Abstract
Implantable neural stimulators represent an advanced treatment adjunct to medication for pharmacoresistant epilepsy and alternative for patients that are not good candidates for resective surgery. Three treatment modalities are currently FDA-approved: vagus nerve stimulation, responsive neurostimulation, and deep brain stimulation. These devices were originally trialed in very similar patient populations with focal epilepsy, but head-to-head comparison trials have not been performed. As such, device selection may be challenging due to large overlaps in clinical indications and efficacy. Here we will review the data reported in the original pivotal clinical trials as well as long-term experience with these technologies. We will highlight differences in their features and mechanisms of action which may help optimize device selection on a case-by-case basis.
Electronic supplementary material
The online version of this article (10.1007/s13311-019-00727-2) contains supplementary material, which is available to authorized users.
Key Words: Neurostimulation, vagus nerve stimulation, VNS, responsive neurostimulation, RNS, deep brain stimulation, DBS, epilepsy, seizures
Introduction
Approximately a third of epileptic individuals will continue to experience seizures despite successive trials of anti-convulsant medications [1]. Among these individuals, only a minority will be candidates for surgical resection. For the remainder of individuals, electrical neurostimulation and neuromodulation is a palliative option that has seen increasing utilization over the past two decades.
The modern era of human brain stimulation in epilepsy began with Sir Victor Horsley in 1886 when he utilized electrical cortical stimulation for mapping to aid in brain resection in a patient with focal epilepsy [2]. Cortical stimulation to define brain function was significantly expanded by the work of Penfield and Jasper in their landmark monograph published in 1954 [3]. These studies ushered in a golden age of cortical mapping and animal neuroscience studies.
Electrical stimulation was first used therapeutically for epilepsy in the 1970s, when the cerebellum became the first therapeutic target for electrical stimulation in human patient epilepsy, with mixed results [4–6]. These initial efforts paved the way towards deep brain stimulation (DBS) in a number of subcortical targets for various neurological disorders. In addition to the cerebellum, the main targets for DBS in epilepsy have been the thalamus (discussed in greater detail below), hippocampus [7–12], and subthalamic nucleus [13–16].
Peripheral nerve stimulation has also proven fruitful for stimulation in epilepsy. Vagus nerve stimulation (VNS) in humans was initially published in 1990 [17], long after earlier stimulation studies performed by Bailey in 1938 in cats reported cortical EEG changes [18]. Observations of cessation of grand mal seizures by applying pressure on the intraorbital trigeminal nerve were first reported in 1976 [19]. Based on common afferent targets of the trigeminal nerve and vagus nerve, the external trigeminal neurostimulator (eTNS) was developed as a non-invasive alternative to VNS [20, 21]. This technology received the European CE Mark in 2012; the device is still investigational in the USA.
The treatment of epilepsy through neocortical stimulation began when Lesser reported that pulse stimulation could terminate afterdischarges induced during cortical stimulation in 1999 [22]. This idea was explored further with closed-loop abortion of seizures in small cohorts of patients undergoing intracranial epilepsy monitoring with subdural grids [23, 24] and eventual multi-site clinical trials (discussed below). Recently, chronic subthreshold cortical stimulation (CSCS) to prevent neuronal recruitment into the epileptic focus prior to macroscopic seizure detection has been applied with promising results in small series [25, 26].
Steady effort and investigation after this early work in peripheral nerve, neocortical, and subcortical stimulation have culminated in FDA approval for VNS (LivaNova, London, UK) in 1997, responsive neurostimulation (RNS, NeuroPace, Mountainview, CA) in 2013, and DBS of the anterior nucleus of the thalamus (ANT-DBS, Medtronic, Minneapolis, MN) in 2018. These three technologies represent the state-of-the-art neurostimulation options that are currently available in the USA for patients with pharmacoresistant epilepsy who are otherwise poor surgical candidates. Physicians have continued to gain experience with the technology and the devices have continued to evolve after their initial efficacy was established in pivotal clinical trials. In this review, we will examine the three FDA-approved devices and review their features, mechanisms of action, and efficacy data. With these considerations, we will offer recommendations on device selection for particular patient populations.
Device Overviews
VNS
Overview
In this technology, stimulating electrodes are coiled around the left vagus nerve and tunneled subcutaneously to connect to an implantable pulse generator (IPG) placed in the left subclavicular region. This open-loop device delivers scheduled electrical stimulation to the vagus nerve, typically every several minutes for 30–60 s. The output current is typically titrated up to at least 1.50 mA over at least 10–12 weeks after implant based on tolerability. The patient is also provided a wrist magnet to swipe the pulse generator and deliver an extra “dose” of stimulation. In 2015, the AspireSR® model was introduced which incorporated tachycardia-based seizure detection to deliver automated stimulation. In 2017, the newest Sentiva™ model was introduced, which includes the ability for differing stimulation during day vs night and tracking prone position and bradycardia (features that are risk factors for SUDEP [27]). As of 2018, more than 100,000 patients have had the VNS implanted, and it is utilized in several countries worldwide [28].
Mechanism of Action
The mechanism of action (MOA) by which VNS exerts its anti-seizure effect was previously considered unclear. But work throughout the years has shed significant light on the underlying mechanisms. Vagal afferents, which comprise 80% of the nerve, synapse mainly onto the nucleus of the solitary tract (NTS). The solitary nucleus’ subsequent projections to the locus coeruleus (LC) and dorsal raphe are thought to mediate therapeutic effects of VNS in epilepsy and depression.
VNS’ main anti-seizure effect is thought due to its influence on the LC and subsequent increase in CNS noradrenergic tone. LC has widespread projections to forebrain, limbic system, and spinal cord. In addition to a general arousing effect, there is a wide body of literature suggesting that NE in the CNS is anti-epileptic [29–33]. Evidence includes attenuation of the VNS anti-seizure effect in animal models of seizure after both chronic lesioning and acute inactivation of LC [34].
Vagal efferents, which comprise 20% of the bulk of the nerve, are thought to mediate the majority of the stimulation-related side effects of VNS for epilepsy. Laryngeal motor dysfunction and paresthesia are common and generally well-tolerated and are due to stimulation of CN X fibers originating from nucleus ambiguus (NA) innervating skeletal laryngeal musculature. Bradycardia or heart block can be caused by stimulation of CN X fibers originating from the NA that innervate parasympathetic cholinergic neurons (the atrioventricular and sinoatrial nodes are predominantly innervated by the left and right CN X, respectively); the incidence of this after VNS implantation is low, however, and only at the level of case reports [35–37]. GI side effects and anorexia arise from stimulation of CN X efferents that originate from the dorsal nucleus of the vagus nerve and synapse onto autonomic ganglia in the viscera.
Efficacy and Tolerability
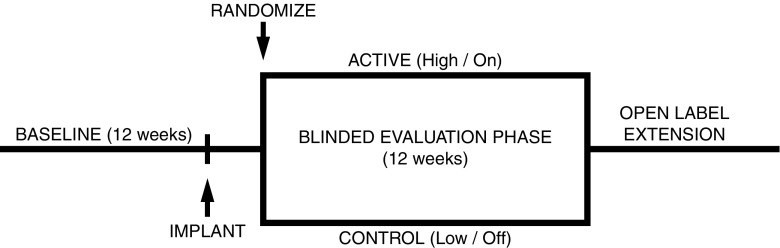
VNS efficacy was most clearly established with two randomized, double-blind, controlled clinical trials known as the E03 (multinational sites) and E05 (US sites only) trials [38–42]. Patients 13 years and older with medically refractory focal epilepsy participated. Study designs (Fig. 1) between these two trials were identical and featured a 12- to 16-week pre-implant evaluation period to establish baseline seizure frequencies, followed by implantation and randomization 2 weeks postoperatively to receive “high” (active, titrated to tolerance) and “low” (control, titrated to the threshold of patient perception) stimulation. Two weeks after this, seizure reduction was measured for a 12-week blinded evaluation phase (BEP).
Fig. 1.
VNS, RNS, and DBS common pivotal trial design. The pivotal trials for VNS, RNS, and DBS featured the common trial design below. The time from implant to randomization (the postoperative recovery period) was typically 4 weeks, but the RNS trial featured an additional 4 weeks for parameter optimization prior to the blinded evaluation phase. RNS and DBS active vs control arms were stimulation on vs off, vs VNS trials’ active and control arms were stimulation high vs low.
The primary endpoint of mean seizure reduction vs controls throughout the BEP was met in both trials and was 18.4% (24.5% active vs 6.1% control) for E03, and 12.7% (27.9% active vs 15.2% control) for E05. A secondary endpoint 50% responder rate was statistically significant in E03 at (31% active vs 13% control), but not E05 (23.4% active vs 15.7% control). Since these studies, VNS use has markedly expanded for patients’ age, with children as young as 3 years old receiving effective and safe therapy from it.
Long-term uncontrolled studies of patients with focal, multifocal, and generalized epilepsy with a variety of seizure types (e.g., tonic-clonic, focal seizures with impaired awareness, absence, and drop attacks) and epilepsy syndromes have demonstrated safety and efficacy of VNS. VNS long-term data were reviewed in an evidence-based guideline in 2013 [43] which highlighted two class III trials. One trial featured median seizure reductions and responder rates of 40% and 43%, respectively, at 3 years [44], and the other 63.8% and 64.4% at 5 years [45]. The largest long-term cohort of VNS had median seizure reduction of 56% with mean follow-up of 4.9 years for the 436 pediatric and adult patients. The median weekly seizure frequency reduced from 4 to 1.5 [46]. A recent large cohort of children and adults in Japan implanted with VNS featured median seizure reduction and 50% responder rates of 66.2% and 58.8% at 3 years [47]. Significant improvement in quality of life (QoL) was also seen in patients undergoing VNS implantation vs best medical therapy alone in a more recent multicenter randomized clinical trial in adults with pharmacoresistant focal epilepsy [48]. One large database study demonstrated that SUDEP risk decreased significantly (i.e., from 2.47 to 1.68 per 1000 patient-years) in the 10 years after VNS initiation [49]. It should be noted that the study was funded by the manufacturer and included co-authors that were employees or consultants for the manufacturer.
One potential benefit of VNS is its improvement of treatment-resistant depression (TRD) independent of seizure benefit. Active or recent depression prevalence is estimated in 13–37% of patients with epilepsy, and the number is higher in uncontrolled epilepsy [50]. The FDA approved VNS in 2007 for TRD, although Medicare and other insurers have not commonly reimbursed VNS for TRD due to their arguments that it is unproven [51]. As of 2018, six clinical trials for VNS in TRD exist and all studies show anti-depressant efficacy with response rates ranging from 30 to 53% [51], while established treatments NOT using VNS demonstrate 1-year responses rates of approximately 10% [52]. From an FDA-mandated registry, Aaronson showed at 5-year follow-up that VNS plus treatment as usual compared to another group with treatment as usual had higher cumulative response rates (68% vs 41%) and remission rates (43% vs 26%) in a cohort of 795 patients with TRD [53].
VNS side effects most commonly occur during electrical pulse delivery. Hoarseness, coughing, and laryngeal paresthesia are commonly reported (20–60%) and tend to improve with parameter reduction and over the long-term [28]. Less commonly, dyspnea or laryngismus (3–15%) occur with stimulation and improve with adjustment and time [41, 42]. Surgical site infection is relatively low and is greater in children than in adults (4% vs 1%) [43]. We expect that rate can be further reduced by fastidious bandage coverage of implantation site in children to avoid picking. Another probable side effect of VNS, though with limited investigation, is worsening of sleep-disordered breathing. A few small series have reported newly diagnosed or worse obstructive sleep apnea (OSA) in patients after VNS therapy initiation. In two studies of 18 and 23 patients, 22–58% had newly diagnosed OSA and 50% had worsening of preexisting OSA [54, 55]. It can be improved with device adjustment and/or continuous positive airway pressure therapy.
RNS
Overview
The RNS is a “closed-loop” device with sensing capabilities to deliver temporally targeted therapy upon detection of an epileptic seizure. It can currently detect and stimulate seizures originating from up to two seizure foci. The IPG is implanted with a craniotomy to allow it to sit continuous with the skull under the scalp. Lead implantation often requires prior intracranial EEG to localize seizure foci for optimal seizure detection and stimulation. Initially rated at 3–4 years’ battery life, the most recent IPG is advertised to remain operational to 8.4 years under “medium” stimulation settings. Patients are given a magnet to swipe the device to record electrocorticograms (ECoG) for physician review; the magnet can also act as a failsafe to temporarily stop stimulation, should the patient think they are experiencing adverse events due to stimulation. They are also given a laptop and telemetry wand to send recorded ECoG clips (including automated detections, scheduled, and magnet-triggered) and stimulation data to a secure Internet database that in turn can be accessed by physicians and company clinical engineers to optimize device parameters. Updated parameters are uploaded to the patient’s device during clinic visits. A notable feature of this technology is that it enables a form of chronic ambulatory EEG monitoring. Patients generally do not detect stimulations at therapeutic settings.
Mechanism of Action
The main MOA by which RNS exerts its anti-seizure effect is broadly via acute electrical disruption of synchronous activity at its origin that is necessary for continuation/propagation of ictal activity. Early in vitro work on hippocampal slices exhibiting epileptiform activity due to penicillin, elevated extracellular potassium, or lower extracellular calcium demonstrated the anti-epileptic effect of pulsed anodal low-amplitude stimulation [56–58]. The mechanism was determined to be due to suppression of intracellular activity due to membrane hyperpolarization caused by a portion of the extracellular currents that cross into the cell, rather than desynchronization or synaptic effects. Another potential mechanism is axonal conduction blockade via high-frequency stimulation [59, 60]. In addition to acute abortive effects, long-term effects are posited to be due to gene expression changes and subsequent synaptic plasticity, cortical reorganization, or neurogenesis induced by chronic stimulation [61–63].
Efficacy and Tolerability
RNS efficacy was established in a placebo-controlled randomized clinical trial carried out in US sites with results published in 2011 [64]. Study design is summarized in Fig. 1. After a 12-week baseline period, patients were implanted with the device. Four weeks after implantation, patients were randomized to active vs control (stimulation OFF) arms, with an additional 4 weeks of stimulation optimization prior to a 12-week BEP. After the BEP, all subjects’ stimulators were turned on for open-label evaluation.
Mean seizure reduction vs controls throughout the BEP was 20.6% (37.9% active vs 17.3% control). Because of a transient implantation effect, however, mean seizure reduction compared to controls improved over the course of the BEP, and the mean seizure reduction vs controls on a month-by-month basis was more robust at 32.1% during the final month of therapy. Median reduction vs controls throughout the BEP, reported in [65], was 8.8% (28% active vs 19.2% control). Fifty percent responder rates during the BEP was 29% and 27% in the treatment and control arms, a meager difference of 2% and not statistically significant.
Long-term effects of RNS were studied in an open-label long-term treatment trial, with results intermittently released for publication [66, 67]. Though largely mitigated by statistical adjustment, the usual caveats of potential survivorship bias due to possibly greater compliance or treatment benefit among those remaining in the study, as well as confounds from concomitant anti-epileptic drug (AED) therapy, may apply. The 1-, 2-, and 5-year median percent seizure reduction was 44%, 53%, and 48–66%, respectively. The manufacturer website advertises 73% median seizure reduction at year 8. Fifty percent responder rates at 1, 2, and 5 years were 44%, 55%, and 50–61%, respectively [66, 67]. QoL improved at 1 year postimplant and improvements were maintained through 5 years postimplant [67].
RNS appeared to be well tolerated, with adverse events only linked to the implantation procedure (hemorrhage in 4.7%, soft-tissue infection in 5.2% during the pivotal trial), without clear side effects attributable to stimulation. Most of the intracranial hemorrhages occurred in the days after implant, but three subjects in the pivotal trial had cerebral hemorrhages associated with strip or depth electrodes 2–3 years after the implant [67]. No differences in cognition or mood were observed during the pivotal trial or at 1- to 2-year follow-up [64].
DBS
Overview
DBS for epilepsy entails implantation of depth electrodes into the ANT to deliver scheduled stimulation. Depth electrodes emerge from the vertex and are tunneled subcutaneously to a pulse generator that is generally implanted in the subclavicular region. The advertised battery life for Medtronic’s current lineup of Activa™ PC or SC IPGs ranges from 3 to 5 years, with reports indicating the shorter end of the range [68, 69], while their rechargeable RC model is replaced after 9 years.
Mechanism of Action
DBS is thought to functionally inactivate stimulated structures, though details are complex. Reports suggest high-frequency stimulation of subcortical structures can replace natural neural activity with spikes time-locked to the stimulation frequency [70, 71], possibly via excitation of axons and antidromic collision of natural spikes with stimulus-driven spikes evoked at high frequency [72]; in this manner, propagation of ictal activity might be blocked.
DBS has its main use in Parkinson’s disease and is a well-known, highly efficacious therapy that modulates dopaminergic basal ganglia circuits. A similar concept of neural circuit modulation is the basis for the anti-epileptic effect of DBS. The strategy of ANT-DBS in particular is to disrupt or modulate limbic circuits which may serve as conduit for propagation of focal seizures that impair awareness. As a primary relay nucleus in the circuit of Papez, the ANT contains afferent input from hippocampal subiculum via the fornix/mammillothalamic tract and has reciprocal connections to the frontal and cingulate cortex. Numerous pre-clinical studies have implicated the ANT and this circuit in the propagation of certain seizure activity [73–76].
ANT-DBS has the strongest data supporting its use in epilepsy, but other interesting results that support the concept of neural circuit modulation are from CMT-DBS [77–83]. The CMT has received input from motor and premotor cortex and basal ganglia and sends projections to the striatum. Perhaps unsurprisingly, CMT-DBS has been observed to abort generalized seizures, but not complex partial seizures, and has been proposed as a target for generalized epilepsy or Lennox-Gastaut syndrome [81, 84]. However, the CMT has not been targeted in larger trials for epilepsy after an early pilot study failed to demonstrate benefit [78].
Efficacy and Tolerability
Class I data from a double-blinded, randomized, controlled clinical trial of ANT-DBS was published in 2010 (a.k.a. SANTE trial, for Stimulation of the Anterior Nucleus of the Thalamus in Epilepsy) [85]. Highly similar to the study design of the VNS and RNS trials (Fig. 1), subjects completed a 12-week baseline phase documenting seizure rates prior to implantation. After a 4-week recovery, patients were randomized to receive stimulation or not. Stimulation consisted of 1-min duration 90-μs pulses of 5.0V at 145 Hz, separated by 5 min. Seizure rates were observed for a 12-week BEP, followed by turning stimulation on for all subjects in a 9-month open-label phase.
Of note, one outlier subject in the DBS trial whose seizures dramatically worsened (210 seizures in 3 days compared to their baseline seizure rate of 19 seizures per month) necessitated outlier analysis to satisfy primary endpoints and played a role in delaying FDA approval for years, until long-term data demonstrated clearer benefit. With this outlier removed, median seizure reduction vs controls throughout the BEP was 13.9% (35.0% active vs 21.1% control). Similar to the RNS trial, there appeared to be an implantation effect with an initial seizure reduction in both groups early on that dissipated by the end of the BEP, resulting in median seizure reduction of 25.9% (40.4% active vs 14.5% control) during the final month. Fifty percent responder rates for active and controls were 29.6% and 25.9%, not statistically significant. A post hoc analysis of median seizure frequency reduction, stratified by lobe of onset, indicated statistically significant reduction for temporal lobe seizures during the BEP (43.9% active vs 29% control), but not for frontal or other lobes [85, 86]. In a similar vein, complex partial seizures were significantly reduced during the BEP compared to simple partial and partial to generalized seizures after outlier exclusion [86].
Long-term data [86, 87] indicate median seizure reduction at 1, 2, 5, and 7 years of 41.4, 55.6, 68.4, and 74.9%, with slight worsening using last-observation-carried forward analysis. Fifty percent responder rates at 1, 2, 5, and 7 years were 43.4, 53.7, 67.8, and 74%. Similar to the BEP, seizure reductions appeared best in the temporal lobe. Like the RNS long-term data, caveats include survival bias, and concomitant AED adjustment possibly accounting for a minority of the reported improvement. However, post hoc analyses comparing seizure frequency reduction by AED status (no change vs changes in AEDs) indicated parallel improvement in efficacy between the two groups, suggesting that stimulation efficacy in at least a portion of subjects continued to improve over time and was not driven by AED changes [86, 87]. QoL was improved 1 year after implant and remained significantly improved 5 years after implant [87].
Adverse events associated with ANT-DBS that occurred at any time over 5 years of long-term follow-up included implant site pain/paresthesias in approximately 20.9%, implant site infection in 12.7%, and lead mis-targeting in 8.2%. As for possible brain stimulation–related adverse effects, depression was reported in 32.7% (with one occurrence of suicide 4 years postimplant), and “non-serious” memory impairment in 25.5% [87]. It should be noted that 66% of the subjects reporting depression had a prior history. Additionally, specific neuropsychological measures for total mood disturbance and subjective cognitive function were not significantly different during the BEP between active and control when averaged over the entire study population [85], and actually improved statistically over the long term [87].
Device Comparison
Pivotal Trial Comparison
The trials above establishing efficacy for VNS, RNS, and DBS featured comparable patient populations (Table 1) and study designs (Fig. 1). Notable differences included only three seizures per month were required for RNS trial entry compared to six for the others; as such, the RNS population had the lowest seizure burden during the baseline phase, though this is probably not clinically significant. Another difference was since patients can perceive VNS, but not generally RNS or DBS, the E03 and E05 trials’ control arms featured “low stimulation” parameters to maintain blinding at the cost of possible reduction of therapeutic signal, while the RNS and SANTE trials had stimulation turned entirely off for control patients. AEDs were held constant during the blinded phase of the trials.
Table 1.
| VNS (E03) | VNS (E05) | RNS | DBS | |
|---|---|---|---|---|
| n | 114 | 198 | 191 | 110 |
| Epilepsy type | Focal | Focal | Focal | Focal |
| Years with epilepsy | 21.8 ± 9.1 | 22.8 ± 11.1 | 19.6 ± 11.4 | 22.3 ± 13 |
| Seizures per month: inclusion criteria | ≥ 6 | ≥ 6 | ≥ 3 | ≥ 6 to ≤ 300 |
| Seizures per month: observation period | 45.0 (median 20.4 to 23.0)* | 35.8 ± 61.5 (median 14.3 to 16.2)** | 34.2 ± 61.9 (median 9.7) | 56.1 ± 101 (median 19.5) |
| Prior surgery | 31% | 22.6% | 32% | 24.5% |
| No. of AEDs | 2.0 ± 0.8 | 2.1 ± 0.7 | 2.9 ± 1.1 | 2.3 ± 0.7 |
Efficacy Comparison
Table 2 lists the total seizure reduction during the entire BEP of the device trials as well as the 50% responder rates. Mean seizure reduction throughout the BEP, compared to controls, was 18.4%, 12.7%, and 20.6% in the VNS E03 and E05, and RNS trials, respectively (note that the reduction based on a generalized estimating equations (GEE) model for RNS, compared to raw calculations in the VNS trials). SANTE utilized median statistics, with a 13.9% (35% vs 21.1%) median seizure reduction in active vs controls during the BEP. Both RNS and SANTE trials GEE models to estimate the number of seizures reduced in the treatment vs sham arms during the BEP. The RNS GEE model predicted a reduction of 5 seizures per month in a subject with 30 seizures per month at baseline. The DBS GEE model predicted a reduction of 3.6 seizures per month (21.1 control vs 17.5 active) with stimulation in a patient with a baseline of 26 seizures per month, after outlier exclusion. Aside from the E03 trial, none of the other trials demonstrated any difference in active vs control 50% responder rates during the BEP.
Table 2.
Pivotal clinical trials—blinded evaluation phase results. All numbers are means unless otherwise indicated. Seizure reduction in VNS trials were based on raw averages, whereas RNS and DBS were based on a GEE model. Note that these results are for the entire 3-month blinded evaluation phases, and to a degree under-estimates a cumulative effect of RNS and DBS as there was month over month separation of activation from control due to implantation effect (the final month of the 3-month BEP featured 41.5 active vs 9.4% control mean reduction in the RNS trial, and 40.4% active vs 14.5% control median reduction in the DBS trial). Results appear largely similar between devices
| n | % seizure reduction vs baseline | 50% responder rate | |||
|---|---|---|---|---|---|
| Active | Control | Active | Control | ||
| VNS (E03) | 114 | 24.5% | 6.1% | 31% | 13% |
| VNS (E05) | 198 | 27.9% | 15.2% | 23.4%NS | 15.7%NS |
| RNS | 191 | 37.9% | 17.3% | 29%NS | 27%NS |
| DBS | 110 | 35.0 (median) | 21.1 (median) | 29.6%NS | 25.9%NS |
NSNot statistically significant
The long-term results for each of the devices, mentioned earlier, are plotted on Fig. 2 for a more direct visual comparison. All devices show improvement in efficacy over time, arguing for possible neuromodulatory effects that gradually change the epileptic network in addition to any acute seizure-abortive effects. Chronic stimulation may induce neural plasticity changes via positive (Hebbian) or negative (homeostatic synaptic plasticity) network regulatory mechanisms, operating on slower time scales, allowing portions of the network to effectively “unlearn” the epileptic state over many years. Overall, results were quite similar between DBS and RNS though DBS appears to continue to increase in efficacy many years out after RNS has plateaued. VNS results are variable depending upon the trial but are either comparable or slightly less efficacious than RNS or DBS over time.
Fig. 2.
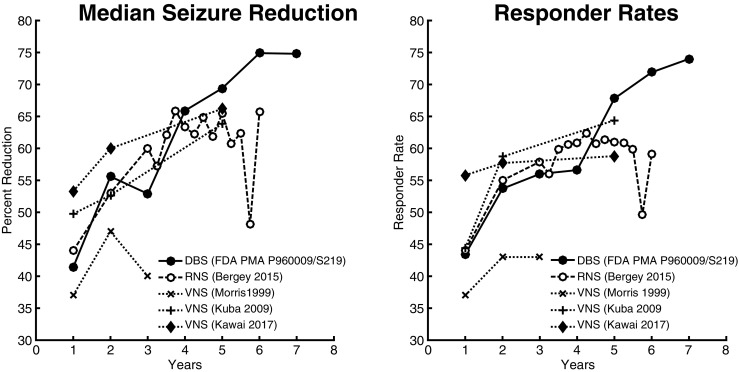
Long-term VNS, RNS, and DBS median seizure reduction and responder rates. Unadjusted long-term seizure reduction and responder rates for VNS, RNS, and DBS [44, 45, 47, 67, 86]. Data from Kuba 2009 were calculated based on the baseline and 1-, 2-, and 5-year number of seizures per month provided in Table 1 of their manuscript, which indicate “mean” numbers, but the body of the manuscript and abstract imply that the Table 1 numbers were medians. DBS appears to have a slight long-term advantage, while VNS long-term results appear equal or slightly worse depending on the cohort. For a fair comparison, note that the numbers used from the original publications to form the graphs below were the unadjusted, declining numbers (and not the last observation carried forward numbers that were reported for some, but not all, studies).
Overall, efficacy appears to be similar between DBS and RNS, with a slight long-term performance edge for DBS. VNS performance trails somewhat, especially after considering implantation effects which may have diluted the BEP numbers for RNS and DBS. Microlesional effects are postulated to disrupt the epileptic network at the site of electrode implantation and has been reported to be responsible for patients experiencing significant improvement in seizure control after intracranial EEG monitoring with either subdural electrodes or depth electrodes [89–91]. This effect would not have been expected to occur with VNS implantation.
There are several developments that may enhance the initially reported efficacy of the anti-epileptic devices. The literature suggests that 82% of patients experience abrupt tachycardia during seizures [92]. Controlled trials providing class I data of magnet stimulation in VNS do not exist, but a comprehensive review of VNS magnet use reported benefit in approximately 45%, for either seizure abortion or reduction with magnet swipe [93]. Limitations to magnet use during seizures include reduced patient awareness and function, unavailability of the magnet, and absence of a caregiver. Our own internal data from patients undergoing epilepsy monitoring suggest that only 13–15% of patients are able to press event buttons to notify nursing of their seizures [94]. If these figures can be extrapolated to VNS magnet use, the VNS autostimulation feature introduced in 2015 (provided with AspireSR® and SenTiva™ models) that deliver stimulation in response to tachycardia may produce added efficacy, in roughly up to 31% of patients compared to older models. Recent retrospective observations of patients experiencing further reduction in seizure burden after updating to an AspireSR® model during VNS battery change appear to support this [95].
Refinement of ANT-DBS surgical targeting and stimulation parameters that have only recently been reported. Increased efficacy may be associated with stimulation of the anterior portion of the ANT containing the anteromedial and the anterior principal subnuclei [96]. Furthermore, exploration of stimulation parameters may lead to increased efficacy with reduced psychiatric side effects [97]. These advances may lead to increased DBS efficacy compared to results reported in SANTE.
Feature Comparison
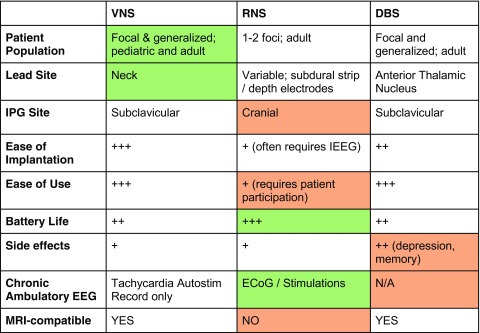
Table 3 lists specific feature comparisons between each VNS, RNS, and DBS. There are no significant feature drawbacks to VNS and it is the least invasive device compared to the others, but the efficacy may be more limited. RNS has several drawbacks, including (1) a frequent requirement for invasive intracranial EEG to localize onset prior to implantation of the device; (2) a cranial IPG implantation site, meaning battery changes are a scalp surgery; (3) inability of patients to get brain MRIs after implantation; and (4) requiring a motivated patient or caregiver (and usually a wired internet connection) for optimal device use. However, RNS has the longest advertised non-rechargeable battery life and is the only device capable of recording chronic ambulatory EEG, which may improve monitoring of seizure burden and possibly lead to palliative epilepsy surgery in bilateral patients whose epilepsy foci are found to produce vastly asymmetric seizure rates. Additionally, there do not appear to be significant neuropsychiatric side effects of therapy. The main advantages to DBS include its ease of use and slightly superior long-term efficacy, but this may be balanced by potential negative mood and memory effects associated with ANT stimulation. All three devices have relatively high theoretical benefit for patients with long-term AED compliance concerns. However, that same compliance concern may make optimal RNS usage difficult with data lost due to lack of uploads from the patient.
Table 3.
VNS, RNS, and DBS features. Relative advantages are labeled in green, whereas relative disadvantages are labeled in red.
Device Selection
As both short- and long-term efficacy appears not too dissimilar between devices, the main considerations for device selection may be MOA and individual device features. Patient preferences and comorbidities may heavily influence the latter considerations. Undergoing intracranial EEG to localize seizure foci prior to RNS implantation entails more morbidity compared to the other two devices. A lower level of motivation or adherence may be a red flag for optimal use of RNS, though the effort involved in data upload and parameter optimization generally becomes less intense over time. ANT-DBS should be used with care in patients at high risk for cognitive changes (e.g., the elderly) or in those with baseline mood disturbance. The latter group may receive benefit from mood-enhancing effects of VNS. VNS should be used in caution in those with, or suspected of having, sleep apnea.
In the absence of direct evidence, it may be a reasonable approach to match the device MOA and focality of treatment to the patient’s epilepsy type and focality of disease. Each device can be differentiated based on the focality of their mechanism of action. The most diffuse is probably VNS, via its widespread influence on CNS noradrenergic projections. Indeed, multiple small open-label studies on VNS in Lennox-Gastaut syndrome and generalized epilepsy appear to demonstrate efficacy [98–101]. The most focal therapy is RNS, both spatially and temporally, interrupting the ictal onset zone during seizure initiation only. Temporal specificity, however, may not be entirely accurate as seizure stimulation due to noisy detection algorithms typically can lead to hundreds of stimulations per day (personal experience, [102]). If the majority of these are indeed false positive stimulations, RNS may possibly also be providing a degree of chronic stimulation of the ictal onset zone, similar to the approach that is being investigated with CSCS. Ideal patients include highly localized focal epilepsy patients with ≤ 2 foci, over eloquent cortex. In the middle ground is ANT-DBS, which disrupts limbic circuitry that may become involved in focal seizures with impairment of awareness. In support of this, patients with temporal lobe epilepsy and complex partial seizures exhibited statistically significant efficacy during the BEP of SANTE, possibly reflecting the role of mesial temporal structures in the circuit of Papez. Patients with strong limbic involvement of their seizures may be ideal candidates for ANT-DBS.
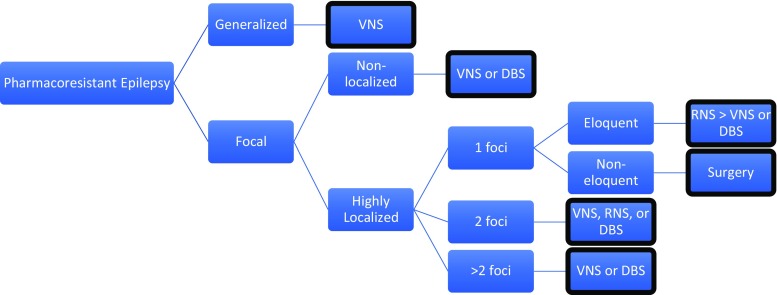
Figure 3 shows a decision flowchart mainly based upon focality of therapy. Note that other promising but investigational treatments such as CMT-DBS for generalized epilepsy, mentioned in the text above, are not considered. Considerations not captured in the flowchart include the specific location of the epileptic foci. For one focus in eloquent cortex, RNS may have an advantage over DBS and VNS, whereas ANT-DBS may be more efficacious in temporal lobe epilepsy and if limbic structures are involved (though the temporal lobe is likely non-eloquent). However, RNS appears to successfully treat bilateral hippocampal epilepsy without significant disruption in memory function [103]. In pure neocortical or frontal lobe epilepsy with up to two foci, RNS may be superior to ANT-DBS. For non-localized focal epilepsy or focal epilepsy greater than 2 foci, RNS would not be advised, and ANT-DBS may have greater efficacy over VNS.
Fig. 3.
Decision algorithm. The following shows a general algorithm for efficacious device selection based on mechanism of action and focality. If only considering efficacy, DBS may have an advantage if seizures are limbic/temporal compared to others at the same node. Other considerations (side effects, device features, and patient preferences) also drive device selection but are not captured in this flowchart.
Conclusions
The addition of new, well-tolerated brain stimulation options for the treatment of pharmacoresistant epilepsy represents a welcome crowding of the anti-epileptic device space. With more choice comes the responsibility to carefully consider each technology on a case-by-case basis for optimal treatment response. Head-to-head trials addressing efficacy and tolerability would be ideal for a more direct comparison between competing technologies. Further randomized controlled clinical trials are necessary to evaluate promising technologies such as eTNS, CMT-DBS, and CSCS, and may further broaden our treatment armamentarium.
Electronic supplementary material
(PDF 515 kb)
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 2.Vilensky JA, Gilman S. Horsley was the first to use electrical stimulation of the human cerebral cortex intraoperatively. Surg Neurol. 2002;58(6):425–6. doi: 10.1016/s0090-3019(02)00920-5. [DOI] [PubMed] [Google Scholar]
- 3.Penfield WJHH, Wilder P. Epilepsy, cerebral l. Epilepsy and the functional anatomy of the human brain. 1. Boston: Little, Brown; 1954. p. 896. [Google Scholar]
- 4.Cooper IS, Amin I, Gilman S. The effect of chronic cerebellar stimulation upon epilepsy in man. Trans Am Neurol Assoc. 1973;98:192–6. [PubMed] [Google Scholar]
- 5.Sramka M, Fritz G, Galanda M, Nadvornik P. Some observations in treatment stimulation of epilepsy. Acta Neurochir 1976;(23 Suppl):257–62. [DOI] [PubMed]
- 6.Van Buren JM, Wood JH, Oakley J, Hambrecht F. Preliminary evaluation of cerebellar stimulation by double-blind stimulation and biological criteria in the treatment of epilepsy. J Neurosurg. 1978;48(3):407–16. doi: 10.3171/jns.1978.48.3.0407. [DOI] [PubMed] [Google Scholar]
- 7.Boex C, Seeck M, Vulliemoz S, Rossetti AO, Staedler C, Spinelli L, et al. Chronic deep brain stimulation in mesial temporal lobe epilepsy. Seizure. 2011;20(6):485–90. doi: 10.1016/j.seizure.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Boon P, Vonck K, De Herdt V, Van Dycke A, Goethals M, Goossens L, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48(8):1551–60. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 9.Cukiert A, Cukiert CM, Burattini JA, Mariani PP, Bezerra DF. Seizure outcome after hippocampal deep brain stimulation in patients with refractory temporal lobe epilepsy: a prospective, controlled, randomized, double-blind study. Epilepsia. 2017;58(10):1728–33. doi: 10.1111/epi.13860. [DOI] [PubMed] [Google Scholar]
- 10.McLachlan RS, Pigott S, Tellez-Zenteno JF, Wiebe S, Parrent A. Bilateral hippocampal stimulation for intractable temporal lobe epilepsy: impact on seizures and memory. Epilepsia. 2010;51(2):304–7. doi: 10.1111/j.1528-1167.2009.02332.x. [DOI] [PubMed] [Google Scholar]
- 11.Velasco AL, Velasco F, Velasco M, Trejo D, Castro G, Carrillo-Ruiz JD. Electrical stimulation of the hippocampal epileptic foci for seizure control: a double-blind, long-term follow-up study. Epilepsia. 2007;48(10):1895–903. doi: 10.1111/j.1528-1167.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- 12.Vonck K, Boon P, Achten E, De Reuck J, Caemaert J. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol. 2002;52(5):556–65. doi: 10.1002/ana.10323. [DOI] [PubMed] [Google Scholar]
- 13.Chabardes S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002;4(Suppl 3):S83–93. [PubMed] [Google Scholar]
- 14.Handforth A, DeSalles AA, Krahl SE. Deep brain stimulation of the subthalamic nucleus as adjunct treatment for refractory epilepsy. Epilepsia. 2006;47(7):1239–41. doi: 10.1111/j.1528-1167.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee KJ, Jang KS, Shon YM. Chronic deep brain stimulation of subthalamic and anterior thalamic nuclei for controlling refractory partial epilepsy. Acta Neurochir Suppl. 2006;99:87–91. doi: 10.1007/978-3-211-35205-2_17. [DOI] [PubMed] [Google Scholar]
- 16.Vesper J, Haak S, Ostertag C, Nikkhah G. Subthalamic nucleus deep brain stimulation in elderly patients--analysis of outcome and complications. BMC Neurol. 2007;7:7. doi: 10.1186/1471-2377-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31(Suppl 2):S40–3. doi: 10.1111/j.1528-1157.1990.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 18.Bailey P, Bremer F. A sensory cortical representation of the vagus nerve (with a note on the effects of low blood pressure on the cortical electrograms) J Neurophysiol. 1938;1:405–12. [Google Scholar]
- 19.Maksimow K. [Interruption of grand mal epileptic seizures by the trigeminal nerve stimulation] Neurol Neurochir Pol. 1976;10(2):205–8. [PubMed] [Google Scholar]
- 20.DeGiorgio CM, Murray D, Markovic D, Whitehurst T. Trigeminal nerve stimulation for epilepsy: long-term feasibility and efficacy. Neurology. 2009;72(10):936–8. doi: 10.1212/01.wnl.0000344181.97126.b4. [DOI] [PubMed] [Google Scholar]
- 21.DeGiorgio CM, Soss J, Cook IA, Markovic D, Gornbein J, Murray D, et al. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology. 2013;80(9):786–91. doi: 10.1212/WNL.0b013e318285c11a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesser RP, Kim SH, Beyderman L, Miglioretti DL, Webber WR, Bare M, et al. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology. 1999;53(9):2073–81. doi: 10.1212/wnl.53.9.2073. [DOI] [PubMed] [Google Scholar]
- 23.Kossoff EH, Ritzl EK, Politsky JM, Murro AM, Smith JR, Duckrow RB, et al. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45(12):1560–7. doi: 10.1111/j.0013-9580.2004.26104.x. [DOI] [PubMed] [Google Scholar]
- 24.Osorio I, Frei MG, Sunderam S, Giftakis J, Bhavaraju NC, Schaffner SF, et al. Automated seizure abatement in humans using electrical stimulation. Ann Neurol. 2005;57(2):258–68. doi: 10.1002/ana.20377. [DOI] [PubMed] [Google Scholar]
- 25.Child ND, Stead M, Wirrell EC, Nickels KC, Wetjen NM, Lee KH, et al. Chronic subthreshold subdural cortical stimulation for the treatment of focal epilepsy originating from eloquent cortex. Epilepsia. 2014;55(3):e18–21. doi: 10.1111/epi.12525. [DOI] [PubMed] [Google Scholar]
- 26.Kerezoudis P, Grewal SS, Stead M, Lundstrom BN, Britton JW, Shin C, et al. Chronic subthreshold cortical stimulation for adult drug-resistant focal epilepsy: safety, feasibility, and technique. J Neurosurg. 2018;129(2):533–43. doi: 10.3171/2017.5.JNS163134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liebenthal JA, Wu S, Rose S, Ebersole JS, Tao JX. Association of prone position with sudden unexpected death in epilepsy. Neurology. 2015;84(7):703–9. doi: 10.1212/WNL.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 28.Wheless JW, Gienapp AJ, Ryvlin P. Vagus nerve stimulation (VNS) therapy update. Epilepsy Behav 2018. [DOI] [PubMed]
- 29.Gellman RL, Kallianos JA, McNamara JO. Alpha-2 receptors mediate an endogenous noradrenergic suppression of kindling development. J Pharmacol Exp Ther. 1987;241(3):891–8. [PubMed] [Google Scholar]
- 30.Barry DI, Wanscher B, Kragh J, Bolwig TG, Kokaia M, Brundin P, et al. Grafts of fetal locus coeruleus neurons in rat amygdala-piriform cortex suppress seizure development in hippocampal kindling. Exp Neurol. 1989;106(2):125–32. doi: 10.1016/0014-4886(89)90085-x. [DOI] [PubMed] [Google Scholar]
- 31.Browning RA, Lanker ML, Faingold CL. Injections of noradrenergic and GABAergic agonists into the inferior colliculus: effects on audiogenic seizures in genetically epilepsy-prone rats. Epilepsy Res. 1989;4(2):119–25. doi: 10.1016/0920-1211(89)90016-8. [DOI] [PubMed] [Google Scholar]
- 32.Ferraro G, Sardo P, Sabatino M, Caravaglios G, La Grutta V. Anticonvulsant activity of the noradrenergic locus coeruleus system: role of beta mediation. Neurosci Lett. 1994;169(1–2):93–6. doi: 10.1016/0304-3940(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 33.Shouse MN, Langer J, Bier M, Farber PR, Alcalde O, Moghimi R, et al. The alpha 2 adrenoreceptor agonist clonidine suppresses seizures, whereas the alpha 2 adrenoreceptor antagonist idazoxan promotes seizures: pontine microinfusion studies of amygdala-kindled kittens. Brain Res. 1996;731(1–2):203–7. doi: 10.1016/0006-8993(96)00594-x. [DOI] [PubMed] [Google Scholar]
- 34.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709–14. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 35.Tatum WO, Moore DB, Stecker MM, Baltuch GH, French JA, Ferreira JA, et al. Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurology. 1999;52(6):1267–9. doi: 10.1212/wnl.52.6.1267. [DOI] [PubMed] [Google Scholar]
- 36.Clark AJ, Kuperman RA, Auguste KI, Sun PP. Intractable episodic bradycardia resulting from progressive lead traction in an epileptic child with a vagus nerve stimulator: a delayed complication. J Neurosurg Pediatr. 2012;9(4):389–93. doi: 10.3171/2011.12.PEDS11124. [DOI] [PubMed] [Google Scholar]
- 37.Kato H, Fujimoto A, Okanishi T, Sugiura R, Ijima K, Enoki H. New onset syncopal events following vagus nerve stimulator implantation might be key to preventing vagus nerve stimulation-induced symptomatic bradycardia - a case report and review. Epilepsy Behav Case Rep. 2018;10:57–60. doi: 10.1016/j.ebcr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):616–26. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 39.Ramsay RE, Uthman BM, Augustinsson LE, Upton AR, Naritoku D, Willis J, et al. Vagus nerve stimulation for treatment of partial seizures: 2. Safety, side effects, and tolerability. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):627–36. doi: 10.1111/j.1528-1157.1994.tb02483.x. [DOI] [PubMed] [Google Scholar]
- 40.George R, Salinsky M, Kuzniecky R, Rosenfeld W, Bergen D, Tarver WB, et al. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on first 67 patients exiting a controlled study. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(3):637–43. doi: 10.1111/j.1528-1157.1994.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 41.A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology 1995;45(2):224–30. [DOI] [PubMed]
- 42.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51(1):48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 43.Morris GL, 3rd, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(16):1453–9. doi: 10.1212/WNL.0b013e3182a393d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris GL, 3rd, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology. 1999;53(8):1731–5. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]
- 45.Kuba R, Brazdil M, Kalina M, Prochazka T, Hovorka J, Nezadal T, et al. Vagus nerve stimulation: longitudinal follow-up of patients treated for 5 years. Seizure. 2009;18(4):269–74. doi: 10.1016/j.seizure.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Elliott RE, Morsi A, Kalhorn SP, Marcus J, Sellin J, Kang M, et al. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: long-term outcomes and predictors of response. Epilepsy Behav. 2011;20(1):57–63. doi: 10.1016/j.yebeh.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Kawai K, Tanaka T, Baba H, Bunker M, Ikeda A, Inoue Y, et al. Outcome of vagus nerve stimulation for drug-resistant epilepsy: the first three years of a prospective Japanese registry. Epileptic Disord. 2017;19(3):327–38. doi: 10.1684/epd.2017.0929. [DOI] [PubMed] [Google Scholar]
- 48.Ryvlin P, Gilliam FG, Nguyen DK, Colicchio G, Iudice A, Tinuper P, et al. The long-term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: the PuLsE (Open Prospective Randomized Long-term Effectiveness) trial. Epilepsia. 2014;55(6):893–900. doi: 10.1111/epi.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryvlin P, So EL, Gordon CM, Hesdorffer DC, Sperling MR, Devinsky O, et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia. 2018;59(3):562–72. doi: 10.1111/epi.14002. [DOI] [PubMed] [Google Scholar]
- 50.Fiest KM, Dykeman J, Patten SB, Wiebe S, Kaplan GG, Maxwell CJ, et al. Depression in epilepsy: a systematic review and meta-analysis. Neurology. 2013;80(6):590–9. doi: 10.1212/WNL.0b013e31827b1ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conway CR, Kumar A, Xiong W, Bunker M, Aaronson ST, Rush AJ. Chronic vagus nerve stimulation significantly improves quality of life in treatment-resistant major depression. J Clin Psychiatry 2018;79(5). [DOI] [PubMed]
- 52.Dunner DL, Rush AJ, Russell JM, Burke M, Woodard S, Wingard P, et al. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry. 2006;67(5):688–95. doi: 10.4088/jcp.v67n0501. [DOI] [PubMed] [Google Scholar]
- 53.Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, et al. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am J Psychiatry. 2017;174(7):640–8. doi: 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
- 54.Zambrelli E, Saibene AM, Furia F, Chiesa V, Vignoli A, Pipolo C, et al. Laryngeal motility alteration: a missing link between sleep apnea and vagus nerve stimulation for epilepsy. Epilepsia. 2016;57(1):e24–7. doi: 10.1111/epi.13252. [DOI] [PubMed] [Google Scholar]
- 55.Salvade A, Ryvlin P, Rossetti AO. Impact of vagus nerve stimulation on sleep-related breathing disorders in adults with epilepsy. Epilepsy Behav. 2018;79:126–9. doi: 10.1016/j.yebeh.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 56.Nakagawa M, Durand D. Suppression of spontaneous epileptiform activity with applied currents. Brain Res. 1991;567(2):241–7. doi: 10.1016/0006-8993(91)90801-2. [DOI] [PubMed] [Google Scholar]
- 57.Kayyali H, Durand D. Effects of applied currents on epileptiform bursts in vitro. Exp Neurol. 1991;113(2):249–54. doi: 10.1016/0014-4886(91)90181-b. [DOI] [PubMed] [Google Scholar]
- 58.Warren RJ, Durand DM. Effects of applied currents on spontaneous epileptiform activity induced by low calcium in the rat hippocampus. Brain Res. 1998;806(2):186–95. doi: 10.1016/s0006-8993(98)00723-9. [DOI] [PubMed] [Google Scholar]
- 59.Jensen AL, Durand DM. High frequency stimulation can block axonal conduction. Exp Neurol. 2009;220(1):57–70. doi: 10.1016/j.expneurol.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng Z, Zheng X, Yu Y, Durand DM. Functional disconnection of axonal fibers generated by high frequency stimulation in the hippocampal CA1 region in-vivo. Brain Res. 2013;1509:32–42. doi: 10.1016/j.brainres.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31(38):13469–84. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stavrinou LC, Boviatsis EJ, Stathis P, Leonardos A, Panourias IG, Sakas DE. Sustained relief after discontinuation of DBS for dystonia: implications for the possible role of synaptic plasticity and cortical reorganization. J Neurol Surg A Cent Eur Neurosurg. 2012;73(3):175–8. doi: 10.1055/s-0032-1313590. [DOI] [PubMed] [Google Scholar]
- 63.Lozano AM, Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77(3):406–24. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 64.Morrell MJ, Group RNSSiES. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77(13):1295–304. [DOI] [PubMed]
- 65.Neuropace RNS System: summary of safety and effectiveness. In: Administration USFD, editor. 2013.
- 66.Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432–41. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84(8):810–7. doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park K, Lim YH, Jang M, Kim A, Kim HJ, Paek SH, et al. Battery life matters in deep brain stimulation. Stereotact Funct Neurosurg. 2018;96(1):65–6. doi: 10.1159/000486686. [DOI] [PubMed] [Google Scholar]
- 69.Hargreaves E, Patel R, DiPaola R, Wong S, Caputo D, Danish S. Deep brain stimulation (DBS) battery longevity of Medtronic Activa SC is briefer than preceding Soletra models, a within subject analysis (abstract) Mov Disord. 2017;32(suppl 2):604. [Google Scholar]
- 70.Garcia L, Audin J, D'Alessandro G, Bioulac B, Hammond C. Dual effect of high-frequency stimulation on subthalamic neuron activity. J Neurosci. 2003;23(25):8743–51. doi: 10.1523/JNEUROSCI.23-25-08743.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia L, D'Alessandro G, Fernagut PO, Bioulac B, Hammond C. Impact of high-frequency stimulation parameters on the pattern of discharge of subthalamic neurons. J Neurophysiol. 2005;94(6):3662–9. doi: 10.1152/jn.00496.2005. [DOI] [PubMed] [Google Scholar]
- 72.Griffin DM, Hudson HM, Belhaj-Saif A, Cheney PD. Hijacking cortical motor output with repetitive microstimulation. J Neurosci. 2011;31(37):13088–96. doi: 10.1523/JNEUROSCI.6322-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mirski MA, Rossell LA, Terry JB, Fisher RS. Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res. 1997;28(2):89–100. doi: 10.1016/s0920-1211(97)00034-x. [DOI] [PubMed] [Google Scholar]
- 74.Oikawa H, Sasaki M, Tamakawa Y, Kamei A. The circuit of Papez in mesial temporal sclerosis: MRI. Neuroradiology. 2001;43(3):205–10. doi: 10.1007/s002340000463. [DOI] [PubMed] [Google Scholar]
- 75.Hamani C, Hodaie M, Chiang J, del Campo M, Andrade DM, Sherman D, et al. Deep brain stimulation of the anterior nucleus of the thalamus: effects of electrical stimulation on pilocarpine-induced seizures and status epilepticus. Epilepsy Res. 2008;78(2–3):117–23. doi: 10.1016/j.eplepsyres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Jefferys JGR, Jiruska P, de Curtis M, Avoli M. Limbic network synchronization and temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. Bethesda (MD). 2012. [PubMed]
- 77.Velasco F, Velasco M, Ogarrio C, Fanghanel G. Electrical stimulation of the centromedian thalamic nucleus in the treatment of convulsive seizures: a preliminary report. Epilepsia. 1987;28(4):421–30. doi: 10.1111/j.1528-1157.1987.tb03668.x. [DOI] [PubMed] [Google Scholar]
- 78.Fisher RS, Uematsu S, Krauss GL, Cysyk BJ, McPherson R, Lesser RP, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33(5):841–51. doi: 10.1111/j.1528-1157.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 79.Velasco F, Velasco M, Velasco AL, Jimenez F, Marquez I, Rise M. Electrical stimulation of the centromedian thalamic nucleus in control of seizures: long-term studies. Epilepsia. 1995;36(1):63–71. doi: 10.1111/j.1528-1157.1995.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 80.Velasco F, Velasco M, Jimenez F, Velasco AL, Brito F, Rise M, et al. Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery. 2000;47(2):295–304. doi: 10.1097/00006123-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 81.Velasco AL, Velasco F, Jimenez F, Velasco M, Castro G, Carrillo-Ruiz JD, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006;47(7):1203–12. doi: 10.1111/j.1528-1167.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 82.Valentin A, Garcia Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013;54(10):1823–33. doi: 10.1111/epi.12352. [DOI] [PubMed] [Google Scholar]
- 83.Son BC, Shon YM, Choi JG, Kim J, Ha SW, Kim SH, et al. Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact Funct Neurosurg. 2016;94(3):187–97. doi: 10.1159/000446611. [DOI] [PubMed] [Google Scholar]
- 84.Laxpati NG, Kasoff WS, Gross RE. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics. 2014;11(3):508–26. doi: 10.1007/s13311-014-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 86.Medtronic DBS therapy for epilepsy: summary of safety and effectiveness. In: Administration USFD, editor. 2018.
- 87.Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017–25. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.VNS Therapy System: summary of safety and effectiveness. In: Administration USFD, editor. 2017.
- 89.Katariwala NM, Bakay RA, Pennell PB, Olson LD, Henry TR, Epstein CM. Remission of intractable partial epilepsy following implantation of intracranial electrodes. Neurology. 2001;57(8):1505–7. doi: 10.1212/wnl.57.8.1505. [DOI] [PubMed] [Google Scholar]
- 90.Schulze-Bonhage A, Dennig D, Wagner K, Cordeiro JG, Carius A, Fauser S, et al. Seizure control resulting from intrahippocampal depth electrode insertion. J Neurol Neurosurg Psychiatry. 2010;81(3):352–3. doi: 10.1136/jnnp.2009.180075. [DOI] [PubMed] [Google Scholar]
- 91.Roth J, Olasunkanmi A, Ma TS, Carlson C, Devinsky O, Harter DH, et al. Epilepsy control following intracranial monitoring without resection in young children. Epilepsia. 2012;53(2):334–41. doi: 10.1111/j.1528-1167.2011.03380.x. [DOI] [PubMed] [Google Scholar]
- 92.Eggleston KS, Olin BD, Fisher RS. Ictal tachycardia: the head-heart connection. Seizure. 2014;23(7):496–505. doi: 10.1016/j.seizure.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 93.Fisher RS, Eggleston KS, Wright CW. Vagus nerve stimulation magnet activation for seizures: a critical review. Acta Neurol Scand. 2015;131(1):1–8. doi: 10.1111/ane.12288. [DOI] [PubMed] [Google Scholar]
- 94.Yum A, Mani R, Kaufman K, Sivaraaman K, Esfahanizadeh A, Wong S. Reiliability of seizure detection in the epilepsy monitoring unit (abstract) Washington, DC: American Epilepsy Society Annual Meeting; 2015. [Google Scholar]
- 95.Hamilton P, Soryal I, Dhahri P, Wimalachandra W, Leat A, Hughes D, et al. Clinical outcomes of VNS therapy with AspireSR((R)) (including cardiac-based seizure detection) at a large complex epilepsy and surgery centre. Seizure. 2018;58:120–6. doi: 10.1016/j.seizure.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 96.Lehtimaki K, Mottonen T, Jarventausta K, Katisko J, Tahtinen T, Haapasalo J, et al. Outcome based definition of the anterior thalamic deep brain stimulation target in refractory epilepsy. Brain Stimul. 2016;9(2):268–75. doi: 10.1016/j.brs.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 97.Jarvenpaa S, Peltola J, Rainesalo S, Leinonen E, Lehtimaki K, Jarventausta K. Reversible psychiatric adverse effects related to deep brain stimulation of the anterior thalamus in patients with refractory epilepsy. Epilepsy Behav. 2018;88:373–9. doi: 10.1016/j.yebeh.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Frost M, Gates J, Helmers SL, Wheless JW, Levisohn P, Tardo C, et al. Vagus nerve stimulation in children with refractory seizures associated with Lennox-Gastaut syndrome. Epilepsia. 2001;42(9):1148–52. doi: 10.1046/j.1528-1157.2001.23900.x. [DOI] [PubMed] [Google Scholar]
- 99.Holmes MD, Silbergeld DL, Drouhard D, Wilensky AJ, Ojemann LM. Effect of vagus nerve stimulation on adults with pharmacoresistant generalized epilepsy syndromes. Seizure. 2004;13(5):340–5. doi: 10.1016/j.seizure.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Kostov K, Kostov H, Tauboll E. Long-term vagus nerve stimulation in the treatment of Lennox-Gastaut syndrome. Epilepsy Behav. 2009;16(2):321–4. doi: 10.1016/j.yebeh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 101.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 2011;115(6):1248–55. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 102.Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics. 2014;11(3):553–63. doi: 10.1007/s13311-014-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geller EB, Skarpaas TL, Gross RE, Goodman RR, Barkley GL, Bazil CW, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58(6):994–1004. doi: 10.1111/epi.13740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 515 kb)