Abstract
Background: Tropomyosin 4 (TPM4) is a member of the tropomyosin family of actin-binding proteins. Abnormal level of TPM4 is found in several cancers, and TPM4 is considered as a potential detecting marker for ovarian cancer, breast cancer, colon cancer, keratoacanthoma and esophageal squamous cell carcinoma. In this paper, the function of TPM4 in lung cancer cell lines was determined.
Materials and methods: TPM4 knockout cells were constructed by CRISPR/CAS9 technique. TPM4 overexpression cells were also constructed based on TPM4 knockout cells. Cell growth ability was detected by MTS assay. The potency of cell motility was investigated using transwell assay and wound scratch assay. The protein levels in lung cancer cells were determined by western-blot. Immunofluorescence technique was used to image the structure of F-actin.
Results: As a result, TPM4 downregulation and TPM4 upregulation cell models were obtained successfully. Cell motility was inhibited by the suppression of TPM4 while cell migration was enhanced in TPM4 upregulated cells. But TPM4 was not involved in cell proliferation and EMT progression. Microfilaments were depolymerized result from the suppression of TPM4 expression. And F-actin assembly was increased when TPM4 was upregulated.
Conclusion: In summary, TPM4 was able to promote cell motility by altering the actin cytoskeleton directly.
Keywords: TPM4, cell migration, F-actin, lung cancer
Introduction
Lung cancer is one of the most commonly diagnosed cancers, ranking as the leading cause of all cancer-related deaths in the world.1,2 Despite some advances in early detection and the use of modern surgical techniques in combination with various treatment modalities, such as targeted therapy, radiotherapy and chemotherapy, the prognosis of the patients with lung cancer still remains poor.3 As the molecular basis involved in lung cancer progression and metastasis is very important for the prognosis and therapy of lung cancer, identifying the potential molecules that promote lung cancer progression and metastasis are urgently needed.
Tropomyosin 4 (TPM4) is a member of the tropomyosin family of actin-binding proteins which is involved in the cytoskeleton of non-muscle cells.4 Tropomyosins can provide stability to the microfilaments and regulate lots of other actin-binding proteins.4–6 In the past decade, abnormal TPM4 expression has been observed in many cancers, including decreasing expression or increasing expression in different tumor tissues.7–9 In ovarian cancer and breast carcinoma, TPM4 was considered as a potential cancer maker according to protein expression profiles.10,11 In human non-small cell lung carcinoma, cDNAs sequencing and quantitative reverse transcription polymerase chain reaction analysis indicated that TPM4 was upregulated during lung development.12 But the TPM4 expression level had no statistical difference between the lung tumor tissues and surrounding non-cancerous tissues from the same patients (NTST).12 And the biological function of TPM4 in lung cancer is still unknown.
In order to investigate the biological function and clinical significance of TPM4 in lung cancer, TPM4 downregulation as well as upregulation cell models were constructed. The effects of TPM4 in lung cancer cell lines were investigated.
Materials and methods
Materials
RPMI 1640, FBS, phallotoxins and trypsin were purchased from Thermo Fisher (Waltham, MA); anti-TPM4, anti-F actin, anti-vimentin, anti-snail, anti-ZEB1 and β-catenin antibodies were obtained from Abcam (Cambridge, England, UK); Bisbenzimide Hoechst 33342 were purchased from Sigma (St. Louis, MO); Transwell® polycarbonate membrane cell culture inserts were from Corning (Tewksbury, MA); Matrigel Matrix was bought from BD Science (San Diego, CA).
Cell culture
Human lung adenocarcinoma cell lines A549 and NCI-H1299 were obtained from the National Infrastructure of Cell Line Resource. Cells were cultured in RPMI 1640 medium supplemented with 10% FBS. The cells were maintained at 37°C in a CO2 incubator.
Construction of recombinant lentiviral vectors and infection into cells
Recombinant lentiviral vectors were designed and packaged in 293T cells by GeneChem (Shanghai, China). The sgRNA sequence designed based on the human TPM4 mRNA sequence was 5ʹ-TCTGCAGGTAGCTCGTAAGC-3.
For cellular infection, cells were subcultured at 6,000 cells/well in 96-well culture plate and infected with recombinant lentivirus. Stable clones were selected and identified by western blot.
MTS assay
Cell growth was assessed using the nonradioactive cell proliferation assay. Briefly, 6,000 cells/well were subcultured in 96-well culture plates. The next day, 20 μL of MTS were added to each well and incubated at 37°C for 3 hrs. The absorbance was recorded at 490 nm (Bio-Rad, Hercules, CA, USA). The measurements carried out for next 3 days. The assay was repeated three times and the data are presented as the mean ± standard error of the mean.
Cell migration assay
The migration assay was performed as in a 24-well transwell with 8.0-μm pore polycarbonate membrane-coated inserts. For migration assay, after starvation for 12 hrs, 5×104 cells with 200 μL serum-free medium was added to the upper compartment of the chamber, while the lower compartment was filled with 600 μL of RPMI 1640 supplemented with 10% FBS. After incubation at 37°C for 24 hrs, the tumor cells remaining inside the upper chamber were removed with cotton swabs. The cells on the lower surface of the membrane were stained with 2% crystal violet. The number of cells on the lower surface of the membrane was counted in five microscopic fields at 200× magnification. At least triplicate samples were tested. The data are presented as mean ± SEM.
Wound scratch assay
Briefly, cells were plated in a 12-well plate and cultured overnight. After serum starvation for 24 hrs, cells were scratched with a pipette tip (20 μL); the wounds were observed at 0–24 hrs along the scratch.
Immunofluorescence microscopy
Cells were cultured on coverslips for 24 hrs. After washing three times in PBS, cells were fixed in 4% paraformaldehyde for 10 mins followed by 0.2% TritonX-100 for 15 mins at room temperature. After blocking of non-specific binding sites in 3% BSA for 30 mins, coverslips were incubated with TPM4 antibodies overnight at −4°C. The staining was completed with Alexa Fluor 488–conjugated second antibody incubation for 1 hr at room temperature. Cells were then stained with rhodamine phalloidin for 20 mins at room temperature. At last, nuclei were stained with Hoechst 33342 for 30 mins. Anti-fade solution was added to the coverslips to prevent quenching. Imaging was performed by confocal microscopes (Leica TCS SP8) through a 40× oil objective.
Western blot analysis
Total proteins (10 μg/well) were separated using 12% SDS-PAGE and then transferred onto NC membranes. After blocked by 5% milk, membranes were incubated with corresponding primary antibodies overnight at 4°C. Then, the membranes were incubated with appropriate HRconjugatedP- secondary antibody for 1 hr at room temperature. Proteins were detected by using the ECL plus reagents.
Statistical analysis
All values are expressed as the mean ± SEM. For comparison between means of 2 groups, statistical differences were tested with unpaired Student t-tests. Statistical significance was tested using SPSS Statistics, version 19.0. P<0.05 (*) was considered different; P<0.01 (**) was considered significantly different.
Results
TPM4 expression was blocked by CRISPR/CAS9 technique in A549 and H1299 cells
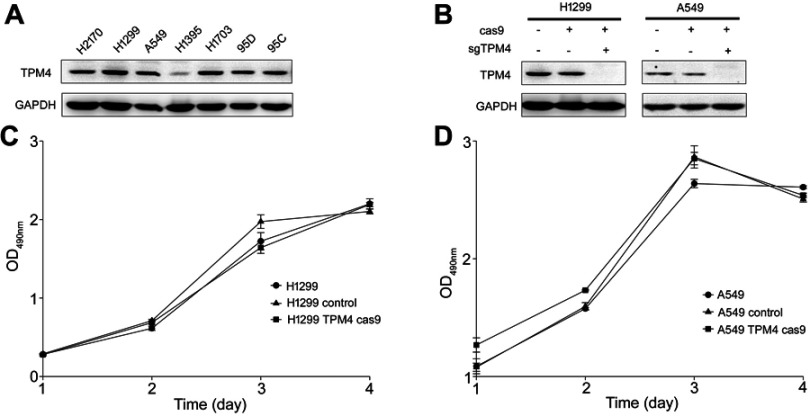
In order to investigate the expression level of TPM4 in lung cancer cell lines, Western blot analysis was used. As indicated in Figure 1A, TPM4 was highly expressed in most non-small cell lung cancer (NSCLC) cell lines except H1395 cells. In order to determine the function of TPM4 in NSCLC cell lines, TPM4 knockout cell strains were constructed by CRISPR/CAS9 technique. The TPM4 knockout clones were identified by WB test. As listed in Figure 1B, TPM4 expression was absolutely inhibited by sgTPM4 in both A549 and H1299 cells.
Figure 1.
TPM4 was not involved in cell proliferation. (A) TPM4 was extensively expressed in lung cancer cells. The level of TPM4 in lung cancer cells was determined by western blot. GAPDH is used as a loading control. (B) TPM4 knockout cell model was constructed by CRISPR/CAS9. TPM4 protein level was detected by Western blot, with GADPH used as the internal loading controls. (C, D) Cells were seeded in 96-well plates and an MTS assay was performed. Absorbance at 490 nm (y axis) was measured at 24-hr intervals. The data are expressed as the mean ± standard error of the mean from three separate experiments.
Abbreviations: CRISPR, clustered, regular lyinterspaced, short palindromic repeats; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
Cell proliferation was not affected by the downregulation of TPM4
The MTS assay is a colorimetric assay for assessing cell metabolic activity which can indicate the cell viability and cell proliferation indirectly. MTS assay was done to determine whether TPM4 was involved in the regulation of cell growth. As shown in Figure 1C and D, cell growth activity was not changed either in A549 cells or in H1299 cells while the expression of TPM4 was suppressed. Colony formation assay and anchorage-independent growth assay were also applicated and similar results were obtained (data not shown). It indicated that TPM4 did not play any role in cell proliferation in lung cancer cells.
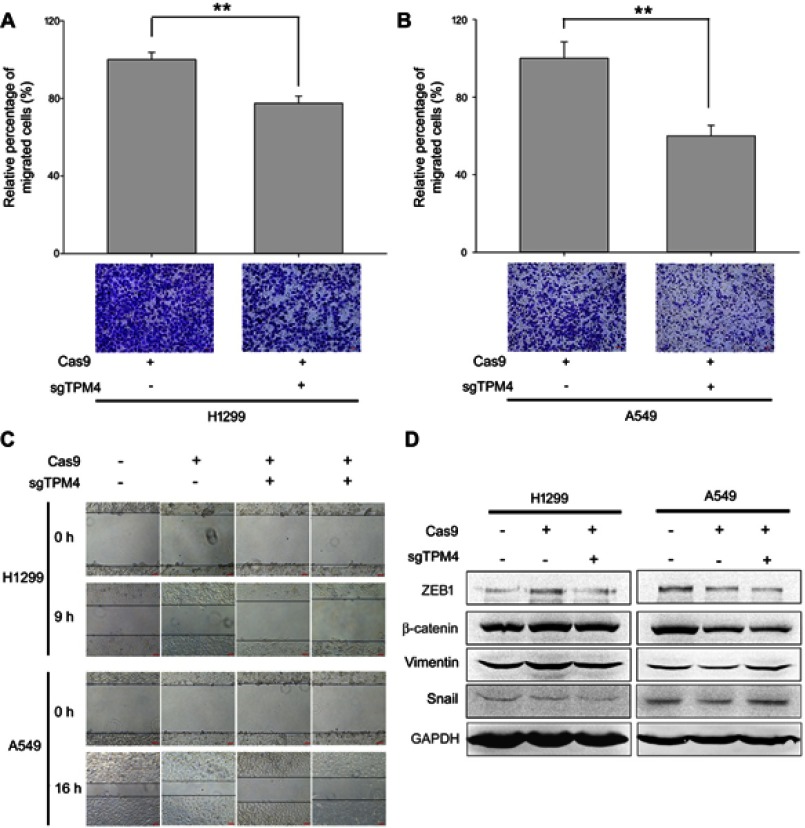
Downregulating TPM4-inhibited cell migration in lung cancer cells
To study whether TPM4 regulate cell migration and invasion, TPM4 knockout clones were used. The role of TPM4 in regulating lung cancer cell motility was determined by transwell assay and scratch assay. As illustrated in Figure 2A and B, the migration ability of H1299 and A549 cells was suppressed because of the knocked out of tpm4 gene. Similar results were also obtained by scratch assay. In order to exclude off-target effect, two TPM4 knockout clones of H1299 and A549 cells were used. As shown in Figure 2C, cell migration was remarkably weakened by the suppression of TPM4 expression in H1299 and A549 cells.Epithelial-mesenchymal cell transformation (EMT) is an important process during normal embryonic development as well as the metastasis of malignant epithelial tumors, showing decreased intracellular adhesion and increased motility.13 In this study, the levels of several EMT markers were detected. As a result, levels of vimentin, transcription factors snail and ZEB1, as well as cytoskeletal markers β-catenin were not affected while the expression of TPM4 was inhibited (Figure 2D). It indicated that TPM4 was not involved in EMT process.
Figure 2.
TPM4 was involved in lung cancer cell migration. (A,B) Transwell migration assay performed. Cells migrated through the chamber was counted in five microscopic fields and standardized by H1299 group and A549 group, respectively. The data is expressed as mean ± SEM. Bars represent 50 μm. ** p<0.01 (unpaired Student's t-test). (C) Wound healing assay was performed with TPM4 knock out cell clones as well as control cells for 0–16 hrs. Bars represent 50 μm. (D) Expression of mesenchymal proteins, vimentin and β-catenin, transcription factors, snail, and ZEB1 was examined by immunoblotting. GAPDH is used as a loading control.
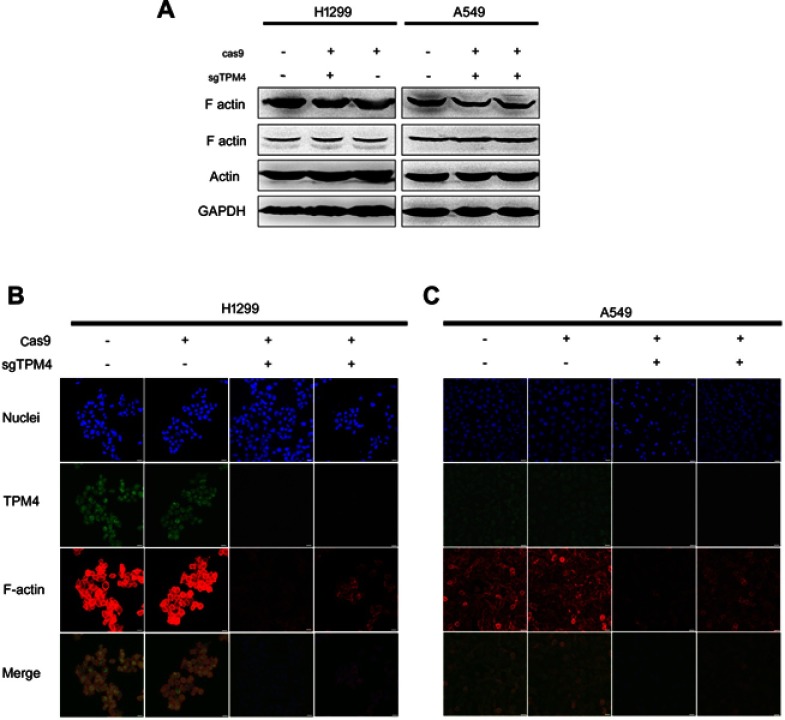
Intracellular cytoskeleton was modulated by TPM4
TPMs can associate with both filamentous actin (F-actin) and actin-binding proteins, thereby mediating the interaction of actin-binding proteins to F-actin as well as providing stability to the microfilaments.14 And cell migration and invasion involves the dynamic and spatial regulation of the cytoskeleton.15,16 The effect of TPM4 on cell microfilaments (F-actin) was determined. Two different antibodies recognizing human filamentous actin were used to detect the expression level of F-actin. It indicated that F-actin level was not affected by the suppression of TPM4 expression (Figure 3A). To observe the morphological changes of microfilaments, F-actin structure was labeled by phalloidin in Figure 3B and 3C. In two TPM4 knockout clones of H1299 and A549 cells, F-actin level was significantly decreased. We thought that microfilaments were depolymerized result from the suppression of TPM4 expression.
Figure 3.
Downregulation of TPM4 led to disruption of actin organization. (A) F-actin expression level was detected by WB. The actin level was also determined as a control, with GADPH used as the internal loading controls. (B, C) F-actin and TPM4 were labeled by immunofluorescence staining. The red signal represents the staining of F-actin, the green signal represents the staining of TPM4, and the blue signal represents the nuclear DNA staining by Hoechst 33342. Bars represent 20 μm.
Abbreviation: WB, Western blot.
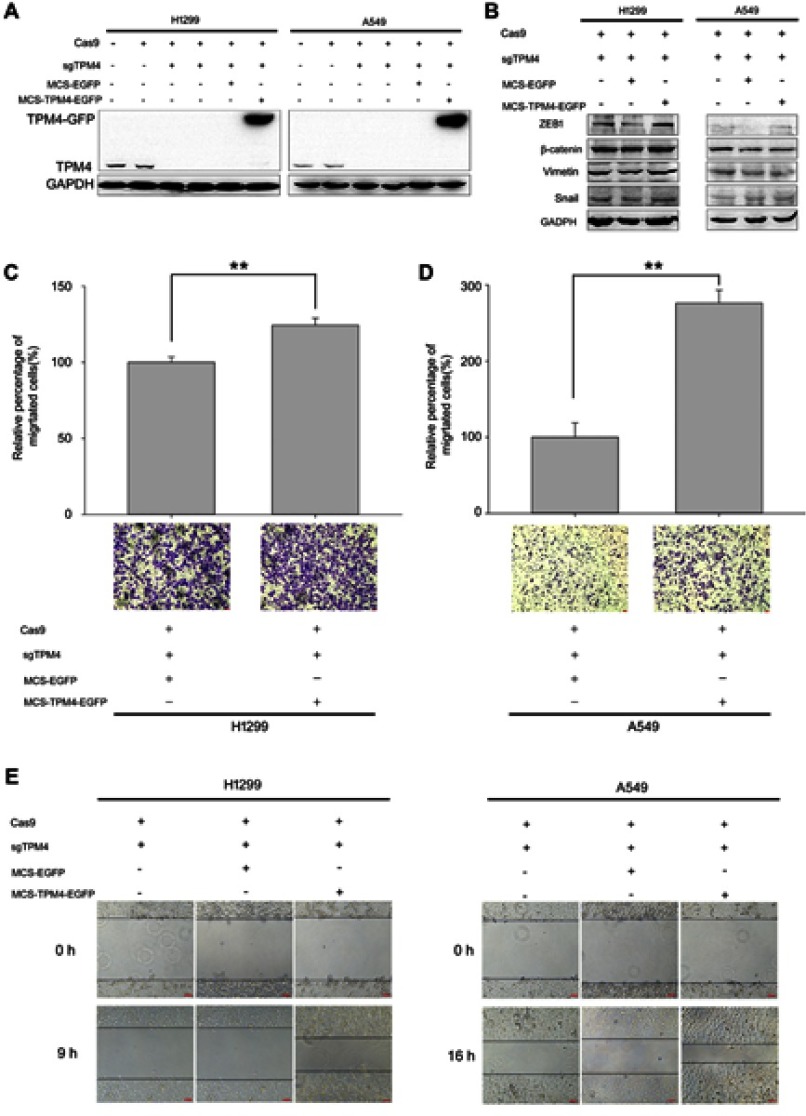
TPM4 is able to promote cell motility by modulating F-actin assembly
In order to illustrate the function of TPM4, TPM4 was re-expressed in TPM4 knock-out cells (Figure 4A). As shown in Figure 4C and D, cell migration ability was enhanced in TPM4 upregulated H1299 and A549 cells. Wound healing assay also indicated that cell motility was regained while TPM4 was reexpressed in H1299 and A549 cells (Figure 4E). And levels of EMT markers were still not affected by the overexpression of TPM4 (Figure 4B).
Figure 4.
TPM4 promoted H1299 and A549 cell migration. (A) TPM4 upregulation cell model was obtained and the expression of TPM4 was determined by WB. GAPDH is used as a loading control. (B) Expression of mesenchymal proteins, vimentin and β-catenin, transcription factors, snail, and ZEB1 was examined by immunoblotting. GAPDH is used as a loading control. (C, D) Transwell migration assay was performed. Cells migrated through the chamber were counted in five microscopic fields and normalized to H1299 group or A549 group, respectively. The data is expressed as mean ± SEM. Bars represent 50 μm. ** p<0.01 (unpaired Student t-test). (E) wound scratch assay was performed. The wounds were observed and recorded at 0 hr, 9 hrs, and 16 hrs along the scratch, respectively. Bars represent 50 μm.
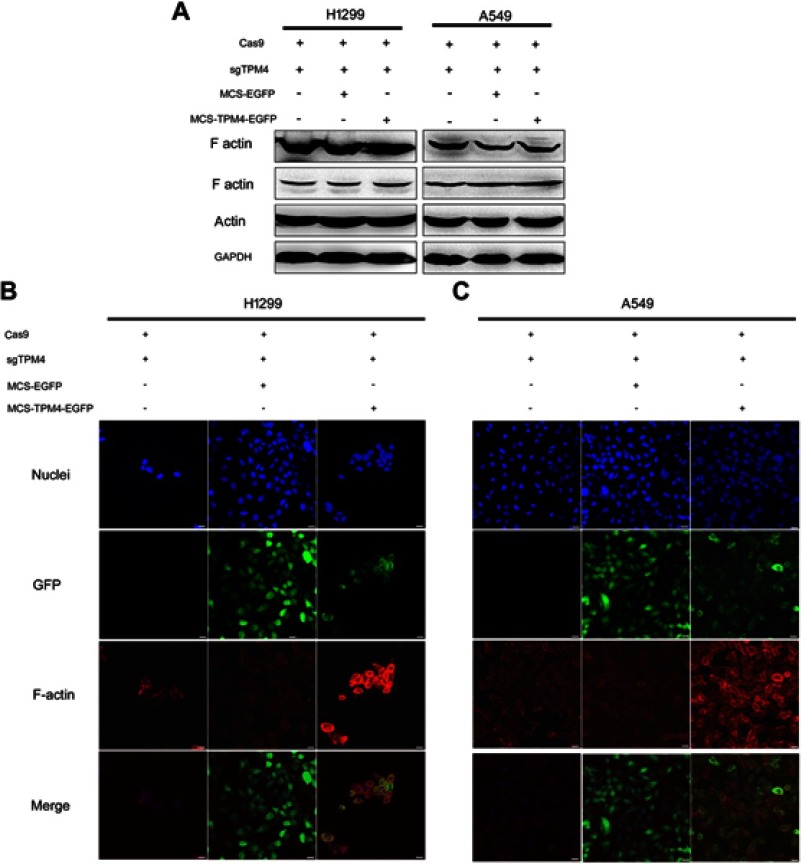
F-actin was also detected by both Western blot analysis and immunofluorescence test. The expression level of F-actin was not affected by the reexpression of TPM4 in H1299 and A549 cells (Figure 5A). But the immunofluorescence test indicated that F-actin level was significantly enhanced by upregulation of TPM4 (Figure 5B). More filopodia were found in TPM4 overexpressed H1299 and A549 cells. Taken together, TPM4 could promote F-actin formation in lung cancer cells.
Figure 5.
F-actin assembly was increased when TPM4 was upregulated. (A) WB technique was used to determine F-actin level in TPM4 upregulating cells as well as control cells. The actin level was used as a control, with GADPH used as the internal loading controls. (B, C) F-actin was labeled by immunofluorescence staining. The red signal represents the staining of F-actin, the green signal represents the fluorescence of GFP, and the blue signal represents the nuclear DNA staining by Hoechst 33342. Bars represent 20 μm.
Abbreviation: WB, Western blot.
Discussion
TPMs together with actin-binding proteins are involved in the regulation of actin’s interaction with binding proteins. In our study, the function of TPM4 in lung cancer cells was determined. TPM4 plays an important role in F-actin formation and cell motility.
In the previous study, TPM4 was considered as a potential marker in several cancers. In breast cancer and ovarian cancer, TPM4 could be a biomarker for the early detection.10,17 TPM4 level was elevated in the serum levels of ovarian cancer patients compared to non-cancer controls.18 But TPM4 plays different roles in different tumors acting as oncogene or anti-oncogene. According to the proteomic or DNA microarray analysis, TPM4 was upegulated in esophageal squamous cell carcinoma (ESCC), keratoacanthoma, and infiltrating ductal breast carcinomas.8,19,20 TPM4 level is decreased in colon cancer tissue and cell lines.7 In colon cancer, TPM4 expression level was obviously associated with clinical TNM stage, and survival analysis indicated that low expression of TPM4 was an unfavorable independent prognostic factor for colon cancer patients.7 In our research, TPM4 was highly expressed in most NSCLC cell lines. But the function of TPM4 in lung cancer was unknown yet.
In colon cancer lines, cell growth was not affected by TPM4 expression.7 In our study, we got similar results, TPM4 had no effect on cell proliferation of H1299 and A549 cells. F-actin plays an important role in cell migration and invasion. Since TPM4 is involved in the stabilization of the actin filament, we speculated that cell motility could be modulated by TPM4. On the basis of the proteome profile, TPM4 level was higher in primary tumors compared to (against that) the lymph node metastatic site.9 Comparative proteomic analysis also indicated that TPM4 was a potential marker for breast cancer metastatic progression, and TPM4 level was associated with the presence of lymph node metastasis, clinical stage.11 In colon cancer, cell migration and invasion were inhibited by upregulation of TPM4.7 Tpm4.1 downregulation in MCF10A cells enhanced cell migration and invasion.21 In lung cancer cell line H1299 and A549 cells, cell migration ability was associated with the expression level of TPM4. As a result, TPM4 expression was able to promote cell migration. However, cell invasion ability was not affected while TPM4 expression was upregulated or downregulated in lung cancer cells (data not shown). Briefly, TPM4 is involved in cell motility regulation and plays different roles in different tumors.
Meanwhile, whether TPM4 was involved in the EMT progression was detected. The levels of EMT markers were not affected by TPM4 expression. In MDA-MB-231 cells, downregulation of Tpm4.1 also led to disruption of actin organization.21 We also determined the effect of TPM4 on F-actin. As a result, F-actin levels as well as filopodia were decreased in TPM4 knockout lung cancer cells while that were increased by overexpression of TPM4. But the expression level of F-actin was not affected by TPM4 in Western blot test. F-actin is a linear polymer microfilament formed by monomer (also called globular-actin, G-actin). The Western blot test recognizes both F-actin and G-actin. It demonstrated that TPM4 could promote the formation of F-actin while the expression of F-actin was not changed. We thought that TPM4 modulates cell motility by altering the actin formation.
In summary, TPM4 was highly expressed in a set of lung cancer cell lines. As an actin-associating protein, TPM4 was able to regulate actin assembly in H1299 and A549 cells. Our findings demonstrated that TPM4 promoted cell migration by modulating F-actin formation.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81602531; No, 81672838; No, 61431019; No, 61671430) and Beijing Natural Science Foundation (No. 7174289).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Sáez B, Pattillo S, Carlos J, Vuletin S, Fernando J. Deformación de la pared torácica como presentación de un blastoma pleuropulmonar, caso clínico. Rev Chil Pediatr. 2018;89:231–235. doi: 10.4067/S0370-41062018000200231 [DOI] [PubMed] [Google Scholar]
- 4.Lin JJ, Eppinga RD, Warren KS, McCrae KR. Human tropomyosin isoforms in the regulation of cytoskeleton functions. Adv Exp Med Biol. 2008;644:201–222. [DOI] [PubMed] [Google Scholar]
- 5.Clayton JE, Pollard LW, Murray GG, Lord M. Myosin motor isoforms direct specification of actomyosin function by tropomyosins. Cytoskeleton (Hoboken, NJ). 2015;72:131–145. doi: 10.1002/cm.v72.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janco M, Bonello TT, Byun A, et al. The impact of tropomyosins on actin filament assembly is isoform specific. Bioarchitecture. 2016;6:61–75. doi: 10.1080/19490992.2016.1201619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang R, Zheng G, Ren D, et al. The clinical significance and biological function of tropomyosin 4 in colon cancer. Biomed Pharmacother. 2018;101:1–7. doi: 10.1016/j.biopha.2018.01.166 [DOI] [PubMed] [Google Scholar]
- 8.Qi Y, Chiu JF, Wang L, Kwong DL, He QY. Comparative proteomic analysis of esophageal squamous cell carcinoma. Proteomics. 2005;5:2960–2971. doi: 10.1002/pmic.200401175 [DOI] [PubMed] [Google Scholar]
- 9.Milioli HH, Santos SK, Kaviski R, et al. Comparative proteomics of primary breast carcinomas and lymph node metastases outlining markers of tumor invasion. Cancer Genomics Proteomics. 2015;12:89–101. [PubMed] [Google Scholar]
- 10.Bailey MJ, Shield-Artin KL, Oliva K, Ayhan M, Reisman S, Rice GE. Stage-specific analysis of plasma protein profiles in ovarian cancer: difference in-gel electrophoresis analysis of pooled clinical samples. J Carcinog. 2013;12:10. doi: 10.4103/1477-3163.114216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li DQ, Wang L, Fei F, et al. Identification of breast cancer metastasis-associated proteins in an isogenic tumor metastasis model using two-dimensional gel electrophoresis and liquid chromatography-ion trap-mass spectrometry. Proteomics. 2006;6:3352–3368. doi: 10.1002/pmic.200500617 [DOI] [PubMed] [Google Scholar]
- 12.Kopantzev EP, Monastyrskaya GS, Vinogradova TV, et al. Differences in gene expression levels between early and later stages of human lung development are opposite to those between normal lung tissue and non-small lung cell carcinoma. Lung Cancer. 2008;62:23–34. doi: 10.1016/j.lungcan.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 14.Suchowerska AK, Fok S, Stefen H, et al. Developmental profiling of tropomyosin expression in mouse brain reveals Tpm4.2 as the major post-synaptic tropomyosin in the mature brain. Front Cell Neurosci. 2017;11:421. doi: 10.3389/fncel.2017.00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24:277–283. doi: 10.1016/j.ceb.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Najm P, El-Sibai M. Palladin regulation of the actin structures needed for cancer invasion. Cell Adh Migr. 2014;8:29–35. doi: 10.4161/cam.28024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pendharkar N, Gajbhiye A, Taunk K, et al. Quantitative tissue proteomic investigation of invasive ductal carcinoma of breast with luminal B HER2 positive and HER2 enriched subtypes towards potential diagnostic and therapeutic biomarkers. J Proteomics. 2016;132:112–130. doi: 10.1016/j.jprot.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 18.Tang HY, Beer LA, Tanyi JL, Zhang R, Liu Q, Speicher DW. Protein isoform-specific validation defines multiple chloride intracellular channel and tropomyosin isoforms as serological biomarkers of ovarian cancer. J Proteomics. 2013;89:165–178. doi: 10.1016/j.jprot.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ra SH, Su A, Li X, et al. Keratoacanthoma and squamous cell carcinoma are distinct from a molecular perspective. Mod Pathol. 2015;28:799–806. doi: 10.1038/modpathol.2015.5 [DOI] [PubMed] [Google Scholar]
- 20.Kabbage M, Trimeche M, Ben Nasr H, et al. Tropomyosin-4 correlates with higher SBR grades and tubular differentiation in infiltrating ductal breast carcinomas: an immunohistochemical and proteomics-based study. Tumour Biol. 2013;34:3593–3602. doi: 10.1007/s13277-013-0939-0 [DOI] [PubMed] [Google Scholar]
- 21.Jeong S, Lim S, Schevzov G, Gunning PW, Helfman DM. Loss of Tpm4.1 leads to disruption of cell-cell adhesions and invasive behavior in breast epithelial cells via increased Rac1 signaling. Oncotarget. 2017;8:33544–33559. doi: 10.18632/oncotarget.16825 [DOI] [PMC free article] [PubMed] [Google Scholar]