be induced by the oncogenic signaling pathways, but it is not necessarily associated with a strong immune cells infiltration [36, 37] . Furthermore, PD-L1 is a dynamic biomarker, which expression could be induced by radiation therapy, chemotherapies [38, 39], or targeted therapies. Data from archived tissue, often used to guide treatment decisions, are not necessarily representative of the tumor microenvironment at the time of ICI initiation. In conclusion, PD-L1 appears to be less reliable in this context and should be combined with other biomarkers more representative of the tumor microenvironment in order to better select accurate candidates.
Tumor mutation burden
Beyond PDL1 expression, simple clinical factors have also been explored as predictors of response to immune checkpoint inhibitors. In particular, a lack of tobacco exposure has been associated with lower responsiveness to PD1 blockade [6••, 40]. One explanation for these findings is that lung cancer in never or minimal smokers is generally associated with a low TMB [10], another predictive biomarker of response to immune checkpoint inhibitors, independent of PDL1 expression [41] . Low TMB results in a lack of immunogenic neo-antigens, and thus non-inflamed (“excluded”) microenvironment. This is the most likely explanation to the most favorable outcomes observed in the KRAS, BRAF non-V600E, and even MET exon14 patients, alterations that are more frequently observed in smokers. This is consistent with other studies reporting low TMB in EGFR, ALK, ROS1 [42], or RET-driven lung cancers [30•], but higher in KRAS or BRAF [42].
TMB is however, like PD-L1, a dynamic biomarker that may be modified by many anti-cancer treatments. In the KEYNOTE-189 and PACIFIC trials, for example, never-smokers derived benefits from immunotherapy [2, 43], likely because chemotherapy and radiation therapy dramatically change the tumor microenvironment, leading to immunogenic cell death with neoantigens release and local and systemic T cells expansions. It is likely that these changes, combined with DNA alterations (and therefore increased TMB), explain the benefit observed in never smokers. TMB analysis can thus be a reliable marker in this population. However, whole-exome sequencing (WES) or the use of broad NGS panels, mandatory for TMB calculation, might be challenging to be translated into routine clinical practice due to the cost, the lack of a standardized panel and cut-off, and the limited availability of tissue [41]. There is thus a need to move beyond TMB and identify specific genetic determinants of response to PD-1 inhibitors, especially since not all point mutations will result in the genesis of highly immunogenic peptides [44].
The best predictive marker of response in this population combined with TMB may in fact be the tumor-infiltrating lymphocytes (TILs), which defines the notion of “hot” inflammatory tumor and “cold,” excluded, tumor.
Combination therapies
To increase the probability to obtain sustained disease control in patients with oncogene addiction using ICI, combination therapies may be a promising approach [45]. Activating the patient’s immune system during the time of tumor reduction and remission may be the best way to ensure that responses are converted into long-term and durable benefits. Targeted therapy could enhance anti-tumor immune responses by releasing neoantigens and synergistically improve of ICI anti-tumor activity [46]. From a clinical point of view, the combination of targeted agents with immunotherapies is of interest, considering that immunotherapy may transform the important tumor responses achieved with small molecule inhibitors to durable and long-lasting remissions. For example, in melanoma it has been proved that BRAF inhibition could have favorable effects in the tumor microenvironment and it becomes more immunogenic [47]. Several clinical trials investigating combination strategies are currently ongoing, especially for EGFR and ALK. First-line therapy combination of nivolumab and erlotinib showed excellent response rates, even in EGFR–TKI pretreated patients, but with a grade 3–4 incidence of adverse-events of 24% [48]. A phase 1 trial showed an ORR of 77.8% with gefitinib + durvalumab (n = 10) vs 80% with gefitinib alone followed by the combination of the two drugs (n = 10) [49]. Osimertinib and durvalumab also showed encouraging outcomes in TKI-naïve or resistant EGFR-mutated patients, but this combination seems to strongly potentialize the risk of interstitial pneumonia.
There is also a clear rational for a synergistic effect of ICI, chemotherapy, and anti-VEGF, even in cases of oncogenic addiction. Indeed, cytotoxic chemotherapy exposes the immune system to a high tumor antigen load and the normalization of tumor microvascularisation by anti-VEGF induces an increase in T lymphocyte infiltration [50]. Such a combination strategy has recently been analyzed in the ImPOWER 150 study (platine-based chemotherapy, bevacizumab, and atezolizumab) and showed significant efficacy in patients with oncogene addictions after failure of all available generations of TKI. The odds ratio for overall survival between the two groups was 0.54 (0.29; 1.03).
Conclusion
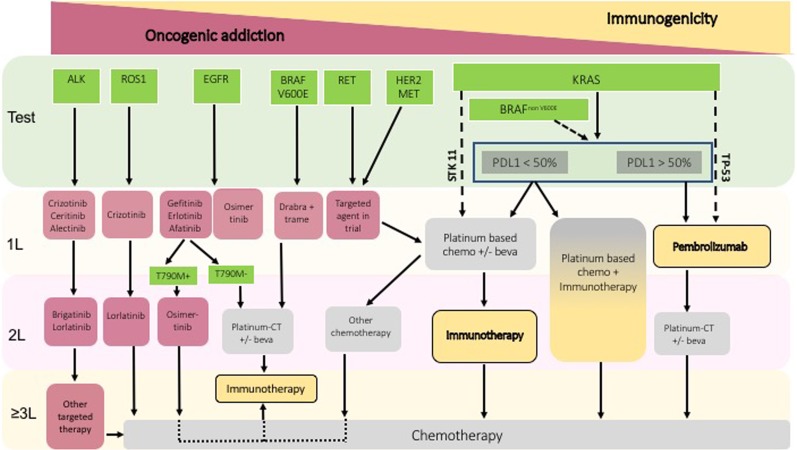
Lung cancer is becoming a more diverse disease with regard to management including a wide range of targets and treatment options. In patients with oncogenic addiction, targeted therapy is clearly the most suitable option. The potential interest of immunotherapy to improve patients’ outcomes and particularly long-term survival has not been defined yet. Based on published work, we suggest that (i) immunotherapy single agent should be proposed only after exhaustion of much validated treatments (an algorithm according to each driver is proposed in Fig. 3), (ii) a combination of immunotherapy with targeted therapy is of interest provided that we can manage toxicity and find the best sequence, (iii) a combination of immunotherapy with chemotherapy may be appealing in patients pretreated with targeted agents. The best way to opt in for the best strategy will depend upon the identification of adequate biomarkers. New basic and clinical research is awaited in this field.
Fig. 3.
Proposed algorithm of integration of immunotherapy in the management of patients with oncogenic addiction.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Lung Cancer
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 2.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M, PACIFIC Investigators Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein IB. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002;297(5578):63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 5.Nagahashi M, Sato S, Yuza K, Shimada Y, Ichikawa H, Watanabe S, Takada K, Okamoto T, Okuda S, Lyle S, Takabe K, Tsuchida M, Wakai T. Common driver mutations and smoking history affect tumor mutation burden in lung adenocarcinoma. J Surg Res. 2018;230:181–185. doi: 10.1016/j.jss.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlesi F, Mazieres J, Merlio J-P, Debieuvre D, Mosser J, Lena H, Ouafik L'H, Besse B, Rouquette I, Westeel V, Escande F, Monnet I, Lemoine A, Veillon R, Blons H, Audigier-Valette C, Bringuier PP, Lamy R, Beau-Faller M, Pujol JL, Sabourin JC, Penault-Llorca F, Denis MG, Lantuejoul S, Morin F, Tran Q, Missy P, Langlais A, Milleron B, Cadranel J, Soria JC, Zalcman G. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387(10026):1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 8.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gainor JF, Rizvi H, Jimenez Aguilar E, Mooradian M, Lydon CA, Anderson D, et al. Response and durability of anti-PD-(L)1 therapy in never- or light-smokers with non-small cell lung cancer (NSCLC) and high PD-L1 expression. J Clin Oncol. 2018;36(15_suppl):9011. doi: 10.1200/JCO.2018.36.15_suppl.9011. [DOI] [Google Scholar]
- 10.Gibbons DL, Byers LA, Kurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res MCR. 2014;12(1):3–13. doi: 10.1158/1541-7786.MCR-13-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jančík S, Drábek J, Radzioch D, Hajdúch M. Clinical relevance of KRAS in human cancers. J Biomed Biotechnol [Internet]. 2010 [cited 2019 Feb 7];2010. Available from:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2896632/ [DOI] [PMC free article] [PubMed]
- 12.Meng D, Yuan M, Li X, Chen L, Yang J, Zhao X, Ma W, Xin J. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: a systematic review with meta-analysis. Lung Cancer Amst Neth. 2013;81(1):1–10. doi: 10.1016/j.lungcan.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Guibert N, Ilie M, Long E, Hofman V, Bouhlel L, Brest P, Mograbi B, Marquette CH, Didier A, Mazieres J, Hofman P. KRAS Mutations in lung adenocarcinoma: molecular and epidemiological characteristics, methods for detection, and therapeutic strategy perspectives. Curr Mol Med. 2015;15(5):418–432. doi: 10.2174/1566524015666150505161412. [DOI] [PubMed] [Google Scholar]
- 14.Tomasini P, Walia P, Labbe C, Jao K, Leighl NB. Targeting the KRAS pathway in non-small cell lung cancer. Oncologist. 2016;21(12):1450–1460. doi: 10.1634/theoncologist.2015-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guibert N, Ilie M, Léna H, Didier A, Hofman P, Mazieres J. KRAS and bronchial adenocarcinoma. Between disappointments and hopes. Rev Mal Respir. 2016;33(2):156–164. doi: 10.1016/j.rmr.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Miao D, Margolis CA, Vokes NI, Liu D, Taylor-Weiner A, Wankowicz SM, Adeegbe D, Keliher D, Schilling B, Tracy A, Manos M, Chau NG, Hanna GJ, Polak P, Rodig SJ, Signoretti S, Sholl LM, Engelman JA, Getz G, Jänne PA, Haddad RI, Choueiri TK, Barbie DA, Haq R, Awad MM, Schadendorf D, Hodi FS, Bellmunt J, Wong KK, Hammerman P, van Allen EM. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet. 2018;50(9):1271–1281. doi: 10.1038/s41588-018-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calles A, Liao X, Sholl LM, Rodig SJ, Freeman GJ, Butaney M, Lydon C, Dahlberg SE, Hodi FS, Oxnard GR, Jackman DM, Jänne PA. Expression of PD-1 and its ligands, PD-L1 and PD-L2, in smokers and never smokers with KRAS-mutant lung cancer. J Thorac Oncol. 2015;10(12):1726–1735. doi: 10.1097/JTO.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 19.Mazieres J, Drilon AE, Mhanna L, Milia J, Lusque A, Cortot AB, et al. Efficacy of immune-checkpoint inhibitors (ICI) in non-small cell lung cancer (NSCLC) patients harboring activating molecular alterations (ImmunoTarget) J Clin Oncol. 2018;36(15_suppl):9010. doi: 10.1200/JCO.2018.36.15_suppl.9010. [DOI] [Google Scholar]
- 20.Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, Buczkowski KA, Liu Y, Awad MM, Denning WL, Diao L, Wang J, Parra-Cuentas ER, Wistuba II, Soucheray M, Thai T, Asahina H, Kitajima S, Altabef A, Cavanaugh JD, Rhee K, Gao P, Zhang H, Fecci PE, Shimamura T, Hellmann MD, Heymach JV, Hodi FS, Freeman GJ, Barbie DA, Dranoff G, Hammerman PS, Wong KK. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 2016;76(5):999–1008. doi: 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, Behrens C, Kadara H, Parra ER, Canales JR, Zhang J, Giri U, Gudikote J, Cortez MA, Yang C, Fan Y, Peyton M, Girard L, Coombes KR, Toniatti C, Heffernan TP, Choi M, Frampton GM, Miller V, Weinstein JN, Herbst RS, Wong KK, Zhang J, Sharma P, Mills GB, Hong WK, Minna JD, Allison JP, Futreal A, Wang J, Wistuba II, Heymach JV. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5(8):860–877. doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.•.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;CD-18-0099. An important work showing the potential role of STK1/LKB1 in resistance to immunotherapy. [DOI] [PMC free article] [PubMed]
- 23.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T, Huang Y, Yi X, Zhang L. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 24.•.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, Coluzzi P, Ledezma B, Mendenhall M, Hunt J, Wolf B, Jones B, Madrigal J, Horton J, Spiegel M, Carroll J, Gukasyan J, Williams T, Sauer L, Wells C, Hardy A, Linares P, Lim C, Ma L, Adame C, Garon EB. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.•.Garassino MC, Cho B-C, Kim J-H, Mazières J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Z-Y, Zhang J-T, Liu S-Y, Su J, Zhang C, Xie Z, Zhou Q, Tu HY, Xu CR, Yan LX, Li YF, Zhong WZ, Wu YL. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145. doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang M, Li G, Wang Y, Wang Y, Zhao S, Haihong P, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017 [cited 2018 Jul 10];7(1). [DOI] [PMC free article] [PubMed]
- 29.Negrao MV, Reuben A, Robichaux JP, Le X, Nilsson MB, Wu C, et al. Association of EGFR and HER-2 exon 20 mutations with distinct patterns of response to immune checkpoint blockade in non-small cell lung cancer. J Clin Oncol. 2018;36(15_suppl):9052. doi: 10.1200/JCO.2018.36.15_suppl.9052. [DOI] [Google Scholar]
- 30.•.Sabari JK, Offin MD, Wu SL, Ni A, Halpenny D, Montecalvo J, et al. RET-rearranged lung cancers: immunophenotype and response to immunotherapy. J Clin Oncol. 2018;36(15_suppl):9034. doi: 10.1200/JCO.2018.36.15_suppl.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.•.Dudnik E, Peled N, Nechushtan H, Wollner M, Onn A, Agbarya A, et al. BRAF mutant lung cancer: programmed death ligand 1 expression, tumor mutational burden, microsatellite instability status, and response to immune check-point inhibitors. J Thorac Oncol. 2018;13(8):1128–1137. doi: 10.1016/j.jtho.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Sabari JK, Montecalvo J, Chen R, Dienstag JA, Mrad C, Bergagnini I, et al. PD-L1 expression and response to immunotherapy in patients with MET exon 14-altered non-small cell lung cancers (NSCLC) J Clin Oncol. 2017;35(15_suppl):8512. doi: 10.1200/JCO.2017.35.15_suppl.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remon J, Hendriks LE, Cabrera C, Reguart N, Besse B. Immunotherapy for oncogenic-driven advanced non-small cell lung cancers: is the time ripe for a change? Cancer Treat Rev. 2018;71:47–58. doi: 10.1016/j.ctrv.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Gettinger S, Politi K. PD-1 Axis inhibitors in EGFR- and ALK-driven lung cancer: lost cause? Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22(18):4539–4541. doi: 10.1158/1078-0432.CCR-16-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khunger M, Hernandez AV, Pasupuleti V, Rakshit S, Pennell NA, Stevenson J, et al. Programmed cell death 1 (PD-1) ligand (PD-L1) expression in solid tumors as a predictive biomarker of benefit from PD-1/PD-L1 axis inhibitors: a systematic review and meta-analysis. JCO Precis Oncol. 2017;1(1):1–15. doi: 10.1200/PO.16.00030. [DOI] [PubMed] [Google Scholar]
- 36.D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112(1):95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ota K, Azuma K, Kawahara A, Hattori S, Iwama E, Tanizaki J, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, Hoshino T, Nakanishi Y, Okamoto I. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21(17):4014–4021. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 38.Han JJ, Kim D-W, Koh J, Keam B, Kim TM, Jeon YK, et al. Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer. 2016;17(4):263–270.e2. doi: 10.1016/j.cllc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou F, Zhou C. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2017;12(7):1085–1097. doi: 10.1016/j.jtho.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Kim HS, Kim BJ. Prognostic value of smoking status in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Oncotarget. 2017;8(54):93149–93155. doi: 10.18632/oncotarget.18703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O’Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, Paz-Ares L. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spigel DR, Schrock AB, Fabrizio D, Frampton GM, Sun J, He J, et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol. 2016;34(15_suppl):9017. doi: 10.1200/JCO.2016.34.15_suppl.9017. [DOI] [Google Scholar]
- 43.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 44.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 45.Karachaliou N, Gonzalez-Cao M, Sosa A, Berenguer J, Bracht JWP, Ito M, Rosell R. The combination of checkpoint immunotherapy and targeted therapy in cancer. Ann Transl Med. 2017;5(19):388. doi: 10.21037/atm.2017.06.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilotto S, Molina-Vila MA, Karachaliou N, Carbognin L, Viteri S, González-Cao M, Bria E, Tortora G, Rosell R. Integrating the molecular background of targeted therapy and immunotherapy in lung cancer: a way to explore the impact of mutational landscape on tumor immunogenicity. Transl Lung Cancer Res. 2015;4(6):721–727. doi: 10.3978/j.issn.2218-6751.2015.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18(5):1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 48.Rizvi NA, Chow LQM, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al. Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol. 2014;32(15_suppl):8022. doi: 10.1200/jco.2014.32.15_suppl.8022. [DOI] [Google Scholar]
- 49.Gibbons DL, Chow LQ, Kim D-W, Kim S-W, Yeh T, Song X, Jiang H, Taylor R, Karakunnel J, Creelan B. 57O efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): a phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. J Thorac Oncol. 2016;11(4 Suppl):S79. doi: 10.1016/S1556-0864(16)30171-X. [DOI] [Google Scholar]
- 50.Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52(Pt 2):117–124. doi: 10.1016/j.semcancer.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol. 2013;31(31):3987–3996. doi: 10.1200/JCO.2012.45.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]