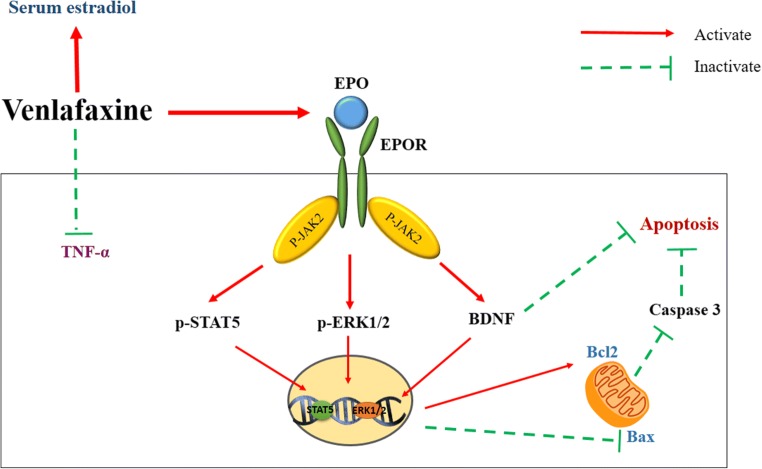
Abstract
Reduced estradiol levels are associated with depression in women during the transition to and after menopause. A considerable number of studies focusing on the theme of treating depression through the activation of erythropoietin (EPO)-induced signaling pathways have been published. Venlafaxine is an approved antidepressant drug that inhibits both serotonin and norepinephrine transporters. The aim of the present study was to investigate the effects of venlafaxine on the depressive-like behaviors and serum estradiol levels in female rats following ovariectomy (OVX) and the possible roles of EPO-induced signaling pathways. Venlafaxine (10 mg/kg/day) was orally administered to OVX rats over a period of 4 weeks using two different treatment regimens: either starting 24 h or 2 weeks after OVX. Venlafaxine showed a superior efficacy in inducing antidepressant-like effects after an acute treatment (24 h post-OVX) than after the delayed treatment (2 weeks post-OVX) and was characterized by a decreased immobility time in the forced swimming test. In parallel, venlafaxine induced EPO and EPO receptor mRNA expression and increased levels of phospho-Janus kinase 2 (p-JAK2), phospho-signal transducer and activator of transcription 5, and phospho-extracellular signal-regulated kinase 1/2 in the hippocampus of OVX rats. Meanwhile, rats exhibited a marked reduction in the hippocampal Bax/Bcl2 ratio, caspase-3 activity, and tumor necrosis factor alpha levels after venlafaxine treatment. Venlafaxine also increased the hippocampal brain-derived neurotrophic factor and serum estradiol levels. Based on these findings, venlafaxine exerts a neuroprotective effect on OVX rats that is at least partially attributed to the activation of EPO/EPOR/JAK2 signaling pathways, anti-apoptotic activities, anti-inflammatory activities, and neurotrophic activities, as well as an increase in serum estradiol level.
Graphical Abstract.
ᅟ
Electronic supplementary material
The online version of this article (10.1007/s13311-018-00680-6) contains supplementary material, which is available to authorized users.
Key Words: Apoptosis, EPO/EPOR, JAK2, ovariectomy, postmenopausal depression, venlafaxine
Introduction
Depression is one of the major causes of morbidity worldwide, with a lifetime prevalence of approximately 15–20% [1]. The condition occurs approximately twice as frequently in women as in men, beginning during adolescence and occurring throughout women’s lives [2]. The decrease in estrogen level during periods of hormonal fluctuation, such as premenstrual, postpartum, and peri- to postmenopausal periods, is associated with the incidence and symptomatology of depression [3]. Ovariectomy (OVX), which removes the primary source of estrogens, produces depressive-like behaviors in rats [4]. Estrogen replacement therapies have been reported to produce antidepressant effects alone or when administered as coadjuvants to facilitate the effects of the clinically used antidepressants in postmenopausal women [5, 6]. However, the main challenge faced by patients using estrogen therapy is severe side effects, including coronary heart disease, stroke, and breast cancer [7]; hence, the debate continues about the best strategies for the management of postmenopausal depression.
Erythropoietin (EPO) is a cytokine that was originally identified to play a role in erythropoiesis but has recently been shown to have multiple targets and actions [8]. Based on accumulating evidence, EPO and its receptor (EPOR) are expressed in the brain, and EPO is produced by neurons [9]. EPOR has been detected in the murine hippocampus and cortex [10]. Existing research recognizes the critical neurotrophic and neuroprotective effects of EPO on increasing the level of the brain-derived neurotrophic factor (BDNF) in the hippocampus and protecting against neuronal apoptosis [11]. Furthermore, a considerable number of studies focusing on the theme of treating depression by activating EPO have been published [12, 13]. In addition, the elevation of serotonin (5-HT) levels was reported to upregulate EPO expression and produce significant improvements in the neuronal differentiation of hippocampal neural progenitor cells [14]. Hence, the EPO pathway represents a possible target for antidepressant drugs, such as selective serotonin reuptake inhibitors (SSRIs).
SSRIs have become the main treatment for female patients with depression, particularly postmenopausal women, due to the presence of fewer adverse drug reactions and superior responses in women than in men [15]. The probable mechanism of these drugs is the enhancement of net serotonergic transmission by blocking the presynaptic 5-HT uptake site [16]. Interestingly, an increase in the serum estrogen level also improves affect and cognition in postmenopausal women [17].
Taken together, a treatment that both activates EPO/EPOR signaling pathways and increases serum estradiol levels may have potential as a therapeutic agent for mitigating postmenopausal depression. Venlafaxine is an approved antidepressant drug that inhibits both 5-HT and norepinephrine transporters and is increasingly used as an alternative to SSRIs [18]. Currently, the relationship between EPO and depression has not received sufficient attention. Drawing upon two strands of research, this study attempted first to unravel the effects of venlafaxine on the depressive-like behaviors and serum estradiol levels following OVX in female rats to reveal the possible role of EPO/EPOR signaling pathways and second to explore whether the post-OVX interval influences the antidepressant potential of venlafaxine. Venlafaxine was chosen due to its faster onset of action and superior clinical efficacy compared to other antidepressant drugs [19].
Materials and Methods
Animals
Young adult (12 months old) female Wistar rats weighing 300–350 g were provided by the animal facility of Faculty of Pharmacy, Cairo University. Animals were housed under controlled environmental conditions at a constant temperature (25 ± 2 °C) on a 12/12-h light/dark cycle. Rats were allowed free access to food and water. The protocols used in this study complied with The Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 2011) and were approved by the Ethics Committee for Animal Experimentation at Faculty of Pharmacy, Cairo University (Permit Number: PT 2099). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Vaginal smears were collected daily between 8 and 9 a.m. to monitor estrous cycles, and animals that did not show more than two consistent 4-day cycles were excluded. All surgical procedures were performed at 9 a.m. during metestrus. Rats in the sham-operated group were subjected to the same surgical procedures as rats in the other experimental groups, except for the removal of the ovaries.
Drugs and Chemicals
Venlafaxine was provided as a gift from the International Drug Agency for Pharmaceutical Industry (Cairo, Egypt) and was freshly prepared daily in a physiological saline solution (0.9%, w/v). Venlafaxine was administered to each animal in a volume of 0.5 mL/300 g body weight. The selection of dose and duration of venlafaxine administration was based on a published study [20]. Rat anti-erythropoietin (anti-EPO) antibodies (AF959, R&D Systems, Minneapolis, USA) were used to neutralize EPO and prevent the EPO-EPOR interaction [21]. Fine chemicals and reagents were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), unless indicated otherwise.
Experimental Design
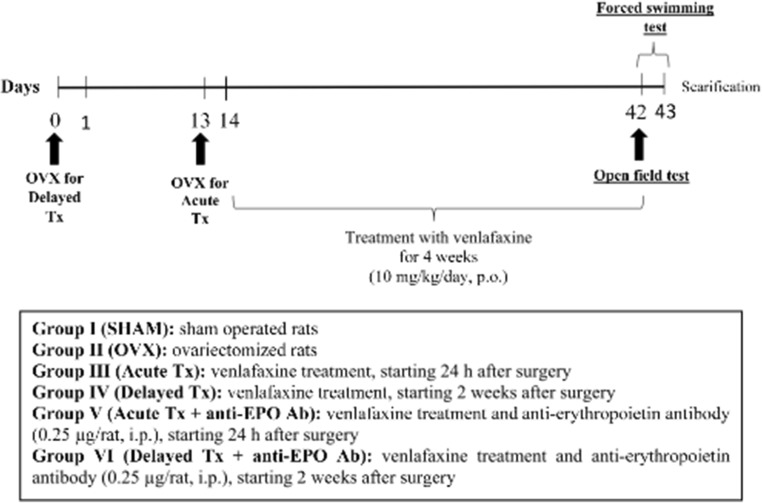
As depicted in Fig. 1, 90 rats were randomly allocated into one of six treatment groups (n = 15) by a technical assistant who was not involved in the analysis. Group size was based on a power analysis (power = 0.8, α = 0.05) using effect sizes previously determined by Ibrahim et al. [22]. Group I (SHAM group): normal sham-operated rats were orally administered normal saline (p.o.) via an oral syringe and served as the control group. Group II (OVX group): rats were bilaterally ovariectomized. Group III (Acute Tx): OVX rats were treated with venlafaxine (10 mg/kg/day, p.o.) [20] over a period of 4 weeks starting 24 h after OVX. Group IV (Delayed Tx): OVX rats were treated with venlafaxine (10 mg/kg/day, p.o.) over a period of 4 weeks starting 2 weeks after OVX. Group V (Acute Tx + anti-EPO Ab): OVX rats were treated with venlafaxine (10 mg/kg/day, p.o.) and the anti-EPO antibody (0.25 μg/rat, i.p.) [23] over a period of 4 weeks starting 24 h after OVX. Group VI (Delayed Tx + anti-EPO Ab): OVX rats were treated with venlafaxine (10 mg/kg/day, p.o.) and an anti-EPO antibody (0.25 μg/rat, i.p.) over a period of 4 weeks starting 2 weeks after OVX. Six weeks after surgery, all animals were subjected to behavioral tests, the open-field test followed by the forced swimming test (FST), arranged in sequence from the least stressful test to the most stressful test with a 2-h rest period between the tests. All testing was conducted during the animal’s light cycle. At the end of the behavioral tests, the animals were anesthetized with thiopental (5 mg/kg, i.p.) and blood samples were collected from the retro-orbital sinus in nonheparinized capillary tubes for serum separation to estimate estrogen levels. Afterwards, the rats in each group were divided into three sets and then euthanized; subsequently, brains were rapidly dissected and washed with ice-cold saline. In the first set (n = 3), brains were fixed with 10% (v/v) formalin for 24 h to perform histopathological staining with hematoxylin and eosin. In the other sets, hippocampi were promptly dissected and stored at − 80 °C. Hippocampi from rats in the second set (n = 6) were homogenized in ice-cold physiological saline to prepare a 10% homogenate for the assessment of caspase-3, BDNF, and tumor necrosis factor alpha (TNF-α) levels. Hippocampi from rats in the third set (n = 6) were used to assess the expression of the EPO and EPOR genes, and levels of the phospho-Janus kinase 2 (p-JAK2), phospho-signal transducer and activator of transcription 5 (p-STAT5), phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2), and Bax and Bcl2 proteins. During the analysis of these measurements, the investigators were blinded to sample identity, and sample coding and decoding were performed by an independent experimenter.
Fig. 1.
Schematic of the experimental design
Surgery
The female rats were anesthetized with a mixture of ketamine hydrochloride (50 mg/kg; i.p) and xylazine (10 mg/kg; i.p.). The lumbar dorsum was shaved and disinfected with 70% ethanol, and then a small incision through the skin, connective tissues, and muscles was made. The ovaries with associated oviduct were exteriorized, a hemostatic clamp was applied around the blood supply to the ovaries, and a suture knot was made below the clamp. Subsequently, the ovaries and part of the oviduct were removed and discarded. The muscle and skin layers were sutured, and the wound was topically treated with povidone–iodine and antibiotic spray. Sham-operated rats underwent the same procedure as the OVX rats, without resection of the ovaries. Rats were also subcutaneously injected with 0.1 mL of diclofenac sodium and 0.1 mL of cefotaxime (100 mg/mL) and were maintained on a soy-free diet to exclude the effects of phytosteroids in the diet.
Behavioral Assessments
Open-Field Test
Spontaneous locomotor behaviors were assessed using the open-field test to confirm that the effects of venlafaxine on the results of the FST were not due to nonspecific effects on locomotor activity. The experiment was performed in a square wooden box of 80 × 80 × 40 cm in size. The walls were painted red and the floor was divided with white lines into a 4 × 4 grid of 16 equal squares. Each rat was individually placed in the central area of the open field, and the locomotor behaviors were video-recorded for 3 min. The test was performed under dim white light in a sound-attenuated room, and the floor and walls of the apparatus were cleaned after each rat was tested to eliminate possible bias due to odors left by previous rats. The ambulation frequency (number of squares crossed) and latency time (time elapsed with a lack of movement during testing) were calculated for each animal [24].
Forced Swimming Test
Rats were subjected to the FST using the method described by Cryan et al. to investigate changes in the depressive-like behaviors of rats in each experimental group [25]. Swimming sessions were conducted by placing individual rats in a cylindrical container (50 cm in height × 20 cm in diameter) filled with water (25 ± 2 °C) to a level of 40 cm. In the first session (day 1), rats were placed in water for 10–15 min of training. Twenty-four hours later (day 2), rats were again placed in the cylinder for 5 min and the immobility time (when no additional movements other than those necessary to keep the rat’s head out of the water) was recorded. The cylinder was cleaned and filled with fresh water between experiments. At the end of the experiment, all animals were dried and placed back in their home cages.
Biochemical Measurements
Enzyme-Linked Immunosorbent Assay
Serum estradiol levels were estimated using a rat ELISA kit (Shanghai Boyun Biotech, Co., Ltd., Shanghai, China). Likewise, hippocampal BDNF, TNF-α, and caspase-3 levels were estimated using rat ELISA kits purchased from Abnova Corporation (Jhongli, Taiwan), R&D Systems Inc. (Minneapolis, USA), and Cusabio Life Science (Wuhan, Hubei, China), respectively. The procedures were performed according to the manufacturer’s instructions. The results are presented as picogram/milligram protein for BDNF and TNF-α and nanogram/milligram protein for caspase-3, and the protein content was quantified according to the method described by Bradford [26].
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from hippocampal tissues using an SV Total RNA Isolation System (Promega, Madison, WI, USA), and the purity of the obtained RNA was confirmed spectrophotometrically by recording the optical density at 260/280 nm. Equal amounts of extracted RNA were then reverse transcribed into cDNAs using an RT-PCR kit (#K1621, Fermentas, Waltham, MA, USA) according to the manufacturer’s instructions. Quantitative RT-PCR was performed to assess the expression of the EPO and EPOR mRNAs using SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. Briefly, 1 μg of total RNA was mixed with 50 μM oligo (dT) 20, 50 ng/μL random primers, and 10 mM dNTP mix in a total volume of 10 μL. The primer sequences used in the present study are listed in Table 1. The thermal cycler protocol consisted of an initial enzyme activation step at 95 °C for 10 min, followed by 45 cycles of 15 s of denaturation at 95 °C and 1 min of annealing/extension at 60 °C. The relative expression of the target gene was calculated using the 2−ΔΔCT formula [27]. All values were normalized to β-actin levels and presented as fold changes.
Table 1.
The primer sequences used for real-time polymerase chain reaction (RT-PCR)
| Gene | Primer sequence |
|---|---|
| EPO |
Forward primer 5′-ACTCCGAACACTCACAGTGGATAC-3′ Reverse primer 5′-GATTCTGAGGCTCTTCTTCTCTGG-3′ |
| EPOR |
Forward primer 5′-TCTCATTCTCGTCCTCATCTCA-3′ Reverse primer 5′-GACCCTCAAACTCATTCTCTGG-3′ |
| β-Actin |
Forward primer 5′-TGCTGGTGCTGAGTATGTCG-3′ Reverse primer 5′-TTGAGAGCAATGCCAGCC-3′ |
Western Blot Analysis
Hippocampal levels of the p-JAK2, p-STAT5, p-ERK1/2, and Bax and Bcl2 proteins were analyzed using the Western blot methodology. After proteins were extracted from hippocampal tissues, equal amounts of proteins were loaded onto 8% sodium dodecyl sulfate-polyacrylamide gels and separated by electrophoresis according to their molecular weights. Following electrophoresis, proteins were transferred to nitrocellulose membranes (Amersham Bioscience, Piscataway, NJ, USA) using a semidry transfer apparatus (Bio-Rad, Hercules, CA, USA). Membranes were blocked with 5% (w/v) skim milk in Tris-buffered saline containing 0.05% Tween 20 (TBST) at 4 °C overnight. Afterwards, membranes were incubated with a 1:1000 dilution of antibodies against rat p-Tyr1007/1008-JAK2, p-Tyr694-STAT5, p-Thr202/Tyr204-ERK1/2, and Bax and Bcl2 (Thermo Fisher Scientific, Inc., Rockford, IL, USA) for 1 h at room temperature with constant shaking. Next, membranes were probed with horseradish peroxidase-conjugated goat anti-mouse immunoglobulins (1:2000; Fluka, St. Louis, MO, USA). Finally, the band intensity was analyzed using a ChemiDoc™ imaging system with Image Lab™ software version 5.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The results are presented in arbitrary units after normalization to levels of the β-actin protein.
Histopathological Examination
Brains were carefully removed from three rats, rinsed with ice-cold saline, and immediately fixed with 10% formalin for 24 h. Samples were dehydrated by incubations in serial dilutions of alcohol, cleared with xylene and embedded in paraffin at 56 °C in a hot air oven for 24 h. Coronal brain sections were processed for paraffin embedding and 4-μm sections were prepared. Sections were then stained with hematoxylin and eosin (H&E) and examined under a light microscope (Olympus CX21, Tokyo, Japan) [28]. A scoring system ranging from 0 to 3 points was used to evaluate the degree of severity of the observed histopathological changes, where 0 = no changes, 1 = mild changes (< 30%), 2 = moderate changes (30–50%), and 3 = severe changes (> 50%) [29].
Statistical Analysis
The data are presented as means ± S.D. Data were analyzed using one-way ANOVA followed by the Tukey–Kramer multiple comparison test, except for the histopathological scores, which were analyzed using Kruskal–Wallis ANOVA followed by Dunn’s multiple comparison test. GraphPad Prism software (version 6; GraphPad Software, Inc., San Diego, CA, USA) was used to perform the statistical analysis and create the graphs. The level of significance was set to p < 0.05 for all statistical tests. Data points were considered outliers only if they failed the Dixon test [30] or if they were greater than four standard deviations from the mean. Finally, Mead’s “Resource Equation” was used to ensure that sample sizes were sufficient to establish a statistically significant difference [31].
Results
Behavioral Changes
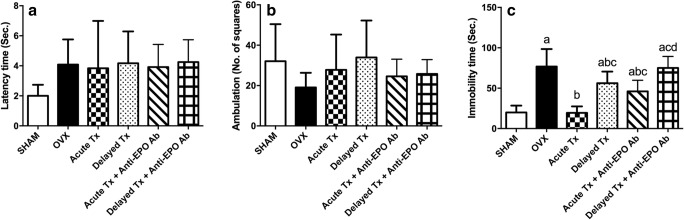
Based on the data shown in Fig. 2, OVX increased the immobility time in the FST approximately fourfold compared with the sham-operated group. Treatment with venlafaxine immediately after OVX (Acute Tx) normalized the immobility time, while delayed treatment with venlafaxine 2 weeks after the operation (Delayed Tx) and acute treatment in the presence of anti-EPO antibodies (Acute Tx + anti-EPO Ab) reduced the immobility time by only 26% and 40%, respectively, compared with the OVX rats. On the other hand, delayed treatment with venlafaxine in the presence of anti-EPO antibodies (Delayed Tx + anti-EPO Ab) failed to modify any of the measured behavioral parameters. Moreover, the length of time between the introduction of the rat into the open-field apparatus until it left the center (latency time) and the ambulation frequency were not affected by OVX or any of the treatments.
Fig. 2.
Effects of the acute and delayed venlafaxine treatments on open-field latency (A) (F(5,66) = 2.337, p = 0.0514), ambulation frequency (B) (F(5,66) = 1.799, p = 0.1251), and immobility time in the forced swimming test (C) (F(5,66) = 38.32, p < 0.0001) in ovariectomized (OVX) rats. Each bar with a vertical line represents the mean of experiments ± S.D. (n = 15). (a) Compared with the sham-operated group, (b) compared with OVX rats, (c) compared with the acute venlafaxine-treated group, and (d) compared with the acute venlafaxine treatment in combination with anti-erythropoietin (anti-EPO) antibodies (statistical analyses were performed using ANOVA followed by Tukey’s post hoc test, and the criterion for statistical significance was set to p < 0.05)
Serum Levels of Estradiol and Erythropoietin-Related Parameters
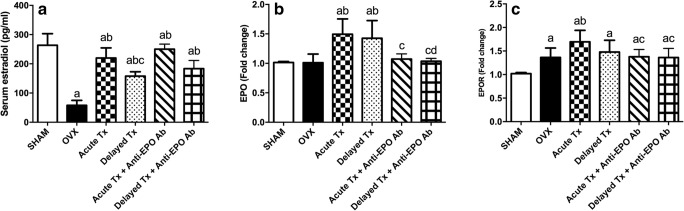
The findings presented in Fig. 3 are quite revealing in several ways. First, OVX markedly reduced serum estradiol levels to approximately 22% and increased hippocampal EPOR mRNA expression to approximately 133% of the values in the sham-operated rats. Second, both acute and delayed treatments in the presence and in the absence of anti-EPO Ab ameliorated the OVX-induced reduction in serum estradiol levels. Third, both regimens administered in the absence of anti-EPO Ab increased EPO mRNA expression to approximately 140% of the level in the OVX group. In the presence of anti-EPO Ab, the effect of the venlafaxine treatment on EPO mRNA levels was significantly reduced to those of both sham-operated and OVX rats. Fourth, only the acute treatment in the absence of anti-EPO Ab managed to increase the expression of EPOR compared to OVX rats.
Fig. 3.
Effects of acute and delayed venlafaxine treatments on serum estradiol levels (A) (F(5,42) = 62.3, p < 0.0001) and EPO (B) (F(5,36) = 10.96, p < 0.0001) and EPOR mRNA expression (C) (F(5,36) = 9.008, p < 0.0001). Each bar with a vertical line represents the mean of experiments ± S.D. (n = 6). (a) Compared with the sham-operated group, (b) compared with OVX rats, (c) compared with the acute venlafaxine-treated group, and (d) compared with the acute venlafaxine treatment in the presence of anti-EPO antibodies (statistical analyses were performed using ANOVA followed by Tukey’s post hoc test; the criterion for statistical significance was set to p < 0.05)
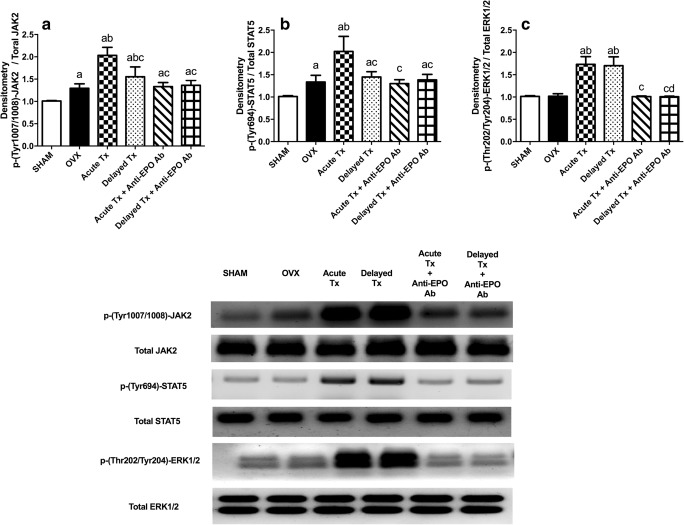
The Janus Kinase 2 Pathway Biomarkers
As shown in Fig. 4, substantial increases in the levels of the p-JAK2 and p-STAT5 proteins were observed in the hippocampus of OVX rats to approximately 130% of the levels in the sham-operated group, but p-ERK1/2 levels were not significantly different between groups. Acute venlafaxine treatment in the absence of anti-EPO Ab increased the levels of these three proteins by approximately 1.5-fold compared with the OVX rats. The delayed administration of venlafaxine increased p-ERK1/2 levels to the same level observed following the acute treatment; however, p-JAK2 levels were increased to a lesser extent. The administration of the anti-EPO Ab abolished most of the venlafaxine-induced changes in the aforementioned JAK2 pathway-related biomarkers.
Fig. 4.
Levels of the JAK2 pathway-related parameters p-(Tyr1007/1008)-JAK2 (A) (F(5,36) = 43.63, p < 0.0001), p-(Tyr694)-STAT5 (B) (F(5,34) = 25.9, p < 0.0001), and p-(Thr202/Tyr204)-ERK1/2 (C) (F(5,36) = 75.25, p < 0.0001). Each bar with a vertical line represents the mean of experiments ± S.D. (n = 6). (a) Compared with the sham-operated group, (b) compared with OVX rats, (c) compared with the acute venlafaxine-treated group, and (d) compared with the acute venlafaxine treatment in the presence of anti-EPO antibodies (statistical analyses were performed using ANOVA followed by Tukey’s post hoc test, and the criterion for statistical significance was set to p < 0.05)
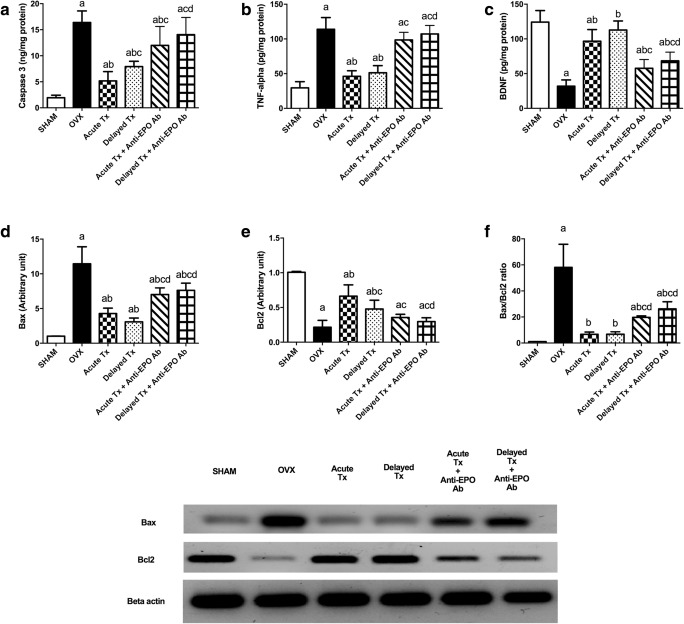
Apoptotic and Inflammatory Biomarkers
Surgical removal of ovaries markedly increased hippocampal caspase-3 and TNF-α levels by approximately 845% and 385%, respectively, and decreased BDNF levels to approximately one quarter of the levels in the sham-operated group. Surprisingly, OVX increased Bax expression and the Bax/Bcl2 ratio by approximately 11- and 58-fold, respectively, compared to the sham-operated group. The operation also reduced the Bcl2 expression to one-fifth the level of the sham-operated group. Both acute and delayed venlafaxine treatments reversed the increases in caspase-3, TNF-α, and Bax levels and the Bax/Bcl2 ratio more efficiently in the absence of anti-EPO Ab. Additionally, the acute venlafaxine treatment tripled both the BDNF level and Bcl2 expression, while the delayed venlafaxine treatment increased BDNF and Bcl2 levels by approximately 3.5- and 2-fold, respectively, compared with the OVX rats. The differences between acute and delayed venlafaxine treatments in the presence and in the absence of anti-EPO Ab are highlighted in Fig. 5.
Fig. 5.
Levels of apoptotic and inflammatory biomarkers after acute and delayed venlafaxine treatments: caspase-3 activity (A) (F(5,42) = 43.65, p < 0.0001), TNF-α (B) (F(5,42) = 78.36, p < 0.0001), BDNF (C) (F(5,42) = 53.36, p < 0.0001), Bax (D) (F(5,36) = 65.3, p < 0.0001), Bcl2 (E) (F(5,36) = 62.54, p < 0.0001) levels, and Bax/Bcl2 ratio (F) (F(5,34) = 56.58, p < 0.0001). Each bar with a vertical line represents the mean of experiments ± S.D. (n = 6). (a) Compared with the sham-operated group, (b) compared with the OVX rats, (c) compared with the acute venlafaxine-treated group, and (d) compared with the acute venlafaxine treatment combined with anti-EPO antibodies (statistical analyses were performed using ANOVA followed by Tukey’s post hoc test, and the criterion for statistical significance was set to p < 0.05)
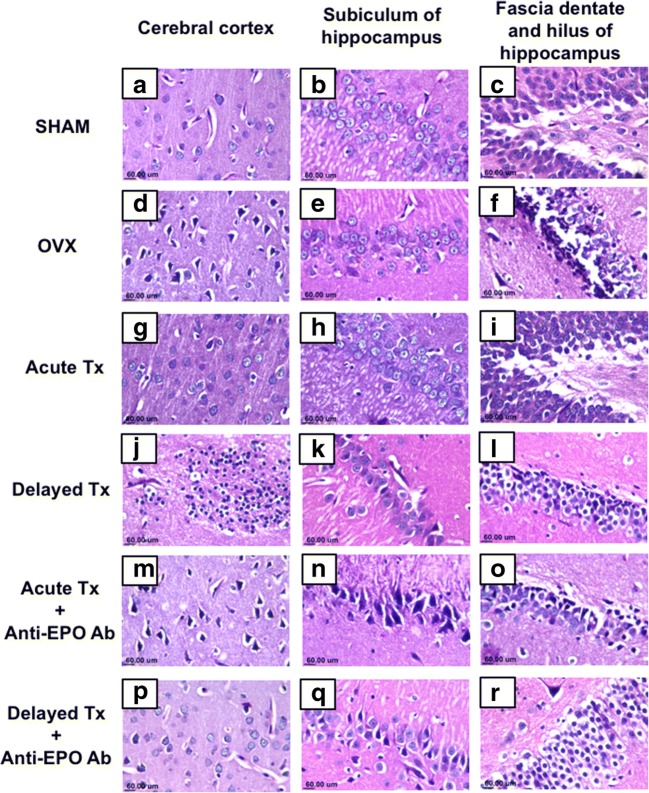
Histopathological Findings
Sections from sham-operated rats revealed no histopathological alterations and normal histological structures of neurons in the cerebral cortex (Fig. 6(A)), subiculum of the hippocampus (Fig. 6(B)), and fascia dentate and hilus of the hippocampus (Fig. 6(C)). On the other hand, OVX rats showed nuclear pyknosis and degeneration of the neurons in the cerebral cortex (Fig. 6(D)) and the fascia dentate and hilus of the hippocampus (Fig. 6(F)). Additionally, intracellular edema was observed in the neurons of the hippocampal subiculum (Fig. 6(E)). Acute treatment with venlafaxine in the absence of anti-EPO Ab ameliorated the neurological damage induced by OVX, as evidenced by the normal histopathological architecture of the cerebral cortical neurons and neurons of the subiculum and fascia dentate of the hippocampus, as shown in Fig. 6(G, H, I). In contrast, delayed treatment with venlafaxine in the absence of anti-EPO Ab failed to correct the damage induced by OVX, as focal gliosis was observed in the cerebral cortex (Fig. 6(J)). Moreover, the neurons of the fascia dentate of the hippocampus showed nuclear pyknosis and intracellular edema (Fig. 6(L)), while the neurons of the hippocampal subiculum were fairly well preserved (Fig. 6(K)). The administration of anti-EPO Ab ablated most of the beneficial effects of the acute treatment with venlafaxine, as evidenced by nuclear pyknosis and degeneration of neurons in the cerebral cortex (Fig. 6(M)), subiculum of the hippocampus (Fig. 6(N)), and fascia dentate and hilus of hippocampus (Fig. 6(O)). On the other hand, co-administration of the delayed venlafaxine treatment with anti-EPO Ab showed a normal histological appearance of the cerebral cortex (Fig. 6(P)), with nuclear pyknosis and degeneration observed in the subiculum of hippocampus (Fig. 6(Q)) and vacuolar degeneration in the fascia dentate (Fig. 6R). These results are also presented in Table 2.
Fig. 6.
Representative photomicrographs of the normal cerebral cortex in the sham-operated group (A) and acute venlafaxine-treated group (G). Sections of the cerebral cortex from ovariectomized rats (D) and delayed Tx (J) groups showed nuclear pyknosis and degeneration. The hippocampal subiculum exhibited a normal morphology in the sham group (B), acute (H), and delayed (K) treated groups, while intracellular edema was only observed in the ovariectomized group (E). The morphology of the fascia dentate and hilus of the hippocampus was normal in the sham-operated (C) and acute Tx (I) groups. However, nuclear pyknosis and neuronal degeneration were observed in both ovariectomized rats (F) and the delayed Tx (L) group (H&E × 80)
Table 2.
Histopathological scoring of nuclear pyknosis and degeneration in different brain regions
| Group | SHAM | OVX | Acute Tx | Delayed Tx | Acute Tx + anti-EPO Ab | Delayed Tx + anti-EPO Ab | |
|---|---|---|---|---|---|---|---|
| Brain region | |||||||
| Nuclear pyknosis and degeneration in | Cerebral cortex | 0 | 3 ± 1a | 0 ± 1b | 2 ± 1 | 2 ± 1ac | 1 ± 1b |
| Hippocampal subiculum | 0 | 3 ± 0a | 0 ± 2b | 1 ± 2b | 2 ± 1ac | 2 ± 1ac | |
| Fascia dentate and hilus of hippocampus | 0 | 3 ± 1a | 0 ± 1b | 2 ± 2 | 2 ± 1ac | 2 ± 1ac | |
Results are presented as the medians and ranges of three rats per group. aCompared with the sham-operated group, bcompared with OVX rats, and ccompared with the acute venlafaxine-treated group (statistical analyses were performed using Kruskal–Wallis ANOVA followed by Dunn’s post hoc test, and the criterion for statistical significance was set to p < 0.05)
Discussion
In an examination of the first set of questions posed by this study, venlafaxine exerted antidepressant-like effects on OVX rats, which were characterized by decreased immobility time in the FST. These effects were accompanied by upregulation of EPO and EPOR mRNA expression; increased levels of the p-JAK2, p-STAT5, p-ERK1/2, and Bcl2 proteins; downregulation of Bax expression; reduced caspase-3 activity and TNF-α levels; and increased BDNF levels in the hippocampus of OVX rats. Our experimental evidence supports the hypothesis that venlafaxine increases the serum estradiol level. Regarding the second research question, the antidepressant effect of venlafaxine depended on the time interval after OVX. Venlafaxine exhibited superior efficacy in mitigating depressive-like behaviors associated with OVX in the acute setting (24 h post-OVX) compared with the delayed setting (2 weeks post-OVX). Notably, researchers have postulated that a chronic hypo-estrogenic state may reduce the response to SSRIs [32, 33]. Consistent with these discoveries, previous studies reported a poorer response of postmenopausal women to antidepressant treatments than premenopausal women [34, 35]. The findings from this investigation were contrary to the results reported by Benmansour et al. [36] who showed that the antidepressant-like effects of SSRIs were not affected by the length of hormone depletion. The inconsistency in these conclusions may be due to the difference in the initiation time of SSRI treatment in relation to OVX onset. Benmansour et al. (36) studied the impact of length of hormone depletion by measuring the effects of SSRIs at 2 weeks, 4 months, or 8 months post-OVX.
Based on accumulating evidence, EPO/EPOR and their downstream signaling pathways are potential targets in the treatment of depression [12]. Interestingly, the data from the present study show that OVX increased EPOR mRNA expression. In parallel, EPOR expression is induced by brain injury [37]. The most notable finding to emerge from the present study is that venlafaxine upregulated EPO expression and potentiated the induction of EPOR mRNA expression levels in the hippocampus of OVX rats, implying that this antidepressant effect is possibly mediated by signaling pathways involving EPO. This finding broadly supports the findings from other studies in this field, as Choi and Son [14] showed that fluoxetine, a SSRI, increased EPO expression in the hippocampus of depressed mice.
EPO/EPOR induces JAK2 phosphorylation, which in turn activates multiple downstream signaling pathways, including STAT5 and ERK1/2 [9, 38]. The substrates of JAK2 phosphorylate STAT5 and ERK1/2, which exert neuroprotective effects by upregulating anti-apoptotic genes and downregulation of apoptotic genes [39, 40]. Here, the venlafaxine treatment increased p-JAK2, p-STAT-5, and p-ERK1/2 levels in the hippocampus of OVX rats. A note of caution is due here because, although the OVX rats exhibited a marked increase in the hippocampal levels of p-JAK2 and p-STAT5, the magnitude of the increase was weaker than observed in venlafaxine-treated OVX rats. Therefore, the activation of JAK2 and STAT5 in OVX rats is likely associated with the self-defense response protecting against hippocampal injury.
Apoptosis and the molecular mechanisms underlying cell death and survival have been suggested as major mechanisms contributing to depression [41]. Postmortem studies revealed increased apoptosis in the hippocampus and cortex of patients with depression [42]. The Bcl2 family has been postulated as one of the critical mechanisms determining apoptosis. Bcl2 and Bax are both Bcl2 family members; the former belongs to the pro-survival family and the latter belongs to the pro-apoptotic family [43]. The ratio of Bax/Bcl2 is a critical indicator of apoptosis [44]. Bcl2 blocks and Bax induces the release of apoptogenic factors, which in turn stimulate initiator caspases, leading to the activation of the executioner caspase-3 [45]. The activation of the caspase-3 protease leads to the formation of cleaved caspase-3, which constitutes a vital step in the apoptotic process by inducing DNA degradation or fragmentation [46]. In the present study, treatment with venlafaxine significantly upregulated Bcl2 expression, downregulated Bax expression, and decreased the caspase-3 level in the hippocampus of OVX rats. Moreover, the observed decrease in Bax/Bcl2 ratio in the current study further verified the anti-apoptotic actions of venlafaxine. These data are consistent with a previous report showing that venlafaxine inhibits apoptosis in hippocampal neurons by regulating the expression of apoptotic genes in a rat depression model [47].
Additionally, EPO was reported to induce the expression of neurotrophic factors such as BDNF [11, 48]. BDNF plays crucial roles not only in synaptic plasticity, neuronal survival, and proliferation but also in neuronal death [49]. BDNF has been suggested to be involved in the pathogenesis of depression [50]. BDNF levels are markedly reduced in the hippocampi of patients with depression [51]. Lower BDNF levels are associated with Bax-induced apoptosis [52]. Furthermore, an infusion of BDNF into the hippocampus produced antidepressant effects on rats [53]. Consistent with these findings, the BDNF level was restored in the hippocampus of OVX rats after venlafaxine administration in the present study. A comparison of this result with the data presented by Huang et al. is encouraging [54]; the authors reported that the inhibitory effect of venlafaxine on hippocampal apoptosis was mediated by the upregulation of BDNF in a rat depression model.
Furthermore, blockade of the EPO receptor mitigated most of the beneficial effects of the venlafaxine treatment, as both the acute and delayed treatments in the presence of anti-EPO Ab showed weaker effects than acute or delayed treatments alone on immobility time; caspase-3, TNF-α, Bcl2, and Bax levels; the Bax/Bcl2 ratio; and the histopathological findings, suggesting that the EPO pathway might be a cornerstone in the antidepressant effects of venlafaxine.
According to these data, we speculated that venlafaxine protects hippocampal neurons in OVX rats from apoptosis by activating EPO/EPOR/JAK2 signaling and downstream anti-apoptotic pathways. The neuroprotective effects of venlafaxine observed in the present study were also confirmed by the marked mitigation of the histological changes observed in the cortex and hippocampus of OVX rats.
Extensive data support the role of inflammation in depression [55, 56]. Patients with depression exhibit all of the cardinal features of an inflammatory response, including increased levels of pro-inflammatory cytokines such as TNF-α [57]. In the present study, venlafaxine alleviated the increase in hippocampal TNF-α levels in OVX rats, signifying its anti-inflammatory effects, which may contribute to its neuroprotective activity. These data are consistent with previous reports showing the anti-inflammatory effects of SSRIs and venlafaxine on rat depression models [20, 58].
Finally, SSRIs have the potential to interfere with estrogen signaling [59, 60]. As shown in the present study, the venlafaxine treatment increased serum estradiol levels in OVX rats. Similarly, a study by Zhou et al. [17] suggested that paroxetine increases the serum estrogen level in postmenopausal women. Furthermore, SSRIs were reported to increase estradiol secretion in vitro, probably by modifying the activities of enzymes involved in steroid synthesis and metabolism [60, 61]. In contrast to our findings, Taylor et al. [58] reported that fluoxetine decreases circulating estrogen levels in OVX females treated with exogenous estrogen. In addition, Rahavi et al. [62] has speculated that the SSRI-induced decrease in estrogen levels may be due to the inhibition of luteinizing hormone. To date, the mechanisms by which SSRIs modulate estrogen levels remain unclear.
In conclusion, our data will pave the way for studies aiming to obtain a better understanding of the mechanisms underlying the neuroprotective effects of venlafaxine on OVX rats. These effects are at least partially attributed to the activation of EPO/EPOR/JAK2 signaling pathways; anti-apoptotic, anti-inflammatory, and neurotrophic activities; and increased serum estradiol levels. This is the first report showing that venlafaxine exhibits superior efficacy in inducing antidepressant-like effects following acute treatment compared to after delayed treatment. Moreover, these findings lay the groundwork for future research focused on treating postmenopausal and perimenopausal depression.
Electronic Supplementary Material
(PDF 1224 kb)
Acknowledgments
The authors are grateful to Dr. Adel M. Bakeer, Professor of Pathology, Faculty of Veterinary Medicine, Cairo University, for the kind assistance with the histopathology.
Authors’ Contributions
Muhammed A. Saad, Ayman E. El-Sahar, Rabab H. Sayed: conceived and designed the experiments
Muhammed A. Saad, Ayman E. El-Sahar, Rabab H. Sayed, Eman M. Elbaz, Hebatullah S. Helmy, Mahmoud A. Senousy: performed the experiments
Muhammed A. Saad, Ayman E. El-Sahar, Rabab H. Sayed: analyzed the data and wrote the manuscript
Eman M. Elbaz, Hebatullah S. Helmy, Mahmoud A. Senousy: contributed reagents and analysis tools
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Compliance with Ethical Standards
The protocols used in this study were approved by the Ethics Committee for Animal Experimentation at Faculty of Pharmacy, Cairo University (Permit Number: PT 2099).
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Noble RE. Depression in women. Metabolism. 2005;54:49–52. doi: 10.1016/j.metabol.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Graziottin A, Serafini A. Depression and the menopause: why antidepressants are not enough? Menopause Int. 2009;15:76–81. doi: 10.1258/mi.2009.009021. [DOI] [PubMed] [Google Scholar]
- 4.Kiss Á, Delattre AM, Pereira SIR, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17β-Estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res. 2012;227:100–108. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 5.Estrada-Camarena E, Lopez-Rubalcava C, Vega-Rivera N, Recamier-Carballo S, Fernandez-Guasti A. Antidepressant effects of estrogens: a basic approximation. Behav Pharmacol. 2010;21:451–464. doi: 10.1097/FBP.0b013e32833db7e9. [DOI] [PubMed] [Google Scholar]
- 6.Zanardi R, Rossini D, Magri L, Malaguti A, Colombo C, Smeraldi E. Response to SSRIs and role of the hormonal therapy in post-menopausal depression. Eur Neuropsychopharmacol. 2007;17:400–405. doi: 10.1016/j.euroneuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Rozenberg S, Vandromme J, Antoine C. Postmenopausal hormone therapy: risks and benefits. Nat Rev Endocrinol. 2013;9:216–227. doi: 10.1038/nrendo.2013.17. [DOI] [PubMed] [Google Scholar]
- 8.Nairz M, Sonnweber T, Schroll A, Theurl I, Weiss G. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 2012;14:238–246. doi: 10.1016/j.micinf.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoetin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1996;76:105–116. doi: 10.1016/S0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 11.Viviani B, Bartesaghi S, Corsini E, Villa P, Ghezzi P, Garau A, Galli CL, Marinovich M. Erythropoietin protects primary hippocampal neurons increasing the expression of brain-derived neurotrophic factor. J Neurochem. 2005;93:412–421. doi: 10.1111/j.1471-4159.2005.03033.x. [DOI] [PubMed] [Google Scholar]
- 12.Ma C, Cheng F, Wang X, Zhai C, Yue W, Lian Y, Wang Q (2016) Erythropoietin pathway: A potential target for the treatment of depression. Int J Mol Sci. : 10.3390/ijms17050677 [DOI] [PMC free article] [PubMed]
- 13.Osborn M, Rustom N, Clarke M, Litteljohn D, Rudyk C, Anisman H, Hayley S (2013) Antidepressant-Like Effects of Erythropoietin: A Focus on Behavioural and Hippocampal Processes. PLoS One. 10.1371/journal.pone.0072813 [DOI] [PMC free article] [PubMed]
- 14.Choi M, Son H. Effects of serotonin on erythropoietin expression in mouse hippocampus. Exp Neurobiol. 2013;22:45–50. doi: 10.5607/en.2013.22.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uher R, Maier W, Hauser J, et al. Differential efficacy of escitalopram and nortriptyline on dimensional measures of depression. Br J Psychiatry. 2009;194:252–259. doi: 10.1192/bjp.bp.108.057554. [DOI] [PubMed] [Google Scholar]
- 16.Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996;16:6394–6401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou B, Xie S, Hu J, Sun X, Guan H, Deng Y. Paroxetine Increased the Serum Estrogen in Postmenopausal Women with Depressive and Anxiety Symptoms. Open J Depress. 2014;3:184–194. doi: 10.4236/ojd.2014.35022. [DOI] [Google Scholar]
- 18.Fenli S, Feng W, Ronghua Z, Huande L. Biochemical mechanism studies of venlafaxine by metabonomic method in rat model of depression. Eur Rev Med Pharmacol Sci. 2013;17:41–48. [PubMed] [Google Scholar]
- 19.De Silva VA, Hanwella R. Efficacy and tolerability of venlafaxine versus specific serotonin reuptake inhibitors in treatment of major depressive disorder: A meta-analysis of published studies. Int Clin Psychopharmacol. 2012;27:8–16. doi: 10.1097/YIC.0b013e32834ce13f. [DOI] [PubMed] [Google Scholar]
- 20.Wang CH, Gu JY, Zhang XL, Dong J, Yang J, Zhang YL, Ning QF, Shan XW, Li Y (2016) Venlafaxine ameliorates the depression-like behaviors and hippocampal S100B expression in a rat depression model. Behav Brain Funct. 10.1186/s12993-016-0116-x [DOI] [PMC free article] [PubMed]
- 21.Flamme I, Ellinghaus P, Urrego D, Krüger T (2017) FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS One. 10.1371/journal.pone.0186979 [DOI] [PMC free article] [PubMed]
- 22.Ibrahim WW, Safar MM, Khattab MM, Agha AM. 17β-Estradiol augments antidepressant efficacy of escitalopram in ovariectomized rats: Neuroprotective and serotonin reuptake transporter modulatory effects. Psychoneuroendocrinology. 2016;74:240–250. doi: 10.1016/j.psyneuen.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K, Piao Y -F, Miyazono K, Urabe A, Takaku F (1989) Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol 140:323–334 [DOI] [PubMed]
- 24.Walsh RN, R a C. The Open-Field Test: a critical review. Psychol Bull. 1976;83:482–504. doi: 10.1037/0033-2909.83.3.482. [DOI] [PubMed] [Google Scholar]
- 25.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 26.BRADFORD MM. Determinacion De Proteinas: Metodo De Bradford. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Bancroft J, Turner A, Stevens D (1996) Theory and practice of histological techniques, 4th ed. New York : Churchill Livingstone
- 29.Kamel AS, Abdelkader NF, Abd El-Rahman SS, Emara M, Zaki HF, Khattab MM (2018) Stimulation of ACE2/ANG(1–7)/Mas Axis by Diminazene Ameliorates Alzheimer’s Disease in the D-Galactose-Ovariectomized Rat Model: Role of PI3K/Akt Pathway. Mol Neurobiol. doi: 10.1007/s12035-018-0966-3 [DOI] [PubMed]
- 30.Dixon WJ. Power Under Normality of Several Nonparametric Tests. Ann Math Stat. 1954;25:610–614. doi: 10.1214/aoms/1177728732. [DOI] [Google Scholar]
- 31.Mead R. The design of experiments: Statistical principles for practical applications. New York, NY, US: Cambridge University Press; 1988. [Google Scholar]
- 32.Thase ME, Entsuah R, Cantillon M, Kornstein SG. Relative Antidepressant Efficacy of Venlafaxine and SSRIs: Sex-Age Interactions. J Women’s Heal. 2005;14:609–616. doi: 10.1089/jwh.2005.14.609. [DOI] [PubMed] [Google Scholar]
- 33.Pinto-Meza A, Usall J, Serrano-Blanco A, Suárez D, Haro JM. Gender differences in response to antidepressant treatment prescribed in primary care. Does menopause make a difference? J Affect Disord. 2006;93:53–60. doi: 10.1016/j.jad.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Pae C-U, Mandelli L, Kim T-S, Han C, Masand PS, Marks DM, A a P, Steffens DC, De Ronchi D, Serretti A. Effectiveness of antidepressant treatments in pre-menopausal versus post-menopausal women: a pilot study on differential effects of sex hormones on antidepressant effects. Biomed Pharmacother. 2009;63:228–235. doi: 10.1016/j.biopha.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Huang CC, Wei IH, Chou YH, Su TP. Effect of age, gender, menopausal status, and ovarian hormonal level on rTMS in treatment-resistant depression. Psychoneuroendocrinology. 2008;33:821–831. doi: 10.1016/j.psyneuen.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Benmansour S, Arroyo LD, Frazer A. Comparison of the Antidepressant-Like Effects of Estradiol and That of Selective Serotonin Reuptake Inhibitors in Middle-Aged Ovariectomized Rats. Front Aging Neurosci. 2016;8:1–13. doi: 10.3389/fnagi.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ott C, Martens H, Hassouna I, et al. Widespread expression of erythropoietin receptor in brain and its induction by injury. Mol Med. 2015;21:803–815. doi: 10.2119/molmed.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon M-S, Kim M-H, Kim S-H, Park K-D, Yoo S-H, Oh I-U, Pak S, Seo Y-J. Erythropoietin exerts cell protective effect by activating PI3K/Akt and MAPK pathways in C6 Cells. Neurol Res. 2014;36:215–223. doi: 10.1179/1743132813Y.0000000284. [DOI] [PubMed] [Google Scholar]
- 39.Correia C, Lee SH, Meng XW, Vincelette ND, Knorr KLB, Ding H, Nowakowski GS, Dai H, Kaufmann SH. Emerging understanding of Bcl-2 biology: Implications for neoplastic progression and treatment. Biochim Biophys Acta - Mol Cell Res. 2015;1853:1658–1671. doi: 10.1016/j.bbamcr.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponce LL, Navarro JC, Ahmed O, Robertson CS. Erythropoietin neuroprotection with traumatic brain injury. Pathophysiology. 2013;20:31–38. doi: 10.1016/j.pathophys.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKernan DP, Dinan TG, Cryan JF. “Killing the Blues”: A role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog Neurobiol. 2009;88:246–263. doi: 10.1016/j.pneurobio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Lucassen PJ, Müller MB, Holsboer F, Bauer J, Holtrop A, Wouda J, Hoogendijk WJG, De Kloet ER, Swaab DF. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsten T, Zong WX, Thompson CB. Defining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist. 2005;11:10–15. doi: 10.1177/1073858404269267. [DOI] [PubMed] [Google Scholar]
- 44.Monsalve DM, Merced T, Fernández IF, Blanco S, Vázquez-Cedeira M, Lazo PA (2013) Human VRK2 modulates apoptosis by interaction with Bcl-xL and regulation of BAX gene expression. Cell Death Dis. 10.1038/cddis.2013.40 [DOI] [PMC free article] [PubMed]
- 45.Cory S, Adams JM. The BCL2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 46.Mnich K, Carleton LA, Kavanagh ET, Doyle KM, Samali A, Gorman AM (2014) Nerve growth factor-mediated inhibition of apoptosis post-caspase activation is due to removal of active caspase-3 in a lysosome-dependent manner. Cell Death Dis. 10.1038/cddis.2014.173 [DOI] [PMC free article] [PubMed]
- 47.Wang Y, Xiao Z, Liu X, Berk M. Venlafaxine modulates depression-induced behaviour and the expression of Bax mRNA and Bcl-xl mRNA in both hippocampus and myocardium. Hum Psychopharmacol. 2011;26:95–101. doi: 10.1002/hup.1177. [DOI] [PubMed] [Google Scholar]
- 48.Aalling N, Hageman I, Miskowiak K, Orlowski D, Wegener G, Wortwein G. Erythropoietin prevents the effect of chronic restraint stress on the number of hippocampal CA3c dendritic terminals—relation to expression of genes involved in synaptic plasticity, angiogenesis, inflammation, and oxidative stress in male rats. J Neurosci Res. 2018;96:103–116. doi: 10.1002/jnr.24107. [DOI] [PubMed] [Google Scholar]
- 49.Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70:735–744. doi: 10.1016/S0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- 50.Cunha ABM, Frey BN, Andreazza AC, Goi JD, Rosa AR, Gonçalves CA, Santin A, Kapczinski F. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett. 2006;398:215–219. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 51.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–58. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Snider WD, Korsmeyer SJ. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17:401–411. doi: 10.1016/S0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 53.Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang X, Mao YS, Li C, Wang H, Ji JL. Venlafaxine inhibits apoptosis of hippocampal neurons by up-regulating brain-derived neurotrophic factor in a rat depression model. Pharmazie. 2014;69:909–916. [PubMed] [Google Scholar]
- 55.Miller AH, Raison CL. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eyre H, Baune BT. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology. 2012;37:1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 58.Horikawa H, Kato TA, Mizoguchi Y, et al. Inhibitory effects of SSRIs on IFN-γ induced microglial activation through the regulation of intracellular calcium. Prog Neuro-Psychopharmacology Biol Psychiatry. 2010;34:1306–1316. doi: 10.1016/j.pnpbp.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Taylor GT, Farr S, Klinga K, Weiss J. Chronic fluoxetine suppresses circulating estrogen and the enhanced spatial learning of estrogen-treated ovariectomized rats. Psychoneuroendocrinology. 2004;29:1241–1249. doi: 10.1016/j.psyneuen.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Hansen CH, Larsen LW, Sørensen AM, Halling-Sørensen B, Styrishave B. The six most widely used selective serotonin reuptake inhibitors decrease androgens and increase estrogens in the H295R cell line. Toxicol Vitr. 2017;41:1–11. doi: 10.1016/j.tiv.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Lupu D, Sjödin M, Varshney M, Lindberg J, Loghin F, Rüegg J (2017) FLUOXETINE MODULATES SEX STEROID LEVELS IN VITRO. Clujul Med. doi: 10.15386/cjmed-868 [DOI] [PMC free article] [PubMed]
- 62.Rehavi M, Attali G, Gil-Ad I, Weizman a (2000) Suppression of serum gonadal steroids in rats by chronic treatment with dopamine and serotonin reuptake inhibitors. Eur Neuropsychopharmacol 10:145–50 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)