Abstract
Objective:
Stressful life events (SLEs) impact the quality of life (QOL) of cancer patients. This study investigated the mediation of the relationship between SLEs and QOL (Model 1: Emotional-EQOL and Model 2: Physical/Functional-PFQOL by three types of coping: Action/Planning, Support/Advise-Seeking, and Disengagement/Denial.
Design and Main Measures:
662 persons with cancer completed a Stressful Life Events Checklist, the Brief COPE scale, the FACT Emotional, Physical, and Functional Scales, and the Physical Impact Scale of the Sickness Impact Profile.
Results:
SLEs were positively associated with Action/Planning (Model 1:B=0.195, 95% CI=[0.089, 0.304]; Model 2:B=0.192, 95% CI=[0.086, 0.289]) and Disengagement/Denial (Model 1:B=0.394, 95% CI=[0.281, 0.513]; Model 2:B=.392, 95% CI=[0.285, 0.508]) but not Support/Advice-Seeking ; however, only Disengagement/Denial was related to Emotional-QOL (Model 1:B=−0.659, 95% CI=[−0.848, −0.498]) and Physical/Functional-QOL (Model 2:B= −1.460, 95% CI=[−1.856, −1.069]). Thus, only Disengagement/Denial mediated the relationship between SLEs and Emotional-QOL and Physical/Functional-QOL.
Conclusions:
The results indicated that SLEs represent a class of events for which there may be only one dominant coping response, disengagement. SLEs may not be controllable or predictable and reduce capacity for active coping with serious illness. However, SLEs may be detected at any point in the cancer trajectory so that supportive services might be provided.
Keywords: cancer, coping, stressful life events, quality of life
In the course of coping with a diagnosis of cancer, enduring treatments and potentially managing concurrent, late, and long-term physical and emotional effects, individuals may be confronted with a variety of challenges and stressors that can impair quality of life and cause distress (Alfano & Rowland, 2006; Bayly & Lloyd-Williams, 2016; Chirico, Lucidi, Alizernini, Merluzzi, Giordano, 2017; Mitchell, Ferguson, Gill, & Symonds, 2013; Stanton, Ganz, & Rowland, 2005; Stein, Syrjala, & Andrykowski, 2008). Given the stress inherent in cancer, it has been conceptualized as a traumatic event and studied as a condition that may invoke post-traumatic stress (Andrykowski & Kangas, 2010; Kangas, Henry & Bryant, 2002; Shelby, Golden Kreutz, & Andersen, 2008). Prevalence estimates for clinical levels of PTSD in cancer patients range from 2.4% to 32% and these estimated prevalence rates vary greatly based factors such as the diagnostic criteria considered, the quality of the measure used, phase in the cancer trajectory, stage at diagnosis, treatments, and side effects (Arnaboldi, Riva, Crico, & Pravettoni, 2017; Mehnert & Koch, 2007; Pérez et al., 2014).
In addition to the trauma that results from the diagnosis, treatment, and long-term effects of cancer, patients and survivors are embedded in lives where cancer is not the only stressful event with which they are contending. Other stressful life events (SLEs) precede, co-occur, and interact with cancer. Whereas SLEs do not account for the etiology of cancer (Butow, Hiller, Price, Thackway, Kricker, & Tennant, 2000; Shoemaker et al., 2016) they may contribute to the progression of disease (Chida, Hamer, Wardle, & Steptoe, 2008) and an individual’s ability to manage its effects. They also have an impact on biomarkers such as telomere length, nucleotide sequences at the end of chromosomes that provide genomic stability, and are associated with cellular aging, disease morbidity and mortality (Osler, Bendix, Rask, & Rod, 2016). Recent evidence, in the context of cancer, has supported the relationship between SLEs and physiological and psychological outcomes such as atypical blunted cortisol reactivity (Costanzo, Stawski, Ryff, Coe, & Almeida, 2012; Couture-Lalande, Lebel, & Bielajew, 2014), distress (Langford et al., 2017) and compromised quality of life (QOL; Golden-Kruetz et al., 2005). Moreover, SLEs prior to a diagnosis of breast cancer may place women at higher risk for physical, psychological, and social problems (Beatty, Lee & Wade, 2009) in the post-diagnosis phase.
It is interesting to note that persons with cancer may not experience more SLEs than healthy controls (Beatty et al., 2009) but they perceive those events as more severe and disruptive (Costanzo et al., 2012). In addition, those events are associated with more negative affect, less positive affect, and more physical symptoms (Costanzo et al, 2012). Moreover, distress and compromised QOL have deleterious personal and health effects such as disruption of personal relationships and roles (Beatty et al., 2009), dysregulation of immune functioning, reduced adherence to medical regimens (Kennard, 2004), longer hospitalizations (Prieto et al., 2002) and dissatisfaction with care (Bui, Ostir, Kuo, Freeman, & Goodwin, 2005). Thus, SLEs do provoke a variety of physical and psychological responses, which have some consequences that may impact adjustment to cancer as well as important quality of life and health outcomes.
A critical issue in the relationship between SLEs and outcomes such as distress, PTSD symptoms, and QOL is variation in the ultimate effects of this process. One approach to accounting for the relationship of SLEs and negative effects is dispositional resilience (Kenne Sarenmalm, Browall, Peeaaon, Fall-Dickson, & Gaston-Johansson, 2013), that is, the extent to which one believes that events are predictable and explicable and one has the capability to meet the challenges posed by the events (Kenne Sarenmalm et al., 2013). Alternatively, there is a process-oriented coping model (Lazarus & Folkman, 1984) in which events are appraised for threat or harm. Responses to those events are constructed according to the resources that a person can put forth to manage the demands of the situation. If a person is generally efficacious in meeting the demands of all SLEs, then dispositional resilience may prevail. However, for most persons with cancer, as the allostatic load or burden (Sterling & Eyer, 1988) increases in the context of coping with a serious disease, its treatments, and side effects, SLEs provide additional challenges to a potentially already overloaded coping system (Langford et al., 2017). Nevertheless, there is a paucity of research on the utility of a coping model to account for the relationship of SLEs with outcomes, such as distress and QOL, by means of coping mechanisms as mediators.
Recently, Langford et al. (2017) elaborated upon Andersen’s (Andersen, Kiecolt Glaser, & Glaser, 1994) bio-behavior model of cancer stress and disease course by evaluating coping mechanisms that may mediate the relationship of SLEs with psychological distress. They proposed two overarching coping mechanisms, engagement and disengagement coping. Engagement coping is characterized by planning and taking action, whereas disengagement coping is characterized by avoidance, withdrawal and denial. Studies in many areas of health psychology have shown that engagement-type coping is associated with more positive outcomes than disengagement-type coping (e.g., Chirico et al., 2017; Roesh et al., 2005). Langford et al. (2017) found that disengagement coping mediated the relationship between the number of SLEs and cancer-related distress. Moreover, although the number of SLEs was related to engagement coping, the path from engagement coping to distress was not significant. Finally, the direct effect from SLEs to distress was not statistical significant indicating that the indirect effects involving disengagement coping alone accounted for distress in that model.
The basic premise of this study, as in Langford et al., (2017) is that SLEs are related to critical quality of life and health outcomes. Moreover, SLEs may not be perceived as particularly controllable, therefore engagement or action planning coping may not be viable options compared to disengagement and withdrawal from the stressor, which are processes that are consistent with models of self-regulation (Carver & Scheier, 1998). In contrast to Langford et al. (2017) the current study included social support/advice seeking as a coping mechanism, which may be a more functional, adaptive option in the context of stressors than either engagement/action planning or disengagement in terms of accounting for variation in quality of life. Also, given the presence of SLEs, the mediational qualities of coping may vary as a function of different types of quality of life outcomes. Langford et al (2017) tested a coping model with one domain of quality of life, emotional distress, in the model. Given the physical impact of SLEs noted above, there may be a differential relationship of SLEs with physical/functional compared to emotional quality of life outcomes, such that SLEs will have an enduring direct impact on physical/functional quality of life even in the context of coping responses.
The aim of the current study was to examine SLEs in the context of the lives of cancer patients by extending Langford et al. (2017) in key ways. The current study included: 1) a viable alternative to only engagement and disengagement coping, namely support/advice seeking; 2) physical and functional quality of life in addition to emotional quality of life to test the differential relationship between SLEs and quality of life outcomes; 3) broadening external validity to a diverse sample with respect to race/ethnicity by including a large subsample of African Americans, whose mortality rates for many cancers are substantially higher than Caucasian Americans (DeSantis et al., 2016). We hypothesized that 1) support/advice seeking and disengagement/denial coping would mediate the relationship between SLEs and quality of life; 2) whereas, disengagement/denial coping would have a negative relationship with quality of life, support/advice seeking would be positively related to quality of life; 3) SLEs might have a stronger relationship with physical/functional compared to emotional quality of life, which would manifest in an significant direct effect of SLEs on physical/functional quality of life in addition to the indirect, mediated effects.
Method
Participants
Inclusion criteria were: > 17 years of age, diagnosis of cancer, in active treatment for cancer, able to read English, and able to give informed consent. Patients were accrued from a community hospital, a clinical oncology practice, support groups, and in response to newspaper ads. A total of 671 patients were accrued; 9 did not complete survey materials sufficiently and were eliminated from the analysis of data.
The majority of patients were female (68.1%) and the highest incidence of cancer types were breast (52.2%), prostate (10.2%), colon/rectal (6.7%) lymphomas (6.3%) and lung (4.9%). A more complete list of the types of cancer and all other demographic and medical information are contained in Table 1. The participants received the following treatment modalities: surgery (76.4%), chemotherapy (63.9%) and radiation (57.4%). Many patients received more than one type of treatment. The mean age of the patients was 59.36 years. Because of accrual goals for the larger project, the sample consisted of 37.9% African-Americans, 60.9% Caucasian-Americans, and a small percentage of people who identified themselves as Asian, American Indian, or other. In terms of income, 32.5% reported earning an annual salary of less than $25,000; 28.5% reported earning between $25,000 and 50,000 and 40% reported earning over $50,000. Many of the patients had attended college or had obtained an undergraduate college degree (44.5%) as their highest level of educational attainment. In addition, 24.4% had a graduate degree or taken graduate level courses, and 31.6% attended high school or completed only high school.
Table 1.
Demographic and Clinical Information for the Total Sample (N=662)
| Characteristic | % |
|---|---|
| Sex | |
| Female | 68.1 |
| Education | |
| Less than High School Degree | 9.5 |
| High School Degree | 22.1 |
| Some College Courses | 27.8 |
| Undergraduate College Degree only | 16.7 |
| Graduate Courses or Degree | 24.4 |
| Employment | |
| Employed | 37.5 |
| Unemployed | 16.8 |
| Retired | 41.1 |
| Full-Time Homemaker | 5.5 |
| On Leave | 3.8 |
| Disabled | .3 |
| Race/Ethnicity | |
| Caucasian | 60.9 |
| African American | 37.9 |
| Other | 1.2 |
| Marital Status | |
| Never Married | 11.6 |
| Married | 58.5 |
| Divorced | 16.5 |
| Separated | 3.5 |
| Widowed | 8.9 |
| Other | 1.1 |
| Type of Cancer | |
| Breast | 52.2 |
| Prostate | 10.2 |
| Colon/Rectal | 6.7 |
| Lymphomas | 6.3 |
| Lung | 4.9 |
| Ovary/Uterus | 3.4 |
| Bone | 2.6 |
| Leukemia/Blood | 2.2 |
| Brain | 1.4 |
| Eye | 1.4 |
| Esophagus/Stomach | 1.1 |
| Other | 10.8 |
Measures
Stressful life events (SLEs).
The Stressful Life Events Checklist consisted of items based on prior work by Forsen (1991) in which he identified and slightly modified the most commonly endorsed SLEs from the Social Readjustment Rating Scale (Noone, 2017) by breast cancer patients. Thus, based on the most stressful events among breast cancer patients in Forsen (1991), a list of 12 items was included in a SLE Checklist for this study. In addition to those items (e.g., death of a spouse, death of a close personal friend, loss of job, divorce or separation from spouse or partner), illness events were included based on interviews with patients, who were not part of this study (e.g., recurrence of cancer, severe reaction to treatments, illness besides cancer that needed complicated treatment). Patients were asked to check those events that occurred in the year prior to completing the checklist. Essentially, an endorsed item represented at least one occurrence of that item in the past year. As in Langford et al. (2017) the total number of SLEs checked was used in the data analyses.
Physical debilitation.
The Physical Impact Scale (ambulation, mobility, body care and movement) of the Sickness Impact Profile (Bergner, Bobbit, Carter, & Gilson, 1981) was used to assess degree of physical impairment or functional status. This scale consists of 45 items, each describing an activity of daily functioning that might be impaired by illness symptoms (e.g., “I stand up only with someone’s help”). The patients checked items that applied to them, then a priori, empirically determined severity-weighted scale values from the manual (Bergner et al., 1991) for each item were applied and the scores were summed to form a total score for the Physical Impact Scale. Internal consistency was based on the severity-weighted scores for checked items. The test-retest reliability for the measure is high (r = .92), as is the internal consistency (α = .94; Bergner et al., 1981). Internal consistency of the Physical Impact Scale scores for the present sample was .92.
Coping.
The Brief COPE Scale (Carver, 1997) was used to assess the coping strategies utilized by patients. Participants rated how frequently they used the strategy described in each of 28 items on a scale from 1 (not at all) to 4 (a lot). Based on the replication in the current study of a factor analysis (principle components, varimax rotation) on a different but comparable sample, items from Brief COPE were combined to calculate scores for three factors: action planning (AP), support/advice seeking (SAS), and disengagement/denial (DD) (Merluzzi, Philip, Zhang, & Sullivan, 2015). The scores for AP consisted of Brief COPE items from the active coping, planning, and using instrumental support subscales (Cronbach’s α = .79). SAS scores were derived from items in the acceptance, venting, and using emotional support scales (Cronbach’s α = .68). Finally, the DD consisted of the items from the denial and behavioral disengagement subscales of the Brief COPE scale (Cronbach’s α = .72).
Quality of life.
The Functional Assessment of Cancer Therapy (FACT, Cella et al., 1993) is a 27-item measure of quality of life that contains four subscales: Physical Well Being, Social/Family Well Being, Emotional Well-Being, and Functional Well-Being. Individuals with cancer respond to a variety of questions by indicating on a scale from 1 (not at all) to 5 (very much) how the items apply to their lives. The authors reported subscale alphas of between .69 and .82 and a total score alpha of .89 in a large heterogeneous sample of cancer patients (Cella et al., 1993). Two indices were used from the FACT: Emotional Well-Being (referred to in this study as emotional quality of life – EQOL) is a scale that assesses the presence of anxiety, depression, and worry, the basic components of distress; the Physical and Functional Well-Being scales were summed to form the Trial Outcome Index, which is used as a clinical trials endpoint (Webster, Cella, & Yost, 2003). The Physical Well-Being scale assesses the presence of typical symptoms such as pain and fatigue and the Functional Well-Being scale assesses the ability to function effectively and satisfaction with life. The Trial Outcome Index is referred to in this study as physical/functional quality of life (PFQOL).
Demographic and medical information.
As part of the materials completed for the study, participants provided demographic information, their diagnosis, date of initial diagnosis, and types of treatments received. Comorbidity information was obtained from the participants’ physician, with their permission, and were included if they were determined to be serious diseases that required constant attention (e.g., advanced diabetes) or were potentially life threatening (e.g., COPD). Up to four conditions were obtained for each patient. Given that the comorbid conditions were considered to be serious, a code of “1” was used to signify that a comorbidity was present and “0” was used to indicate that it was absent for up to 4 serious comorbidities. Thus, the comorbidity score ranged from 0 to 4.
Procedure
This study was approved by the Institutional Review Boards of the University of Notre Dame and Memorial Hospital of South Bend, Indiana. All participants were treated in accordance with the Ethical Standards of the American Psychological Association and the regulations of the Health Information Portability and Accountability Act.
Participants were from the Midwest, South, and West regions of the United States. They were recruited via public announcements, newspaper advertisements, support groups, and e-mails to health organizations. In addition, physicians’ referrals were received from a large oncology practice affiliated with a community hospital. Participants received materials via mail, completed the surveys at home, returned them by mail, and were compensated $40. Medical information was verified by contact with participants’ physicians in accordance with HIPAA procedures.
Data Analysis
Descriptive statistics and statistical tests on the SLEs were accomplished with SPSS. The mediation models were tested using the R Statistical Package (bmem;Zhang & Wang, 2013a) to estimate model parameters. In addition to parameter estimations, 95% bias corrected confidence intervals from bootstrap resampling procedures were computed to confirm the significance of the model parameters. The parameters were significant if the 95% confidence interval did not include “0”. Missing data were handled by using the two-stage Maximum Likelihood Model (Zhang & Wang, 2013b). The mediation models consisted of the total number of SLEs, three distinct types of coping, and either EQOL or PFQOL (FACT Trial Outcome Index). The model parameters were computed while controlling for physical debilitation (SIP), age, education, and income. In addition, because time since diagnosis and comorbidities were related to PFQOL (but not EQOL), they were also included as covariates in the mediation model with PFQOL as the dependent variable. Finally, power analyses indicated that the N of 662 was more than adequate to test the two regression-based models.
Results
Demographic, Medical, and Stressful Life Events (SLE) Data.
Table 2 includes the list of SLEs based on the percent of endorsement by the participants. Events involving close relationships and work constituted the more frequently experienced SLEs. Table 3 contains the means and standard deviations of SLEs for sex, race, income, employment and marital status. Compared to Langford et al., (2017), there were no significant sex differences (p =.417), however, consistent with Langford et al. (2017), African Americans reported significantly more SLEs than Caucasian Americans (p =.005). Age was negatively correlated with the number of SLEs, when considering the prior year as the time period for SLEs; generally, with increasing age there was a modest decline in the number of SLEs (r = −0.238; p =.01). Langford et al. (2017) found no correlation with age. The total SLE score was related to income with those earning less than $15,000 reporting significantly more SLEs than all income levels greater than $15,000 (p =.001). Also, those earning between $25,000 and $39,999 reported more SLEs than all income levels greater than $39,999 (Table 3). Patients who were never married reported significantly more SLEs than (p =.001) than those who were married or widowed. Those who reported being married also reported significantly less SLEs than those who never married, divorced or separated from a partner (p =.001). For income and marital/partnered status, these results were similar to Langford et al. (2017). Finally, those who were unemployed or on leave from employment had significantly more SLEs than all other employment categories.
Table 2.
Frequency of Stressful Life Events Based on the Percentage Endorsement by Patients (N=662)
| Stressor | % |
|---|---|
| Problems in the family | 37.4 |
| A close relative suffering accident or sickness | 37.0 |
| Major negative change in financial state | 33.7 |
| Death in family (other than spouse/partner) | 26.2 |
| Death of a close personal friend | 22.4 |
| Job change or work conditions that caused difficulties and/or uncomfortable situation | 19.6 |
| Conclusion of a meaningful task or work activity | 19.0 |
| Illness (besides cancer) that needed complicated treatment or caused serious disability | 18.1 |
| Loss of an important relationship | 14.9 |
| Move to a new home | 11.4 |
| Recurrence of cancer (in the original site) | 8.9 |
| Severe reaction to treatments | 8.6 |
| Children, on whom you have depended, moved away from home | 7.3 |
| Discovery of distal (metastatic disease) | 5.9 |
| Dismissed from job after many years in service | 5.3 |
| Invasion of privacy from society | 4.9 |
| Divorce or separation from spouse/partner | 4.3 |
| Divorce of a child | 3.9 |
| Death of spouse | 1.6 |
Table 3.
Means (SDs) of Total Life Events for Demographic Characteristics and Correlations with Age and Functional Status
| Characteristic | Total number of stressors endorsed | |
|---|---|---|
| Sex | Mean (SD) | p |
| Male | 3.06 (2.50) | p=.417 |
| Female | 3.23 (2.44) | |
| Race | ||
| African American | 3.51 (2.56) | p=.005 |
| Caucasian American | 2.96 (2.28) | |
| Income | ||
| <$15,000 | 4.64 (2.92) | p=.001 |
| $15-24,999 | 3.09 (2.49) | 1>2-71 |
| $25-39,999 | 3.50 (2.55) | 3>4,6,72 |
| $40-49,999 | 2.58 (1.99) | |
| $50-50,999 | 2.90 (1.93) | |
| $60-69,999 | 2.77 (2.22) | |
| >$70,000 | 2.55 (1.93) | |
| Marital Status | ||
| Never Married | 4.07 (2.79) | p=.001 |
| Married | 2.84 (2.24) | 1>2,53 |
| Divorced | 3.52 (2.68) | 2<1,3,44 |
| Separated | 4.52 (3.07) | |
| Widowed | 3.00 (2.05) | |
| Employment | ||
| Employed | 2.94 | p=.001 |
| Unemployed | 4.83 | 2,5>1,3,4,65 |
| Retired | 2.67 | |
| Full-Time Homemaker | 2.62 | |
| On Leave | 5.38 | |
| Disabled | 4.00 | |
| Comorbidities | ||
| Present | 3.79 (2.57) | p=.006 |
| Absent | 3.07 (2.42) | |
| Correlation | ||
| Age (M=59.36; SD=12.8) | r=−0.238 | p=.01 |
| SIP (Functional Status) | r=0.351 | p=.001 |
Note: SIP=Sickness Impact Profile (Physical Impact Scale).
The SLE score in the first income category is greater than all other categories.
The SLE score in the third category is significantly greater than those in categories 4, 6, and 7.
The SLE score in the first income category is greater than categories 2 and 5.
The SLE score in the second category is significantly greater than those in categories 1, 3, and 4.
The SLE score in categories 2 and 5 are significantly greater than those in categories 1, 3, 4, & 6.
In terms of illness-related variables there was a relationship between SIP scores, which measured functional status related to mobility and self-care, and SLEs (r =.351, p =.001; see Table 4). Thus, higher SIP scores (i.e., lower functional status) were associated with a greater number of SLEs. Also, comorbidity was related to SLEs. The presence of comorbidities (M= 3.79) was associated with significantly more SLEs than their absence (M= 3.07; p =.006; see Table 3). These results for functional status and comorbidity were similar to Langford et al. (2017). In addition, the number of comorbidities was also correlated with PFQOL (r = −.123; p <.01) but not with EQOL (r = .009; ns), and, similarly, time since diagnosis was correlated with PFQOL (r = .165; p <.01) but not with EQOL (r = .043; ns). Finally, similar to Langford et al. (2017), there were no differences in total SLEs between those who had metastatic disease at diagnosis and those who did not. Generally, for most demographic data and disease-related data, the results of this study replicated Langford et al. (2017), thus setting the stage for extending their findings by including support/advice seeking coping and testing coping mediated models for EQOL and PFQOL outcomes in the context of SLEs.
Table 4.
Correlations Among Variables in the Mediation Models
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 SIP | 1 | |||||||||||
| 2 Age | −.050 | 1 | ||||||||||
| 3 Income | −.365** | −.060 | 1 | |||||||||
| 4 Education | −.233** | .012 | .497** | 1 | ||||||||
| 5 Time Since Diagnosis | .049 | .188** | .001 | .046 | 1 | |||||||
| 6 Comorbidities | .219** | .180** | −.186** | −.085* | .076 | 1 | ||||||
| 7 Total Life Events | .351** | −.238** | −.233** | −.075 | −.069 | .145** | 1 | |||||
| 8 Action Planning | .039 | −.133** | .043 | .128** | −.032 | .006 | .169** | 1 | ||||
| 9 Support Advice | .021 | −.082* | .133** | .080* | −.133** | −.007 | .004 | .425** | 1 | |||
| 10 Disengagement | .324** | −.215** | −.221** | −.208** | .075 | .063 | .415 | .166** | −.073 | 1 | ||
| 11 EQOL | −.186** | .239** | .046 | .070 | .043 | .009 | −.182** | −.014 | .080* | −.326** | 1 | |
| 12 PFQOL | −.617** | .175** | .305** | .195** | .165** | −.123** | −.433** | −.115** | −.041 | −.507** | .342** | 1 |
Note: SIP=Sickness Impact Profile; Comorbidities = number of comorbid conditions; Coping Factors: Action Planning, Support/Advice Seeking, Disengagement/Denial; EQOL=Emotional Quality of Life; PFQOL=Physical/Functional Quality of Life (Trail Outcome Index). SIP, Age, Income, and Education were covariates in the EQOL and PFQOL mediation models. Time Since Diagnosis and Comorbidities were also covariates in the PFQOL mediation model.
p=.05
p=.01
Mediated Model Testing
Emotional quality of life.
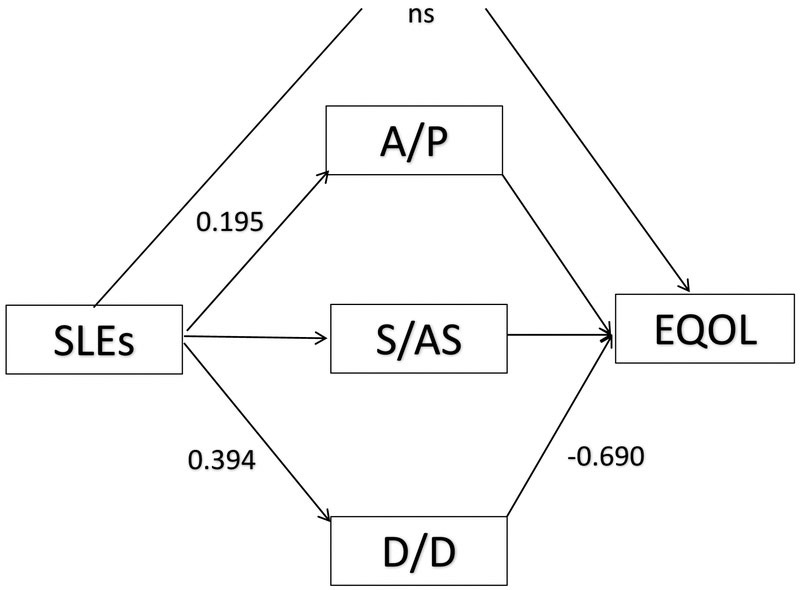
Identical to Langford et al. (2017), only DD coping significantly mediated the relationship between SLEs and EQOL (Table 5). The 95% bootstrap confidence intervals for the indirect effects involving AP, SAS and DD coping were (−0.017, 0.056), (−0.039, 0.023) and (−0.374, −0.174), respectively, confirming DD as the sole mediator of the relationship between SLEs and EQOL (Figure 1). Moreover, consistent with Langford et al, the direct effect from SLEs to EQOL was not significant indicating that the indirect effects accounted for variation in EQOL in this model
Table 5.
Emotional Quality of Life (EQOL) Outcome Mediation Model: Parameter Estimates, Standard Errors, and Bootstrapped 95% Confidence Intervals
| Path | Model Parameter |
Estimate | SE | 2.5% | 97.5% |
|---|---|---|---|---|---|
| a11 | SLE→AP | 0.195 | 0.054 | 0.089 | 0.304 |
| a2 | SLE→SAS | −0.003 | 0.059 | −0.119 | 0.109 |
| a31 | SLE→DD | 0.394 | 0.060 | 0.281 | 0.513 |
| b1 | AP→EQOL | 0.097 | 0.080 | −0.113 | 0.230 |
| b2 | SAS→EQOL | 0.183 | 0.180 | −0.085 | 0.619 |
| b31 | DD→EQOL | −0.659 | 0.088 | −0.848 | −0.498 |
| c | SLE→EQOL | 0.023 | 0.242 | −0.335 | 0.644 |
| a1*b1 | SLE→AP * AP→EQOL | 0.019 | 0.017 | −0.017 | 0.056 |
| a2*b2 | SLE→SAS * SAS→EQOL | −0.001 | 0.015 | −0.039 | 0.023 |
| a3*b31 | SLE→DD * DD→EQOL | −0.260 | 0.051 | −0.374 | −0.174 |
Note: SLE=Stressful Life Events; AP=Action Planning Coping; SAS=Support/Advice Seeking Coping; DD=Disengagement/Denial Coping; EQOL=Emotional Quality of Life; SE: Boot= Average standard error based on bootstrap resampling.
denotes significant parameter estimates.
Figure 1.
Mediated model with statistically significant path coefficients based on 95% CIs (p<.05). The direct effect of stressful life events (SLEs) on emotional quality of life (EQOL) was not statistically significant, however the mediating effect of disengagement/denial coping (D/D) was significant. The total indirect effect for mediation (SLEs→>DD→EQOL) was significant (−0.260, CI 95% = −0.374, −0.174).
Physical/functional quality of life.
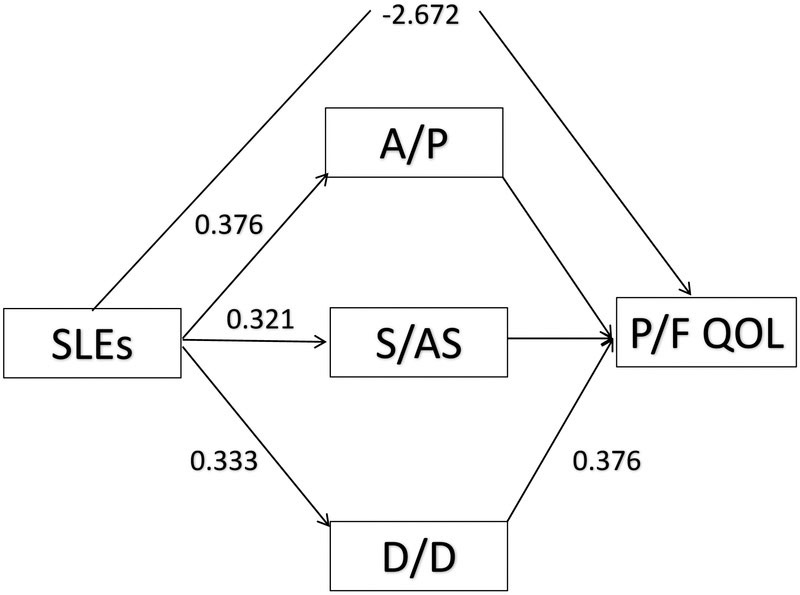
Extending beyond Langford et al. (2017) by using PFQOL as an outcome variable, the 95% bootstrap confidence intervals for the indirect effects of AP, SAS and DD were (−0.066, 0.060), (−0.021, 0.047) and (−0.831, −0.393), respectively, confirming DD as the sole mediator (Table 6). SLEs had a positive relationship with other forms of coping such as AP and SAS, but those types of coping were unrelated to the PFQOL (Figure 2). The direct effect involving SLEs and PFQOL was also significant (−1.295, −0.399).
Table 6.
Physical/Functional Quality of Life (PFQOL) Outcome Mediation Model: Parameter Estimates, Standard Errors, and Bootstrapped 95% Confidence Intervals
| Path | Model Parameter |
Estimate | SE | 2.5% | 97.5% |
|---|---|---|---|---|---|
| a11 | SLE→AP | 0.197 | 0.052 | 0.104 | 0.307 |
| a2 | SLE→SAS | −0.013 | 0.058 | −0.121 | 0.099 |
| a31 | SLE→DD | 0.396 | 0.062 | 0.268 | 0.519 |
| b1 | AP→PFQOL | −0.023 | 0.160 | −0.349 | 0.286 |
| b2 | SAS→PFQOL | −0.221 | 0.165 | −0.525 | 0.127 |
| b31 | DD→PFQOL | −1.460 | 0.201 | −1.860 | −1.083 |
| c1 | SLE→PFQOL | −0.844 | 0.241 | −1.297 | −0.360 |
| a1*b1 | SLE→AP * AP→PFQOL | −0.005 | 0.032 | −0.070 | 0.055 |
| a2*b2 | SLE→SAS * SAS→PFQOL | 0.003 | 0.017 | −0.025 | 0.048 |
| a3*b31 | SLE→DD * DD→PFQOL | −0.577 | 0.118 | −0.832 | −0.370 |
Note: SLE=Stressful Life Events; AP=Action Planning Coping; SAS=Support/Advice Seeking Coping; DD=Disengagement/Denial Coping; EQOL=Emotional Quality of Life; SE: Boot = Average standard error based on bootstrap resampling.
denotes significant parameter estimate
Figure 2.
Mediated model with statistically significant path coefficients based on 95% CIs (p<.05). The direct effect of stressful life events (SLEs) on Physical/Functional quality of life (PFQOl) was statistically significant as was the indirect effect of disengagement/denial coping (D/D). The total indirect effect for mediation (SLEs→>DD→PFQOL) was significant (−0.529, CI 95% = −1.206, −0.085). Also, the total indirect and direct effect (SLEs→>DD→PFQOL + SLES→PFQOL) was significant (−3.201, CI 95% = −5.000, −1.878)
Recapitulation of the Hypotheses
Contrary to the hypothesized mediation of support/advice seeking (Hypothesis 1), no paths involving SAS with either SLEs or outcomes (EQOL or PFQOL) were significant. Also, the path from SLEs to AP coping was significant in both mediation models, however, neither the path from AP to EQOL nor to PFQOL was significant. Thus, only DD emerged as a significant mediator between SLEs and quality of life and, as hypothesized, its relationship with both EQOL and PFQOL was negative (Hypothesis 2).
With respect to Hypothesis 3, which proposed no direct effect from SLEs to EQOL in the mediated model and, in contrast, a significant direct effect from SLEs to PFQOL in the mediated model, there was confirmation. There was a parallel between the Langford et al. (2017) findings regarding distress and the mediation model in the current study that included EQOL. In both instances, the direct path from SLEs to the outcomes (distress in Langford et al. (2017 and EQOL in the current study) was not significant in the mediation model. In contrast, the direct effect of SLEs was significant in the mediation model with PFQOL. Thus, the indirect effect involving DD coping in both Langford et al. (2017) and in this study accounted for variation in outcome (distress and EQOL). Alternatively, with PFQOL as the outcome, the direct effects of SLEs were significant as were the indirect effects involving DD coping. Finally, it is interesting to note that whereas DD coping was the sole mediator between SLEs and both EQOL and PFQOL, the means for the three approaches to coping indicated that Support/Advice Seeking (M = 3.78) was used to a greater extent than Action Planning (M = 2.82) and Disengagement/Denial (M = 2.87), but only the latter was related to both SLEs and quality of life.
Discussion
Based on the results of the current study and Langford et al. (2017), SLEs take their toll on emotional and physical functional QOL, and the only coping mechanism that mediated the relationship between SLEs with these outcomes, namely disengagement/denial, was negatively related to QOL. This suggested that for those individuals who are managing both a cancer diagnosis and SLEs, the sole mediating role of DD may place them at risk of poor psychosocial and disease outcomes. Perhaps during the treatment phase there is diminished capacity for active coping given the demands of the disease, its treatments, and SLEs. Thus, the significance of DD as a coping mechanism may indicate that SLEs are perceived as an organized class of events (Rudy & Merluzzi, 1984) that are uncontrollable or unpredictable and tend to increase the challenges already present in coping with cancer. One hypothesis to account for the absence of a mediating effect for AP coping in this study and engagement coping in Langford et al. (2017) is that the events are in the past and perceived as unchangeable and, therefore, must be endured. If that were the case, then we might expect SAS to have a more robust effect. Whereas in Langford et al. that type of coping was not tested apart from engagement and disengagement coping, in the current study in which that type of coping was tested, no mediating role emerged. Also, in contrast to Langford et al. (2017) who included more tradition trauma-related SLEs, the current study included SLEs related to illness (e.g., recurrence of cancer); however, there was a consistency in the findings, indicating that SLEs of all types are problematic if they accumulate in lives of cancer patients.
The major difference between, on the one hand, Langford et al. (2017) and the model in this study testing mediating effects on EQOL and, on the other hand, the model testing mediation with PFQOL in the current study, is the direct effect of SLEs on the outcome. That direct relationship of SLEs to QOL only prevailed in the mediation model with PFQOL. The direct effect in the model with PFQOL versus EQOL may reflect qualitative differences in the outcomes. Emotional reactivity may be more amenable to change by coping, whereas symptoms and functional abilities may be somewhat less malleable by coping and more directly tied to the amount of burden vested in the SLEs. Unfortunately, for both types of quality of life, the outcome is negatively related to disengagement coping with no offset by other types coping that may counterbalance negative outcomes.
There are some limitations to the current study. Although it was based on a theoretically driven model (Lazarus & Folkman, 1984), the design is cross-sectional, and therefore, causal arguments are not viable. Longitudinal research will be needed to provide a more dynamic approach to the effects of coping in the relationship between SLEs and outcomes such as distress and quality of life. Also, both Langford et al. (2017) and this study used a checklist format for assessing SLEs. Future work might include other methods of assessing SLEs such as blind interview assessments (Harkness & Monroe, 2016), a structured interview format in which the interviewer is blind to any information about the participant. That more nuanced assessment of SLEs might include an analysis of the intensity and controllability of each SLE as well as personality characteristics (e.g., hardiness, optimism) and resources other than coping (e.g., social support, health insurance). Finally, an emerging concept in stress resistance, regulatory flexibility (Bonanno, Papa, Lalande, Westphal, & Coifman, 2004), which involves meta-analytic skills in perceiving stressors, having an elaborate coping repertoire, and utilizing feedback effectively, would be applicable to the challenges inherent in coping with cancer. Regulatory flexibility may also benefit from an integration with a resource such as emotional intelligence (Salovey, Stroud, Woolery & Epel, 2002), which involves attention to as well as discernment and regulation of emotion.
This study draws attention to what might be termed despondent disengagement, which was associated with a decrease in functioning. However, there are other types of disengagement that may be more functional and adaptive such as rational disengagement, which is tantamount to goal modification and acceptance and also acknowledging some limitations on controlling outcomes (Wrosch, Scheier, Miller, Schulz, & Carver, 2003; Wrosch, Miller, Scheier, & de-Pontet, 2007). Also, letting go, that is relinquishing control of outcomes, in the context of cancer may provide some relief from the anxiety related to the uncertainty of outcomes. There is some evidence that faith-based perspectives on letting go may foster higher quality of life (Merluzzi & Philip, 2017; Salsman et al., 2015) than assuming personal control of outcomes. Thus, alternatives to despondent disengagement and denial may be fruitful avenues to explore in terms of giving patients options for coping with SLEs that may not result in decrements in functioning.
The results of this study have direct clinical implications. Screening for SLEs as well as personal and social resources is easily accomplished in a clinical setting; however, future research should seek to develop standard instruments that are appropriate for the cancer setting and consider both general SLEs and illness related events. The Stressful Life Events Questionnaire (Goodman et al., 1998) is an example of a standard SLE questionnaire designed for use with PTSD. However, even in PTSD research there are a plethora of instruments, making it difficult to establish precise metrics on screening as to how much exposure to SLEs or trauma is critical in determining referral for stress management (Tsai, Pietrzak, Hoff, & Harpaz-Rotem, 2016). Finally, future research could test coping models with PTSD and subsyndromal symptoms as the outcome as well as the additive impact of SLEs and comorbidities (Shelby, Golden Kreutz, & Andersen, 2008) on those outcomes.
The association found between DD coping and quality of life in the current study may also help guide more effective efforts to identify vulnerable individuals and intervene in their lives. Screening measures that utilize technology and modern psychometrics are enabling more comprehensive and rapid assessment of individuals’ distress and supportive care needs (Verdonck-de Leeuw et al., 2009). Such measures could also incorporate a brief assessments of a patients’ broad coping tendencies and exposure to SLEs and thus further refine comprehensive cancer care.
In sum, the current study provides further insight into the relationship between SLEs, coping and quality of life, among a vulnerable patient population. The results broadly support those reported previously by Langford et al. (2017) while extending those findings to other domains of QOL. These findings can help further guide the development of psychosocial screening measures, as well as inform the day-to-day supportive care practices of mental health professionals in medical settings.
Acknowledgements
This research was supported by a grant from the National Cancer Institute (CA94914). The authors would like to thank the patients from around the country who participated in this study as well as the physicians and staff of Michiana Hematology-Oncology, the Northern Indiana Cancer Research Consortium, and Memorial Hospital’s Regional Cancer Center, South Bend, IN, USA.
Footnotes
Conflict of Interest
The authors report no conflicts of interest in the conduct of this research.
Contributor Information
Thomas V. Merluzzi, Department of Psychology, University of Notre Dame, 121 Haggar Hall, Notre Dame IN USA, 46556.
Andrea Chirico, Department of Psychology of Developmental and Socialization Processes, "Sapienza" University of Rome, Via dei Marsi, 80 - 00185 Roma, Italy.
Samantha Serpentini, Veneto Institute of Oncology IOV - IRCCS, Via Gattamelata, 64 – 35128 Padova (Italy); Facebook: SamanthaDottssaSerpentini.
Miao Yang, Department of Psychology, University of Notre Dame, 218A Haggar Hall, Notre Dame, IN 46556.
Errol J. Philip, Laboratory for Psychooncology Research, University of Notre Dame, 220 Haggar Hall, Notre Dame IN USA
References
- Alfano CM, & Rowland JH (2006). Recovery issues in cancer survivorship: A new challenge for supportive care. Cancer Journal, 12, 432–443. 10.1097/00130404200609000-00012 [DOI] [PubMed] [Google Scholar]
- Andersen BL, Kiecolt Glaser JK, & Glaser R (1994). A biobehavioral model of cancer stress and disease course. The American Psychologist, 49(5), 389–404. doi: 10.1037/0003-066X.49.5.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrykowski MA & Kangas M (2010). Posttraumatic stress disorder associated with cancer diagnosis and treatment In Holland JC, Breitbart WS, Jacobsen PB, Lederberg MS, Loscalzo MJ, R. & McCorklen R, (eds.). Oxford Textbook of Psycho-Oncology (2nd ed; pp. 348–357). New York: Oxford University Press. [Google Scholar]
- Arnaboldi P, Riva S, Crico C, & Pravettoni G (2017). A systematic literature review exploring the prevalence of post-traumatic stress disorder and the role played by stress and traumatic stress in breast cancer diagnosis and trajectory. Breast Cancer, 9, 473–485. doi: 10.2147/BCTT.S111101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly JL, & Lloyd-Williams M (2016). Identifying functional impairment and rehabilitation needs in patients newly diagnosed with inoperable lung cancer: A structured literature review. Supportive Care in Cancer, 24, 2359–2379. 10.1007/s00520-015-3066-1 [DOI] [PubMed] [Google Scholar]
- Beatty L, Lee C, & Wade T (2009). A prospective examination of perceived stress as a mediator of the relationship between life-events and QOL following breast cancer. British Journal of Health Psychology, 14(4), 789–804. doi: 10.1348/135910709X412459 [DOI] [PubMed] [Google Scholar]
- Bergner M, Bobbit RA, Carter WB, & Gilson BS (1981). The Sickness Impact Profile: Development and final revision of a health status measure. Medical Care, 19, 787–805. doi: 10.1097/00005650-198108000-00001 [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Papa A, Lalande K, Westphal M, & Coifman K (2004). The importance of being flexible: The ability to enhance and suppress emotional expression predicts long-term adjustment. Psychological Science, 157, 482–487. [DOI] [PubMed] [Google Scholar]
- Butow PN, Hiller JE, Price MA, Thackway SV, Kricker A, & Tennant CC (2000). Epidemiological evidence for a relationship between life events, coping style, and personality factors in the development of breast cancer. Journal of Psychosomatic Research, 49(3), 169–181. doi: 10.1016/S0022-3999(00)00156-2 [DOI] [PubMed] [Google Scholar]
- Bui Q, Ostir G, Kuo Y, Freeman J, & Goodwin J (2005). Relationship of depression to patient satisfaction: Findings from the barriers to breast cancer study. Breast Cancer Research and Treatment, 89(1), 23–28. doi: 10.1007/s10549-004-1005-9 [DOI] [PubMed] [Google Scholar]
- Carver CS (1997). You want to measure coping but your protocol’s too long: Consider the Brief COPE. International Journal of Behavioral Medicine, 4, 92–100. 10.1207/s15327558ijbm0401_6 [DOI] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, … & Brannon J (1993). The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. Journal of Clinical Oncology, 11, 570–579. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Wardle J & Steptoe A (2008). Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature: Clinical Practice, Oncology, 5, 466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- Chirico A, Lucidi F, Alivernini F, Merluzzi TV, & Giordano A (2017). A meta-analytic review of the relationship of cancer coping self-efficacy with distress and quality of life. Oncotarget, doi: 10.18632/oncotarget.15758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo ES, Stawski RS, Ryff CD, Coe CL, & Almeida DM (2012). Cancer survivors’ responses to daily stressors: Implications for quality of life. Health Psychology, 11, 360–370. doi: 10.1037/a0027018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture-Lalande M, Lebel S, & Bielajew C (2014). Analysis of the cortisol diurnal rhythmicity and cortisol reactivity in long-term breast cancer survivors. Breast Cancer Management, 3, 465–476. [Google Scholar]
- DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, & Jemal A (2016). Progress and opportunities in reducing racial disparities cancer statistics for African Americans, CA: A Cancer Journal for Clinicians, 290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- Forsen A (1991). Psychosocial stress as a risk for breast cancer. Psychotherapy and psychosomatics, 55, 176–185. [DOI] [PubMed] [Google Scholar]
- Golden Kreutz D, Thornton L, Wells-Di Gregorio S, Frierson G, Jim H, Carpenter K, . . . Andersen B (2005). Traumatic stress, perceived global stress, and life events: Prospectively predicting quality of life in breast cancer patients. Health Psychology, 24(3), 288–296. doi: 10.1037/0278-6133.24.3.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman LA, Corcoran C, Turner K, Yuan N, & Green BL (1998). Assessing traumatic event exposure: General issues and preliminary findings for the stressful life events screening questionnaire. Journal of Traumatic Stress, 11(3), 521–542. doi: 10.1023/A:1024456713321 [DOI] [PubMed] [Google Scholar]
- Harkness KL & Monroe SM (2016). The assessment and measurement of adult life stress: Basic premises, operational principles, and design requirements. Journal of Abnormal Psychology, 125, 727–745. 10.1037/abn0000178 [DOI] [PubMed] [Google Scholar]
- Kangas M, Henry J, & Bryant RA (2002). Posttraumatic stress disorder following cancer: A conceptual and empirical review. Clinical Psychology Review, 22, 499–524. doi: 10.1016/S0272-7358(01)00118-0 [DOI] [PubMed] [Google Scholar]
- Kennard B, et al. (2004). Nonadherence in adolescent oncology patients: preliminary data on psychological risk factors and relationships to outcome. Journal of Clinical Psychology, 11, 30–39. [Google Scholar]
- Kenne Sarenmalm E, Browall M, Persson L, Fall Dickson J, & Gaston Johansson F (2013). Relationship of sense of coherence to stressful events, coping strategies, health status, and quality of life in women with breast cancer. Psycho-Oncology, 22(1), 20–27. doi: 10.1002/pon.2053 [DOI] [PubMed] [Google Scholar]
- Langford DJ, Cooper B, Paul S, Humphreys J, Keagy C, Conley YP, … & Dunn LB (2017). Evaluation of coping as a mediator of the relationship between stressful life events and cancer-related distress. Health Psychology. Advance online publication. 10.1037/hea0000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping. New York, NY: Springer, Inc. [Google Scholar]
- Mehnert A, & Koch U (2007). Prevalence of acute and post-traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: A prospective study. Psycho-Oncology, 16(3), 181–188. doi: 10.1002/pon.1057 [DOI] [PubMed] [Google Scholar]
- Merluzzi T, & Philip E (2017). “Letting go”: From ancient to modern perspectives on relinquishing personal control: A theoretical perspective on religion and coping with cancer. Journal of Religion and Health, doi: 10.1007/s10943-017-0366-4 [DOI] [PubMed] [Google Scholar]
- Merluzzi TV, Philip EJ, Zhang Z, & Sullivan C (2015). Perceived discrimination, coping, and quality of life for African-American and Caucasian persons with cancer. Cultural Diversity and Ethnic Minority Psychology, 21(3), 337–44. doi: 10.1037/a0037543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Ferguson DW, Gill J, Paul J, & Symonds P (2013). Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncology, 14, 721–732. 10.1016/S14702045(13)70244-4 [DOI] [PubMed] [Google Scholar]
- Noone P (2017). The Holmes-Rahe stress inventory. Occupational Medicine, 67(7), 581–582. doi: 10.1093/occmed/kqx099 [DOI] [PubMed] [Google Scholar]
- Pérez S, Galdón M, Andreu Y, Ibáñez E, Durá E, Conchado A, & Cardeña E (2014). Posttraumatic stress symptoms in breast cancer patients: Temporal evolution, predictors, and mediation. Journal of Traumatic Stress, 27(2), 224–231. doi: 10.1002/jts.21901 [DOI] [PubMed] [Google Scholar]
- Osler M, Bendix L, Rask L, & Rod N (2016). Stressful life events and leucocyte telomere length: Do lifestyle factors, somatic and mental health, or low-grade inflammation mediate this relationship? Results from a cohort of Danish men born in 1953. Brain, Behavior, and Immunity, 58, 248–253. doi: 10.1016/j.bbi.2016.07.154 [DOI] [PubMed] [Google Scholar]
- Prieto JM, Blanch J, Atala J, Carreras E, Rovira M, Cirera E, … Gasto C (2002). Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. Journal of Clinical Oncology, 20, 1907–1917. doi: 10.1200/JCO.2005.05.751 [DOI] [PubMed] [Google Scholar]
- Roesch SC, Adams L, Hines A, Palmores A, Vyas P, Tran C, . . .Vaughn AA (2005). Coping with prostate cancer: A meta-analytic review. Journal of Behavioral Medicine, 28, 281–293. 10.1007/s10865-005-4664-z [DOI] [PubMed] [Google Scholar]
- Rudy TE & Merluzzi TV (1984). Recovering social cognitive schemas In Kendall PC (Ed.), Advances in cognitive behavioral research and therapy (Vol. 3, pp. 61–102). New York: Academic Press. [Google Scholar]
- Salsman J, Pustejovsky J, Jim HSL, Munoz A, Merluzzi T, George L, . . . Fitchett G (2015). A meta-analytic approach to examining the correlation between religion/spirituality and mental health in cancer. Cancer, 121(21), 3769–3778. doi: 10.1002/cncr.29350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby R, Golden Kreutz D, & Andersen B (2008). PTSD diagnoses, subsyndromal symptoms, and comorbidities contribute to impairments for breast cancer survivors. Journal of Traumatic Stress, 21(2), 165–172. doi: 10.1002/jts.20316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker MJ, Jones ME, Wright LB, Griffin J, McFadden E, Ashworth A, & Swerdlow A (2016). Psychological stress, adverse life events and breast cancer incidence: A cohort investigation in 106,000 women in the United Kingdom. Breast Cancer Research, 18:72 DOI 10.1186/s13058-016-0733-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL, Ganz PA, Rowland JH, Meyerowitz BE, Krupnick JL, & Sears SR (2005). Promoting adjustment after treatment for cancer. Cancer, 104(Suppl. 11), 2608–2613. 10.1002/cncr.21246 [DOI] [PubMed] [Google Scholar]
- Stein KD, Syrjala KL, & Andrykowski MA (2008). Physical and psychological long-term and late effects of cancer. Cancer, 112(Suppl. 11), 2577–2592. 10.1002/cncr.23448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P& Eyer J (1988). Allostasis: A new paradigm to explain arousal pathology In Fisher S, Reason JT (Eds.), Handbook of life stress, cognition, and health, (pp. 629–649). New York: Wiley [Google Scholar]
- Tsai J, Pietrzak RH, Hoff RA, & Harpaz-Rotem I (2016). Accuracy of screening for posttraumatic stress disorder in specialty mental health clinics in the U.S. Veterans Affairs Healthcare System. Psychiatry Research, 240, 157–162. doi: 10.1016/j.psychres.2016.04.036 [DOI] [PubMed] [Google Scholar]
- Verdonck-de Leeuw IM, de Bree R, Keizer AL, Houffelaar T, Cuijpers P, van der Linden M, & Leemans CR (2009). Computerized prospective screening for high levels of emotional distress in head and neck cancer patients and referral rate to psychosocial care. Oral Oncology, 45, 129–33. doi: 10.1016/j.oraloncology.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Wan C, Couture Lalande M, Lebel S, & Bielajew C (2017). The role of stressful life events on the cortisol reactivity patterns of breast cancer survivors. Psychology and Health, 10, 1–17. doi: 10.1080/08870446.2017.1346194 [DOI] [PubMed] [Google Scholar]
- Webster K, Cella D, & Yost K (2003). The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health and Quality of Life Outcomes, 1, 79 10.1186/1477-7525-1-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrosch C, Miller G, Scheier M, & de-Pontet SB (2007). Giving up on unattainable goals: Benefits for health? Personality Social Psychology Bulletin, 33(2), 251–265. doi: 10.1177/0146167206294905 [DOI] [PubMed] [Google Scholar]
- Wrosch C, Scheier M, Miller G, Schulz R, & Carver C (2003). Adaptive self-regulation of unattainable goals: Goal disengagement, goal reengagement, and subjective well-being. Personality Social Psychology Bulletin, 29(12), 1494–1508. doi: 10.1177/0146167203256921 [DOI] [PubMed] [Google Scholar]
- Zhang Z, & Wang L (2013a). bmem: Mediation analysis with missing data using bootstrap. R package version 1.5. https://cran.r-project.org/web/packages/bmem/index.html [Google Scholar]
- Zhang Z, & Wang L (2013b). Methods for mediation analysis with missing data. Psychometrika, 78(1), 154–184. doi: 10.1007/s11336-012-9301-5 [DOI] [PubMed] [Google Scholar]