Abstract
Background
Mycobacterium avium subspecies paratuberculosis (M. paratuberculosis) is a facultative intracellular pathogen that resides within host macrophages during infection of ruminant animals. We examined survival of M. paratuberculosis infections within cultured macrophages to better understand the interplay between bacterium and host.
Results
Serial plating of M. paratuberculosis infected macrophage lysates on Herold's egg yolk medium showed that mycobacterial replication takes place between 0 and 24 hours post-infection. This initial growth phase was followed by a steady decline in viability over the next six days. Antibodies against M. paratuberculosis were affinity purified and used in conjunction with transmission electron microscopy to track the development of intracellular bacilli. Immunogold labeling of infected macrophages with antibody against M. paratuberculosis showed degraded intracellular mycobacteria that were unrecognizable by morphology alone. Conversely, when macrophages were heavily infected with M. paratuberculosis, no degraded forms were observed and macrophages were killed.
Conclusions
We present a general description of M. paratuberculosis survival within cultured macrophages using transmission electron microscopy and viability counts. The results of this study provides further insight surrounding M. paratuberculosis-macrophage infections and have implications in the pathogenesis of M. paratuberculosis, a pathogen known to persist inside cattle for many years.
Background
Johne's disease, also called paratuberculosis, is a chronic granulomatous enteritis of ruminant animals caused by Mycobacterium avium subspecies paratuberculosis (M. paratuberculosis). While Johne's disease can end in death of the animal, the economic impact of this disease is much more significant [1,2]. Losses are estimated to be $200/clinically infected cow/year and are a result of animal culling, reduced milk production, poor reproductive performance, and reduced carcass value [1,3]. Research on the pathogenesis and immunology of M. paratuberculosis infections of cattle is necessary to allow design of more rational diagnostic and control procedures.
A small number of specialized microorganisms can survive inside macrophages designed specifically to kill bacteria. However, a hallmark of mycobacterial pathogenesis is their ability to survive, and even replicate, within macrophages. These include Mycobacteria, Salmonella, Listeria, Coxiella and Corynebacteria. Different mechanisms are employed as survival strategies and the mycobacteria are exceptional in the duration and persistence of this interaction. Survival of pathogenic mycobacteria is attributed to the fact that the mycobacterial phagosome does not fuse with lysosomes [4-6]. The mechanism that prevents phagosome maturation is still unknown as are any mycobacterial genes that contribute to the delayed maturation.
Several studies surrounding the interactions of M. paratuberculosis with macrophages have been published because of its importance in pathogenesis. These studies include entry into J774 macrophages [7,8], an electron microscopic examination of goat tissue [9,10] and an assessment of intracellular fate of M. paratuberculosis within bovine monocytes/macrophages [11,12]. However, many assumptions regarding M. paratuberculosis interactions with macrophages are based on analogies to M. tuberculosis[13] or M. avium[14]. In this communication, we present an analysis of M. paratuberculosis survival within J774 macrophages using transmission electron microscopy to show temporal events early during infection.
Results
Viability of M. paratuberculosis within resting J774 macrophages
The growth of M. paratuberculosis was measured at early stages during infection of non-activated macrophages. Growth was measured by bacterial cell counts following serial dilutions on HEYM slants. The cell counts from three independent experiments showed a slow decline in M. paratuberculosis viability over 7 days (Fig. 1). After infection, an initial growth phase occurred until 24 hours postinfection where mycobacterial counts began to decline. In two of the three experiments, an increase in bacterial counts occurred after 70 hours postinfection up until 95 hours where a second decline in viable mycobacteria occurred. These data suggest that while M. paratuberculosis survives much longer in macrophages than some pathogens [15,16] including other species of mycobacteria [17], there remains a significant decrease in viability over time.
Figure 1.
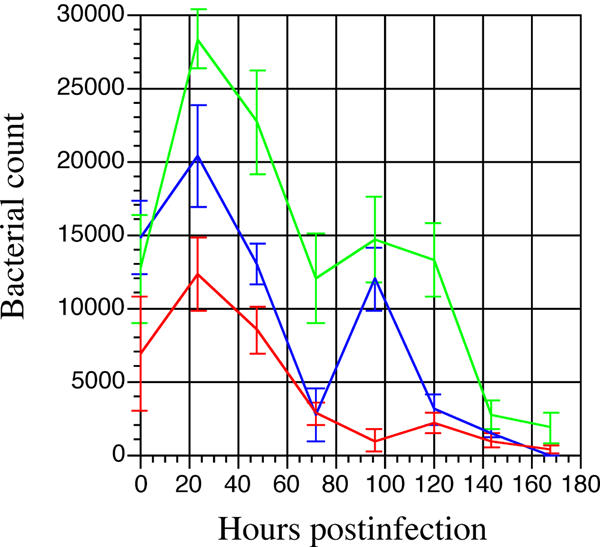
Growth and survival of M. paratuberculosis in murine macrophages. Resting J774 macrophages were infected with M. paratuberculosis at time zero. Infected lysates were harvested and plated in triplicate on HEYM slants at 0, 24, 48, 72, 96, 120, 144, and 168 hours postinfection. The results from three independent experiments are shown. All platings were performed in triplicate. Error bars denote standard deviation.
Immunoelectron microscopy of intracellular M. paratuberculosis
Because the viability of M. paratuberculosis decreased with time, it was of interest to examine the progression of M. paratuberculosis infection by immunoelectron microscopy. However, a reliable method to label intracellular mycobacteria needed to be developed first. Therefore rabbit antibodies against a whole cell sonicated lysate of M. paratuberculosis were affinity purified. These purified antibodies labeled the outer periphery of Middlebrook 7H9 cultured M. paratuberculosis (Fig. 2C). This purified antibody preparation was then used to label M. paratuberculosis within infected macrophages (Fig. 2A and 2B). The purified antibody was highly specific as all gold particles are associated with the mycobacteria and no labeling of the surrounding background or macrophages was observed. Control preparations of uninfected macrophages failed to label with antibody against M. paratuberculosis (data not shown). Note the mycobacterial morphology remained constant when comparing intracellular with extracellular bacilli (compare Fig 2B with Fig 2C).
Figure 2.
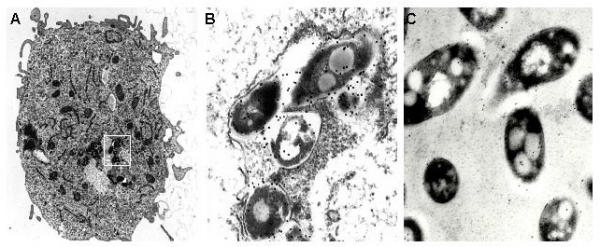
Immunogold labeling of intracellular and extracellular M. paratuberculosis. A single macrophage is shown at 48-h postinfection (A). (B) An enlargement of the boxed region in panel A showing immunogold labeling of intracellular M. paratuberculosis. (C) Preparation of M. paratuberculosis cultured in Middlebrook 7H9 medium. Gold particle labeling was seen predominantly at the periphery of the mycobacterial cells. Magnification: 11,180 × (A); 104,000 × (B); 88,400 × (C).
Temporal events during M. paratuberculosis infection of J774 macrophages
Macrophages were infected with M. paratuberculosis at a 5:1 ratio and fixed in glutaraldehyde at various time points to examine the development of mycobacteria in this environment (Fig. 3). Vacuoles harboring mycobacteria appeared tightly arranged and not spacious as previously observed in Coxiella burnetii containing vacuoles [18,19]. The mycobacteria themselves were in very close contact with each other. The size, number, and morphology of the mycobacteria appeared to remain relatively constant throughout the observed time period. At all times postinfection, mycobacteria were mostly found as groups inside vacoules. Occasionally, single bacilli were observed within a tight vacuole. An increase in the percentage of degraded mycobacteria was observed with time. There were two degraded bacilli per 25 fields at 24 hour postinfection and 12 degraded bacilli per 13 fields at 72 hours postinfection. Similar data were obtained in two independent experiments. Degraded mycobacteria were morphologically unrecognizable, but did label with immunogold, indicating the presence of M. paratuberculosis antigen (Fig. 4). These data indicate that macrophages can kill and degrade a percentage of mycobacterial cells in a given infection.
Figure 3.
Transmission electron micrographs of M. paratuberculosis-containing vacuoles in J774 macrophages. Infected macrophages were processed for transmission electron microscopy at 24 (A), 48 (B), 72 (C), and 96 (D) hours postinfection. The left column shows the infected macrophage and the right column shows an enlargement of the mycobacterial vacuole. Image magnification is shown beneath each column. Arrows point to the same mycobacterial cell in each magnification.
Figure 4.
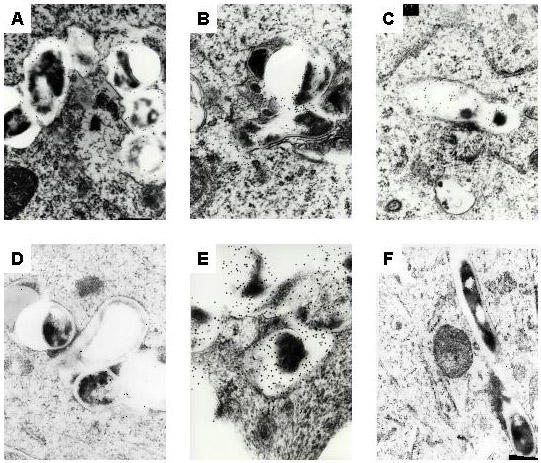
Some mycobacteria are degraded within macrophages. Infected macrophages were processed for transmission electron microscopy at 24 (A), 48 (B), 72 (C), 96 (D), and 120 (E) hours postinfection. Degraded, unrecognizable mycobacteria were labeled with immunogold particles (A-E). Healthy M. paratuberculosis bacilli at 72 hours postinfection were seen in (F). Magnification: 104,000 × (A-C), 104,000 × (D), 165,000 × (E), 88,400 × (F).
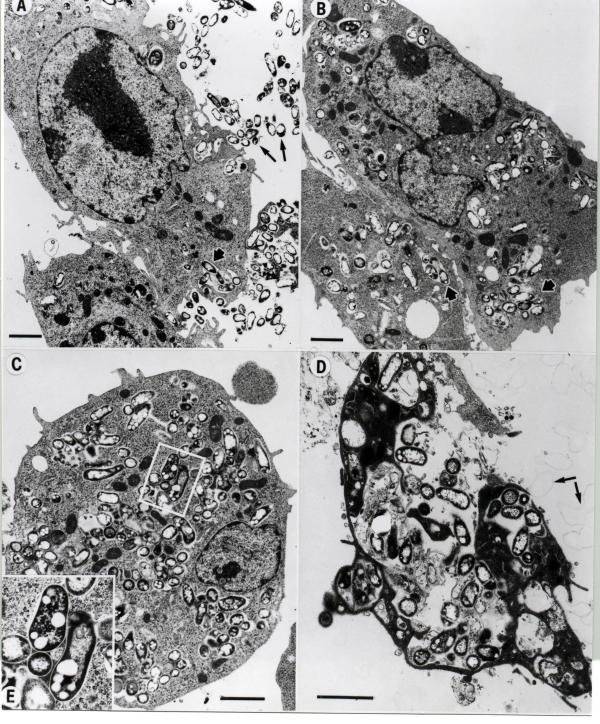
We next examined the outcome of mycobacteria within heavily infected macrophages. J774 macrophages were infected with M. paratuberculosis at high multiplicities of infection (30:1) and examined at various times post-infection (fig. 5). Extracellular mycobacteria were phagocytosed via actin rearrangement and remain within phagosomal vacuoles dispersed throughout the cytoplasm (fig. 5A and 5B). At least 20 infected macrophages were examined at each time point. Note the electron transparent lipid vesicles present within M. paratuberculosis. These electron transparent vesicles were seen at all time points examined. At 48 hr postinfection (fig. 5C), the cytoplasm of the macrophage is loaded with mycobacterial-containing vacuoles. By 72 hr postinfection, the macrophage cytoplasm is dark and the cell appears necrotic, possibly due to the heavy mycobacterial load or apoptosis. Severe membrane sloughing or blebbing seen to the right of the macrophage and identified by the arrows in Fig. 5D is a sign of apoptosis [20-22]. No degraded mycobacteria were observed in these experiments. Therefore, at high levels of infection, it appears that mycobacteria survive while the host macrophage is killed. Whereas the outcome is reversed at lower moi.
Figure 5.
Heavy M. paratuberculosis infection of J774 macrophages. Macrophages were infected at a 30:1 ratio and processed for transmission electron microscopy at 0 (A), 24 (B), 48 (C), and 72 (D) hours. Long arrows in A identify extracellular M. paratuberculosis whereas short arrows in A and B show intracellular M. paratuberculosis within vacuoles, presumably phagosomes. Membrane protrusions indicative of actin rearrangements involving phagocytosis can be seen in the top center of panel A. The inset (E) shows the intracellular detail of selected bacilli. Arrows in (D) identify membrane blebs. Scale bars = 1 μm.
Discussion
Growth and survival of mycobacterial species within macrophages has been an area of intensive study because of its implications in pathogenicity. For example, M. bovis has been shown to grow within macrophages whereas BCG strains do not [23]. This observation is controversial since there are reports of immunocompromised patients with disseminated BCG. Although multi-species studies are complex and multi-factorial, M. avium appears to be able to survive in secondary lysosomes better than does M. tuberculosis[18]. In macrophages co-infected with Coxiella burnetii, an intracellular pathogen known to inhabit and replicate within secondary lysosomes [19], M. avium growth was not impaired [18]. Whereas M. tuberculosis bacilli that co-localized with C. burnetii containing vacuoles, did show reduced growth [18]. A recent report by Thomsen et al. [24] showed a higher percentage of degraded M. paratuberculosis in euthymic as compared to athymic mice. The in vivo study by Thomsen supports data from our in vitro study, indicating that J774 cultured macrophages are a good model for pathogenesis studies.
Many bacteria such as Staphylococcus aureus are rapidly endocytosed and digested by macrophages within a few hours postinfection [25]. The present study showed an initial 25 hour intracellular growth period of M. paratuberculosis followed by a slow decline in the viability within macrophages over a period of 7 days. This survival curve is similar to that observed by Zhao et al. [12] which showed M. paratuberculosis intracellular growth in the first six days followed by killing after day six. The experiments described herein were performed using the type strain of M. paratuberculosis, which may have affected the survivability within macrophages since it is not a recent field isolate. However, M. paratuberculosis 6783 [17] and M. paratuberculosis BO45 [12] are field isolates that have both showed a decline in viability inside macrophages over time, although much slower than observed with the type strain. No attempt has been made to activate the macrophages in this study, although several laboratories have shown that macrophages activated with cytokines such as interferon-γ increase the ability to kill mycobacteria [26-30].
A very recent communication of M. paratuberculosis interactions with macrophages was published during the course of our experiments [17]. This comprehensive study showed that M. paratuberculosis-containing vacuoles were mildly acidified (pH 6.3) as compared to latex beads (pH 5.2). In addition, the M. paratuberculosis phagosome was characterized by the presence of LAMP 1 and absence of LAMP 2 lysosomal membrane protein markers as well as other endocytic tracer molecules. These cell biology studies clearly add to our understanding of M. paratuberculosis interactions with macrophages.
This study has also provided a general description of early events in M. paratuberculosis infection of cultured macrophages. Several intracellular pathogens such as Chlamydia or Coxiella undergo readily distinguishable morphological changes during infection of host cells [31,32]. This is clearly not the case for M. paratuberculosis or other mycobacteria as they remain morphologically similar at least up until 4 days postinfection. However, an increased percentage of degraded mycobacterial forms was observed over time. These degraded forms appeared by 24 hours postinfection and were hardly recognizable morphologically, but did label with immunogold particles indicating the presence of mycobacterial antigen. At low moi, the bacilli were tightly clustered into a single vacuole throughout the observed time period. This mycobacterial vacuole is most likely characteristic of a late endosome, based on previous studies with M. avium, M. tuberculosis[33,34] and M. paratuberculosis[17].
All intracellular bacterial pathogens enter host cells surrounded by a membrane bound vacuole [35,36]. Some pathogens in heavily infected cells collect into a single vacuole within the same cell [37]. J774 macrophages infected at a moi of 5:1 showed separate M. paratuberculosis-containing vacuoles within the same macrophage even after 48 hours postinfection (Figure 2). Likewise, M. tuberculosis and M. avium appear to remain in distinct phagosomes that do not harbor more than one bacilli per vacuole (see Figures 1A and 4B in [38] for example). However, in M. avium-infected macrophages, one of the first phenotypic alterations following activation with cytokines is the coalescence of individual M. avium-containing vacuoles into communal vacuoles with many bacilli [26]. The significance of separate M. paratuberculosis-containing vacuoles observed in this study is still unclear.
There appears to be a tenuous relationship to gain control between M. paratuberculosis and the macrophage with survival at stake. The macrophage can control growth and even kill M. paratuberculosis. However, the mycobacteria are cytotoxic to macrophages or induce apoptosis at high moi. The mechanism that enables M. paratuberculosis to persist within cattle for several years remains unclear as high moi are not likely observed at early stages of Johne's disease.
Conclusions
In vitro assays to quantify survival of bacteria in macrophages provide useful insights into host-pathogen relations. The results of this study provides further insight surrounding M. paratuberculosis-macrophage infections and have implications in the pathogenesis of M. paratuberculosis, a pathogen known to persist inside cattle for many years. Further studies need to address the tenuous relationship between mycobacteria and macrophage in relation to disease outcome.
Materials and methods
Bacterial strains and growth conditions
M. paratuberculosis ATCC19698 was grown in Middlebrook 7H9 broth (pH 6.0) supplemented with oleic acid albumin dextrose complex (Becton Dickinson Microbiology) and 0.05% Tween 80 and ferric mycobactin J (2 mg/L). For viability counts, M. paratuberculosis was cultured on Herold's egg yolk medium (HEYM) prepared as described elsewhere [39].
Culture of M. paratuberculosis in macrophages
The murine macrophage cell line, J774.16, was cultured in Dulbecco's modified Eagle medium (MEM; Life Technologies) supplemented with 10% defined fetal calf serum (Hyclone Laboratories) at 37°C in 5% CO2. Because phagocytosis of mycobacteria was shown to be enhanced with complement protein C3 [40], macrophages were infected with M. paratuberculosis in the presence of 10% serum from a clinical cow with Johne's disease. Multiplicities of infection (moi) ranged between 2:1 and 30:1 (bacteria: macrophage ratio) in PBS. The number of macrophages was 3.0 × 106/well as determined on an Angel CellTrak 3B cell counter. The input inoculum was 3.0 × 107mycobacteria/well for a 10:1 moi experiment. After 2 hours, extracellular bacteria were removed by washing the monolayers twice with fresh media. Infected macrophages were then incubated in MEM at 37°C and in 5% CO2. Medium was exchanged every third day during the course of the infection. To determine survival of M. paratuberculosis within macrophages, 24-well plates of macrophages were infected at an moi of 10:1. Infected macrophages were lysed in cold sterile distilled H2O at 0, 24, 48, 72, 96, 120, 144, and 168 hours postinfection. Following lysis, serial platings of infected macrophage lysates on HEYM slants were performed. Lysates from three separate wells were plated for each time point.
Antibodies
Colloidal gold-conjugated goat anti-rabbit IgG was purchased from Ted Pella, Inc., Redding, CA. Two New Zealand white rabbits were immunized with sonicated preparations of M. paratuberculosis as previously described [41]. Antibodies directed at a whole cell sonicated extract of M. paratuberculosis were affinity purified using AminoLink columns (Pierce Chemical Company, Rockford, IL, USA). Briefly, the sonicated extract of M. paratuberculosis was coupled by reductive amination to a 4% agarose support column (Pierce Chemical Company). Sera from rabbits immunized with a killed preparation of M. paratuberculosis (1–2 ml) were passed over the column followed by three washes and elution according to the instructions of the manufacturer. Eluted fractions were evaluated by spectrophotometry and immunoblot analysis [42]. Fractions with the highest absorbance at 280 nm and the strongest reactivity by immunoblot were neutralized in 1 M Tris-HCl (pH 9.5) buffer and stored at 4°C.
Electron microscopy
All fixation and staining procedures were conducted at room temperature. Noninfected and M. paratuberculosis-infected macrophages were cultured on membrane inserts for times indicated in each experiment described below. Cells were fixed for 2–4 h in 2.5% glutaraldehyde in 0.1 M Cacodylate buffer, pH 7.4. Fixed cells were washed in the same buffer three times and were postfixed in 1% OsO4 in 0.1 M cacodylate buffer, pH 7.4, for 2 h. After washing in the same buffer, cells were incubated with 30% ethanol for 10 min. The cells were further dehydrated with a graded series of ethanol and embedded in epoxy resin (Embed 812). Ultrathin sections for immunoelectron microscopy were washed in buffer 15 min three times and etched with saturated sodium metaperiodate for 15 min. Cells were then blocked with 5% BSA for 30 min at room temperature. Cells were treated with affinity purified rabbit IgG against M. paratuberculosis (diluted 1:20) in the blocking solution for 2 h at room temperature. Cells were washed in Tris buffer containing 0.1% Tween 20 and 0.1% BSA four times for 10 min each and then incubated with goat anti-rabbit IgG conjugated to colloidal gold (10 nm diameter) in Tris buffer for 2 h. Immunolabeled sections were washed in Tris buffer four times and fixed with 1% glutaraldehyde in Tris for 10 min. All ultrathin sections were double stained with uranyl acetate and Reynolds lead citrate and then observed under a Philips 410 microscope.
Acknowledgments
Acknowledgements
The expert technical assistance of Janis Hansen and Trudy Bosworth is appreciated. We gratefully acknowledge Judy Stasko for the embedding and staining of samples for electron microscopy.
Contributor Information
John P Bannantine, Email: jbannant@nadc.ars.usda.gov.
Judith R Stabel, Email: jstabel@nadc.ars.usda.gov.
References
- Ott SL, Wells SJ, Wagner BA. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev Vet Med. 1999;40:179–192. doi: 10.1016/S0167-5877(99)00037-9. [DOI] [PubMed] [Google Scholar]
- Johnson-Ifearulundu Y, Kaneene JB, Lloyd JW. Herd-level economic analysis of the impact of paratuberculosis on dairy herds. J Am Vet Med Assoc. 1999;214:822–825. [PubMed] [Google Scholar]
- Wells SJ, Wagner BA, Dargatz DA. Proceedings of the Sixth International Colloquium on Paratuberculosis; Melbourne, Australia.; 1999. Factors associated with M. a. paratuberculosis infection in U.S. dairy herds. pp. 62–65. [Google Scholar]
- Armstrong JA, Hart PD. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C, de Chastellier C, Lang T, Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986;52:252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PD, Young MR. Ammonium chloride, an inhibitor of phagosome-lysosome fusion in macrophages, concurrently induces phagosome-endosome fusion, and opens a novel pathway: studies of a pathogenic mycobacterium and a nonpathogenic yeast. J Exp Med. 1991;174:881–889. doi: 10.1084/jem.174.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethe R, Kuehnel MP, Darji A, Weiss S, Rohde M, Gerlach G-F, Valentin-Weigand P. Proceedings of the Sixth International Colloquium on Paratuberculosis; Melbourne, Australia.; 1999. Interactions of Mycobacterium avium subsp. paratuberculosis with murine macrophages: intracellular survival and modulation of macrophage functions. pp. 635–637. [Google Scholar]
- Rastogi N, Blom-Potar MC, David HL. Comparative intracellular growth of difficult-to-grow and other mycobacteria in a macrophage cell line. Acta Leprol. 1989;7:156–159. [PubMed] [Google Scholar]
- Paliwal OP, Rehbinder C. Ultrastructural studies of paratuberculosis (Johne's disease) in goats. Acta Vet Scand. 1981;22:180–188. doi: 10.1186/BF03547507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigur-Dardottir OG, Press CM, Evensen O. Uptake of Mycobacterium avium subsp. paratuberculosis through the distal small intestinal mucosa in goats: an ultrastructural study. Vet Pathol. 2001;38:184–189. doi: 10.1354/vp.38-2-184. [DOI] [PubMed] [Google Scholar]
- Bendixen PH, Bloch B, Jorgensen JB. Lack of intracellular degradation of Mycobacterium paratuberculosis by bovine macrophages infected in vitro and in vivo: light microscopic and electron microscopic observations. Am J Vet Res. 1981;42:109–113. [PubMed] [Google Scholar]
- Zhao BY, Czuprynski CJ, Collins MT. Intracellular fate of Mycobacterium avium subspecies paratuberculosis in monocytes from normal and infected, interferon-responsive cows as determined by a radiometric method. Can J Vet Res. 1999;63:56–61. [PMC free article] [PubMed] [Google Scholar]
- Russell DG, Sturgill-Koszycki S, Vanheyningen T, Collins H, Schaible UE. Why intracellular parasitism need not be a degrading experience for Mycobacterium. Philos Trans R Soc Lond B Biol Sci. 1997;352:1303–1310. doi: 10.1098/rstb.1997.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohagheghpour N, van Vollenhoven A, Goodman J, Bermudez LE. Interaction of Mycobacterium avium with human monocyte-derived dendritic cells. Infect Immun. 2000;68:5824–5829. doi: 10.1128/IAI.68.10.5824-5829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastellier C, Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994;62:543–553. doi: 10.1128/iai.62.2.543-553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read RC, Zimmerli S, Broaddus C, Sanan DA, Stephens DS, Ernst JD. The (alpha2–>8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect Immun. 1996;64:3210–3217. doi: 10.1128/iai.64.8.3210-3217.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehnel MP, Goethe R, Habermann A, Mueller E, Rohde M, Griffiths G, Valentin-Weigand P. Characterization of the intracellular survival of Mycobacterium avium ssp. paratuberculosis: phagosomal pH and fusogenicity in J774 macrophages compared with other mycobacteria. Cell Microbiol. 2001;3:551–566. doi: 10.1046/j.1462-5822.2001.00139.x. [DOI] [PubMed] [Google Scholar]
- Gomes MS, Paul S, Moreira AL, Appelberg R, Rabinovitch M, Kaplan G. Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect Immun. 1999;67:3199–3206. doi: 10.1128/iai.67.7.3199-3206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen RA, Scidmore MA, Rockey DD, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Gan YH, Peng SQ, Liu HY. Molecular mechanism of apoptosis induced by ricin in HeLa cells. Acta Pharmacol Sin. 2000;21:243–248. [PubMed] [Google Scholar]
- Saafi EL, Konarkowska B, Zhang S, Kistler J, Cooper GJ. Ultrastructural evidence that apoptosis is the mechanism by which human amylin evokes death in RINm5F pancreatic islet beta-cells. Cell Biol Int. 2001;25:339–350. doi: 10.1006/cbir.2000.0643. [DOI] [PubMed] [Google Scholar]
- Aldwell FE, Wedlock DN, Slobbe LJ, Griffin JF, Buddle BM, Buchan GS. In vitro control of Mycobacterium bovis by macrophages. Tuberculosis. 2001;81:115–123. doi: 10.1054/tube.2000.0280. [DOI] [PubMed] [Google Scholar]
- Thomsen BV, Steadham EM, Gallup JM, Ackerman MR, Brees DJ, Cheville NF. T cell-dependent inducible nitric oxide synthase productional and ultrastructural morphology in BALB/c mice infected with Mycobacterium avium subspecies paratuberculosis. Journal of Comparative Pathology. 2001;125:137–144. doi: 10.1053/jcpa.2001.0491. [DOI] [PubMed] [Google Scholar]
- Schmitt-Slomska J, Michailova L, Ivanova E, Toshkov A. Adhesion and phagocytosis of Staphylococcus aureus L-forms. J Basic Microbiol. 1986;26:429–440. doi: 10.1002/jobm.3620260712. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- Xing Z, Zganiacz A, Santosuosso M. Role of IL-12 in macrophage activation during intracellular infection: IL-12 and mycobacteria synergistically release TNF-alpha and nitric oxide from macrophages via IFN-gamma induction. J Leukoc Biol. 2000;68:897–902. [PubMed] [Google Scholar]
- Bonay M, Bouchonnet F, Pelicic V, Lagier B, Grandsaigne M, Lecossier D, Grodet A, Vokurka M, Gicquel B, Hance AJ. Effect of stimulation of human macrophages on intracellular survival of Mycobacterium bovis Bacillus Calmette-Guerin. Evaluation with a mycobacterial reporter strain. Am J Respir Crit Care Med. 1999;159:1629–1637. doi: 10.1164/ajrccm.159.5.9807021. [DOI] [PubMed] [Google Scholar]
- Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- Zurbrick BG, Follett DM, Czuprynski CJ. Cytokine regulation of the intracellular growth of Mycobacterium paratuberculosis in bovine monocytes. Infect Immun. 1988;56:1692–1697. doi: 10.1128/iai.56.7.1692-1697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen RA, Hackstadt T, Samuel JE. Developmental biology of Coxiella burnettii. Trends Microbiol. 1999;7:149–154. doi: 10.1016/S0966-842X(99)01475-4. [DOI] [PubMed] [Google Scholar]
- Rockey DD, Matsumoto A. The chlamydial developmental cycle. In: Brun YV, Shimkets LJ, editor. Prokaryotic Development Washington, D.C. American Society for Microbiology; 2000. pp. 403–425. [Google Scholar]
- Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. Embo J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- Small PL, Ramakrishnan L, Falkow S. Remodeling schemes of intracellular pathogens. Science. 1994;263:637–639. doi: 10.1126/science.8303269. [DOI] [PubMed] [Google Scholar]
- Garcia-del Portillo F, Finlay BB. The varied lifestyles of intracellular pathogens within eukaryotic vacuolar compartments. Trends Microbiol. 1995;3:373–380. doi: 10.1016/S0966-842X(00)88982-9. [DOI] [PubMed] [Google Scholar]
- Sinai AP, Joiner KA. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu Rev Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I, Allen P, Russell DG. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]
- Merkal RS, Curran BJ. Growth and metabolic characteristics of Mycobacterium paratuberculosis. Appl Microbiol. 1974;28:276–279. doi: 10.1128/am.28.2.276-279.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- Stabel JR, Ackermann MR, Goff JP. Comparison of polyclonal antibodies to three different preparations of Mycobacterium paratuberculosis in immunohistochemical diagnosis of Johne's disease in cattle. J Vet Diagn Invest. 1996;8:469–473. doi: 10.1177/104063879600800412. [DOI] [PubMed] [Google Scholar]
- Bannantine JP, Stabel JR. Identification of two Mycobacterium avium subspecies paratuberculosis gene products differentially recognised by sera from rabbits immunised with live mycobacteria but not heat-killed mycobacteria. J Med Microbiol. 2001;50:795–804. doi: 10.1099/0022-1317-50-9-795. [DOI] [PubMed] [Google Scholar]