ABSTRACT
Background
Micronutrients are important for reproductive health and pregnancy, but the status of multiple vitamins and minerals is rarely measured in women before pregnancy.
Objectives
We aimed to assess the status and concurrent deficiencies of micronutrients among women before pregnancy and their relation with common health indicators.
Methods
This was a cross-sectional study that recruited women who expected to become pregnant within the next 6 mo in Asesewa, Ghana, a semi-urban community. Women self-reported demographics and health history. We measured blood pressure, height, and weight and conducted a blood draw and hemoglobin assessment (n = 98). We measured serum/plasma concentrations of ferritin, iron, total iron binding capacity, zinc, copper, retinol, and 25-hydroxyvitamin D, in addition to markers of inflammation. We used established cutoffs for deficiency and insufficiency/low status for each micronutrient after adjusting ferritin, zinc, and retinol for inflammation. We compared biomarker distributions by common health indicators.
Results
Forty percent of women had overweight/obesity, 33% were anemic, and 23% had elevated blood pressure. Overall, 27% had ≥1 deficiencies, whereas only 4% had 2 deficiencies. Fifty-eight percent of women had ≥1 insufficiencies and 18% had ≥2 insufficiencies. Prevalence of individual deficiencies was 12%, 7%, 7%, 4%, and 0% and prevalence of individual insufficiencies was 18%, 12%, 29%, 13%, and 13% for iron, copper, vitamin A, zinc, and vitamin D, respectively. Iron biomarkers and retinol concentrations differed by anemia status, and copper was higher in those with elevated blood pressure. Micronutrient concentrations were not associated with self-reported medical history (parity or history of anemia, malaria, or night blindness).
Conclusions
In Asesewa, Ghana, there was a relatively low prevalence of individual micronutrient deficiencies, but the majority of women were insufficient in ≥1 micronutrients. Iron and vitamin A status was lower in those with anemia, but otherwise, micronutrient status did not relate to common health markers.
Keywords: prepregnancy, co-occurring, concurrent, deficiency, micronutrients, status, biomarker
Introduction
Micronutrients, particularly iron, zinc, iodine, and vitamin A, are essential for the overall health and development of women of reproductive age (WRA) (1). These micronutrients play important biological roles during the reproductive years and are vital in preparing a woman for pregnancy. Deficiencies in these micronutrients have been associated with preterm deliveries, low birth weight, fetal growth restriction, congenital abnormalities, and cognitive impairments, among other health issues (2–9). It is important for women to obtain adequate stores of these nutrients prior to pregnancy both to improve reproductive health and to meet the increased demand
during the pregnancy period. Unfortunately, numerous reports indicate a high prevalence of micronutrient deficiencies in WRA worldwide, especially in low- and middle-income countries (1, 10, 11).
Several studies, including meta-analyses, have examined a range of individual micronutrient deficiencies, including zinc, retinol, iron, iodine, folate, vitamin B-12, and vitamin A, in WRA (12–16). Meta-analyses, however, have not reported which of these micronutrient deficiencies co-occur because it is rare that co-occurrence is reported in individual studies. In reality, it is likely that individuals in developing countries often experience multiple micronutrient deficiencies simultaneously. Few studies have assessed concurrent micronutrient deficiencies; only 1 focused on nonpregnant women (17), whereas others assessed pregnant women (18, 19) and lactating mothers (20). Some grouped anemia with specific micronutrients to report concurrence, and insufficiencies were not quantified. As such, the extent of the burden of co-occurring micronutrient deficiencies is unclear and is mostly speculated (14, 15, 17). In addition, data on micronutrient status specific to prepregnancy (women expecting to become pregnant) are lacking.
Public health nutritionists have a limited set of tools to screen for potential micronutrient deficiencies in an inexpensive, quick manner. Examples include the assessment of goiter for iodine deficiency, night blindness for vitamin A, and anemia (which requires a small blood sample) as a proxy for iron deficiency. Dietary intake assessments have been used as a proxy for micronutrient status (21), but in addition to requiring extensive training to properly collect such data, there is often low correlation between the measured dietary intake of a nutrient and biochemically measured micronutrient status in an individual. Currently, simple screeners for micronutrient deficiencies are lacking.
Given the limited information on simultaneously occurring micronutrient deficiencies (and complete lack of data on insufficiencies), coupled with the lack of noninvasive tools to screen for micronutrient deficiencies, we aimed to assess the micronutrient status of Ghanaian women planning to become pregnant and to determine which of these micronutrient deficiencies co-occurred. Furthermore, we explored the relations between several commonly collected noninvasive health measures and micronutrient status.
Methods
Study setting and population
We conducted a cross-sectional study in Asesewa, a semi-urban setting in the eastern region of Ghana. The initial purpose of the study was to assess micronutrient status in WRA in the area and to pilot field methods to identify women before a pregnancy. Asesewa is the administrative capital of Upper Manya Krobo District, a predominantly farming district. More than 73% of the district population is employed in the agriculture sector (22). We recruited a convenience sample of women from churches, mosques, and women's networking groups. We worked together with religious leaders to plan the timing of visits to the churches and mosques in the study area. Trained research assistants and field supervisors addressed congregants during church services and after Muslim prayers, after which interested women were screened. Similar arrangements were made with leaders of women's groups. Later in the study, we added a door-to-door recruitment approach to enable us to reach women who did not belong to religious or women's groups. Inclusion criteria were nonpregnant females, aged 18–35 y, planning/expecting to get pregnant within 6 mo, and residing in the Asesewa area. Specifically, we asked women, “Are you planning to become pregnant in the next 6 months? Or do you expect that you could become pregnant in the next 6 months?” Women were excluded if they had been pregnant in the previous 12 mo. Eligible women were invited to the Asesewa Government Hospital for study consent and 1 visit that included data collection and a blood draw. Visits took place between October 2015 and April 2016. The study was reviewed and approved by the institutional review boards of The Pennsylvania State University and the Noguchi Memorial Institute for Medical Research, University of Ghana. Written informed consent was obtained from each woman prior to data collection. Women were reimbursed for travel costs and received a bar of soap as a token of appreciation for their voluntary participation.
Data collection
Eligible women were consented and enrolled at the beginning of the study visit, which occurred in the morning or early afternoon depending on the woman's availability. Data collection for enrolled women consisted of questions that were administered orally by trained study staff, and responses were recorded on a Samsung Galaxy Tab 4 (Model SM-T230NU; Samsung Electronics) using Open Data Kit 2.0 software (23). We adopted a questionnaire that has been used to assess household socioeconomic status and food insecurity in the study area (24). We added questions on pregnancy and health history and slightly adapted some questions related to socioeconomic status based on the interests of the current study.
Height was measured to the nearest 0.1 cm using the Seca 217 stadiometer (Seca GmbH), and weight was measured to the nearest 0.1 kg using the Seca 874 flat scale (Seca GmbH). Participants wore minimal or light clothing; they were also asked to remove shoes and any heavy clothing or objects, such as jewelry, before weighing. Each measurement was repeated twice, and the mean was calculated. Women provided a small urine sample, and a URIT 2 V reagent strip (URIT Medical Electronic Group) was read 30 s after dipping in the urine. By color, it indicated a range of 4 categories of protein: negative/trace, 30 mg/dL, 100 mg/dL, and ≥300 mg/dL.
Blood collection and processing
Women were not asked to fast before the blood draw; therefore, postprandial time was variable. A trained phlebotomist at the hospital completed the blood draws using a butterfly blood collection system. Blood was collected from an antecubital vein into 2 different 4-mL BD vacutainer venous blood collection tubes—SST serum separation tubes and lithium heparin-coated tubes (VWR). A drop of blood was transferred to a HemoCue cuvette from the butterfly tubing and placed into a HemoCue 201 analyzer to measure hemoglobin concentration. The HemoCue machine was calibrated each morning before fieldwork using control cuvettes. Lithium heparin-coated tubes were centrifuged within 30 min of blood collection at 1300 g for 15 min to obtain plasma. SST serum separation tubes were allowed to stand for 30–60 min to allow for clotting and were then centrifuged in the same way as the plasma samples to obtain serum. Plasma and serum were aliquoted into sterile cryovials and were initially frozen in a liquid nitrogen tank in the hospital for 1–2 wk before transporting to the Noguchi Memorial Institute for Medical Research in Accra, where they were stored at −80°C until the study was completed. Samples were then shipped on dry ice to The Pennsylvania State University and stored in a −80°C freezer until analysis. To prevent vitamin A degradation from light, which could alter the measured retinol concentration, 1 of the plasma cryovials was wrapped with aluminum foil.
Biochemical analyses
We assayed plasma samples for zinc, copper, and retinol, and we assayed serum samples for 25-hydroxyvitamin D [25(OH)D], iron, ferritin, total iron binding capacity (TIBC), and the inflammation markers α1-acid glycoprotein (AGP) and C-reactive protein (CRP). We measured serum ferritin by ELISA (Ramco Laboratories), calibrated against WHO standards, and serum iron and TIBC by colorimetric methods (25). Transferrin saturation (TSAT) was calculated as serum (iron/TIBC) × 100. For zinc and copper assays, plasma samples were diluted 5-fold as previously described (26, 27) with 0.1 N nitric acid (trace element grade; EMD Millipore), and concentrations of each were measured using flame atomic absorption spectroscopy on an AAnalyst 400 spectrometer (Perkin Elmer). Plasma retinol concentration was measured using ultra-performance LC (ACQUITY UPLC System; Waters Corporation) in Catharine Ross’ laboratory. For zinc, copper, and retinol measurements, we used the National Institute of Standards and Technology reference material (SRM 1950) as control samples for our analyses. AGP and CRP were measured using radial immunodiffusion tests (Kent Laboratories) and used to adjust for micronutrient concentrations when inflammation was present. The intra-assay coefficient of variation for the biomarkers was 2.1% for TIBC, 3.1% for copper, 3.3% for zinc, 4.0% for ferritin, and 4.0% for iron.
Serum aliquots were shipped to the Analytical Facility for Bioactive Molecules, The Hospital for Sick Children, Toronto, Canada; this laboratory is certified by Vitamin D External Quality Assessment Scheme for the measurement of 25(OH)D concentrations. Samples were analyzed by LC–tandem MS using an Agilent 1290 HPLC interfaced with an AB Sciex 5500 QTRAP mass spectrometer (28, 29). 25(OH)D3 and the C3 epimer of 25(OH)D3 were quantified separately; these are the main circulating forms of the intermediate metabolite.
Anemia was classified as a hemoglobin concentration <12.0 g/dL; moderate anemia was <11.0 and severe was <8.0 g/dL (30, 31). The following cutoffs were used to define micronutrient deficiency and insufficiency/low status, respectively: adjusted serum ferritin <15.0 µg/L and <20.0 µg/L for iron (30), adjusted plasma retinol <20 µg/dL and <30 µg/dL for vitamin A (32), adjusted plasma zinc <66 µg/dL and <70 µg/dL for zinc (33, 34), plasma copper <70 µg/dL and <90 µg/dL for copper (34), and serum 25(OH)D (without the C3 epimer) <30 nmol/L and <50 nmol/L for vitamin D (35).
We defined signs of inflammation as high CRP (>5.0 mg/L) and/or AGP (>100 mg/L) (36). We classified women into 4 groups of inflammation/infection status: 1 (reference/healthy), normal CRP and AGP; 2 (incubation), high CRP and normal AGP; 3 (early convalescence), high CRP and high AGP; and 4 (late convalescence), normal CRP and high AGP (37). We then used correction factors for ferritin (37), zinc (38), and retinol (39) to adjust for the measured concentrations (reported as “adjusted” throughout). Correction factors were as follows (always 1 for the reference group): ferritin: 0.77, 0.53, and 0.75; retinol: 1.14, 1.31, and 1.12; and zinc: 1.08, 1.14, and 1.12 (each for groups 2, 3, and 4, respectively). Due to the small sample size, we did not internally calculate correction factors or use proposed regression methods (40).
After biomarker data were collected, we compiled results for each woman into a letter and delivered the letters to as many women as we could re-contact. Letters were written in English because it is the national language and is taught in all schools. A dietitian was available at the Nutrition Research Center in Asesewa for women who wanted to discuss their micronutrient status (∼10 women used this resource).
Statistical analyses
We estimated the prevalence of single and co-occurring micronutrient deficiencies/insufficiencies as the percentage of women below established cutoffs for each biomarker (using inflammation-adjusted concentrations for ferritin, retinol, and zinc). For continuous biomarker concentrations, we visually examined the normality of each distribution using kernel density and quantile–quantile plots. We used bivariate analysis to examine differences in nutrient status (biomarker concentration) for several measured and self-reported health indicators divided into dichotomous categories. If a biomarker distribution was normal, we tested for equal variance (i.e., SDs) between groups. We tested for a difference between means using a 2-sample independent t test, with or without an “unequal” option in Stata to relax the equal variance assumption, depending on the variance test. If the biomarker distribution was not normal, we tested the difference using the Wilcoxon–Mann–Whitney test. Stata 15 (Stata) was used for analyses, and a P value <0.05 was considered statistically significant, without adjusting for multiple testing.
Results
We enrolled 100 eligible women, and analysis was conducted for 98 women with complete data. Just over half of households of women owned their home, and a third owned agricultural land (Table 1). Almost 20% of households had a female head. Most women drank commercially packaged water (either bottled or “sachet”). Almost every household had electricity and a mobile phone, yet few had a flush toilet or a specific place for handwashing. Although <40% of women reported being married (government process), almost all other women were in a traditional/customary marriage. Just over half of women reported ≤1 previous births. Few women reported being diagnosed with high blood pressure or other heart problems, but a similar number of women had been diagnosed with anemia or night blindness (14% and 15%, respectively). More than half of women had malaria diagnosed in the past. Only 2 women reported taking a vitamin or mineral supplement.
TABLE 1.
Sociodemographic, pregnancy history, and health characteristics of women of reproductive age and their households in Asesewa, Ghana 1
| Characteristics | |
| Household sociodemographics | |
| Own home, % | 52.0 |
| Own agricultural land, % | 32.7 |
| Female head of household, % | 19.4 |
| Participant is head, % | 13.3 |
| Number of adults in household2 | 2 [2, 4] |
| 1, % | 7.1 |
| 2, % | 48.0 |
| ≥3, % | 44.9 |
| Number of children in household2 | 1 [0, 3] |
| 0, % | 29.6 |
| 1, % | 24.5 |
| ≥2, % | 45.9 |
| Drinking water source,3 % | |
| Piped/public tap | 42.9 |
| Sachet | 55.1 |
| Toilet facility, % | |
| Flush | 17.4 |
| Ventilated improved pit latrine | 36.7 |
| Pit latrine or other | 45.9 |
| Specific place to wash hands, % | 17.4 |
| Has refrigerator/freezer, % | 43.9 |
| Has mosquito net(s), % | 82.7 |
| Participant sociodemographics | |
| Age, y | 26.5 ± 5.1 |
| Education,4 y | 9 [8, 12] |
| Married,5 % | 38.8 |
| Generates income, % | 70.4 |
| Pregnancy history, % | |
| Parity | |
| 0 | 46.9 |
| 1 | 26.5 |
| ≥2 | 26.5 |
| Spontaneous miscarriage (≥1) | 19.3 |
| Induced abortion (≥1) | 24.6 |
| Self-reported health history, % | |
| Anemia | 14.3 |
| Malaria | 55.1 |
| High blood pressure | 3.1 |
| Other heart problems | 9.2 |
| Night blindness | 15.3 |
| Measured health markers | |
| Height,6 cm | 160.0 ± 6.3 |
| BMI, kg/m2 | 23.8 [21.5, 29.0] |
| Normal (18.5 to <25.0), % | 60.2 |
| Overweight (25.0 to <30.0), % | 21.4 |
| Obese (≥30.0), % | 18.4 |
| Systolic blood pressure, mm Hg | 109.5 ± 11.6 |
| Diastolic blood pressure, mm Hg | 70.2 ± 10.4 |
Values are percentages for categorical variables, means ± SDs for continuous variables with a normal distribution, or medians [IQRs] for continuous variables with a nonnormal distribution; n = 98.
Highest number of adults and children reported in household was 7 and 8, respectively.
Two women reported “tube well/borehole.”
Six women reported 0 y of schooling; 11 women reported completing senior secondary school; no one reported completing higher education (university or polytechnics).
Although few women reported being “married,” 96 women reported being in a committed relationship; “customary marriage” is the most common/traditional union in Ghana but is often not called “marriage.”
Minimum and maximum were 141 cm and 176 cm, respectively.
Forty percent of women were overweight or obese (Table 1), and only 1 woman was classified as underweight. Four women had blood pressure in the hypertensive range; however, none of these women reported being diagnosed with high blood pressure previously. All women had negative/trace results for urine protein except 1 woman with a 30 mg/dL result. One-third of women were anemic; of these, 36% (n = 12) were moderately anemic, and 1 woman was severely anemic. For inflammation status, 4% of women were in an “incubation” stage, and 11% of women were in the “late convalescence” stage (no one was in “early convalescence”).
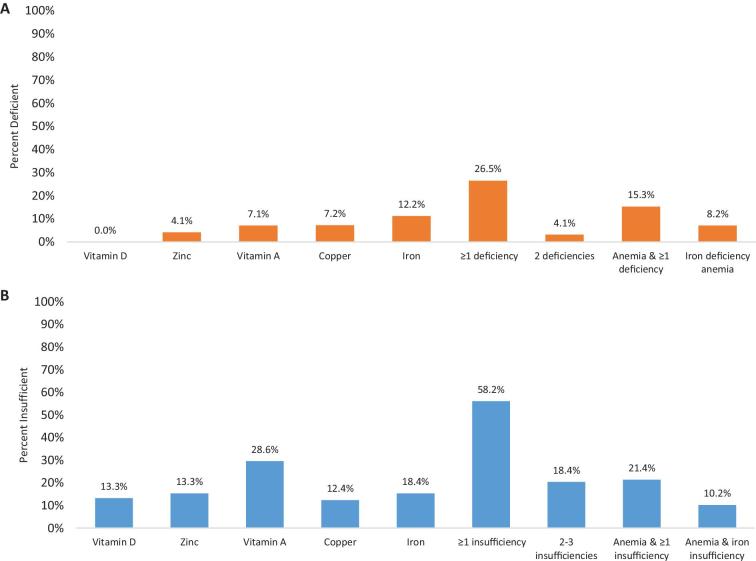
Using deficiency cut points, there were no women with vitamin D deficiency, whereas 4% of women had zinc deficiency, 7% of women had copper or vitamin A deficiency, and 12% had iron deficiency (Figure 1). Using other classifications for iron deficiency, 29% of women had TSAT <16%, and 7% of women had both ferritin <15 µg/L and TSAT <16%. Eight percent of women had a ferritin concentration of <12 µg/L, another common cut point for iron deficiency. Although 27% of women had ≥1 deficiencies, there was not much overlap, and only 4% had 2 deficiencies (none had >2).
FIGURE 1.
Prevalence of micronutrient deficiencies (A) and insufficiencies (B) of women expecting to become pregnant in Ghana (n = 98). Cutoffs used to define deficiency and insufficiency, respectively: 25-hydroxyvitamin D <30 nmol/L and <50 nmol/L (vitamin D); adjusted zinc <66 µg/dL and <70 µg/dL; adjusted retinol <20 µg/dL and <30 µg/dL (vitamin A); copper <70 µg/dL and <90 µg/dL (n = 97); and adjusted ferritin <15 µg/L and <20 µg/L. Adjustments were done using correction factors from references 37–39.
There was some overlap between women who were classified as anemic and those with a micronutrient deficiency: 45% of women with anemia also had ≥1 of the 5 deficiencies we examined (Supplemental Figure 1). On the other hand, 17% of women who were not anemic had ≥1 deficiencies. Thus, hemoglobin was not a good predictor for the presence or absence of these 5 micronutrient deficiencies. The prevalence of iron deficiency anemia was low. Only 24% of all anemia (8 of 33 cases) appeared to be due to iron deficiency (using ferritin <15 µg/L; 48% if using TSAT <16%).
Using cut points for insufficient/low status, the prevalence of poor micronutrient status was higher, with low vitamin A the most common at 29% (Figure 1). More than half of women had low status in ≥1 micronutrients, 18% had at least 2 insufficiencies, and 9 women had 3 insufficiencies. The prevalence of each deficiency and insufficiency was similar across overweight and obesity status, with no meaningful differences (P > 0.05) except that vitamin A insufficiency was higher in normal-weight than in overweight/obese women (36% compared with 18%, P = 0.04).
We also examined the prevalence of the concurrence of 2 insufficiencies to identify which micronutrients were overlapping in insufficient status (Supplemental Table 1). There was low overlap overall; however, vitamin A had the highest prevalence of co-occurrence with iron (7% of women had both) and, surprisingly, with zinc (5%), copper (6%), and vitamin D (6%) as well. Anemia was slightly more predictive of insufficiencies compared with deficiencies: 64% of those with anemia had ≥1 insufficiencies (Supplemental Figure 1).
We presented conventional summary statistics for each biomarker concentration (Table 2) along with distributions by kernel density (Supplemental Figure 2). Differences in nutrient biomarkers by measured (Table 3) and self-reported health (Table 4) indicators are shown by means or medians. Few biomarkers were associated with health indicators. As expected, there was lower TSAT and higher TIBC in those with anemia, but in this study, ferritin and iron concentrations did not differ by anemia classification (Table 3). However, anemia was associated with lower retinol concentrations. AGP concentrations were higher in those with anemia and those classified as overweight. Higher copper concentrations were observed in women with elevated blood pressure. Self-reported health characteristics—parity and history of anemia, malaria, or night blindness—were not related to biomarker concentrations except that AGP was higher among women who reported being diagnosed with anemia (Table 4).
TABLE 2.
Micronutrient and inflammation blood biomarker concentrations of women expecting to become pregnant in Asesewa, Ghana1
| Biomarker | Mean (SD) | Geometric mean (95% CI) | Median [IQR] | Range |
|---|---|---|---|---|
| Serum | ||||
| α1-acid glycoprotein,2 mg/dL | 68.9 (41.3) | 60.5 (54.7, 66.8) | 58.0 [42.1, 81.0] | 18.2–306.9 |
| Iron, µg/dL | 77.4 (42.2) | 65.8 (57.8, 74.8) | 75.0 [48.0, 96.0] | 3.0–275.0 |
| Ferritin, µg/L | 74.4 (60.6) | 51.4 (42.3, 62.4) | 51.5 [32.7, 101.6] | 1.4–284.1 |
| Adjusted ferritin,3 µg/L | 71.3 (57.9) | 49.2 (40.4, 59.9) | 51.5 [31.5, 98.5] | 1.4–284.1 |
| Total iron binding capacity, µg/dL | 312.8 (71.7) | 306.0 (293.6, 318.9) | 302.5 [271.0, 334.0] | 164.0–721.0 |
| Transferrin saturation, % | 25.5 (13.6) | 21.1 (18.2, 24.3) | 25.5 [15.0, 33.0] | 1.0–67.0 |
| 25(OH)D,4 nmol/L | 65.1 (14.2) | 63.5 (60.7, 66.5) | 65.0 [53.9, 73.1] | 31.0–102.6 |
| 25(OH)D C3 epimer, nmol/L | 2.9 (1.4) | 2.6 (2.4, 2.9) | 2.7 [1.9, 3.7] | 0.8–7.3 |
| Total 25(OH)D, nmol/L | 68.0 (15.0) | 66.3 (63.4, 69.4) | 68.7 [56.1, 75.9] | 32.1–107.6 |
| Plasma | ||||
| Copper,5 µg/dL | 114.8 (25.2) | 111.9 (106.7, 117.2) | 114.0 [101.8, 127.4] | 60.2–195.6 |
| Zinc, µg/dL | 81.6 (12.0) | 80.7 (78.4, 83.1) | 79.6 [71.6, 91.6] | 59.2–119.6 |
| Adjusted zinc,6 µg/dL | 83.1 (13.9) | 82.0 (79.4, 84.7) | 79.9 [72.1, 93.2] | 59.2–134.0 |
| Retinol, µg/dL | 37.3 (13.8) | 35.1 (32.7, 37.6) | 35.6 [28.8, 43.4] | 14.4–105.2 |
| Adjusted retinol,7 µg/dL | 38.0 (14.2) | 35.7 (33.3, 38.3) | 36.2 [29.0, 44.3] | 14.4–105.2 |
| Whole blood | ||||
| Hemoglobin, g/dL | 12.3 (1.3) | 12.2 (11.9, 12.5) | 12.4 [11.7, 13.2] | 6.1–14.8 |
n = 98. 25(OH)D, 25-hydroxyvitamin D.
Marker of inflammation; 11 α1-acid glycoprotein values were >100 mg/dL.
Adjustment using correction factors from Thurnham et al. (37).
Marker of vitamin D status; total 25(OH)D is 25(OH)D + the C3 epimer fraction.
n = 97 due to 1 missing value.
Adjustment using correction factors from Mburu et al. (38).
Marker of vitamin A status; adjustment using correction factors from Thurnham et al. (39).
TABLE 3.
Micronutrient blood biomarker concentrations of women expecting to become pregnant in Asesewa, Ghana, by common health indicators measured in the study1
| Hemoglobin | BMI | Blood pressure | ||||
|---|---|---|---|---|---|---|
| ≥12.0 g/dL | <12.0 g/dL | <25 kg/m2 | ≥25 kg/m2 | Normal | Elevated | |
| Biomarker | (n = 65) | (n = 33) | (n = 59) | (n = 39) | (n = 75) | (n = 23) |
| Serum | ||||||
| α1-acid glycoprotein,2 mg/dL | 51.9 [42.1, 78.9]* | 78.9 [51.9, 86.2]* | 51.9 [41.6, 68.5]* | 78.9 [51.9, 86.2]* | 51.9 [42.1, 81.0] | 64.0 [51.9, 95.6] |
| Iron, µg/dL | 81.2 (33.9) | 70.0 (54.9) | 81.2 (47.3) | 71.7 (32.6) | 78.1 (43.3) | 75.2 (39.0) |
| Adjusted ferritin,3 µg/L | 55.4 [41.0, 100.9] | 36.7 [16.1, 86.1] | 47.4 [31.5, 87.5] | 66.5 [24.5, 122.2] | 49.8 [31.5, 96.8] | 66.5 [19.1, 115.1] |
| TIBC, g/dL | 301.0 [261.0, 315.0]* | 329.0 [285.0, 383.0]* | 306.0 [278.0, 342.0] | 301.0 [261.0, 329.0] | 301.0 [264.0, 334.0] | 313.0 [285.0, 363.0] |
| Transferrin saturation, % | 28.0 (12.1)* | 20.4 (15.2)* | 26.3 (14.9) | 24.3 (11.5) | 25.8 (13.9) | 24.3 (12.9) |
| 25(OH)D,4 nmol/L | 65.3 (14.9) | 64.7 (12.9) | 65.0 (15.3) | 65.3 (12.5) | 64.4 (14.5) | 67.4 (13.0) |
| 25(OH)D C3 epimer, nmol/L | 2.9 (1.3) | 3.0 (1.4) | 2.9 (1.4) | 2.9 (1.4) | 2.8 (1.4) | 3.1 (1.4) |
| Total 25(OH)D, nmol/L | 68.2 (15.8) | 67.7 (13.6) | 67.8 (16.3) | 68.3 (13.1) | 67.2 (15.4) | 70.5 (13.7) |
| Plasma | ||||||
| Copper,5 µg/dL | 115.8 (25.2) | 112.6 (25.4) | 115.6 (24.9) | 113.4 (25.9) | 111.6 (23.8)* | 124.9 (27.4)* |
| Adjusted zinc,6 µg/dL | 84.2 (13.0) | 80.9 (15.6) | 81.1 (12.6) | 86.1 (15.3) | 83.6 (14.0) | 81.3 (13.8) |
| Adjusted retinol,7 µg/dL | 40.2 (15.3)* | 33.6 (10.8)* | 35.6 (11.5) | 41.7 (17.0) | 37.2 (12.4) | 40.6 (19.0) |
| Whole blood | ||||||
| Hemoglobin, g/dL | 13.0 (0.7)* | 10.9 (1.1)* | 12.1 (1.4) | 12.5 (1.1) | 12.2 (1.4) | 12.4 (0.9) |
Data are presented as arithmetic means (SDs) or medians [IQRs]; n = 98. *P < 0.05 for t test or Wilcoxon–Mann–Whitney test if distribution was normal or nonnormal, respectively. TIBC, total iron binding capacity; 25(OH)D, 25-hydroxyvitamin D.
Markers of inflammation; 11 α1-acid glycoprotein values >100 mg/L.
Adjustment using correction factors from Thurnham et al. (37).
Marker of vitamin D status.
n = 97 due to 1 missing value.
Adjustment using correction factors from Mburu et al. (38).
Marker of vitamin A status; adjustment using correction factors from Thurnham et al. (39).
TABLE 4.
Micronutrient blood biomarker concentrations of women expecting to become pregnant in Asesewa, Ghana, by self-reported health history1
| Parity | Anemia2 | Malaria2 | Night blindness3 | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | ≥1 | No | Yes | No | Yes | No | Yes | |
| Biomarker | (n = 46) | (n = 52) | (n = 83) | (n = 14) | (n = 44) | (n = 54) | (n = 83) | (n = 15) |
| Serum | ||||||||
| α1-acid glycoprotein,4 mg/dL | 55.0 [41.6, 81.0] | 59.7 [43.8, 81.0] | 51.9 [42.1, 81.0]* | 81.0 [58.0, 133.4]* | 58.0 [43.8, 81.0] | 61.5 [42.1, 81.0] | 61.5 [42.1, 81.0] | 51.9 [42.1, 64.0] |
| Iron, µg/dL | 82.2 (48.4) | 73.2 (35.7) | 76.6 (44.0) | 82.4 (32.3) | 77.6 (49.9) | 77.2 (35.1) | 76.7 (38.7) | 81.3 (59.5) |
| Adjusted ferritin,5 µg/L | 59.7 [34.0, 91.6] | 49.4 [30.2, 106.1] | 50.6 [24.5, 101.0] | 58.2 [47.4, 91.7] | 50.8 [31.6, 117.9] | 52.1 [28.8, 89.4] | 51.3 [31.7, 100.9] | 51.7 [24.1, 94.0] |
| TIBC, g/dL | 301.0 [268.0, 334.0] | 306.0 [280.5, 337.0] | 306.0 [271.0, 341.0] | 301.0 [283.0, 306.0] | 315.0 [281.5, 341.5] | 301.0 [268.0, 329.0] | 301.0 [271.0, 334.0] | 306.0 [268.0, 342.0] |
| Transferrin saturation, % | 26.3 (14.4) | 24.8 (13.0) | 25.0 (14.0) | 28.7 (11.9) | 24.2 (14.6) | 26.6 (12.8) | 26.2 (14.2) | 21.3 (9.2) |
| 25(OH)D,6 nmol/L | 63.2 (14.5) | 66.8 (13.8) | 65.1 (14.4) | 65.7 (13.6) | 66.0 (15.6) | 64.4 (13.1) | 64.8 (14.1) | 67.0 (15.0) |
| 25(OH)D C3 epimer, nmol/L | 3.0 (1.4) | 2.8 (1.3) | 3.0 (1.4) | 2.6 (1.4) | 2.9 (1.4) | 2.9 (1.4) | 2.8 (1.2) | 3.6 (1.8) |
| Total 25(OH)D, nmol/L | 66.2 (15.5) | 69.6 (14.5) | 68.1 (15.3) | 68.4 (14.0) | 68.9 (16.4) | 67.3 (13.9) | 67.5 (14.9) | 70.7 (16.0) |
| Plasma | ||||||||
| Copper,7 µg/dL | 116.5 (20.5) | 113.2 (28.7) | 114.8 (25.1) | 115.9 (26.7) | 115.6 (20.8) | 114.1 (28.4) | 115.3 (25.1) | 111.6 (26.2) |
| Adjusted zinc,8 µg/dL | 82.4 (11.9) | 83.7 (15.6) | 81.9 (12.5) | 90.6 (19.4) | 80.9 (11.3) | 84.8 (15.6) | 83.4 (14.0) | 81.3 (13.4) |
| Adjusted retinol,9 µg/dL | 37.7 (12.9) | 38.3 (15.5) | 36.9 (12.9) | 44.4 (20.2) | 38.4 (17.6) | 37.7 (11.0) | 37.7 (14.0) | 39.4 (15.8) |
| Whole blood | ||||||||
| Hemoglobin, g/dL | 12.3 (1.3) | 12.2 (1.4) | 12.3 (1.2) | 11.9 (1.9) | 12.2 (1.6) | 12.3 (1.1) | 12.3 (1.4) | 12.3 (1.1) |
Data are presented as arithmetic means (SDs) or medians [IQRs]; n = 98. *P < 0.05 for t test or Wilcoxon–Mann–Whitney test if distribution was normal or nonnormal, respectively. TIBC, total iron binding capacity; 25(OH)D, 25-hydroxyvitamin D.
Response to the question, “Has a health professional ever told you that you have . . .?”; 1 response was “don't know” and is not included in the table.
Response to the question, “Have you ever experienced night blindness?”
Marker of inflammation; 11 α1-acid glycoprotein values >100 mg/L.
Adjustment using correction factors from Thurnham et al. (37).
Marker of vitamin D status.
n = 97 due to 1 missing value.
Adjustment using correction factors from Mburu et al. (38).
Marker of vitamin A status; adjustment using correction factors from Thurnham et al. (39).
Discussion
In this cross-sectional study in a semi-urban area of Ghana, we examined the status of 5 micronutrients in WRA who were expecting to become pregnant in the next 6 mo. The prevalence of each individual deficiency was low, but more than one-fourth of women had an overt deficiency. We also found that more than half of women were insufficient in ≥1 micronutrients, and one-third had anemia. In examining health factors that related to micronutrient status, there were few associations beyond anemia's relation to iron and vitamin A status biomarkers, yet anemia had a low positive predictive value (45%) for micronutrient deficiencies. This is a setting in which most women are raising food at their homesteads, malaria is endemic, and the overall number of births per woman is moderate. Recent projects designed to reduce food insecurity, including Nutrition Links (24), have raised awareness and availability of animal source foods, and have increased nutritional knowledge in this area. The effect of these projects may account for the relatively low prevalence of micronutrient deficiencies we observed in this area compared with other low-resource settings.
Although there are numerous studies examining micronutrient status in women of reproductive age, few have used blood-based biomarkers, and of those, it is rare that studies report multiple micronutrients and concurrent deficiencies. There are 3 studies, similar to ours, that measured a relatively small number of micronutrients (3–6 each) on a small number of women (109–283 women) (17, 19, 20) in India and Indonesia. Another more comprehensive study was conducted in Nepal on >1100 pregnant women and measured the status of 11 vitamins and minerals (18). Overall, similar cut points were used to define deficiency, but insufficiency/low status was not reported in addition to deficiency prevalence (with the exception of low vitamin A status being reported in the Nepal study). In general, the prevalence of deficiencies, and concurrent deficiencies, in the women in Ghana was lower than that reported in the other 4 studies; we could not compare the prevalence of insufficiencies to the larger context because they are so rarely reported. It was quite surprising to us that so few studies have reported the overlap of biomarker-based micronutrient status, and we find it urgent and important that more studies measure and report the concurrence of multiple micronutrient deficiencies in WRA. Future reviews and meta-analyses of micronutrient deficiencies should examine the potential impact of publication bias (i.e., Are papers only published if prevalence is high?) on reporting.
Here, as we aimed to draw attention to the issues of co-occurring deficiencies, we also highlight that although often mentioned as a major concern, the negative health consequences of overlapping micronutrient deficiencies are not well documented. It is known that when mineral deficiencies are extreme or overlapping, there is a higher risk of lead, arsenic, or other toxic heavy metal uptake, causing a double hit of the burden of deficiencies (41, 42). When both calcium and vitamin D are deficient, there is a much higher risk for poor bone health outcomes (43). Vitamin A, zinc, and other micronutrient deficiencies can exacerbate iron deficiency anemia by disrupting iron transport or hematopoiesis, and ultimately they can cause continued anemia even with iron repletion (44, 45). In this study in Ghana, vitamin A insufficiency was most commonly co-occurring with other insufficiencies (iron, zinc, and vitamin D). In the study of pregnant Nepalese women, zinc had the highest overlap with other deficiencies (vitamin A, vitamin B-6, vitamin B-12, riboflavin, and iron) (18). Yet, none of the studies we found connected co-occurring deficiencies with specific health outcomes in WRA or during pregnancy. Because poor reproductive and pregnancy outcomes (e.g., miscarriage, stillbirth, preterm birth, and fetal growth restriction) remain an enormous public health burden, understanding the relation between multiple deficiencies and higher risk of adverse outcomes is paramount. On a positive note, there is now substantial evidence to show a benefit to healthy pregnancy outcomes when multiple micronutrients are given as a supplement to women during pregnancy (compared with only iron and folic acid) (46, 47).
Because there are regional and local-level differences in nutritional status, it is important to be able to assess the micronutrient status of individuals and communities. Although this study was small, we did not find common health indicators or self-reported markers that would provide insight into micronutrient status, aside from the known relations between anemia, iron, and vitamin A. As part of the vitamin D status assessment, we examined the C3 epimer fraction of 25(OH)D and did not find that its inclusion impacted our results (we chose to report it because of the emerging interest in the potential differences in concentrations by age and race/ethnicity). When iron biomarkers are not measured, it is typically estimated that half of anemia is due to iron deficiency. Here, we found a lower ratio of iron deficiency within anemia (depending on the classification for iron deficiency, 24–48% of those anemic) and in general found anemia status as a poor positive or negative predictor of deficiencies or insufficiencies. Simple, common health screening data, similar to those reported in this article, should be tested in populations with a higher prevalence of micronutrient deficiencies. Because it is difficult and costly to measure and interpret micronutrient biomarkers, we recommend that resources be focused on identifying potential screening tools in addition to developing inexpensive and less invasive methods for more directly measuring micronutrient status.
In this study, we were most interested in examining the prepregnancy health and nutritional status of women who might become pregnant soon. Because this study was planned to pilot field methods for identifying women to follow through to pregnancy, the sample size was small and we did not measure dietary intake. We did, however, use rigorous methods for assessing micronutrient status—by examining multiple biomarkers in blood (rather than relying on dietary intake), by accounting for inflammation, and by using robust lab methods [including measurement of several iron biomarkers and the C3 epimer of 25(OH)D]. We also assessed both deficiencies and insufficiencies. We were limited in resources (funds and the amount of blood collected) and were only able to assess 5 micronutrients; ideally, most essential vitamins and minerals would be examined, particularly others that cause anemia (e.g., folate and vitamin B-12). Yet, micronutrients are essential and have multiple roles in the body; and all of those we examined have been found to be important before and during pregnancy (48–50). Malaria is endemic in the study area, but we did not test for it in participants; thus, we could not examine associations with micronutrients, nor account for it in adjusting relevant biomarker concentrations. Of note, the Biomarkers Reflecting Inflammation and Nutrition Determinants of Anemia project found that accounting for malaria did not make a meaningful impact on the prevalence of iron deficiency (40).
The majority of women in this semi-urban area in Ghana were insufficient in ≥1 of 5 micronutrients assessed (iron, zinc, copper, vitamin A, and vitamin D), and more than one-fourth were deficient in at least 1 micronutrient. Although the true burden of deficiency in the population is expected to be much higher, because we measured only 5 of ∼30 essential or semi-essential vitamins and minerals, even the prevalence observed could have a large impact in this unique set of women expecting to become pregnant within the next 6 mo. Numerous studies have found that micronutrient deficiencies are high in women and children (5, 16, 51, 52), recognized as “hidden hunger” because it is more difficult to identify and measure compared with energy deficiency. However, quantifying concurrent deficiencies and their collective impact on health has not been given enough attention. Assessments are often limited by the complexity of collecting and measuring biomarker concentrations in a larger sample of blood. Moving forward, we strongly urge development of easier but accurate methods for assessing more biomarkers and the evaluation and reporting of concurrent deficiencies in all studies in which multiple micronutrients are assessed.
Supplementary Material
Acknowledgments
We thank Claudia Ewa for helping set up and oversee the fieldwork. We thank Boateng Bannerman for his immense work on setting up the tablets and data sets for data collection. We appreciate the early help of Moses Klevor in establishing logistics and supplies as well as connecting us with community, hospital, and religious group leaders. We are grateful to A. Catharine Ross for her expert input on vitamin A status and to Nan-qian Li and Cheng-Hsin (Gina) Wei for determining retinol concentrations. Finally, we thank Suzanne Simons for her expert help and oversight for iron biomarker measurements. The authors’ responsibilities were as follows—ADG, SA, RP, EKC, and LEM-K: designed the research; ADG, SA, RP, and LEM-K: conducted the research; ADG: analyzed the data; ADG, SA, and RP: wrote the article; ADG: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This study was partially funded by the Africana Research Center at The Pennsylvania State University (grant LMRYKB14FF).
Author disclosures: ADG, SA, RP, EKC, and LEM-K, no conflicts of interest.
Supplemental Figures 1 and 2 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: AGP, α1-acid glycoprotein; CRP, C-reactive protein; TIBC, total iron binding capacity; TSAT, transferrin saturation; WRA, women of reproductive age; 25(OH)D, 25-hydroxyvitamin D.
References
- 1. Ramakrishnan U. Prevalence of micronutrient malnutrition worldwide. Nutr Rev 2002;60(Suppl 5):S46–52. [DOI] [PubMed] [Google Scholar]
- 2. Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 2000;71(5):1280S–4S. [DOI] [PubMed] [Google Scholar]
- 3. Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 2006;64(5 Pt 2):S34–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black RE. Micronutrients in pregnancy. Br J Nutr 2001;85(S2):S193. [DOI] [PubMed] [Google Scholar]
- 5. Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab 2015;66(2):22–33. [DOI] [PubMed] [Google Scholar]
- 6. Benton D. Vitamins and neural and cognitive developmental outcomes in children. Proc Nutr Soc 2012;71(1):14–26. [DOI] [PubMed] [Google Scholar]
- 7. Fenech MF. Dietary reference values of individual micronutrients and nutriomes for genome damage prevention: current status and a road map to the future. Am J Clin Nutr 2010;91(5):1438S–54S. [DOI] [PubMed] [Google Scholar]
- 8. UNICEF. Micronutrient initiative (2004) vitamin and mineral deficiency: a global progress report. New York: UNICEF; 2004. [Google Scholar]
- 9. Ward E. Addressing nutritional gaps with multivitamin and mineral supplements. Nutr J 2014;13(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hwalla N, Al Dhaheri A, Radwan H, Alfawaz H, Fouda M, Al-Daghri N, Zaghloul S, Blumberg J. The prevalence of micronutrient deficiencies and inadequacies in the Middle East and approaches to interventions. Nutrients 2017;9(3):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Investing in the future—A united call to action on vitamin and mineral deficiencies: global report 2009. New Delhi (India): Micronutrient Initiative; 2009. [Google Scholar]
- 12. Gernand AD, Schulze KJ, Stewart CP, West KP, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol 2016;12(5):274–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alaofè H, Burney J, Naylor R, Taren D. Prevalence of anaemia, deficiencies of iron and vitamin A and their determinants in rural women and young children: a cross-sectional study in Kalalé district of northern Benin. Public Health Nutr 2017;20(7):1203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asare GA, Nani A.. Serum levels of Cu, Se, and Zn in adult rural/urban residents in Ghana: paradigm shift? Biol Trace Elem Res 2010;137(2):139–49. [DOI] [PubMed] [Google Scholar]
- 15. Harika R, Faber M, Samuel F, Kimiywe J, Mulugeta A, Eilander A. Micronutrient status and dietary intake of iron, vitamin A, iodine, folate and zinc in women of reproductive age and pregnant women in Ethiopia, Kenya, Nigeria and South Africa: a systematic review of data from 2005 to 2015. Nutrients 2017;9(10):1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R et al.. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet North Am Ed 2013;382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 17. Menon KC, Skeaff SA, Thomson CD, Gray AR, Ferguson EL, Zodpey S, Saraf A, Das PK, Toteja GS, Pandav CS. Concurrent micronutrient deficiencies are prevalent in nonpregnant rural and tribal women from central India. Nutrition 2011;27(4):496–502. [DOI] [PubMed] [Google Scholar]
- 18. Jiang T, Christian P, Khatry SK, Wu L, West KP Jr.. Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J Nutr 2005;135(5):1106–12. [DOI] [PubMed] [Google Scholar]
- 19. Pathak P, Kapil U, Kapoor SK, Saxena R, Kumar A, Gupta N, Dwivedi SN, Singh R, Singh P. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr 2004;71(11):1007–14. [DOI] [PubMed] [Google Scholar]
- 20. Dijkhuizen MA, Wieringa FT, West CE, Muherdiyantiningsih, Muhilal. Concurrent micronutrient deficiencies in lactating mothers and their infants in Indonesia. Am J Clin Nutr 2001;73(4):786–91. [DOI] [PubMed] [Google Scholar]
- 21. Torheim LE, Ferguson EL, Penrose K, Arimond M. Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J Nutr 2010;140(11):2051S–8S. [DOI] [PubMed] [Google Scholar]
- 22. Ghana Statistical Service. 2010 population and housing census: the district analytical report for the Upper Manya Krobo District. Accra (Ghana): Ghana Statistical Service; 2014. [Google Scholar]
- 23. Brunette W, Sundt M, Dell N, Chaudhri R, Breit N, Borriello G. Open data kit 2.0: expanding and refining information services for developing regions. Proceedings of the 14th Workshop on Mobile Computing Systems and Applications. New York: ACM; 2013:10. [Google Scholar]
- 24. Marquis GS, Colecraft EK, Kanlisi R, Aidam BA, Atuobi-Yeboah A, Pinto C, Aryeetey R. An agriculture-nutrition intervention improved children's diet and growth in a randomized trial in Ghana. Matern Child Nutr 2018;14(Suppl 3):e12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 26. Smith JCJ, Butrimovitz GP, Purdy WC. Direct measurement of zinc in plasma by atomic absorption spectroscopy. Clin Chem 1979;25(8):1487–91. [PubMed] [Google Scholar]
- 27. Kiilerich S, Christiansen C, Naestoft J. Determination of zinc in serum and urine by atomic absorption spectrophotometry: relationship between serum levels of zinc and proteins in 104 normal subjects. Clin Chim Acta 1980;105:231–9. [DOI] [PubMed] [Google Scholar]
- 28. Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov E, McCoy LF, Pfeiffer CM. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta 2011;412(17–18):1594–9. [DOI] [PubMed] [Google Scholar]
- 29. Yazdanpanah M, Bailey D, Walsh W, Wan B, Adeli K. Analytical measurement of serum 25-OH-vitamin D(3), 25-OH-vitamin D(2) and their C3-epimers by LC-MS/MS in infant and pediatric specimens. Clin Biochem 2013;46(13–14):1264–71. [DOI] [PubMed] [Google Scholar]
- 30. WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System [Internet]. Geneva (Switzerland): WHO; 2011[cited 26 July, 2017]. Available from: http://www.who.int/vmnis/indicators/serum_ferritin.pdf. [Google Scholar]
- 31. CDC. Recommendations to prevent and control iron deficiency in the United States. MMWR 1998;47(RR-3):1–36. [PubMed] [Google Scholar]
- 32.de Pee S, Dary O.. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr 2002;132(Suppl):2895S–901S. [DOI] [PubMed] [Google Scholar]
- 33. Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull 2007;28(3):S403–S29. [DOI] [PubMed] [Google Scholar]
- 34. Institute of Medicine , Food and Nutrition Board. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicone, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 35. Institute of Medicine , Food and Nutrition Board. Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 36. WHO/CDC. Assessing the iron status of populations: report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level, 6–8 April, 2004. [Internet]. Geneva (Switzerland):WHO; 2007[cited 21 July, 2017]. Available from: https://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107/en/. [Google Scholar]
- 37. Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010;92(3):546–55. [DOI] [PubMed] [Google Scholar]
- 38. Mburu A, Thurnham D, Mwaniki D, Muniu E, Alumasa F. The influence of inflammation on plasma zinc concentration in apparently healthy, HIV+ Kenyan adults and zinc responses after a multi-micronutrient supplement. Eur J Clin Nutr 2010;64(5):510. [DOI] [PubMed] [Google Scholar]
- 39. Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003;362:2052–58. [DOI] [PubMed] [Google Scholar]
- 40. Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ et al.. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 2017;106(Suppl 1):359s–71s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahaffey KR. Environmental lead toxicity: nutrition as a component of intervention. Environ Health Perspect 1990;89:75–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang H, Shi H, Chang L, Zhang X, Li J, Yang Y, Jiang Y. Association of blood lead with calcium, iron, zinc and hemoglobin in children aged 0–7 years: a large population-based study. Biol Trace Elem Res 2012;149(2):143–7. [DOI] [PubMed] [Google Scholar]
- 43. Pettifor JM. Nutritional rickets: deficiency of vitamin D, calcium, or both? Am J Clin Nutr 2004;80(6):1725S–9S. [DOI] [PubMed] [Google Scholar]
- 44. Cañete A, Cano E, Muñoz-Chápuli R, Carmona R. Role of vitamin A/retinoic acid in regulation of embryonic and adult hematopoiesis. Nutrients 2017;9(2):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kraemer K., Zimmermann MB, eds. Nutritional anemia. Basel (Switzerland): Sight and Life Press; 2007. [Google Scholar]
- 46. Smith ER, Shankar AH, Wu LS, Aboud S, Adu-Afarwuah S, Ali H, Agustina R, Arifeen S, Ashorn P, Bhutta ZA et al.. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob Health 2017;5(11):e1090–e100. [DOI] [PubMed] [Google Scholar]
- 47. Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 2019;3:CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cetin I, Berti C, Calabrese S. Role of micronutrients in the periconceptional period. Hum Reprod Update 2010;16(1):80–95. [DOI] [PubMed] [Google Scholar]
- 49. Darnton-Hill I, Mkparu UC.. Micronutrients in pregnancy in low- and middle-income countries. Nutrients 2015;7(3):1744–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Simpson JL, Bailey LB, Pietrzik K, Shane B, Holzgreve W. Micronutrients and women of reproductive potential: required dietary intake and consequences of dietary deficiency or excess. Part II: vitamin D, vitamin A, iron, zinc, iodine, essential fatty acids. J Matern Fetal Neonatal Med 2011;24(1):1–24. [DOI] [PubMed] [Google Scholar]
- 51. Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, Calvo MS, Cashman KD, Combs G, De-Regil LM et al.. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci 2018;1430(1):44–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. WHO. Global prevalence of vitamin A deficiency in populations at risk 1995–2005: WHO Global Database on Vitamin A Deficiency. Geneva (Switzerland): WHO; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.