Abstract
The major challenges of current mesenchymal stem cell (MSC)-based therapeutics are their low differentiation potential into specialized cell types and their homing ability to sites of injury. Therefore, many researchers have directed their efforts toward finding a novel stimulatory factor that can significantly enhance the therapeutic effects of MSCs. Colony-stimulating factor 2 (CSF-2) is previously known as a hematopoietic growth factor involved in the differentiation of various myeloid cells from hematopoietic progenitor cells. In addition to this canonical hematopoietic function, we identified for the first time that CSF-2 is actively secreted by stem cells, in response to various types of injuries, as an endogenous damage signal that promotes the therapeutic effects of MSCs by enhancing their multi-lineage differentiation and migratory capacities, possibly through its receptor CD116. Our results also revealed that CSF-2 exerts its stimulatory effects on MSCs via PI3K/Akt- and/or FAK/ERK1/2-signaling pathways. More importantly, we also found that MSCs stimulated with CSF-2 show markedly enhanced differentiation and migratory capacities and subsequent in vivo therapeutic effects in an endometrial ablation animal model. Collectively, our findings provide compelling evidence for a novel non-hematopoietic function of CSF-2 in promoting multiple beneficial functions of MSCs via a non-canonical mechanism as an endogenous damage signal.
Keywords: CSF-2, MSCs, growth, migration, stemness, differentiation, Akt, ERK1/2
Graphical Abstract
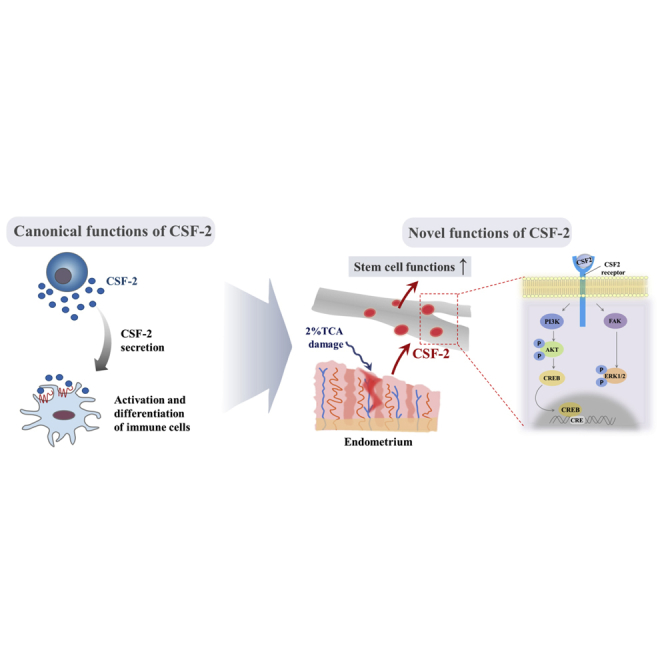
In addition to its canonical function, CSF-2 is actively secreted by stem cells, in response to various types of injuries, as an endogenous damage signal that promotes the therapeutic effects of stem cells by enhancing their multi-lineage differentiation and migratory capacities via PI3K/Akt- and/or FAK/ERK1/2-signaling pathways in vitro and in vivo.
Introduction
Until recently it was believed that colony-stimulating factor 2 (CSF-2), also known as granulocyte-macrophage colony-stimulating factor (GM-CSF), is a hematopoietic growth factor involved in the differentiation of various myeloid cells, such as dendritic cells, granulocytes, and macrophages from hematopoietic progenitor cells.1 Interestingly, in addition to its hematopoietic function, several recent studies suggested that CSF-2 and its receptor are also found on multiple types of non-hematopoietic cells, including neuronal cells,2 intestinal epithelial cells,3 and keratinocytes.4 In this context, special attention has been devoted recently to the non-hematopoietic functions of CSF-2 as a novel secretory factor in multiple human diseases, because it was released selectively under certain disease conditions and improved the symptoms of these diseases.5, 6, 7 Therefore, in the current study, we focused our attention on discovering the previously unidentified roles of CSF-2 as a non-hematopoietic cytokine that appears to stimulate multiple beneficial functions of mesenchymal stem cells (MSCs), because of previous observations showing that CSF-2 enhances the proliferation of neural precursor cells8 and exerts pro-neurogenic effects on neural progenitor cells.9
MSCs have been used for many years to treat a number of diseases in the fields of regenerative medicine, because of their therapeutic potential to promote growth and differentiation into various lineages of endogenous stem or progenitor cells.10, 11 Moreover, MSCs can be activated and recruited to damaged sites in response to chemotactic signals of cellular damage and, subsequently, promote tissue repair.12, 13, 14 However, the molecular mechanisms by which MSCs sense danger signals, are recruited to the sites of tissue injury, and mediate beneficial effects in tissue regeneration still remain largely unclear. In the current study, we hypothesized that CSF-2 may be actively secreted from injured tissue as an endogenous danger signal and, subsequently, stimulate tissue regeneration by promoting the migration and/or differentiation potential of MSCs. Here, we demonstrated for the first time that CSF-2 is actively released by non-hematopoietic cells in response to injury signals, both in vitro and in vivo, and then acts as a potent facilitating factor that promotes the migratory and differentiation capacities of stem cells.
Subsequently, we explored the possible mechanism underlying the promoting effects of CSF-2 on the migratory and differentiation capacities of MSCs. Importantly, CSF-2 significantly activates multifunctional pathways, such as ERK1/2- and PI3K/Akt-signaling cascades, which are involved in multiple biological functions, including stem cell migration,15, 16 differentiation,16, 17, 18 and survival.19, 20 Additionally, suppression of these signaling pathways with a specific inhibitor markedly attenuated CSF-2-mediated promoting effects on the migration and differentiation of MSCs. Our results suggest that CSF-2 enhances the differentiation potential and homing ability of stem cells through ERK1/2- and/or PI3K/Akt-signaling pathways. Another important finding from the current study is that the therapeutic effects of stem cells could be markedly enhanced when they were pre-stimulated with CSF-2 before administration in an endometrial ablation animal model. Taken together, these results suggest that, in addition to its well-known hematopoietic functions, CSF-2 is actively secreted in response to tissue injury as an endogenous danger signal, and, subsequently, it enhances the in vivo therapeutic effects of stem cells by stimulating differentiation and migratory potential through ERK1/2 and/or PI3K/Akt signaling.
Results
CSF-2 Is Actively Secreted in Response to Multiple Injury Signals In Vitro and In Vivo
Human MSCs were successfully isolated from adipose tissues (Figure S1A), and then their properties were characterized by the following conjugated monoclonal antibody combinations: negative (CD34 and CD45) and positive (CD44, CD73, and CD105) MSC surface markers (Figure S1B). Next, their multiple lineage differentiation potential was evaluated by inducing in vitro osteogenic, adipogenic, and chondrogenic differentiation (Figure S1C). To investigate whether CSF-2 is actively secreted from injured or stressed cells in response to various damage signals, MSCs were exposed to multiple damage conditions, such as radiation damage, oxidative stress, and serum depletion. Secreted proteins in the culture supernatant were precipitated using a 10% trichloroacetic acid (TCA) protocol, as previously described.21
To evaluate whether H2O2 treatment actually induces oxidative stress in stem cells, the expression levels of reactive oxygen species (ROS) modulator 1 (ROMO1), which is one of the well-known mediators of oxidative stress, were measured in both mitochondrial and cytosolic fractions. As expected, ROMO1 expression levels were significantly increased by H2O2 treatment in both mitochondrial and cytosolic fractions (Figure S2), suggesting that H2O2 treatment successfully induced oxidative stress. Additionally, to evaluate whether 4-Gy exposure actually induces growth inhibition, the expression levels of tumor suppressor protein p53 and cell cycle stages were analyzed by western blotting and flow cytometry, respectively. As expected, the protein levels of p53 were significantly increased by 4-Gy exposure (Figure S3A). The 4-Gy exposures also induced G2/M cell-cycle arrest in MSCs (Figure S3B). These results indicated that acute irradiation significantly induced cell growth inhibition of stem cells. To evaluate whether serum deprivation induces cell-cycle arrest at G0/G1, the cell cycle stages were also analyzed by flow cytometry. As expected, serum deprivation also significantly induced G0/G1 cell-cycle arrest in MSCs, indicating that serum deprivation significantly induced cell-cycle arrest at G0/G1 in MSCs (Figures S4A and S4B).
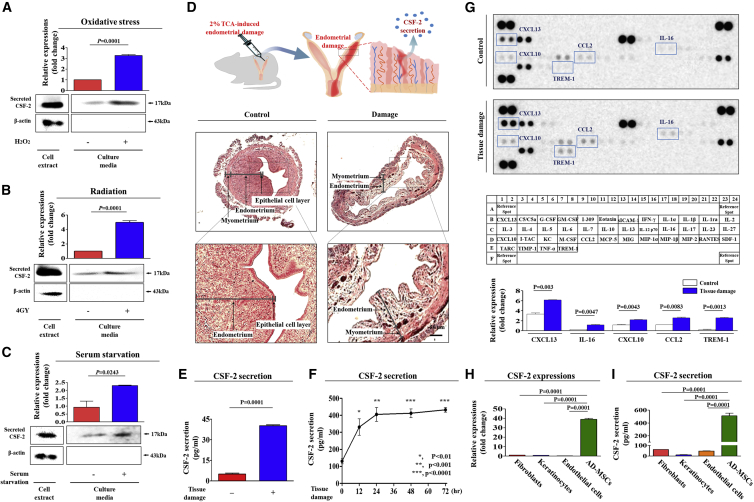
As shown in Figures 1A–1C, MSCs actively secreted CSF-2 into the culture medium in response to various damage signals or stress in vitro. Cocuzza et al.22 have developed an animal model of endometrial ablation by administering acidic solution into the uterine cavity. Therefore, to further verify in vivo whether tissue injury can induce CSF-2 secretion into the blood circulation to restore a damaged region, systemic CSF-2 levels in peripheral blood samples from mice were examined following acidic TCA solution-induced uterine endometrial damage. Histological examination revealed that acidic solutions distinctively impaired and narrowed the endometrial functional layer with degenerative changes and a loss of superficial gland column compared to control groups (Figure 1D). The endometrial damage resulted in a significant increase in CSF-2 secretion into the peripheral circulation of mice in vivo, as determined by ELISA (Figure 1E). To further determine entry points of CSF-2 secretion after injuries, mice were exposed to endometrial tissue damage, and then peripheral bloods were sampled at many time points and we performed ELISA analysis. Injured endometrial tissues secreted CSF-2 into the peripheral circulation in response to damage after 12 h. The observed CSF-2 secretion kinetics showed this in a clearly time-dependent manner, with a peak release 24 h after injury (Figure 1F).
Figure 1.
CSF-2 Is Actively Secreted by Stem Cells in Response to Various Injury Signals In Vitro and In Vivo
(A) Mesenchymal stem cells (MSCs) were incubated in standard culture medium with or without H2O2 (10 mM) for 30 min, after which the medium was replaced with serum-free medium, and the cells were cultured for 48 h. (B) MSCs were exposed to acute irradiation at a dose of 4 Gy (X-ray), after which the medium was replaced with serum-free medium, and the cells were cultured for 48 h. (C) MSCs were cultured with or without serum for 48 h. (D) The 2% TCA treatment (150 μL, administered directly into the uterine horn) produced significant histological uterine endometrial ablation. (E) Increased CSF-2 concentrations by TCA treatment in the serum samples were also detected by ELISA. (F) TCA-induced acute endometrial ablation resulted in a clearly time-dependent manner, with a peak release 24 h after injury. (G) Cytokine/growth factor assay was performed using damaged and non-damaged samples. The membrane was printed with antibodies for 40 growth factors, cytokines, and receptors, with four positive and four negative controls in the upper and lower left corners. Five growth factors or related proteins (CXCL13, IL-16, CXCL10, CCL2, and TREM-1) were markedly enriched in the damaged groups compared with those in the control groups. The expression and secretion levels of CSF-2 in multiple cell types, such as fibroblasts, keratinocytes, endothelial cells, and MSCs, were detected by qPCR (H) and ELISA (I), respectively. β-actin was used as the internal control. The results represent the mean ± SD from three independent experiments.
We also conducted an additional set of cytokine/growth factor assays using peripheral blood samples from mice, with or without acid solution-induced uterine endometrial damage, to examine whether the endometrial damage can induce the secretion of other cytokines/chemokines. In this experiment, we detected the changes in 40 proteins in both damaged and non-damaged blood samples. Interestingly, the expression levels of six growth factors, namely, CXCL13, interleukin-6 (IL-6), CXCL10, CCL2, and TREM-1, were secreted substantially by endometrial damage, whereas the levels of other growth factors showed only minor changes (Figure 1G). Additionally, to further determine whether CSF-2 is preferentially secreted from stem cells compared to other differentiated cell types, the expression and secretion levels of CSF-2 in multiple cell types were quantified using real-time PCR and ELISA, respectively. Interestingly, both real-time PCR and ELISA results showed that stem cells exhibited significantly higher CSF-2 expression (Figure 1H) and secretion (Figure 1I) than other terminally differentiated somatic cells, such as endothelial cells, fibroblasts, and keratinocytes, indicating that CSF-2 likely acts as a stem cell-specific secretory factor.
CSF-2 Significantly Enhances Various Beneficial Functions of Stem Cells In Vitro
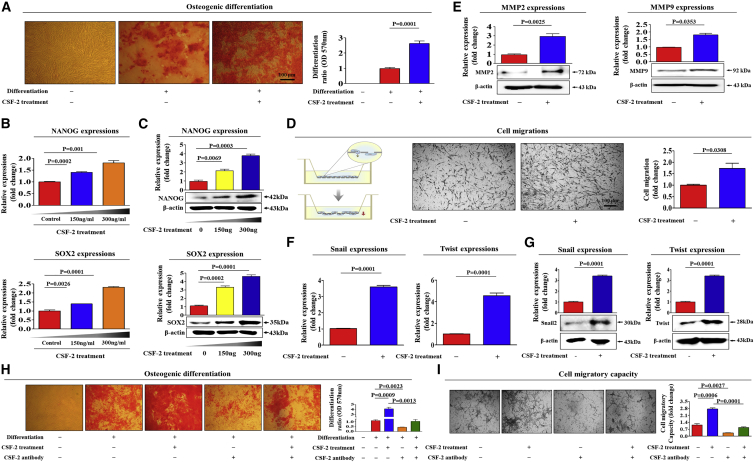
As CSF-2 might play a role as an endogenous danger signal in response to tissue damage, we investigated whether CSF-2 can promote multiple beneficial functions of stem cells to repair damaged tissue. Importantly, CSF-2 markedly enhanced the differentiation capacities of stem cells toward osteoblasts in vitro (Figure 2A). Both mRNA and protein levels of the most commonly used pluripotency-associated transcription factors, NANOG and SOX2, were also significantly enhanced by CSF-2 treatment (Figures 2B and 2C). Differentiation potential and migratory capacity to the sites of tissue damage of stem cells are independently important for their therapeutic potential. We therefore also investigated whether CSF-2 can promote migratory capacity of stem cells. Importantly, CSF-2 significantly enhanced the migratory capacity of stem cells in vitro (Figure 2D). To further evaluate the promoting effect of CSF-2 on the migratory capability of stem cells, western blot analysis was used to assess the expression levels of matrix metalloproteinase 2/9 (MMP-2/9), which play a crucial role in regulating cell migration and tissue regeneration (Figure 2E). It is also important to compare CSF-2 with another well-known migration-stimulating factor (FGF2). Interestingly, CSF-2 more effectively increased the migratory capacity of stem cells than the well-known migration-stimulating factor FGF2 (Figure S5).
Figure 2.
CSF-2 Promotes the Differentiation and Migratory Capacities of MSCs In Vitro
Confluent MSCs were cultured in osteogenic medium with or without CSF-2 (300 ng/mL). The effects of CSF-2 on osteoblast differentiation were determined by alizarin red staining. (A) The relative quantification of calcium mineral content was determined by measuring the absorbance at 570 nm. Real-time PCR (B) and western blotting (C) results demonstrating changes in the expression of the stem cell markers NANOG and SOX2 after CSF-2 treatment for 24 h. (D) MSCs were treated with CSF-2 for 24 h, after which the effect of CSF-2 on MSC migration ability was evaluated using the transwell migration assay. CSF-2 treatment significantly increased MSC migration across the membrane compared with the negative control. (E) The relative expression levels of key positive regulators of cell migration (MMP-2/9) were assessed using western blotting. Real-time PCR (F) and western blotting (G) results demonstrating changes in the expression of various EMT (epithelial-to-mesenchymal transition) markers, such as snail, and twist. MSCs were treated with 300 ng/mL CSF-2 alone or were concomitantly treated with neutralizing antibody targeting CSF-2; subsequent changes in osteoblast differentiation (H) and migratory capacity (I) were measured with alizarin red staining and transwell assay, respectively. β-actin was used as the internal control. The results represent the mean ± SD from three independent experiments.
Previous studies have revealed that reorganization and turnover of the actin cytoskeleton can be associated with cell migration by pushing or pulling on the substrate beneath the plasma membrane at the front of the cell.23 Consistently, phalloidin staining of actin cytoskeleton revealed a significant correlation between the CSF-2 treatment and dynamic actin filament rearrangement (Figure S6), suggesting that the markedly enhanced migratory ability of CSF-2-treated stem cells may be associated with actin filament rearrangement. Additionally, CSF-2 significantly increased mRNA and protein expression levels of various EMT (epithelial-to-mesenchymal transition) markers, such as snail and twist (Figures 2F and 2G). To further substantiate our hypothesis, we performed additional experiments using a CSF-2-neutralizing antibody to block the activity of CSF-2. Importantly, the CSF-2-induced multi-lineage differentiation potential toward osteoblasts (Figure 2H) and migratory capacity (Figure 2I) were significantly attenuated by CSF-2-neutralizing antibody.
CSF-2 Enhances Multiple Stem Cell Functions through Its Cognate Receptor CD116
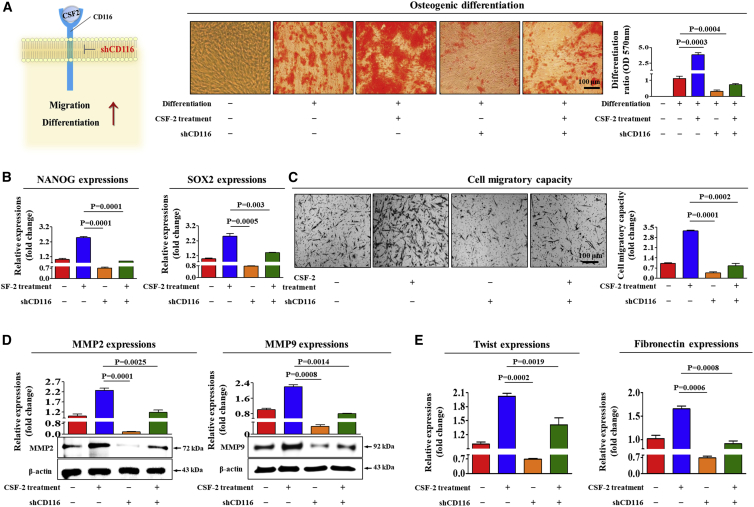
The functions of CSF-2 in multiple types of hematopoietic cells are mediated by a cell surface receptor, CD116, belonging to the colony-stimulating hematopoietic growth factor receptor family.24 Therefore, to confirm whether CD116 acts as a functional receptor for CSF-2 in human adipose tissue-derived MSCs, MSCs were stably transduced with small hairpin RNA (shRNA) 1, 2, 3, 4, or 5 targeting CD116 or with a non-targeting control shRNA. CD116 shRNA construct 5, hereafter referred to as CD116 shRNA, was the most efficient at knockdown. Successful knockdown of CD116 was verified based on RNA and protein levels in MSCs (Figures S7A–S7C). Importantly, the CSF-2-induced multi-lineage differentiation potential toward osteoblasts (Figure 3A) and expressions of the pluripotency-associated factors NANOG and SOX2 (Figure 3B) were significantly attenuated by CD116 depletion. In addition, we found that CSF-2-induced effects on migratory ability (Figure 3C) and MMP-2/9 expression (Figure 3D) were markedly disrupted by CD116 knockdown. Consistently, the stimulatory effects of CSF-2 on actin cytoskeleton disorganization (Figure S8) and on the expressions of various EMT markers (Figure 3E) were also significantly attenuated by CD116 depletion.
Figure 3.
CSF-2 Stimulates Multiple Beneficial Functions of MSCs through CD116
MSCs were treated with 300 ng/mL CSF-2 alone or were concomitantly transfected with shRNA targeting CD116; subsequent changes in osteoblast differentiation (A) and in the expression of pluripotency-associated factors NANOG and SOX2 (B) were measured with alizarin red staining and real-time PCR, respectively. The relative quantification of calcium mineral content was determined by measuring the absorbance at 570 nm. The inhibitory effect of CD116 knockdown on CSF-2-induced changes in the migratory capacity were measured by the transwell assay (C) and western blotting for MMP-2 and MMP-9 (D). (E) The attenuating effects of CD116 depletion on CSF-2-induced expression of fibronectin and twist in MSCs were measured by real-time PCR. β-actin was used as the internal control. The data represent the mean ± SD from three independent experiments.
PI3K/Akt and/or ERK1/2 Signaling Mediates CSF-2-Induced Multiple Beneficial Functions of Stem Cells
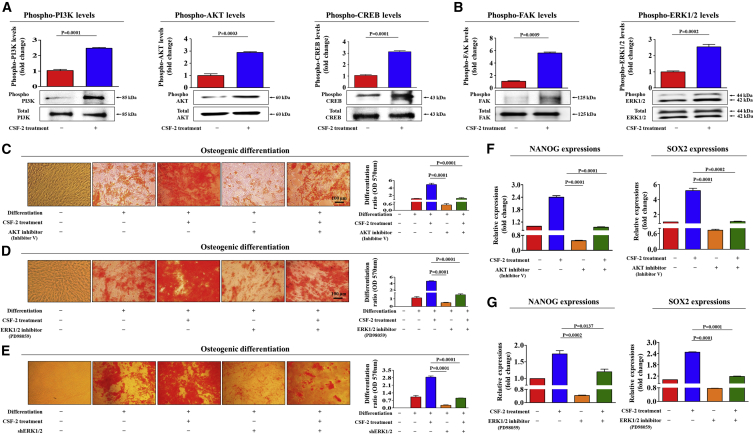
To explore the underlying mechanisms of the stimulatory effects of CSF-2 on multiple stem cell functions, we investigated the effects of CSF-2 on PI3K/Akt- or ERK1/2-signaling components, which have been related to the differentiation,25 maintenance,26 and migration27 of various stem cells. Using western blotting, we investigated whether the PI3K/Akt- and FAK/ERK1/2-signaling cascades are activated in CSF-2-treated stem cells. Importantly, the phosphorylation levels of these signaling components were significantly increased in response to CSF-2 treatment (Figures 4A and 4B).
Figure 4.
CSF-2-Induced Stimulatory Effects on Stem Cells Are Mediated through PI3K/Akt and/or FAK/ERK1/2 Signaling
(A and B) MSCs were stimulated for 10 min with or without CSF-2 (300 ng/mL). The cells were then lysed, and the protein contents were analyzed by western blotting using antibodies targeting the phosphorylated forms of PI3K, AKT, and CREB (A) and FAK and ERK1/2 (B). The phosphorylation levels of these signaling molecules were significantly increased in cells treated with CSF-2. (C and D) MSCs were pretreated with inhibitor V (C; 10 μM) or PD98059 (D; 20 μM) for 1 h prior to the additional treatment with 300 ng/mL CSF-2; subsequent changes in osteoblast differentiation were determined by alizarin red staining. (E) MSCs were treated with 300 ng/mL CSF-2 alone or were concomitantly transfected with shRNA targeting ERK1/2; subsequent changes in osteoblast differentiation were measured with alizarin red staining. (F and G) The expression of pluripotency-associated factors NANOG and SOX2 were determined by real-time PCR. The relative quantification of calcium mineral content was determined by measuring the absorbance at 570 nm. β-actin was used as the internal control. The data represent the mean ± SD from three independent experiments.
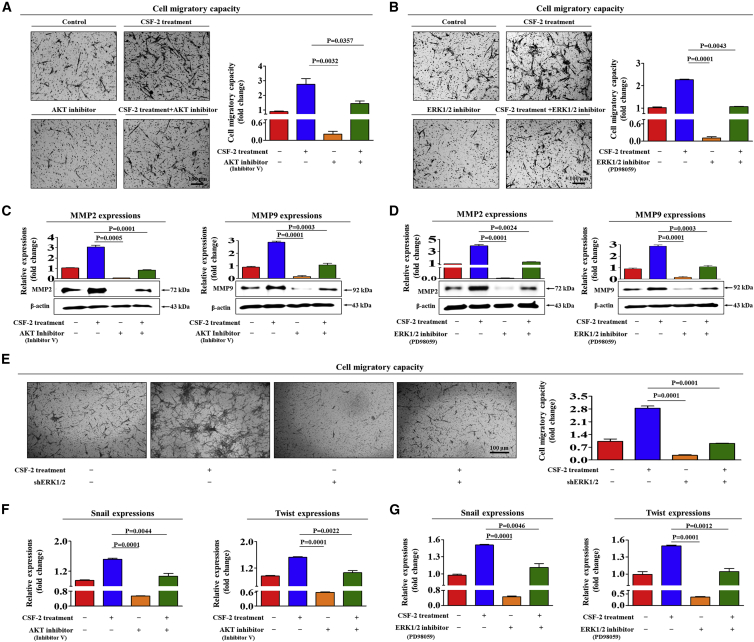
Next, to determine whether the blockade of these signaling components with a specific inhibitor attenuates CSF-2-induced stimulatory effects on multiple beneficial functions of stem cells, we assessed the effects of the Akt inhibitor V or the ERK1/2 inhibitor PD98059 on multi-lineage differentiation potential and on the expression of the pluripotency-associated factors NANOG and SOX2, with or without CSF-2 treatment. Indeed, the CSF-2-induced effects on multi-lineage differentiation potential toward osteoblasts were significantly attenuated by pretreatment with inhibitor V (Figure 4C) or PD98059 (Figure 4D). Additionally, to further confirm whether ERK1/2 signaling can regulate CSF-2-mediated functions, we knocked down both ERK1 and 2 expressions using a specific shRNA in stem cells. Successful knockdown of both ERK1 and ERK2 was verified based on RNA and protein levels in stem cells (Figures S9A–S9D). Consistently, the CSF-2-mediated differentiation potential (Figure 4E) was also significantly attenuated by both ERK1 and ERK2 knockdown. CSF-2-induced stimulatory effects on the expressions of the pluripotency-associated factors NANOG and SOX2 were significantly attenuated by pretreatment with inhibitor V (Figure 4F) or PD98059 (Figure 4G).
In addition, pretreatment with inhibitor V or PD98059 resulted in disruption of the CSF-2-induced migration (Figures 5A and 5B) and MMP-2/9 expression (Figures 5C and 5D) of stem cells. Consistently, the CSF-2-mediated migratory capacities (Figure 5E) were also significantly attenuated by both ERK1 and ERK2 knockdown. The stimulatory effects of CSF-2 on the expressions of various EMT markers were also significantly attenuated by pretreatment with inhibitor V or PD98059 (Figures 5F and 5G). These results indicate that the PI3K/Akt- and/or FAK/ERK1/2-signaling pathways may be associated with the CSF-2-mediated multiple beneficial functions of stem cells.
Figure 5.
Inhibition of Akt or ERK1/2 with Specific Inhibitors Attenuated the CSF-2-Induced Migratory Capacity of MSCs
(A–D) MSCs were pretreated with inhibitor V (A and C, 10 μM) or PD98059 (B and D, 20 μM) for 1 h prior to an additional 48-h treatment with 300 ng/mL CSF-2; subsequent changes in migratory capacity were measured via the transwell assay (A and B) and western blotting for MMP-2 and MMP-9 (C and D). (E) MSCs were treated with 300 ng/mL CSF-2 alone or were concomitantly transfected with shRNA targeting ERK1/2; subsequent changes in the migratory capacity were measured by the transwell assay. (F and G) MSCs were pretreated with inhibitor V (F, 10 μM) or PD98059 (G, 20 μM) for 1 h prior to an additional 48-h treatment with 300 ng/mL CSF-2; subsequent changes in the expressions of multiple EMT markers, such as snail and twist (F and G), were measured by real-time PCR. β-actin was used as the internal control. The data represent the mean ± SD from three independent experiments.
Profiling CSF-2-Induced Expressions of Multiple Growth Factors and Their Interconnected Signaling Networks
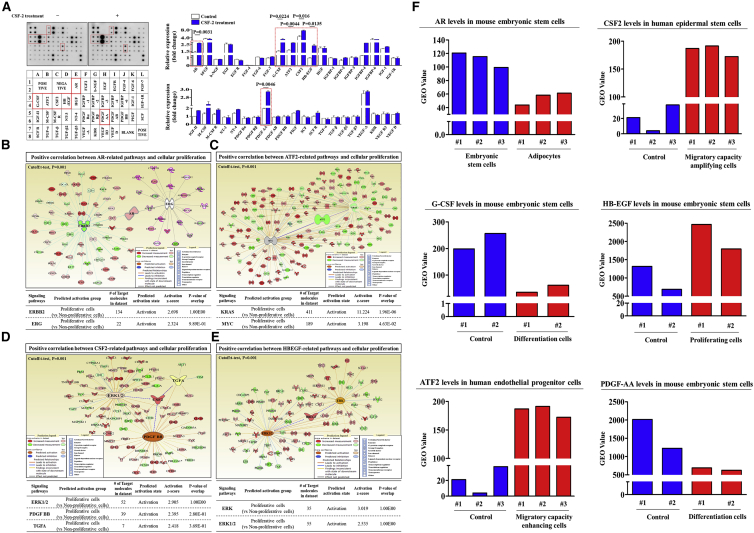
To identify the major growth factors and signaling networks responsible for the stimulatory effects of CSF-2, we analyzed the CSF-2-induced expression of multiple cytokines/growth factors using antibody arrays. In this experiment, we detected the changes in 40 proteins in both CSF-2-treated stem cells and non-treated control stem cells. The expression levels of six growth factors, namely, AR (androgen receptor),28 G-CSF (granulocyte colony-stimulating factor),29 GDNF (glial cell-derived neurotrophic factor),30 GM-CSF,31 HB-EGF (heparin-binding epidermal growth factor-like growth factor),32 and PDGF (platelet-derived growth factor),33 were elevated substantially by CSF-2 treatment, whereas the levels of other growth factors showed only minor changes (Figure 6A). Consistent with our results, these growth factors can function as potent upstream signaling proteins of PI3K/Akt or ERK1/2 signaling. This result indicates that these six growth factors may be at least partly responsible for CSF-2-induced PI3K/Akt or ERK1/2 signaling and the subsequent beneficial effects on stem cells.
Figure 6.
CSF-2-Induced Multiple Growth Factors Are Associated with PI3K/Akt and/or FAK/ERK 1/2 Signaling
Human growth factor antibody array analysis was performed using CSF-2-treated and control samples. The membrane was printed with antibodies for 40 growth factors, cytokines, and receptors, with four positive and four negative controls in the upper and lower left corners. (A) Six growth factors or related proteins (AR, G-CSF, GDNF, GM-CSF, HB-EGF, and PDGF) were markedly enriched in the CSF-2-treated groups compared with those in the control groups. (B–E) Differentially expressed genes from proliferative cells and nonproliferative cells were applied to ingenuity pathway analysis (IPA) software (https://www.qiagenbioinformatics.com/) to predict the activation state (either activated or inhibited) of six growth factors and their related signaling pathways. Big data were analyzed using the Seiber dataset (GEO: GSE63074, GSE62564, GSE63074, and GSE28878) from R2: Genomics Analysis and Visualization Platform (https://hgserver1.amc.nl/cgi-bin/r2/main.cgi). The gene datasets were filtered by the expression profiles of six growth factors in proliferative versus nonproliferative cells. (F) The GEO database (https://www.ncbi.nlm.nih.gov/geo/) was analyzed to further verify the expression levels of these growth factors in various aspects of stem cell functions. The results represent the means ± SD from three independent experiments.
To further confirm whether sonic hedgehog (SHH)-signaling integrity declines with aging, we examined the gene expression profiles in a large clinical database using ingenuity pathway analysis (IPA) software. Positive regulators of AR, ATF2, CSF-2, and HB-EGF were activated in nonsenescent proliferative cells (Figures 6B–6E). The GEO database was analyzed to verify the expression levels of these growth factors in various aspects of stem cell functions. Interestingly, the levels of ATF2, CSF-2, and HB-EGF were markedly increased in proliferating or migratory capacity-enhanced cells, and the levels of AR, G-CSF, and PDGF-AA were decreased in differentiated cells (Figure 6F).
Additionally, we also analyzed the activation state of PI3K/Akt or ERK1/2 signaling and the expression levels of AR, G-CSF, GDNF, GM-CSF, HB-EGF, and PDGF using GeneMANIA (http://genemania.org/) to evaluate interconnected signaling networks regulating cell growth, migration, and pluripotency-associated phenotypes (Figure S10). To find interactions of signaling networks, we considered several biological factors, including co-localization, co-expression, pathways, and genetic interactions. These results suggested a strong positive relationship between the activation state of PI3K/Akt or ERK1/2 signaling and the enhancement of the six most prominent factors, AR, G-CSF, GDNF, GM-CSF, HB-EGF, and PDGF.
CSF-2 Significantly Enhances the Therapeutic Potential of Stem Cells In Vivo by Stimulating Differentiation and Migratory Capacities
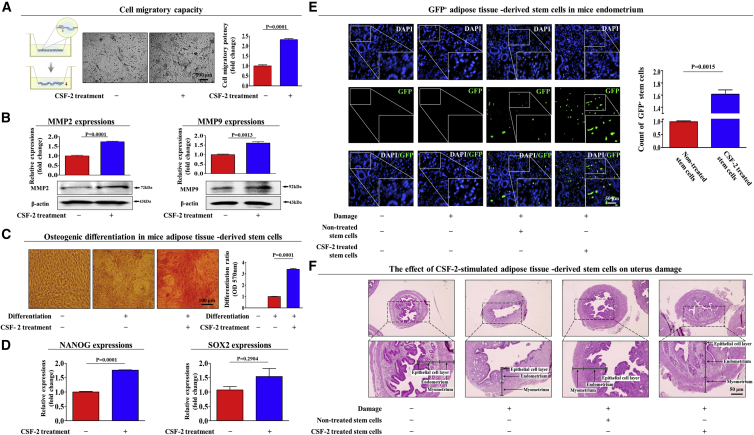
Our in vitro results indicated that CSF-2 may act as an injury-inducible danger signal that enhances multiple beneficial functions of stem cells, such as their differentiation and migratory capabilities. Therefore, we further investigated whether CSF-2 enhances various beneficial functions of stem cells in vivo and their subsequent therapeutic potential. Mice were injected intravenously (i.v.) with CSF-2 (0.5 mg/kg) on 10 consecutive days, and then MSCs were isolated from adipose tissue. Consistent with our in vitro data, the transwell migration assay (Figure 7A) and western blot analysis (Figure 7B) also showed the stimulatory effect of CSF-2 on the migratory ability of stem cells in vivo. CSF-2 significantly enhanced the multi-lineage differentiation potential of stem cells toward osteoblasts in vivo (Figure 7C). Consistently, the expression levels of the pluripotency-associated transcription factors NANOG and SOX2 were significantly increased by CSF-2 in vivo (Figure 7D).
Figure 7.
CSF-2 Improves the Therapeutic Potential of MSCs by Stimulating Differentiation and Migratory Capacities in a TCA-Induced Endometrial Ablation Animal Model
Schematic representation of the experimental protocol as described in the Materials and Methods. Mice were treated daily for 10 days with CSF-2 (0.5 mg/kg, intravenously) or vehicle (PBS). Stem cells were isolated from mouse adipose tissue, and the changes in migratory capacity were measured via the transwell assay (A) and western blotting for MMP-2 and MMP-9 (B). The changes in osteoblast differentiation were determined by alizarin red staining. (C) The relative quantification of calcium mineral content was performed by measuring the absorbance at 570 nm. (D) Real-time PCR results showed the changes in the expression of the mouse stem cell markers NANOG and SOX2 after CSF-2 treatment in vivo. (E) The 2% TCA treatment (150 μL, administered directly into the uterine horn) produced significant histological uterine endometrial ablation compared with the vehicle (PBS) control. CSF-2-treated or non-treated MSCs (1 × 106 cells) were labeled with GFP and injected intravenously into the tail veins of 7-week-old immunodeficient NSG mice with acute TCA-induced endometrial ablation. The mice were sacrificed 7 days after the GFP-labeled MSCs were injected. Green fluorescent images of consecutive sections revealed the presence of GFP-labeled cells. (F) Uterine endometrial tissue was collected and subjected to H&E staining. TCA-induced loss of the endometrial functional layer with degenerative changes was significantly more relieved by the transplantation of CSF-2-pretreated MSCs. β-actin was used as the internal control. The data represent the mean ± SD from eight independent experiments.
Furthermore, we investigated whether CSF-2 can stimulate not only the in vivo homing of stem cells to sites of injury but also their subsequent therapeutic potential in an endometrial ablation animal model. To investigate the effect of CSF-2 on the homing potential of stem cells to the site of injury, we investigated the in vivo migratory capability of stem cells treated with CSF-2 using a GFP system, which allows the monitoring of transplanted cells in animals. Stem cells were stably transfected with a GFP-expressing vector (Figure S11). CSF-2-prestimulated stem cells were intravenously injected into NOD scid gamma (NSG) mice, and their homing ability to the injured sites was monitored. The number of GFP-labeled cells on 5-μm-thick sections was counted and quantified using a fluorescent microscope. Importantly, there was significantly increased recruitment of CSF-2-prestimulated stem cells to the site of endometrial injury compared with that of non-stimulated stem cells (Figure 7E), indicating that CSF-2 treatment significantly enhanced the in vivo homing potential of stem cells to the injured site. Consistent with this result, the symptoms of acidic solution (TCA)-induced loss of the endometrial functional layer with degenerative changes were significantly more relieved by transplantation with CSF-2-prestimulated stem cells than with non-treated stem cells (Figure 7F). Taken together, these results suggest that CSF-2 significantly enhances the therapeutic potential of stem cells in vivo by stimulating differentiation and migratory capacities.
Discussion
MSCs have been proposed as a possible therapeutic alternative for various degenerative diseases due to their capacity to differentiate into multi-lineages (ectodermal34 and mesodermal35 cells) and their remarkable immunomodulatory ability in vivo.36 Despite a considerable number of promising initial results, several limitations of MSCs need to be addressed. The major challenges of current MSC-based therapeutics are their low differentiation potential into specialized cell types and their homing ability to sites of injury.37 Therefore, many researchers have directed their efforts toward finding a novel stimulatory factor that can significantly increase the homing and multi-lineage differentiation capabilities of MSCs.
Recently, special attention has been devoted to investigating the non-canonical functions of CSF-2 as a novel non-hematopoietic factor in various human diseases, such as Crohn’s disease5 and Alzheimer’s disease,38 as it is selectively released under certain disease conditions and ameliorates the severity of these diseases. Moreover, CSF-2 can promote neuronal differentiation of adult neural stem cells in vitro as a neuronal growth factor.9 In this context, we therefore focused our attention on the previously unidentified function of CSF-2 as an endogenous danger signal that can facilitate the mobilization and recruitment of stem cells to sites of injury in accordance with the observations of Deng et al.,39 who showed that CSF-2 can effectively mobilize CD34-positive bone marrow stem cells in patients with acute myocardial infarction. A similar observation has also been described by Egea et al.,5 who demonstrated that CSF-2 can be secreted by non-hematopoietic epithelial cells in response to injury of the colon crypt epithelium and subsequently facilitate epithelial cell proliferation and ulcer healing.
Indeed, in the current study, we revealed for the first time that CSF-2 is actively secreted by stem cells in response to various injury signals, such as radiation damage, oxidative stress, and serum depletion, in vitro (Figures 1A–1C) and in vivo (Figures 1D–1F) as an endogenous damage signal. Importantly, we also found that CSF-2 significantly enhances the therapeutic capability of stem cells by promoting multi-lineage differentiation (Figure 2A) and migratory capability (Figures 2D and 2E) in vitro. In addition, CSF-2-treated stem cells actively secreted many known growth factors for promoting tissue regeneration, specifically, AR,40 G-CSF,41 GDNF,42 GM-CSF,9 HB-EGF,43 and PDGF44 (Figure 6). These results suggest that the CSF-2-mediated beneficial effects of stem cells on tissue regeneration were due, at least in part, to the various secretory factors that regulate diverse biological functions, including cell growth, wound healing, and differentiation.
A functional receptor through which CSF-2 exerts its non-hematopoietic activity on stem cells has not previously been identified. In this study, CSF-2 was shown to exert its non-hematopoietic effects on stem cells through its interaction with CD116. It is normally detected on neutrophils and monocytes and is partially expressed in bone marrow myeloid progenitor cells.45 Interestingly, the CSF-2-induced multi-lineage differentiation potential (Figures 3A and 3B) and migratory ability (Figures 3C and 3D) of MSCs was significantly attenuated by CD116 depletion. These results indicate that CD116 may act as a functional receptor to mediate the various beneficial effects of CSF-2 on stem cells. Because CD116 is known to be involved in regulating diverse biological functions, including apoptosis,46 cell growth,47 and multi-lineage differentiation,48 the role of CSF-2 in activating CD116 implies its potential role in the regulation of stem cell migratory and differentiation capabilities.
Because CD116 is known to activate the PI3K/Akt- and ERK1/2-signaling pathways for the regulation of multiple cellular functions,49, 50 we also examined whether CSF-2 activates these signaling pathways when CSF-2 binds to CD116 in stem cells. It has been shown that the PI3K/Akt-51 or ERK1/2-52 signaling pathway is frequently activated by mitogenic growth factors or cytokines in many cell types. Indeed, our results also revealed that CSF-2 significantly activated PI3K/Akt- and FAK/ERK1/2-signaling pathways in MSCs (Figures 4A and 4B). The role of CSF-2 in activating these signaling cascades is not surprising, considering the results of a previous in vitro study on endothelial progenitor cells (EPCs), which revealed that CSF-2 accelerates EPC proliferation and colony formation through PI3K/Akt and ERK1/2 signaling.53 Blockage of these signaling pathways with specific inhibitors significantly attenuated the CSF-2-mediated osteogenic differentiation (Figures 4C and 4D) and migratory capacities (Figures 5A and 5B) of MSCs, suggesting that CSF-2 exerts its stimulatory effects on stem cells via PI3K/Akt and/or FAK/ERK1/2 signaling. We also conducted the additional set of experiments to evaluate whether AKT or ERK inhibitor itself suppresses proliferation and/or induces cell-cycle arrest of stem cells. Interestingly, inhibitor-only treatments barely affected cell cycle stages of MSCs in our treatment concentrations (Figure S12). These results suggest that attenuating effects of the Akt inhibitor V or the ERK1/2 inhibitor PD98059 on CSF-2-mediated effects may be at least partly responsible for AKT or ERK inhibitor itself-induced inhibition of stem cell growth and/or toxicity.
Taken together, these findings indicate that, in addition to its previously reported hematopoietic functions, CSF-2 is actively secreted in response to various types of injuries as an endogenous danger signal and subsequently promotes the therapeutic effects of stem cells, by enhancing their multi-lineage differentiation and migratory capacities via PI3K/Akt- and/or FAK/ERK1/2-signaling cascades.
Materials and Methods
Isolation and Culture of Human Adipose Tissue-Derived MSCs
Biopsies (10 mL) of adipose tissue away from tumor border were obtained at the time of initial surgery. Human adipose tissue-derived MSCs were isolated from adipose tissues of breast cancer patients with written informed consent from the patients and approval of the Gachon University Institutional Review Board (IRB: GAIRB2015-104). All of the human-related experiments were approved and conducted in accordance with the Gachon University Institutional Review Board (IRB: GAIRB2015-104). Adipose tissue was minced into small pieces, and then the small pieces were digested in DMEM containing 10% fetal bovine serum (FBS) and 250 U/mL type I collagenase for 5 h at 37°C. The digestion mixture was then filtered through a 40-μm cell strainer. Isolated cells were then cultured in EBM-2 medium (Lonza) with EGM-2 supplements at 37°C and 5% CO2.
ELISA
Specific levels of CSF-2 secreted by human dermal fibroblasts (ATCC, PCS-201-012), human epidermal keratinocytes (ATCC, PCS-200-011), human vascular endothelial cells (ATCC, CRL-1730), and adipose tissue-derived MSCs were quantified using ELISA kits (R&D Systems), according to the manufacturer’s instructions. The optical density of each well was then read on a microplate reader at 450-nm wavelength, and CSF-2 concentrations in each sample were determined using the standard curve. Experiments were carried out in triplicate.
In Vitro Cell Migration Assay
Cells were plated at 1 × 105 cells/well in 200 μL culture medium in the upper chambers of transwell-permeable supports (Corning, Corning, NY, USA) to track the migration of cells. The transwell chambers had 8.0-μm pores in 6.5-mm diameter polycarbonate membranes and used a 24-well plate format. After 12 h, the inserts were removed and non-invading cells on the inner side of each membrane were properly wiped. Migrated cells on the lower surface of each membrane were fixed with 4% paraformaldehyde for 5 min and stained with hematoxylin for 15 min. Later, the number of migrated cells was counted in three randomly selected fields of the wells under a light microscope at 50× magnification. To calculate the chemotactic index, the number of cells that migrated in response to the treatment of CSF-2 was divided by the number of spontaneously migrating cells (non-treated control groups).
Osteogenic Differentiation
MSCs were incubated in DMEM high-glucose medium supplemented with 0.1 μM dexamethasone, 10 mM β-glycerophosphate, 50 μM ascorbate, and 10% FBS, with or without CSF-2. MSCs were grown for 3 weeks, with medium replacement twice a week. Differentiated cells were stained with alizarin red S to detect de novo formation of bone matrix. To quantify the alizarin red staining, 10% cetylperidinium chloride was added and cells were incubated for 20 min to elute the stain. The alizarin red S in samples was quantified by measuring the optical density (OD) of the solution at 570 nm with a spectrophotometer.
Adipogenic Differentiation
MSCs were incubated in DMEM low-glucose medium supplemented with 500 μM metylxanthine, 5 μg/mL insulin, and 10% FBS, with or without CSF-2. MSCs were grown for 3 weeks, with medium replacement twice a week. Lipid droplet formation was confirmed by oil red O staining. The relative quantification of lipid droplet formation was determined by absorbance measurement at 500 nm.
Immunofluorescent Staining
Samples were fixed with 4% paraformaldehyde for fluorescent staining. Samples were permeabilized with 0.4 M glycine and 0.3% Triton X-100, and nonspecific binding was blocked with 2% normal swine serum (Dako, Glostrup, Denmark). Staining was performed as described previously,54 using the primary anti-Phalloidin (Cytoskeleton) antibody. Samples were examined by fluorescence microscopy (Zeiss LSM 510 Meta). The calculation of expression was based on green fluorescence area and density divided by cell number, as determined from the number of DAPI-stained nuclei, in three randomly selected fields for each sample from a total of three independent experiments.
Evaluation of CSF-2 Effects on Normal and TCA-Induced Endometrial Ablation Animal Models
All of the animal experiments were approved and conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) (LCDI-2018-0025 and LCDI-2018-0034) of the Lee Gil Ya Cancer and Diabetes Institute of Gachon University. The mice were randomly divided into control (vehicle) and CSF-2 treatment groups. To exactly mimic clinical conditions, Institute of Cancer Research (ICR) mice were exposed to CSF-2 (0.5 mg/kg) or vehicle (PBS) through intraperitoneal injection for 10 consecutive days. The mice were anesthetized and exsanguinated by cardiac puncture, and then MSCs were isolated from adipose tissues. Additionally, 7-week-old immunodeficient NSG mice were subjected to treatment with 2% TCA (150 μL, administered directly into uterine horn) to induce uterine endometrial ablation or with sterilized PBS vehicle as a control. CSF-2-treated or non-treated MSCs (1 × 106 cells) were labeled with GFP and injected intravenously into the tail veins of 7-week-old NSG mice with acute TCA-induced endometrial ablation. The mice were sacrificed 7 days after the GFP-labeled MSCs were injected. Mice of each group were sacrificed by cervical dislocation. Uterine endometrial tissue was collected and subjected to H&E staining.
Flow Cytometry
Fluorescence-activated cell sorting (FACS) analysis and cell sorting were performed using FACS Calibur and FACS Aria machines (Becton Dickinson, Palo Alto, CA), respectively. FACS data were analyzed using FlowJo software (Tree Star, Ashland, OR). Antibodies to the following proteins were used: allophycocyanin (APC)-conjugated CD44 (BD Bioscience, 559942, dilution 1/40), phycoerythrin (PE)-conjugated CD133 (magnetic-activated cell sorting [MACS]; Miltenyi Biotec, Sunnyvale, CA, 130-080-081, dilution 1/40), CD34 (MACS; Miltenyi Biotec, Sunnyvale, CA, 30-081-002), CD44 (MACS; Miltenyi Biotec, Sunnyvale, CA, 130-095-180), CD45 (MACS; Miltenyi Biotec, Sunnyvale, CA, 130-080-201), CD73 (MACS; Miltenyi Biotec, Sunnyvale, CA, 130-095-182), and CD105 (MACS; Miltenyi Biotec, Sunnyvale, CA, 130-094-941). The FACS gates were established by staining with an isotype antibody or secondary antibody.
Protein Isolation and Western Blot Analysis
The protein expression levels were determined by western blot analysis as previously described.55 Cells were lysed in a buffer containing 50 mM Tris, 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% NP 40, and 0.2 mM PMSF. Samples containing equal amounts of protein were separated via SDS-PAGE and then transferred onto nitrocellulose membranes (Bio-Rad). Then, the membranes were incubated with primary antibodies against β-actin (Abcam, MA, USA, ab189073), MMP-2 (Cell Signaling Technology, 4022), MMP-9 (Cell Signaling Technology, 13667), total PI3K (Cell Signaling Technology, 4292), phospho-PI3K (Cell Signaling Technology, 4228), total Akt (Cell Signaling Technology, 4491), phospho-Akt (Cell Signaling Technology, 4060), total ERK1/2 (Cell Signaling Technology, 9012), phospho-ERK1/2 (Cell Signaling Technology, 9101), Ki67 (Novus Biologicals, NB500-170), total FAK (Santa Cruz Biotechnology, sc-558), and phospho-FAK (Santa Cruz Biotechnology, sc-11765) overnight at 4°C and then with horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) (BD Pharmingen, San Diego, CA, USA, 554021) and goat anti-mouse IgG (BD PharMingen, 554002) secondary antibodies for 60 min at room temperature (RT). Antibody-bound proteins were detected using enhanced chemiluminescence (ECL) reagents.
Real-Time PCR
Total RNA from skin cells was extracted using TRIzol reagent (Invitrogen), according to the manufacturer’s protocol. Real-time PCR was performed using a Rotor-Gene Q (QIAGEN). The reaction was subjected to amplification cycles of 95°C for 20 s, 60°C for 20 s, and 72°C for 25 s. The relative mRNA expression of the selected genes was normalized to that of peptidyl prolyl isomerase A (PPIA) and quantified using the ΔΔCT method. The sequences of the PCR primers are listed in Table 1.
Table 1.
Primer Sequences for qRT-PCR
| Gene | GenBank No. | Direction | Primer Sequence |
|---|---|---|---|
| Human CD116 | NM_001161529.1 | F | 5′-GACTCCAGGACGATGAAT-3′ |
| R | 5′-CACGACTCTGTTCTTCTTG-3′ | ||
| Human CSF-2 | NM_000758 | F | 5′-ATGATGGCCAGCCACTACAA-3′ |
| R | 5′-AGCAGTCAAAGGGGATGACA-3′ | ||
| Human fibronectin | NC_000002.12 | F | 5′-GAGAATGGACCTGCAAGCCCA-3′ |
| R | 5′-GTGCAAGTGATGCGTCCGC-3′ | ||
| Human NANOG | NM_024856 | F | 5′-ACATGCAACCTGAAGACGTGTG-3′ |
| R | 5′-CATGGAAACCAGAACACGTGG-3′ | ||
| Human PPIA | NM_021130 | F | 5′-TGCCATCGCCAAGGAGTAG-3′ |
| R | 5′-TGCACAGACGGTCACTCAAA-3′ | ||
| Human Snail2 | NC_000008.11 | F | 5′-TACCGCTGCTCCATTCCAGC-3′ |
| R | 5′-CATGGGGGTCTGAAAGCTTG-3′ | ||
| Human SOX2 | NM_003106.3 | F | 5′-AAATGGGAGGGGTGCAAAAGAGGAG-3′ |
| R | 5′-CAGCTGTCATTTGCTGTGGGTGATG-3′ | ||
| Human twist | NC_000007.14 | F | 5′-CCTGCGCAAGATCATCCCCA-3′ |
| R | 5′-GCTGCAGCTTGCCATCTTGGA-3′ | ||
| Mouse C-MYC | NM_010849 | F | 5′-CGCACACACAACGTCTTGGA-3′ |
| R | 5′-AGGATGTAGGCGGTGGCTTT-3′ | ||
| Mouse HPRF | NM_013556 | F | 5′-GCCTAAGATGAGCGCAAGTTG-3′ |
| R | 5′-TACTAGGCAGATGGCCACAGG-3′ | ||
| Mouse KLF4 | NM_010637 | F | 5′-GGTGCAGCTTGCAGCAGTAA-3′ |
| R | 5′-AAAGTCTAGGTCCAGGAGGT-3′ | ||
| Mouse OCT4 | NM_013633 | F | 5′-GCATTCAAACTGAGGCACCA-3′ |
| R | 5′-AGCTTCTTTCCCCATCCCA-3′ | ||
| Mouse SOX2 | NM_011443 | F | 5′-GAAGCGTGTACTTATCCTTCTTCAT-3′ |
| R | 5′-GAGTGGAAACTTTTGTCCGAGA-3′ |
F, forward; R, reverse.
GeneMANIA Algorithm-Based Bioinformatics Analysis
To further analyze genes that interact with or directly regulate PI3K/Akt or FAK/ERK1/2 signaling, we imported all identified genes and their corresponding accession numbers into GeneMANIA (http://genemania.org/). To find gene interactions, we considered several factors, including co-expression, co-localization, and genetic interactions. From this list, we selected the genes GDNF, AR, G-CSF, GDNF, GM-CSF, HB-EGF, and PDGF to test their involvement in regulating CSF-2-induced PI3K/Akt or FAK/ERK1/2 signaling.
Growth Factor Antibody Array
The assay was performed following the manufacturer’s protocol (Abnova AA0089). Briefly, cells were treated with or without 300 ng/mL CSF-2 for 24 h and then harvested. Protein samples were extracted and incubated with antibody membranes overnight at 4°C. After washing 3 times with wash buffer, the membranes were incubated with biotin-conjugated anti-cytokine antibodies overnight at 4°C. The membranes were then washed 3 times and incubated with HRP-conjugated streptavidin. Chemiluminescence was used to detect signals of the growth factors spotted on the nitrocellulose membrane.
Statistical Analysis
All the statistical data were analyzed in GraphPad Prism 5.0 (GraphPad, San Diego, CA) and evaluated using two-tailed Student’s t tests. Values of p < 0.05 were considered to indicate statistical significance.
Author Contributions
S.-R.P., A.C., and J.-W.K. conducted the experiments. H.-Y.L. and I.-S.H. designed the experiments and wrote the paper.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1A2A3074613 and NRF-2018R1C1B6003442).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.03.010.
Contributor Information
Hwa-Yong Lee, Email: hylee@jwu.ac.kr.
In-Sun Hong, Email: hongstem@gachon.ac.kr.
Supplemental Information
References
- 1.Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989;339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 2.Ha Y., Kim Y.S., Cho J.M., Yoon S.H., Park S.R., Yoon D.H., Kim E.Y., Park H.C. Role of granulocyte-macrophage colony-stimulating factor in preventing apoptosis and improving functional outcome in experimental spinal cord contusion injury. J. Neurosurg. Spine. 2005;2:55–61. doi: 10.3171/spi.2005.2.1.0055. [DOI] [PubMed] [Google Scholar]
- 3.Egea L., Hirata Y., Kagnoff M.F. GM-CSF: a role in immune and inflammatory reactions in the intestine. Expert Rev. Gastroenterol. Hepatol. 2010;4:723–731. doi: 10.1586/egh.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pastore S., Fanales-Belasio E., Albanesi C., Chinni L.M., Giannetti A., Girolomoni G. Granulocyte macrophage colony-stimulating factor is overproduced by keratinocytes in atopic dermatitis. Implications for sustained dendritic cell activation in the skin. J. Clin. Invest. 1997;99:3009–3017. doi: 10.1172/JCI119496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egea L., McAllister C.S., Lakhdari O., Minev I., Shenouda S., Kagnoff M.F. GM-CSF produced by nonhematopoietic cells is required for early epithelial cell proliferation and repair of injured colonic mucosa. J. Immunol. 2013;190:1702–1713. doi: 10.4049/jimmunol.1202368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirata Y., Egea L., Dann S.M., Eckmann L., Kagnoff M.F. GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell Host Microbe. 2010;7:151–163. doi: 10.1016/j.chom.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y., Hunt N.H., Bao S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 2008;18:1220–1229. doi: 10.1038/cr.2008.310. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi K., Ohta S., Kawakami Y., Toda M. Activation of dendritic-like cells and neural stem/progenitor cells in injured spinal cord by GM-CSF. Neurosci. Res. 2009;64:96–103. doi: 10.1016/j.neures.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Krüger C., Laage R., Pitzer C., Schäbitz W.R., Schneider A. The hematopoietic factor GM-CSF (granulocyte-macrophage colony-stimulating factor) promotes neuronal differentiation of adult neural stem cells in vitro. BMC Neurosci. 2007;8:88. doi: 10.1186/1471-2202-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 11.Gong W., Han Z., Zhao H., Wang Y., Wang J., Zhong J., Wang B., Wang S., Wang Y., Sun L., Han Z. Banking human umbilical cord-derived mesenchymal stromal cells for clinical use. Cell Transplant. 2012;21:207–216. doi: 10.3727/096368911X586756. [DOI] [PubMed] [Google Scholar]
- 12.Yellowley C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep. 2013;2:300. doi: 10.1038/bonekey.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honczarenko M., Le Y., Swierkowski M., Ghiran I., Glodek A.M., Silberstein L.E. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 14.Ringe J., Strassburg S., Neumann K., Endres M., Notter M., Burmester G.R., Kaps C., Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J. Cell. Biochem. 2007;101:135–146. doi: 10.1002/jcb.21172. [DOI] [PubMed] [Google Scholar]
- 15.Tang J.M., Yuan J., Li Q., Wang J.N., Kong X., Zheng F., Zhang L., Chen L., Guo L.Y., Huang Y.H. Acetylcholine induces mesenchymal stem cell migration via Ca2+ /PKC/ERK1/2 signal pathway. J. Cell. Biochem. 2012;113:2704–2713. doi: 10.1002/jcb.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forte G., Minieri M., Cossa P., Antenucci D., Sala M., Gnocchi V., Fiaccavento R., Carotenuto F., De Vito P., Baldini P.M. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 17.Zheng B., Wang C., He L., Xu X., Qu J., Hu J., Zhang H. Neural differentiation of mesenchymal stem cells influences chemotactic responses to HGF. J. Cell. Physiol. 2013;228:149–162. doi: 10.1002/jcp.24114. [DOI] [PubMed] [Google Scholar]
- 18.Song B.Q., Chi Y., Li X., Du W.J., Han Z.B., Tian J.J., Li J.J., Chen F., Wu H.H., Han L.X. Inhibition of Notch Signaling Promotes the Adipogenic Differentiation of Mesenchymal Stem Cells Through Autophagy Activation and PTEN-PI3K/AKT/mTOR Pathway. Cell. Physiol. Biochem. 2015;36:1991–2002. doi: 10.1159/000430167. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H., Li D., Shi C., Xin T., Yang J., Zhou Y., Hu S., Tian F., Wang J., Chen Y. Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci. Rep. 2015;5:12898. doi: 10.1038/srep12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N., Yan Y.L., Fu S., Li R.J., Zhao P.F., Xu X.Y., Yang J.P., Damirin A. Lysophosphatidic acid enhances human umbilical cord mesenchymal stem cell viability without differentiation via LPA receptor mediating manner. Apoptosis. 2017;22:1296–1309. doi: 10.1007/s10495-017-1399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park M.C., Kang T., Jin D., Han J.M., Kim S.B., Park Y.J., Cho K., Park Y.W., Guo M., He W. Secreted human glycyl-tRNA synthetase implicated in defense against ERK-activated tumorigenesis. Proc. Natl. Acad. Sci. USA. 2012;109:E640–E647. doi: 10.1073/pnas.1200194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocuzza M.A., Cocuzza M., Maciel G.A., da Motta E.V., Cardoso A.P., Soares J.M., Jr., Baracat E.C. Development of an animal model for endometrial ablation using trichloroacetic acid. Fertil. Steril. 2011;95:2418–2421. doi: 10.1016/j.fertnstert.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi H., Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 25.Müller P., Langenbach A., Kaminski A., Rychly J. Modulating the actin cytoskeleton affects mechanically induced signal transduction and differentiation in mesenchymal stem cells. PLoS ONE. 2013;8:e71283. doi: 10.1371/journal.pone.0071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong L., Hughes O., Yung S., Hyslop L., Stewart R., Wappler I., Peters H., Walter T., Stojkovic P., Evans J. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 27.Gao F., Hu X., Xie X., Liu X., Wang J. Heat shock protein 90 stimulates rat mesenchymal stem cell migration via PI3K/Akt and ERK1/2 pathways. Cell Biochem. Biophys. 2015;71:481–489. doi: 10.1007/s12013-014-0228-6. [DOI] [PubMed] [Google Scholar]
- 28.Basualto-Alarcón C., Jorquera G., Altamirano F., Jaimovich E., Estrada M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med. Sci. Sports Exerc. 2013;45:1712–1720. doi: 10.1249/MSS.0b013e31828cf5f3. [DOI] [PubMed] [Google Scholar]
- 29.Furmento V.A., Marino J., Blank V.C., Roguin L.P. The granulocyte colony-stimulating factor (G-CSF) upregulates metalloproteinase-2 and VEGF through PI3K/Akt and Erk1/2 activation in human trophoblast Swan 71 cells. Placenta. 2014;35:937–946. doi: 10.1016/j.placenta.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Yuan M., Wen S.J., Yang C.X., Pang Y.G., Gao X.Q., Liu X.Q., Huang L., Yuan Q.L. Transplantation of neural stem cells overexpressing glial cell line-derived neurotrophic factor enhances Akt and Erk1/2 signaling and neurogenesis in rats after stroke. Chin. Med. J. (Engl.) 2013;126:1302–1309. [PubMed] [Google Scholar]
- 31.Jeong W., Kim J., Bazer F.W., Song G. Proliferation-stimulating effect of colony stimulating factor 2 on porcine trophectoderm cells is mediated by activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase. PLoS ONE. 2014;9:e88731. doi: 10.1371/journal.pone.0088731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaviglio A.L., Knelson E.H., Blobe G.C. Heparin-binding epidermal growth factor-like growth factor promotes neuroblastoma differentiation. FASEB J. 2017;31:1903–1915. doi: 10.1096/fj.201600828R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farooqi A.A., Siddik Z.H. Platelet-derived growth factor (PDGF) signalling in cancer: rapidly emerging signalling landscape. Cell Biochem. Funct. 2015;33:257–265. doi: 10.1002/cbf.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dezawa M., Takahashi I., Esaki M., Takano M., Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 2001;14:1771–1776. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- 35.Dezawa M., Ishikawa H., Itokazu Y., Yoshihara T., Hoshino M., Takeda S., Ide C., Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 36.Abdi R., Fiorina P., Adra C.N., Atkinson M., Sayegh M.H. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mezey E., Chandross K.J., Harta G., Maki R.A., McKercher S.R. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 38.Boyd T.D., Bennett S.P., Mori T., Governatori N., Runfeldt M., Norden M., Padmanabhan J., Neame P., Wefes I., Sanchez-Ramos J. GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J. Alzheimers Dis. 2010;21:507–518. doi: 10.3233/JAD-2010-091471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng Z., Yang C., Deng H., Yang A., Geng T., Chen X., Ma A., Liu Z. Effects of GM-CSF on the stem cells mobilization and plasma C-reactive protein levels in patients with acute myocardial infarction. Int. J. Cardiol. 2006;113:92–96. doi: 10.1016/j.ijcard.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Di Donato M., Giovannelli P., Cernera G., Di Santi A., Marino I., Bilancio A., Galasso G., Auricchio F., Migliaccio A., Castoria G. Non-genomic androgen action regulates proliferative/migratory signaling in stromal cells. Front. Endocrinol. (Lausanne) 2015;5:225. doi: 10.3389/fendo.2014.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J., Yao L., Zhao S., Zhang X., Yin J., Zhang Y., Chen X., Gao M., Ling E.A., Hao A., Li G. Granulocyte-colony stimulating factor promotes proliferation, migration and invasion in glioma cells. Cancer Biol. Ther. 2012;13:389–400. doi: 10.4161/cbt.19237. [DOI] [PubMed] [Google Scholar]
- 42.Uesaka T., Nagashimada M., Enomoto H. GDNF signaling levels control migration and neuronal differentiation of enteric ganglion precursors. J. Neurosci. 2013;33:16372–16382. doi: 10.1523/JNEUROSCI.2079-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su Y., Besner G.E. Heparin-binding EGF-like growth factor (HB-EGF) promotes cell migration and adhesion via focal adhesion kinase. J. Surg. Res. 2014;189:222–231. doi: 10.1016/j.jss.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li A., Xia X., Yeh J., Kua H., Liu H., Mishina Y., Hao A., Li B. PDGF-AA promotes osteogenic differentiation and migration of mesenchymal stem cell by down-regulating PDGFRα and derepressing BMP-Smad1/5/8 signaling. PLoS ONE. 2014;9:e113785. doi: 10.1371/journal.pone.0113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannistra S.A., Groshek P., Garlick R., Miller J., Griffin J.D. Regulation of surface expression of the granulocyte/macrophage colony-stimulating factor receptor in normal human myeloid cells. Proc. Natl. Acad. Sci. USA. 1990;87:93–97. doi: 10.1073/pnas.87.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han X., Gilbert S., Groschwitz K., Hogan S., Jurickova I., Trapnell B., Samson C., Gully J. Loss of GM-CSF signalling in non-haematopoietic cells increases NSAID ileal injury. Gut. 2010;59:1066–1078. doi: 10.1136/gut.2009.203893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y., Zhao Z., Chen Y., Lv Z., Ding X., Wang R., Xiao H., Hou C., Shen B., Feng J. An epithelial-to-mesenchymal transition-inducing potential of granulocyte macrophage colony-stimulating factor in colon cancer. Sci. Rep. 2017;7:8265. doi: 10.1038/s41598-017-08047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greter M., Helft J., Chow A., Hashimoto D., Mortha A., Agudo-Cantero J., Bogunovic M., Gautier E.L., Miller J., Leboeuf M. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–1046. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schäbitz W.R., Krüger C., Pitzer C., Weber D., Laage R., Gassler N., Aronowski J., Mier W., Kirsch F., Dittgen T. A neuroprotective function for the hematopoietic protein granulocyte-macrophage colony stimulating factor (GM-CSF) J. Cereb. Blood Flow Metab. 2008;28:29–43. doi: 10.1038/sj.jcbfm.9600496. [DOI] [PubMed] [Google Scholar]
- 50.Bozinovski S., Jones J.E., Vlahos R., Hamilton J.A., Anderson G.P. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J. Biol. Chem. 2002;277:42808–42814. doi: 10.1074/jbc.M207840200. [DOI] [PubMed] [Google Scholar]
- 51.Chen J., Crawford R., Chen C., Xiao Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng. Part B Rev. 2013;19:516–528. doi: 10.1089/ten.TEB.2012.0672. [DOI] [PubMed] [Google Scholar]
- 52.Huang C., Jacobson K., Schaller M.D. MAP kinases and cell migration. J. Cell Sci. 2004;117:4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 53.Qiu C., Xie Q., Zhang D., Chen Q., Hu J., Xu L. GM-CSF induces cyclin D1 expression and proliferation of endothelial progenitor cells via PI3K and MAPK signaling. Cell. Physiol. Biochem. 2014;33:784–795. doi: 10.1159/000358652. [DOI] [PubMed] [Google Scholar]
- 54.Dong H.J., Jang G.B., Lee H.Y., Park S.R., Kim J.Y., Nam J.S., Hong I.S. The Wnt/β-catenin signaling/Id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells. Sci. Rep. 2016;6:22966. doi: 10.1038/srep22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi E.S., Jung J.Y., Lee J.S., Park J.H., Cho N.P., Cho S.D. Myeloid cell leukemia-1 is a key molecular target for mithramycin A-induced apoptosis in androgen-independent prostate cancer cells and a tumor xenograft animal model. Cancer Lett. 2013;328:65–72. doi: 10.1016/j.canlet.2012.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.