Abstract
Abstract
Metastatic paraganglioma treatment options are limited. Peptide receptor radionuclide therapy (PRRT) has been introduced as a novel management option for metastatic neuroendocrine tumors demonstrating safety, efficacy, and increased quality of life. We present two cases of marked progression of metastatic paraganglioma following initial partial response to PRRT. Given their positivity on 68Ga-DOTATATE PET/CT and 111In-octreotide SPECT, they underwent PRRT. Imaging following treatment revealed significant improvement in size and intensity, with some foci nearly completely resolved in one patient, and disease regression with a decrease in the number and size of bone and liver lesions in the second patient. Within months, repeat imaging in both patients revealed extensive metastatic disease with new lesions, which eventually lead to their deaths. The mechanism for rapid disease progression after partial response is not well understood, although it could be related to initially high Ki-67 levels or 18F-FDG PET/CT SUVmax values. However, naturally rapid disease progression despite PRRT response cannot be excluded. This finding warrants the importance of proper patient counseling along with early and accurate pre-PRRT assessment, taking into consideration the above potential risk factors for therapy response in order to personalize treatment regimens and achieve maximum patient benefit.
ClinicalTrials.gov Identifier
Keywords: Paraganglioma, PRRT, 177Lu-DOTATATE, 68Ga-DOTATATE, 18F-FDG, SSTR
Introduction
Pheochromocytomas (PHEOs) and paragangliomas (PGLs) are neuroendocrine tumors derived from neural crest cells of the adrenal medulla or extra-adrenal PGL, respectively [1, 2]. The prevalence of malignancy strongly depends on the underlying genetic background, with a higher risk of malignancy associated in patients with mutations in succinate dehydrogenase subunit (SDH) B gene [3, 4]. In the presence of PHEO/PGL-associated metastases, patients have a 50% 5-year overall survival [5]. With less than 40% of patients with malignant PHEO/PGL responding to current therapeutic modalities such as 131I-metaiodobenzylguanidine (MIBG) and chemotherapy, attention has shifted to developing novel systemic antineoplastic therapies [1]. Over the last several decades, peptide receptor radionuclide therapy (PRRT) has been introduced as a treatment option for patients with metastatic neuroendocrine tumors (NETs) [6, 7]. Recent data has shown that PRRT is a promising treatment option with improved patient quality of life [6, 8, 9]. Here, we present two cases that show rapid progression of disease following successful partial treatment response with 177Lu/90Y-DOTATOC and 177Lu-DOTATATE.
Case Report
(Patient A) A 53-year old Caucasian female presented to the National Institutes of Health (NIH) following a workup for flu-like symptoms 3 months prior. Outside computed tomography (CT) and ultrasound of the abdomen revealed an abdominal mass, which was subsequently biopsied and histologic findings were consistent with PGL. Initial symptoms included hypertension accompanied by diffuse diaphoresis, palpitations, weight loss, and increasing levels of anxiety over 4 years. Her endocrinologist recommended further evaluation with a full biochemical workup. Elevated laboratory results led her to seek a specialized consult for PHEO/PGL.
Upon presentation to the NIH in March 2014, she underwent further imaging studies, including whole body magnetic resonance imaging (MRI), CT, and functional imaging using [18F]-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET/CT), [18F]-dihydroxyphenyalanine (18F-FDOPA) PET/CT, and [68Ga]-(DOTA)-[Tyr3]-octreotate (68Ga-DOTATATE) PET/CT, which all revealed a large, heterogeneous 6.0-centimeter (cm) retroperitoneal mass enveloping the aorta with a maximal standardized uptake (SUVmax) value on 18F-FDG PET/CT of 48.9 (Fig. 1a), as well as multiple bone and bone marrow metastases. The SUVmax of metastases in the left iliac bone, T5, and L2 ranged from 36.0 to 51.2. Biochemical evaluation revealed elevated metanephrine and catecholamine values upwards of 40 times above the upper reference limit (URL) (Table 1, Fig. 2). Genetic testing was positive for an SDHA gene nonsense pathogenic mutation: (c.1534C > T (p.Arg512*)), but there was no family history of PHEO/PGL or other NETs.
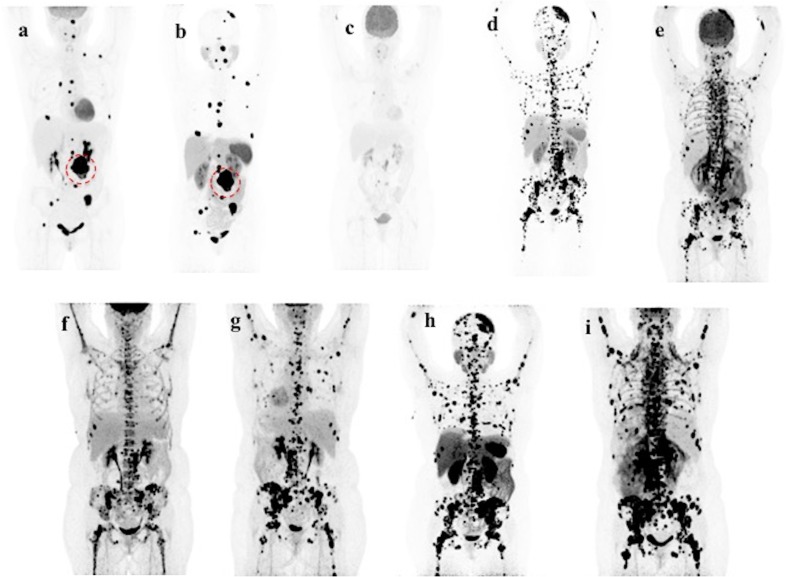
Fig. 1.
Patient A’s course of disease. Upon Patient A’s first presentation to the NIH in March 2014, her 18F-FDG PET/CT (a) and 68Ga-DOTATATE PET/CT (b) showed similar, multiple skeletal lesions and a 6.0cm large, heterogeneous left retroperitoneal mass (dotted circle in a and b), which was surgically removed in April 2014. She returned to the NIH for functional imaging after PRRT in April 2015. 18F-FDG PET/CT (c) revealed significant improvement in the size and intensity of many lesions, with some foci nearly completely resolved, in comparison to her 18F-FDG PET/CT from March 2014 (a). She returned to the NIH in October 2015. 68Ga-DOTATATE PET/CT (d) and 18F-FDG PET/CT (e) both revealed extensive progression of skeletal metastatic involvement with the highest tumor burden in the axial skeleton. Following completion of 6 cycles of CVD, which began in December 2015, 18F-FDG PET/CT in February 2016 revealed evidence of partial response to chemotherapy treatment and stable disease (f). She returned to the NIH in August 2016. 18F-FDG PET/CT (g) and 68Ga-DOTATATE PET/CT (h) showed disease progression owing to worsening bone metastases and new small hypermetabolic lung metastases developed. She returned to the NIH in October 2016 after initiation of bortezomib and clofarabine experimental therapy in September 2016. 18F-FDG PET/CT (i) showed disease progression owing to development of new bone marrow lesions
Table 1.
Biochemical data and clinical characteristics across all time points for Patient A
| March 2014 Presentation |
April 2015 Post-treatment |
September 2015 Follow-up |
February 2016 Follow-up |
October 2016 Follow-up |
|
|---|---|---|---|---|---|
|
Plasma epinephrine [URL 50 pg/mL] |
< 20 (< 1.0×) |
< 20 (< 1.0×) |
33 (1.7×) |
24 (1.2×) |
< 20 (< 1.0×) |
|
Plasma Norepinephrine [URL 750 pg/mL] |
14,755 (19.7×) |
745 (< 1.0×) |
9387 (12.5×) |
5148 (6.9×) |
18,043 (24.1×) |
|
Plasma Dopamine [URL 29 pg/mL] |
221 (7.6×) |
54 (1.9×) |
196 (6.8×) |
147 (5.1×) |
205 (7.1×) |
|
Plasma normetanephrine [URL 112 pg/mL] |
4620 (41.2×) |
176 (1.6×) |
7278 (65.0×) |
3118 (27.9×) |
17,106 (152.7×) |
|
Plasma 3-methoxytyramine [URL 20 pg/mL] |
110 (5.0×) |
< 20 (< 1.0×) |
25 (1.3×) |
165 (8.3×) |
256 (12.8×) |
|
Chromogranin A [URL 93 ng/mL] |
646 (7.0×) |
171 (1.8×) |
1312 (14.1×) |
1260 (13.6×) |
N.A. |
|
Blood pressure [mm Hg] |
150/65 | 127/63 | 149/70 | 110/64 | 118/63 |
|
Heart rate [bpm] |
107 | 83 | 77 | 86 | 109 |
Abbreviations: URL, upper reference limit; mm Hg, milimeters of mercury; bpm, beats per minute; N.A., not available
Values in parentheses represent multiples of the upper reference limit
Fig. 2.
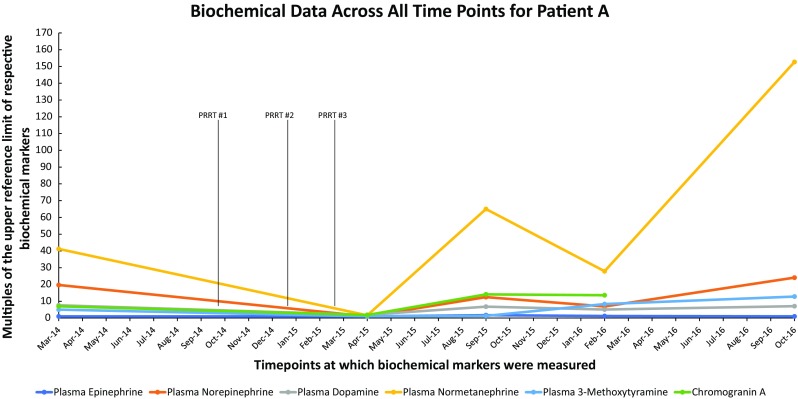
Biochemical data across all time points for Patient A. A graph showing multiples of the upper reference limit of biochemical markers on the Y-axis and various timepoints at which biochemical markers were measured on the X-axis in patient A
In April 2014, the patient underwent an exploratory laparotomy to resect the left retroperitoneal mass, which measured 9.0 cm. Surgical pathology confirmed the lesion was PGL. Her 3- and 4-month post-operative MRI follow-ups showed multiple bone lesions located in the spine, pelvis, ribs, and left parietal skull, which had progressed. Due to the 68Ga-DOTATATE PET/CT from March 2014 (Fig. 1b) showing scattered skeletal lesions with intense uptake as high as an SUVmax of 224.0, she was referred outside of the NIH for PRRT, where she received one cycle of 90Y-DOTATOC (160 milicurie (mCi)) followed by two cycles of 177Lu-DOTATOC (200 mCi) over a 10-week timespan.
The patient returned to the NIH in April 2015 following completion of her last PRRT treatment in February of the same year. Biochemical workup revealed an 83-fold decrease in elevated urine norepinephrine (99 mcg/24 h, previously 8255 mcg/24 h) and an 18-fold decrease in normetanephrine (1055 mcg/24 h, previously 18,754 mcg/24 h), as well as a decrease in plasma normetanephrine (Table 1, Fig. 2). Functional imaging with 18F-FDG PET/CT revealed significant improvement in the size and intensity of many lesions, with near complete resolution of some foci and a decrease in SUVmax to 17.5 of the residual retroperitoneal tumor (Fig. 1c). Based on biochemical workup and functional imaging, the patient was assessed as having responded very well to treatment.
In October of 2015, the patient returned to the NIH with recurring daily symptoms of sweating, elevated blood pressure, chest tightness, and pain radiating to the back. CT and MRI reports revealed extensive metastatic bone involvement with interval progression of existing disease and multiple new lesions. A repeat 68Ga-DOTATATE PET/CT (Fig. 1d) and 18F-FDG PET/CT (Fig. 1e) likewise showed marked progression of skeletal metastatic involvement. On 18F-FDG PET/CT, the SUVmax of the residual retroperitoneal tumor was 64.7, more than 3 times higher than her results 6 months prior (Fig. 1e). Based on her progression, she began six cycles of chemotherapy with cyclophosphamide, vincristine, and dacarbazine (CVD) in November 2015. In February 2016, 18F-FDG PET/CT revealed an SUVmax of 44.5 in the residual retroperineal tumor, indicating stable disease and partial response to CVD (Fig. 1f). However, in May 2016, CVD chemotherapy was aborted after the 10th cycle due to pancytopenia and subsequent development of myelodysplastic syndrome. In August 2016, further disease progression was appreciated on 18F-FDG PET/CT (Fig. 1g) and 68Ga-DOTATATE PET/CT (Fig. 1h). While the 18F-FDG SUVmax of the retroperitoneal tumor remained stable at 43.0, there were increased bone metastases and several newly developed lung metastases. Experimental therapy with bortezomib and clofarabine began in September 2016, but was discontinued in October 2016 after 18F-FDG PET/CT demonstrated new bone marrow lesions and an increase in the SUVmax of the residual retropertineal tumor to 75.5 (Fig. 1i). As the patient desired to continue her treatment plan, she was started on lanreotide and combination capecitabine and temozolomide. However, the patient died in May 2017.
(Patient B) A 50-year old Caucasian female was evaluated by a neurologist at her local hospital for cervical radiculopathy presenting as pain in her left shoulder radiating to her arm and fingers. She lacked symptoms of headache, sweating, or palpitations and her blood pressure was normal. An MRI of the spine revealed a lesion in her C6 vertebrae with invasion of the neuroforamen and epidural space as well as cervical myelopathy. Additional bone lesions were seen in the right ilium, the T6 vertebra, and the left proximal femur. Furthermore, a 10-cm retroperitoneal mass was found adjacent to the aorta and the duodenum, as well as a 2.2-cm liver lesion suspicious for metastasis. A fine-needle aspiration biopsy of the retroperitoneal mass was performed and histological examination revealed a PGL. She was referred to the Radboud University Medical Center in Nijmegen, The Netherlands, for further evaluation.
Biochemical examination showed completely normal plasma levels of metanephrines and 3-methoxytyramine, consistent with biochemically silent PGL (Table 2, Fig. 3). However, her chromogranin A level was elevated over 8.5× the upper reference limit at 865 mcg/L (URL < 100 mcg/L, Table 2, Fig. 3). Genetic testing revealed a deletion of exon 3 in the SDHB gene (c.201-4429_287-934del), a Dutch founder mutation. Her family history was negative for PGL, but her mother had surgery of a non-functional pituitary adenoma at age 32.
Table 2.
Biochemical data across all time points for patient B
| June 2014 Presentation |
October 2014 Pre-treatment |
March 2016 Post-treatment |
April 2016 Follow-Up |
|
|---|---|---|---|---|
|
Plasma metanephrine [URL 450 pmol/L] |
123 (< 1.0×) |
158 (< 1.0×) |
149 (< 1.0×) |
182 (< 1.0×) |
|
Plasma normetanephrine [URL 747 pmol/L] |
314 (< 1.0×) |
315 (< 1.0×) |
244 (< 1.0×) |
543 (< 1.0×) |
|
Plasma 3-methoxytyramine [URL 100 pmol/L] |
< 100 (< 1.0×) |
< 100 (< 1.0×) |
106 (1.1×) |
147 (1.5×) |
|
Chromogranin A [URL 100 mcg/mL] |
865 (8.7×) |
958 (9.6×) |
2780 (27.8×) |
13,020 (130.2×) |
Abbreviations: URL, upper reference limit
Values in parentheses represent multiples of the upper reference limit
Fig. 3.
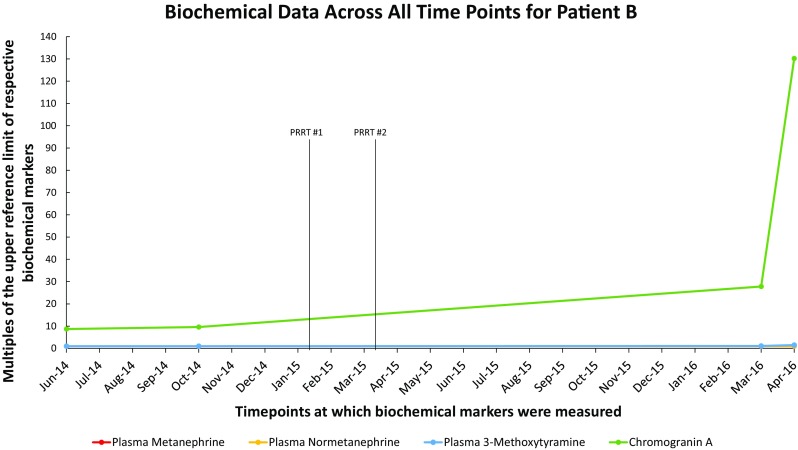
Biochemical data across all time points for Patient B. A graph showing multiples of the upper reference limit of biochemical markers on the Y-axis and various timepoints at which biochemical markers were measured on the X-axis in patient B
An 18F-FDG PET/CT in July 2014 showed high metabolic activity of both the retroperitoneal tumor and liver and bone metastases including bone marrow lesions (Fig. 4a). After vascular embolization, debulking of the C6 metastasis was performed. In September 2014, the patient underwent a debulking surgery to resect the para-aortal retroperitoneal mass which measured 9.0 cm. Histopathology of both sites was consistent with PGL.
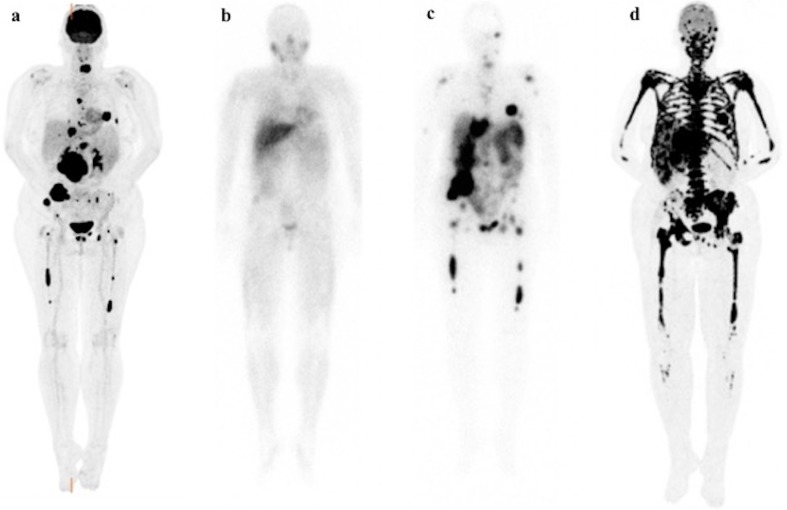
Fig. 4.
Patient B’s course of disease. Functional imaging scans for Patient B. 18F-FDG PET/CT (a) scan before 177Lu-DOTATATE therapy showing high metabolic activity of both the retroperitoneal tumor and liver and bone metastases including bone marrow lesions. 123I-MIBG scintigraphy (b) before 177Lu-DOTATATE therapy showing weak uptake in only part of the lesions, therefore excluding 131I-MIBG therapy as a treatment option. 111In-octreotide SPECT scan (c) before 177Lu-DOTATATE therapy showing all avid uptake of this tracer by all the lesions demonstrated on 18F-FDG PET/CT scan (a) favoring 177Lu-DOTATATE therapy. 18F-FDG PET/CT scan (d) after 2 cycles of 177Lu-DOTATATE therapy showing severe disease progression with multiple new liver and diffuse skeletal involvement
In November 2014, follow-up scanning showed progression of liver and bone metastasis. She underwent 123I-MIBG scintigraphy, which showed weak uptake in only part of the lesions, therefore excluding 131I-MIBG therapy as a treatment option (Fig. 4b). Somatostatin receptor scintigraphy was performed using 111In-octreotide single photon emission tomography (SPECT) and all metastases showed avid uptake of this tracer (Fig. 4c). To control bone pain, external beam radiotherapy was performed on the cervical, thoracic, and pelvic lesions (6 fractions, total dose 4 Gray). In January 2015, follow-up examination again showed progression, with lytic bone lesions and pathological fractures of the pelvic and pubic bone. Chromogranin A increased to 1701 mcg/L, now 17× the URL of normal. She was referred to the Erasmus Medical Center in Rotterdam, the Netherlands, for PRRT where she received two cycles of 177Lu-DOTATATE within a 6-week interval (200 mCi and 197 mCi, respectively). A planned third cycle was postponed due to mild thrombocytopenia (platelet count 96 × 109/L, URL 150-400 × 109/L). The patient was followed with CT scans every 3 months, which initially showed stable disease, but later displayed regression in July and November 2015, with a decrease in the number and size of bone and liver lesions (not pictured). Her chromogranin A decreased to 988 mcg/L, now 9.9× the URL of normal. Her platelet count fluctuated between 60 and 90 × 109/L.
An 18F-FDG PET/CT scan in March 2016, revealed severe progression of disease with multiple new liver and diffuse skeletal involvement (Fig. 4d). In April 2016, she was admitted to Radboud University Medical Center because of quickly progressive, generalized weakness, increased bone pain, and spontaneous hematomas. Laboratory investigations showed a hemoglobin of 7.6 g/dL, leukocytes at 2.3 × 109/L, and platelets at 12 × 109/L. Her chromogranin A level vastly increased to 13,020 mcg/L, now 130.2× the URL of normal (Table 2, Fig. 3). Plasma metanephrines and 3-methoxytyramine remained normal during her disease course except mild elevation in plasma 3-methoxytyramine when she demonstrated progressive disease (Table 2, Fig. 3). A bone marrow aspirate and biopsy, including immunophenotyping, was consistent with displacement of the bone marrow by PGL cells, with no signs of myelodysplasia. Repeated erythrocyte and platelet transfusions were administered. Further systemic treatment was considered impossible because of her poor general performance status and pancytopenia. Palliative supportive care was offered, including erythrocyte and platelet transfusions at 7–10 days intervals. In July of 2016, the patient died of opiate-induced respiratory arrest.
Discussion
Previous studies have reported significant therapeutic responses and increased progression free survival in patients with various neuroendocrine tumors, including metastatic PHEO/PGL, undergoing PRRT [6, 10–15]. Currently, PRRT is performed according to different protocols: a combination of 90Y-DOTATOC and 177Lu-DOTATOC or monotherapy with 177Lu-DOTATATE. 177Lu-DOTATOC or 90Y-DOTATOC are also performed with 2–4 treatment cycles as part of one treatment session. Mild adverse events resulting from this type of therapy include nausea, vomiting, fatigue, fever, and diarrhea [6]. Greater hematological and renal toxicities have been also reported, particularly with 90Y-DOTATOC versus 177Lu-DOTATATE [16]. While most symptoms are described as manageable, several studies have shown resultant myelodysplastic syndrome, acute leukemia, or other cancers, which have also been reported in other standard of care radiotherapies, such as with 131I-MIBG [13, 16–21]. Here, we show two cases where the patients received either 177Lu/90Y-DOTATOC or 177Lu-DOTATATE and demonstrated remarkable response rates with almost complete resolution of several foci. Within the following months, both patients presented with marked progression and extensive metastatic disease to the bone and eventual death of the patients. The median progression free survival of these 2 patients was 14.5 months which is much lower than the 39 months in the retrospective study by Kong et al. in 20 patients who underwent a median of four cycles of 177Lu-DOTATATE therapy [14]. In 2017, Kong et al. described a PGL patient who initially responded to PRRT, but subsequently developed multiple osseous metastases. After additional cycles of PRRT, the patient responded clinically, biochemically, and scintigraphically once more. However, disease relapse occurred 16 months later and the patient died [14]. Kong and colleagues attributed the relapse to the patient’s functional hypertensive disease, which can give way to catecholamine crisis or tumor lysis syndrome [14]. The mechanism for rapid disease progression after achieving partial response is not well understood. However, it may indicate the more naturally aggressive behavior of poorly differentiated tumors, especially those with high Ki-67 values, as previously reported in other neuroendocrine tumors [22–25]. For patient A, the Ki-67 value was overall elevated with 15% in focal areas and a mitotic count greater than 15 per 50 high power fields. For patient B, the Ki-67 values ranged from 20 to 30% in focal areas, with as low as 2% seen elsewhere in the tissue. This is likewise supported by highly positive 18F-FDG PET/CT scans with significantly higher SUVmax values, which correlates to clinically aggressive cases and is a predictor of rapid progressive disease and poor prognosis [26–32]. High mitotic counts combined with increased glucose turnover may be linked to the pathogenesis of rapidly progressive disease following initial partial response to PRRT. Additionally, PRRT-induced immunosuppression could result in rapid progression of a tumor, in this case, metastatic PGL.
Overall, international experience has shown that most patients undergoing PRRT respond very positively with either disease stabilization or regression. A recent study from Strosberg et al. likewise demonstrated that PRRT is strongly supported in metastatic neuroendocrine tumors [7]. While the above two cases could represent the natural history of the disease, their rapid progression could also be related to their high primary tumor Ki-67 values or elevated 18F-FDG PET/CT SUVmax, particularly in their bone marrow lesions. In patient B, prior radiotherapy could also be one of the reasons of rapid progression which has been associated with myelotoxicity apart from age > 70 years, impaired renal function, baseline cytopenias, prior number of therapies, and prior chemotherapy (alkylating agents) [21]. In 2016, Basu et al. performed a retrospective analysis on 5 neuroendocrine tumor patients with bone marrow involvement who underwent 177Lu-DOTATATE therapy, which reported no hematological toxicity in any of their patients [33]. However, 18F-FDG PET/CT uptake in the bone marrow lesions was non-existent, barring just two patients who showed low-grade 18F-FDG uptake [33]. In the two cases we present here, both patients had very high SUVmax values of their bone marrow lesions prior to PRRT therapy. This finding could explain why they responded poorly to PRRT, while 4 of the 5 patients from Basu et al. had no significant relapse in their clinical course after therapy. Additionally, both patients had high Ki-67 values, which were recently studied by Yordanova et al. who reported their results on the safety of repeated cycles of 177Lu-DOTATATE therapy in patients with various neuroendocrine tumors. While no life-threatening adverse events occurred during the treatment, they described that the patients who died suddenly after the last administration of PRRT had significantly higher Ki-67 values than their living counterparts [34]. The risk factors of poor response to PRRT apart from higher Ki-67 index and high uptake on 18F-FDG have been found to be higher tumor burden, involvement of more than two organ systems, poor performance status (Karnofsky performance scale score), and identified progressive disease after first PRRT [35, 36]. Furthermore, several results have also shown that when PRRT is delivered as salvage therapy after chemotherapy in particular, there was a tendency for progression free survival to decline and a greater tendency for long-term hematologic consequences [6, 16, 21]. Therefore, following relapse, the tumor could become increasingly aggressive or its sensitivity to radiation could have diminished, which may warrant a repeat tumor biopsy to reassess Ki-67 levels after recurrence of the disease [34]. This potential finding and risk factors of poor response to PRRT warrants the importance of proper patient counseling before beginning treatment, and early and accurate assessment of radiotherapy responses, including post-therapy 68Ga-DOTA-peptide or 18F-FDG PET/CT scans to not only identify good or poor responders, but to also detect patients who are at risk for rapid progression.
Acknowledgements
We express our sincere gratitude to the patients and their family members for their participation in this study.
Funding Information
This study was funded by the National Institutes of Health (grant number Z1AHD008735) awarded to Karel Pacak. This work was supported, in part, by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development and was supported, in part, by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute.
Compliance with Ethical Standards
Conflict of Interest
Katherine I. Wolf, Abhishek Jha, Anouk van Berkel, Damian Wild, Ingo Janssen, Corina M. Millo, M. J. R. Janssen, Melissa Gonzales, Henri J.K.M. Timmers, and Karel Pacak have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from the individuals who participated in this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Katherine I. Wolf and Abhishek Jha contributed equally to this work.
References
- 1.Pacak K, Del Rivero J, et al. In: Pheochromocytoma. De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, et al., editors. South Dartmouth: Endotext; 2000. [Google Scholar]
- 2.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423–436. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- 4.Turkova H, Prodanov T, Maly M, Martucci V, Adams K, Widimsky J, Jr, et al. Characteristics and outcomes of metastatic Sdhb and sporadic pheochromocytoma/paraganglioma: an National Institutes of Health study. Endocr Pract. 2015;22:302–314. doi: 10.4158/EP15725.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishbein L. Pheochromocytoma and paraganglioma: genetics, diagnosis, and treatment. Hematol Oncol Clin North Am. 2016;30:135–150. doi: 10.1016/j.hoc.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Delpassand ES, Samarghandi A, Zamanian S, Wolin EM, Hamiditabar M, Espenan GD, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014;43:518–525. doi: 10.1097/MPA.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 7.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of (177)Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marinova M, Mucke M, Mahlberg L, Essler M, Cuhls H, Radbruch L, et al. Improving quality of life in patients with pancreatic neuroendocrine tumor following peptide receptor radionuclide therapy assessed by EORTC QLQ-C30. Eur J Nucl Med Mol Imaging. 2018;45:38–46. doi: 10.1007/s00259-017-3816-z. [DOI] [PubMed] [Google Scholar]
- 9.Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with (177)Lu-Dotatate in the phase III NETTER-1 trial. J Clin Oncol. 2018;36:2578–2584. doi: 10.1200/JCO.2018.78.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinato DJ, Black JR, Ramaswami R, Tan TM, Adjogatse D, Sharma R. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med Oncol. 2016;33:47. doi: 10.1007/s12032-016-0737-9. [DOI] [PubMed] [Google Scholar]
- 11.Puranik AD, Kulkarni HR, Singh A, Baum RP. Peptide receptor radionuclide therapy with (90)Y/ (177)Lu-labelled peptides for inoperable head and neck paragangliomas (glomus tumours) Eur J Nucl Med Mol Imaging. 2015;42:1223–1230. doi: 10.1007/s00259-015-3029-2. [DOI] [PubMed] [Google Scholar]
- 12.Zovato S, Kumanova A, Dematte S, Sansovini M, Bodei L, Di Sarra D, et al. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL) Horm Metab Res. 2012;44:411–414. doi: 10.1055/s-0032-1311637. [DOI] [PubMed] [Google Scholar]
- 13.Strosberg J, Krenning E. 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:1391–1392. doi: 10.1056/NEJMoa1607427. [DOI] [PubMed] [Google Scholar]
- 14.Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O, et al. Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma. J Clin Endocrinol Metab. 2017;102:3278–3287. doi: 10.1210/jc.2017-00816. [DOI] [PubMed] [Google Scholar]
- 15.Nastos K, Cheung VTF, Toumpanakis C, Navalkissoor S, Quigley AM, Caplin M, et al. Peptide receptor radionuclide treatment and (131)I-MIBG in the management of patients with metastatic/progressive phaeochromocytomas and paragangliomas. J Surg Oncol. 2017;115:425–434. doi: 10.1002/jso.24553. [DOI] [PubMed] [Google Scholar]
- 16.Bodei L, Kidd M, Prasad V, Modlin IM. Peptide receptor radionuclide therapy of neuroendocrine tumors. Front Horm Res. 2015;44:198–215. doi: 10.1159/000402936. [DOI] [PubMed] [Google Scholar]
- 17.Sabet A, Ezziddin K, Pape UF, Ahmadzadehfar H, Mayer K, Poppel T, et al. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2013;54:1857–1861. doi: 10.2967/jnumed.112.119347. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald PA, Goldsby RE, Huberty JP, Price DC, Hawkins RA, Veatch JJ, et al. Malignant pheochromocytomas and paragangliomas: a phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG) Ann N Y Acad Sci. 2006;1073:465–490. doi: 10.1196/annals.1353.050. [DOI] [PubMed] [Google Scholar]
- 19.Gedik GK, Hoefnagel CA, Bais E, Olmos RA. 131I-MIBG therapy in metastatic phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2008;35:725–733. doi: 10.1007/s00259-007-0652-6. [DOI] [PubMed] [Google Scholar]
- 20.Lam MG, Lips CJ, Jager PL, Dullaart RP, Lentjes EG, van Rijk PP, et al. Repeated [131I]metaiodobenzylguanidine therapy in two patients with malignant pheochromocytoma. J Clin Endocrinol Metab. 2005;90:5888–5895. doi: 10.1210/jc.2004-2290. [DOI] [PubMed] [Google Scholar]
- 21.Kesavan M, Turner JH. Myelotoxicity of peptide receptor radionuclide therapy of neuroendocrine tumors: a decade of experience. Cancer Biother Radiopharm. 2016;31:189–198. doi: 10.1089/cbr.2016.2035. [DOI] [PubMed] [Google Scholar]
- 22.Dhall D, Mertens R, Bresee C, Parakh R, Wang HL, Li M, et al. Ki-67 proliferative index predicts progression-free survival of patients with well-differentiated ileal neuroendocrine tumors. Hum Pathol. 2012;43:489–495. doi: 10.1016/j.humpath.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 23.McCall CM, Shi C, Cornish TC, Klimstra DS, Tang LH, Basturk O, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37:1671–1677. doi: 10.1097/PAS.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boninsegna L, Panzuto F, Partelli S, Capelli P, Delle Fave G, Bettini R, et al. Malignant pancreatic neuroendocrine tumour: lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur J Cancer. 2012;48:1608–1615. doi: 10.1016/j.ejca.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 26.Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009;50:858–864. doi: 10.2967/jnumed.108.057505. [DOI] [PubMed] [Google Scholar]
- 27.Chang CA, Pattison DA, Tothill RW, Kong G, Akhurst TJ, Hicks RJ, et al. (68)Ga-DOTATATE and (18)F-FDG PET/CT in paraganglioma and pheochromocytoma: utility, patterns and heterogeneity. Cancer Imaging. 2016;16:22. doi: 10.1186/s40644-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. F-18-Fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–985. doi: 10.1158/1078-0432.CCR-09-1759. [DOI] [PubMed] [Google Scholar]
- 29.Bahri H, Laurence L, Edeine J, Leghzali H, Devillers A, Raoul JL, et al. High prognostic value of F-18-FDG PET for metastatic Gastroenteropancreatic neuro endocrine tumors: a long-term evaluation. J Nucl Med. 2014;55:1786–1790. doi: 10.2967/jnumed.114.144386. [DOI] [PubMed] [Google Scholar]
- 30.Ezziddin S, Adler L, Sabet A, Poppel TD, Grabellus F, Yuce A, et al. Prognostic stratification of metastatic gastroenteropancreatic neuroendocrine neoplasms by F-18-FDG PET: feasibility of a metabolic grading system. J Nucl Med. 2014;55:1260–1266. doi: 10.2967/jnumed.114.137166. [DOI] [PubMed] [Google Scholar]
- 31.Chan DLH, Pavlakis N, Schembri GP, Bernard EJ, Hsiao E, Hayes A, et al. Dual somatostatin receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: proposal for a novel grading scheme with prognostic significance. Theranostics. 2017;7:1149–1158. doi: 10.7150/thno.18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thapa P, Ranade R, Ostwal V, Shrikhande SV, Goel M, Basu S. Performance of Lu-177-DOTATATE-based peptide receptor radionuclide therapy in metastatic gastroenteropancreatic neuroendocrine tumor: a multiparametric response evaluation correlating with primary tumor site, tumor proliferation index, and dual tracer imaging characteristics. Nucl Med Commun. 2016;37:1030–1037. doi: 10.1097/MNM.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 33.Basu S, Ranade R, Thapa P. Metastatic neuroendocrine tumor with extensive bone marrow involvement at diagnosis: evaluation of response and hematological toxicity profile of PRRT with (177)Lu-DOTATATE. World J Nucl Med. 2016;15:38–43. doi: 10.4103/1450-1147.165353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yordanova A, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP, Essler M, et al. Safety of multiple repeated cycles of 177Lu-octreotate in patients with recurrent neuroendocrine tumour. Eur J Nucl Med Mol Imaging. 2017. [DOI] [PubMed]
- 35.Ezzeddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grünwald F, et al. Predictors of long-term outcome in patients with well-differentiated gastrenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2014;55:183–190. doi: 10.2967/jnumed.113.125336. [DOI] [PubMed] [Google Scholar]
- 36.Gabriel M, Nilica B, Kaiser B, Virgolini IJ. Twelve-year follow-up after peptide receptor radionuclide therapy (PRRT). J Nucl Med. 2018. [DOI] [PubMed]