Abstract
Pulmonary sclerosing pneumocytoma (PSP) is a rare benign neoplasm of the lung that shows a slow growing pattern. Corresponding contrast-enhancements on chest computed tomography (CT) vary widely in both patterns and degrees. However, gross intratumoral radiolucencies, attributable to cyst formation, necrosis, or intratumoral hematoma, were rarely reported in PSP cases. We herein report on a case involving a 61-year-old Japanese women with PSP demonstrating CT-defined intratumoral radiolucency. A chest CT scan revealed a solitary and well-circumscribed nodule that showed a substantial growth over a 7-year period. The tumor was composed of a solid portion visualized with contrast-enhancement and a central radiolucency on a chest CT scan. A positron emission tomography scan revealed high uptake of fluorodeoxyglucose on the solid portion of the tumor, but the radiolucent portion showed negative uptake. The examination of a tumor specimen obtained by a percutaneous core needle biopsy aided in determining a pathological diagnosis of PSP, and the patient subsequently received a right lower lobectomy of the lung. The portion of central radiolucency on the CT scan corresponding to the surgical specimen was pathologically proven to be gross hematoma.
Keywords: Computed tomography, Hematoma, Positron emission tomography, Pulmonary sclerosing pneumocytoma, Tumor doubling time
1. Introduction
Pulmonary sclerosing pneumocytoma (PSP), formerly called as sclerosing hemangioma, is a rare form of benign neoplasm of the lung that predominantly occurs in middle-aged women. This tumor typically demonstrates as a well-circumscribed solitary nodule on a computed tomography (CT) scan and grows slowly. Contrast-enhancements on CT scans differ among cases, whether homogeneous or heterogeneous. However, the presence of intratumoral radiolucency, attributable to cyst formation, necrosis, or hematoma, is unusual.
In the present study, we describe a PSP case that showed substantial tumor growth over a period of 7 years. The tumor was accompanied by gross intratumoral hematoma on a CT scan, which was confirmed in the resected specimen.
2. Material and methods
2.1. Case presentation
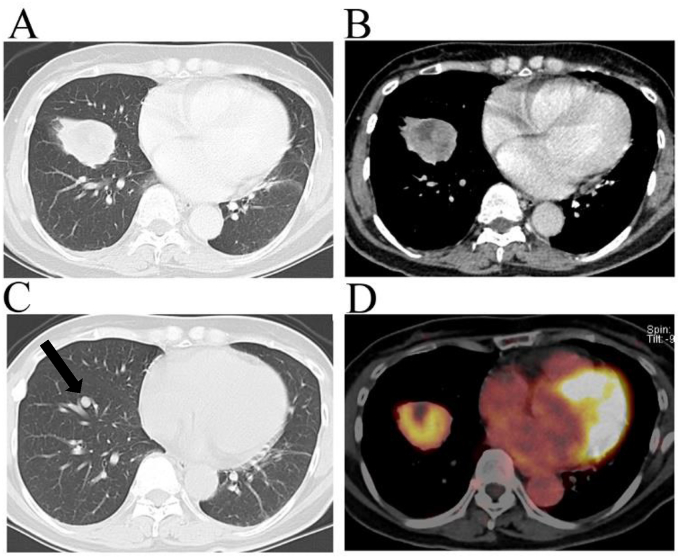
A 61-year-old woman visited the Hino Municipal Hospital complaining of palpitation, which disappeared soon after her visit. Incidentally, on an X-ray examination, a lung nodule on the right side was pointed out to her, although there were not any related symptoms. A contrast-enhanced chest CT scan revealed a solitary and well-circumscribed nodule (36 × 37 × 40 mm in diameter) in the peripheral region of the right lower lobe (Fig. 1A). While a solid part of the tumor was homogeneously enhanced, the tumor included an irregular-shaped low-density area (19 mm in maximum diameter) that was free from contrast-enhancement (Fig. 1B). Lymph node enlargement or pleural effusion was absent. Her medical record showed that a small lung nodule was previously recognized on a CT scan in our hospital when she was 54 years old (Fig. 1C). A further CT scan had been scheduled, but she missed her follow-up. The initial CT scan when her was 54 years old showed a small nodule (7.5 × 7.9 × 8.7 mm in diameter) in the right lower lobe (Fig. 1C, an arrow mark), which corresponded with the grown tumor in the present study. The tumor doubling time was estimated to be 374 days. She had never smoked and was taking medicines for hypertension.
Fig. 1.
A chest computed tomography scan revealed a solitary nodule accompanying a central radiolucency in the right lower lobe when the patient was 61 years old (A and B), which had apparently grown compared to its size when she was 54 years old (arrow mark) (C). A fluorodeoxyglucose (FDG)-positron emission tomography scan when her was 61 years old revealed a high maximum standardized uptake value of 5.2 on the solid portion of the tumor while a central portion showed negative FDG uptake (D).
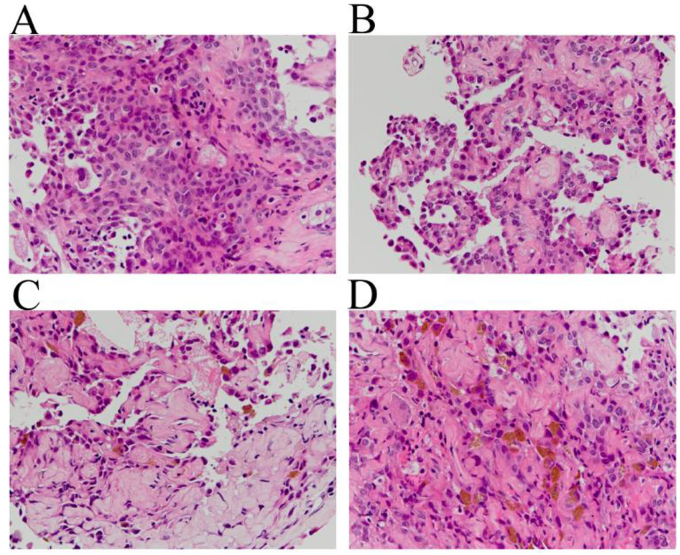
The patient was suspected of having a stage IB primary lung cancer after an evaluation of her whole body via contrast-enhanced CT and magnetic response imaging scans. A fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan revealed a high maximum standardized uptake value (SUVmax) of 5.2 on the solid part of the lung nodule while a portion of intratumoral radiolucency did not show significant FDG uptake (Fig. 1D). Following this, a diagnostic CT-guided transcutaneous core needle biopsy was scheduled. The biopsy specimen obtained from the solid part of the tumor was composed of two major pathological patterns, a solid proliferation of round cells and papillary growth of type Ⅱ pneumocyte-like cuboidal cells overlying a core comprised of round cells (Fig. 2A and B). Sclerotic and hemorrhagic patterns were also evident in minor regions (Fig. 2C and D). All of the four characteristic components necessary for a diagnosis of PSP were pathologically proven in a biopsy specimen.
Fig. 2.
A microscopic examination of the pathological specimen obtained by a transcutaneous core needle biopsy predominantly showed solid (A) and papillary (B) patterns, in concomitance with the minor findings of sclerosing (C) and hemorrhagic (D) components (hematoxylin and eosin staining).
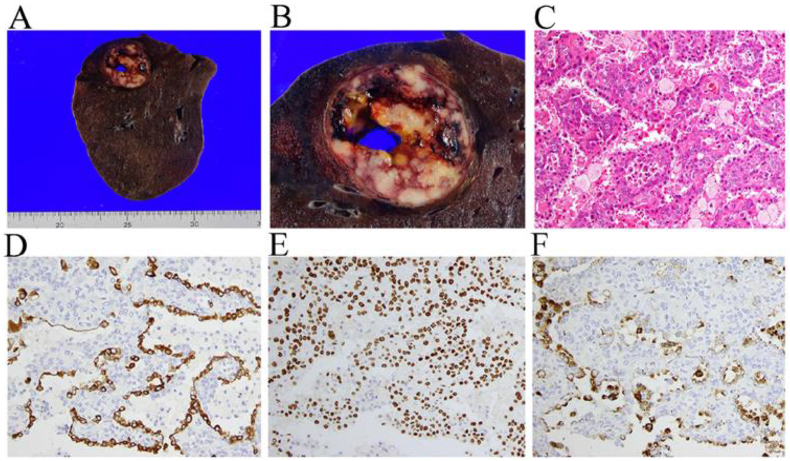
Since the tumor doubling time was relatively short in this case, she received a right lower lobectomy and a lymphadenectomy at the Federation of National Public Service Personnel Mutual Aid Associations, Tachikawa Hospital. An examination of the gross features of the fixed lung revealed an encapsulated tumor (30 × 35 × 35 mm in size) underneath the visceral pleura in the right lower lobe accompanying a cavitary space filled with bloody content (Fig. 3A and B). The cavity formation was pathologically attributed to a gross hematoma but not to the hemorrhagic cyst nor to tumor necrosis. The microscopic examination of a solid part of the resected tumor confirmed a diagnosis of PSP, predominantly showing a papillary growth pattern with a portion of solid and sclerotic patterns (Fig. 3C). Immune-staining was positive for cytokeratin 7 (Fig. 3D), thyroid transcription factor-1 (TTF-1) (Fig. 3E), and napsin A (Fig. 3F) on the papillary-growing type Ⅱ pneumocyte-like cuboidal cells whereas solid-growing round cells were positive only for TTF-1 (Fig. 3E). Together, the immune staining patterns were consistent with those related to PSP. Lymph node metastasis was pathologically absent in the surgical specimens.
Fig. 3.
An examination of the gross features of the surgical specimen revealed an encapsulated tumor underneath the visceral pleura with intratumoral gross hematoma (A and B). A microscopic examination predominantly showed papillary growths of cuboidal and round cells on hematoxylin and eosin staining (C). The surface cuboidal cells stained positive for cytokeratin-7 (D), thyroid transcription factor-1 (TTF-1) (E), and napsin A (F), while the round cells were positive only for TTF-1 (Fig. 3E).
3. Results and discussion
PSP is a rare benign neoplasm that was first described as sclerosing hemangioma by Liebow and Hubbell in 1956 [1]. While this tumor was previously believed to arise from endothelial cells, the results of the current research using electron microscopy and immune staining suggest that the tumor cells were originated from type Ⅱ pneumocytes.
Radiological findings in PSP cases are quite nonspecific; typically, an examination would reveal a well-circumscribed solitary nodule via contrast-enhancement with varying degrees and patterns on a chest CT scan. The heterogeneous internal texture of this type of tumor was observed in 25–59% of PSP cases [2,3], and larger sized PSP tumors were more likely to show heterogeneous enhancements [3]. Intratumoral radiolucency, attributable to cyst formation, necrosis, or hematoma, was an unusual finding [2,3] on a CT scan; however, a rare case of PSP demonstrating CT-defined intratumoral cystic formation was described [4]. In our case, a chest CT scan revealed a homogeneously-enhanced solid tumor, despite the relatively large tumor size (40 mm in maximum diameter). Importantly, the tumor was accompanied by a gross intratumoral radiolucency that was pathologically proven to be hematoma. A radiolucent portion revealed on the CT scan had a negative FDG uptake on a PET scan, showing true hematoma but not hemorrhagic/hemangiomatous tissue components. To the best of our knowledge, the present case report was the first to describe CT-defined intratumoral hematoma in PSP. Since a solitary lung nodule with central radiolucency closely mimics a primary lung cancer with central necrosis, we should be aware of such rare radiological findings in PSP cases.
In most cases, PSP is diagnosed with a tumor measuring a size of less than 3 cm in diameter [5]. While lymph node metastasis or pulmonary metastasis were rarely described in case reports [6,7], PSP mostly shows a slow-growing pattern without malignant behavior. In the present case, the tumor size was relatively large, and the tumor doubling time was 374 days, which was much shorter than a median of 965 days (range: 450–2384 days) as previously reported [4,8]. We presumed that the intratumoral hematoma was also a causative factor for the large tumor size in the present case. A case series study by Lee et al. showed that the size of a tumor was positively correlated with a higher SUVmax on a PET-CT scan in PSP cases [9]; therefore, they implied a higher SUVmax was potentially associated with a rapid growth rate. In this regard, the present case interestingly showed a relationship between higher than normal SUVmax and a shorter tumor doubling time in PSP.
PSP is pathologically composed of a mixture of four major components, papillary, solid, sclerosing, and hemorrhagic/hemangiomatous, out of which two or more components are needed for an accurate diagnosis. Thus, hemorrhagic foci in association with a hemorrhagic/hemangiomatous component were frequently found under microscopic (76 in 100 cases) or macroscopic (20 in 100 cases) observations of surgical specimens in PSP cases [5]. Nevertheless, a formation of gross intratumoral hematoma, with a detectable size on a CT scan, is an exclusively rare occurrence and has not been previously described in the literature.
In summary, we described a case report of PSP with intratumoral hematoma. Physicians should be aware of rare radiological findings like this one associated with PSP cases.
4. Conclusions
We experienced a rare PSP case demonstrating intratumoral hematoma.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author's contribution
All of the authors ensure to task force for preparing the manuscript and approved the final version of the manuscript. All authors contributed the clinical care for the patient in specialized settings.
Consent for publication
Written consent was obtained from the patient for publication of this case report and for a use of accompanying images.
Declaration of interest
None of the authors has any conflicts of interest or any financial ties to disclose.
Submission declaration and verification
The present study was not published or is not currently submitted to any other journal.
Competing interests
None of the authors has any conflicts of interest or any financial ties to disclose.
Acknowledgement
We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Liebow A.A., Hubbell D.S. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer. 1956 Jan-Feb;9(1):53–75. doi: 10.1002/1097-0142(195601/02)9:1<53::aid-cncr2820090104>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Shin S.Y., Kim M.Y., Oh S.Y., Lee H.J., Hong S.A., Jang S.J., Kim S.S. Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Medicine (Baltim.) 2015 Jan;94(4):e498. doi: 10.1097/MD.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q.B., Chen Y.Q., Shen J.J., Zhang C., Song B., Zhu X.J., Zhang B. Sixteen cases of pulmonary sclerosing haemangioma: CT findings are not definitive for preoperative diagnosis. Clin. Radiol. 2011 Aug;66(8):708–714. doi: 10.1016/j.crad.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Sugio K., Yokoyama H., Kaneko S., Ishida T., Sugimachi K. Sclerosing hemangioma of the lung: radiographic and pathological study. Ann. Thorac. Surg. 1992 Feb;53(2):295–300. doi: 10.1016/0003-4975(92)91336-8. [DOI] [PubMed] [Google Scholar]
- 5.Devouassoux-Shisheboran M., Hayashi T., Linnoila R.I., Koss M.N., Travis W.D. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am. J. Surg. Pathol. 2000 Jul;24(7):906–916. doi: 10.1097/00000478-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Pokharel S., Dhillon S.S., Ylagan L., George S., Yendamuri S. Sclerosing pneumocytoma with lymph node metastasis. J. Thorac. Oncol. 2016 Oct;11(10):1802–1804. doi: 10.1016/j.jtho.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu T., Fukuse T., Wada H., Sakurai T. Pulmonary sclerosing hemangioma with pulmonary metastasis. Thorac. Cardiovasc. Surg. 2006 Aug;54(5):348–349. doi: 10.1055/s-2005-872976. [DOI] [PubMed] [Google Scholar]
- 8.Oka S., Ono K., Kuwata T., Nagata Y., Baba T., Shigematsu Y., Shimokawa H., Uramoto H., Hanagiri T., Tanaka F. Surgical treatment for patients with pulmonary sclerosing hemangioma. J. UOEH. 2011 Mar;33(1):41–45. doi: 10.7888/juoeh.33.41. [DOI] [PubMed] [Google Scholar]
- 9.Lee E., Park C.M., Kang K.W., Goo J.M., Kim M.A., Paeng J.C., Lee H.J., Park H.S., Chung D.H. 18F-FDG PET/CT features of pulmonary sclerosing hemangioma. Acta Radiol. 2013 Feb 1;54(1):24–29. doi: 10.1258/ar.2011.110474. [DOI] [PubMed] [Google Scholar]