Abstract
Nociception is an important type of perception that has major influence on daily human life. There are some descending pathways related to pain management and modulation, which are collectively known as the descending antinociceptive system (DAS). Noradrenalin (NA) in the locus coeruleus (LC) and serotonin (5-HT) in the rostral ventromedial medulla (RVM) are components of the DAS. Most 5-HT neurons in the dorsal raphe (DR) have ascending projections rather than descending projections, and they project to the thalamus that modulates nociception. Both the DAS and the DR are believed to be involved in pain-emotion symptoms. In this study, we utilized a fiber photometry system to specifically examine the activity of LC NA neurons and RVM/DR 5-HT neurons using mice carrying tetracycline-controlled transactivator transgene (tTA) under the control of either a dopamine β-hydroxylase promoter or a tryptophan hydroxylase-2 promoter and site-specific infection of an adeno-associated virus carrying a TetO G-CaMP6 gene. After confirmation of specific expression of G-CaMP6 in the target populations, changes in green fluorescent signal intensity were recorded in awake mice upon exposure to acute nociceptive stimulation consisting of a pinch and application of heat (55 °C) to the tail. Both stimuli resulted in rapid and transient (<15 s) increases in the activity of LC NA neurons and RVM/DR 5-HT neurons while the control stimuli did not induce any changes. The present results clearly indicate that acute nociceptive stimuli increase the activity of LC NA neurons and RVM/DR 5 H T neurons and suggest a possible therapeutic target for pain treatment.
Abbreviations: DAS, descending antinociceptive system; NA, noradrenalin; 5-HT, serotonin; LC, locus coeruleus; PAG, periaqueductal gray; RVM, rostral ventromedial medulla; DR, dorsal raphe; CaM, calmodulin; DBH, Dopamine beta hydroxylase; TPH, tryptophan hydroxylase; AAV, adeno associated virus; PFA, paraformaldehyde; PBS, phosphate-buffered saline; PMT, photomultiplier tube; SEM, standard error of the mean; SNRI, serotonin noradrenalin reuptake inhibitor
Keywords: G-CaMP6, Fiber photometry, Locus coeruleus (LC), Rostral ventromedial medulla (RVM), Dorsal raphe (DR)
1. Introduction
Physical activity and mental activity are influenced by a variety of forms of perception. Nociception is one of the most important among them and has a major influence on daily human life. In the modern clinical field, pain symptoms caused by physical and mental disorders have become increasingly prevalent and can become a major problem. In general, when a painful stimulus is applied to peripheral tissues, the nociceptive input is transmitted via nerves to the spinal dorsal horn where they synapse with specific types of ascending neurons. This ascending neuronal pathway transports the nociceptive information via the contralateral funiculus to supraspinal structures. It is then transmitted through the thalamus to the cerebral cortex where it is finally recognized as a painful sensation.
In addition to the ascending nociceptive pathway, there are also some descending pathways related to pain management (Melzack and Wall, 1965; Millan, 2002). These pathways regulate nociceptive inflow and are known collectively as the descending antinociceptive system (DAS). Two of the primary neurotransmitters responsible for modulating the nociceptive input are noradrenalin (NA) and serotonin (5-HT) (Carlsson et al., 1963; Jacobs et al., 2002; Jordan et al., 2008) which are considered major components of the DAS (Ochi and Goto, 2000; Yaksh et al., 1981). The circuit consisting of the locus coeruleus (LC) and the spinal dorsal horn includes the noradrenergic system, and the circuit consisting of the periaqueductal gray (PAG), rostral ventromedial medulla (RVM), and the spinal dorsal horn includes the serotonergic system. Descending noradrenergic and serotonergic terminals are distributed in the spinal dorsal horn (Yoshimura and Furue, 2006) and are important for transmission and control of nociceptive signals (Millan, 2002). Noxious peripheral nociceptive stimulus has been shown to induce an increase in NA within the spinal dorsal horn (Tyce and Yaksh, 1981) and LC (Sajedianfard et al., 2005). It also increases 5-HT within the spinal dorsal horn (Weil-Fugazza et al., 1984; Taguchi and Suzuki, 1992), and causes activity in RVM 5-HT neurons in anesthetized animals (Chiang and Gao, 1986; Wessendorf and Anderson, 1983). In addition, activation of LC NA neurons increases NA in the spinal cord and exhibits antinociceptive effects (Hentall et al., 2003; Hickey et al., 2014; Ren and Dubner, 2008). Antinociceptive effects result from direct stimulation of the RVM (Bardin, 2011; Lovick and Wolstencroft, 1979) and direct stimulation of the PAG (Fardin et al., 1984; Reynolds, 1969) as well.
These findings indicate that NA neurons in the LC and 5-HT neurons in the RVM play a critical role in controlling the DAS. However, there is no report describing the neuronal responses to nociceptive stimulus with a time resolution of seconds in awake animals. In the present study, we used a fiber photometry system (Futatsuki et al., 2018; Moriya et al., 2018), which can evaluate specific neuronal activity in real time without anesthesia, to evaluate whether the results will be similar in awake animals to those obtained from anesthetized animals.
In addition to NA neurons in the LC and 5-HT neurons in the RVM, we also focused on 5-HT neurons in the dorsal raphe (DR) because it is one of the major sources of the serotonergic system in the brain. Most 5-HT neurons in the DR have ascending projections rather than descending projections, and they project to the thalamus that modulates nociception (Andersen and Dafny, 1983). Also, the DR receives projections from NA neurons in the LC and is thus involved in cognition and mood modulation (Commons, 2016; Lechin et al., 2006). Both the DAS and the DR are believed to be involved in pain-emotion symptoms (Alba-Delgado et al., 2013; Borges et al., 2014; Commons, 2016; Lechin et al., 2006; Rea et al., 2014; Seifert and Maihofner, 2009; Zhang et al., 2005). Therefore, we considered it meaningful to evaluate the activity of DR 5-HT neurons along with those of LC NA neurons and RVM 5-HT neurons when acute nociceptive stimulation is applied. In this study, we exposed awake mice to acute nociceptive stimuli and examined the activity of LC NA neurons and RVM/DR 5-HT neurons under real time conditions by utilizing a fiber photometry system.
2. Materials and methods
2.1. Ethical approval
All experimental procedures were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Use Committee of Kagoshima University (MD15075, MD17105).
2.2. Animals
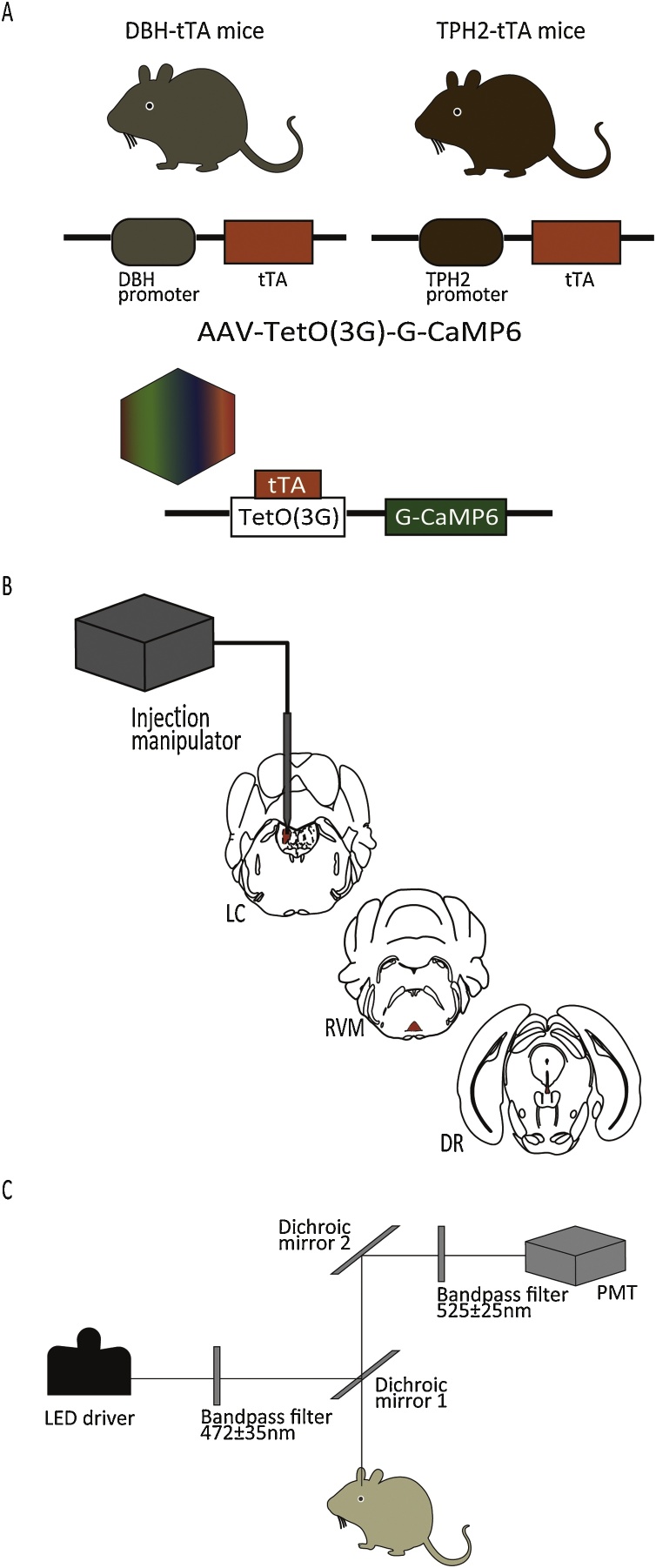
Transgenic mice carrying a tetracycline-controlled transactivator transgene (tTA) under the control of either a dopamine β-hydroxylase (DBH) promoter (n = 27) or a tryptophan hydroxylase-2 (TPH2) promoter (n = 35) (Fig. 1A) (Ikoma et al., 2018; Ohmura et al., 2014) were used.
Fig. 1.
The recording of the activity of LC NA neurons and RVM/DR 5-HT neurons with the fiber photometry system.
(A) Development of DBH- and TPH2-specfiic expression of G-CaMP6 using the tTA/tetO system. (B) AAV injection into LC, RVM and DR regions. (C) Schema of apparatus for the fiber photometry system.
DBH-tTA BAC transgenic mice were generated by transferring the mouse BAC DNA (clone RP23-112F24) into the SW105 Escherichia coli strain (a kind gift from Dr. Neal Copeland, NCI, USA). A cassette containing mammalianized tTA-SV40 polyadenylation signal-FRT-Neo-FRT (Inamura et al., 2012) was inserted into the translation initiation site of the DBH gene using BAC recombination. The FRT-flanking Neo selection marker was removed using FRT-flippase-mediated recombination in SW105 cells. Modified BAC DNA was linearized by PI-SceI enzyme digestion (NEB, USA) and injected into fertilized eggs from CBA/C57BL6 mice.
To check specificity of tTA expression, we crossed DBH-tTA mouse with a reporter mouse which have tetO-GCaMP6 knockin sequence in the downstream region of beta actin gene (B6;129-Actb < tm3.1(tetO-GCaMP6)Kftnk>, RBRC09552, RIKEN Bioresource Research Center, Tsukuba, Japan) (Tanaka et al., 2012). Specificity of TPH2-tTA expression has been reported elsewhere (Ikoma et al., 2018).
Ten to fourteen week old male mice were used in this experiment. Mice were maintained in the laboratory at the standard conditions, which included a 12/12-hr cycle (lights on at 7:00 AM and off at 7:00 PM), a temperature of 24 ± 1 °C, and food and water ad libitum. Efforts were made to minimize animal suffering and to reduce the number of animals used.
2.3. Stereotaxic AAV injection
AAV vectors were produced using the AAV Helper-Free system (Agilent Technologies, Inc., Santa Clara, CA, USA) and purified as described previously (Futatsuki et al., 2018; Inutsuka et al., 2016; Moriya et al., 2018) (Fig. 1A). Surgeries for AAV injections were performed under 2–3% isoflurane anesthesia using a stereotaxic instrument (ST-7, Narishige, Tokyo, Japan). AAV-TetO(3 G)-G-CaMP6 (Serotype:DJ; 1 μl/injection, 4 × 1013 copies/ml) (Ohkura et al., 2012) was slowly taken up into a glass microtube (1B150F-3, World Precision Instruments, Inc., Sarasota, FL, USA), which was connected to a nitrogen pressure source through polyethylene tubing and to an injection manipulator (I-200 J, Narishige) (Fig. 1B). AAV was unilaterally injected into the LC region in DBH-tTA mice (n = 18) and the RVM or DR region in TPH2-tTA mice (n = 16 for each region). Injection sites were as follows: LC: -5.35 mm from bregma, lateral +1.28 mm, ventral -3.65 mm from the surface of the brain; RVM: -5.64 mm from bregma, lateral 0.0 mm on the midline, ventral -5.30 mm from the surface of the brain; DR: -4.20 mm from bregma, lateral 0.0 mm on the midline, ventral -3.00 mm from the surface of the cranium. After AAV injection, the microtube was left in place for ten minutes before being slowly withdrawn. Experiments were carried out after at least fourteen days (two weeks) because it takes approximately that long for G-CaMP6 to fully express.
In an additional experiment (we call this as experiment-2 in this manuscript) to confirm that observed changes in fluorescence was actually from a change in calcium but not from artifact, we injected AAV-TetO(3 G)-mCherry together with AAV-TetO(3 G)-G-CaMP6 (Futatsuki et al., 2018) into the LC region in DBH-tTA mice (n = 3) and the RVM or DR region in TPH2-tTA mice (n = 3 for each region). Fluorescence of mCherry is not affected by neuronal activity and thus can be used as an indicator of total stability of the fiber photometry system.
2.4. Immunohistochemistry
Mice (n = 8 for LC region in DBH-tTA mice and n = 6 for RVM or DR region in TPH2-tTA mice) were transcardially perfused with 20 ml of phosphate-buffered saline (PBS) and 20 ml of 4% paraformaldehyde (PFA) solution under anesthesia with urethane (1.6 g/kg, ip) at least two weeks post AAV injection. The brain was removed and post-fixed with 4% PFA and immersed in 30% sucrose in PBS for two days. We produced serial 30 μm coronal sections with a cryostat (Cryotome FSE, Thermo Scientific, Yokohama, Japan) and every 4th section was used for immunostaining. The brain sections were immersed in blocking solution (1% normal horse serum and 0.3% Triton-X in 0.01 M PBS) for one hour at room temperature. The sections were then incubated with primary antibodies overnight. Primary antibodies were diluted in blocking solution and the conditions were as follows: LC: anti-Tyrosine Hydroxylase rabbit antibody (AB152, EMD Millipore Corp., Temecula, CA, USA) at 1:500; RVM/DR: anti-Tryptophan Hydroxylase sheep antibody (AB1541, EMD Millipore Corp.) at 1:1000. The following day, the sections were washed with PBS three times and then incubated with secondary antibodies at room temperature for two hours. Secondary antibodies were also diluted in blocking solution and the conditions were as follows: LC: CF568-conjugated anti-rabbit antibody raised in donkey (20015, Biotium Inc., Hayward, CA, USA) at 1:200; RVM/DR: CF568-conjugated anti-sheep antibody raised in donkey (20095, Biotium, Inc.) at 1:200. After secondary incubation, the sections were washed once and mounted upon microscope slides (PRO-02, Matsunami, Osaka, Japan) and covered with a micro cover glass (C024601, Matsunami). We observed the sections with a fluorescence microscope (BZ-X700, Keyence, Osaka, Japan), and created the images with Adobe Photoshop CC (Adobe Systems, Inc., San Jose, CA, USA). G-CaMP6 was identified by its own fluorescence.
2.5. In vivo fiber photometry system
A fiber photometry system previously developed in our lab (Futatsuki et al., 2018; Inutsuka et al., 2016; Moriya et al., 2018) (Fig. 1C) was used. In short, blue excitation light (470 nm, 0.5 mW at the tip of the silica fiber) is produced by a high-power LED driver (LEDD1B/M470F3, Thorlabs, Inc., Newton, NJ, USA) which then passes through an excitation bandpass filter (472 ± 35 nm) and is reflected by dichroic mirror-1 and into a single silica fiber (diameter: 400 μm). The green fluorescent signal of G-CaMP6 is detected and collected through the same fiber. The signal passes back through dichroic mirror-1, is reflected by dichroic mirror-2, goes through the bandpass emission filter (525 ± 25 nm), and is guided to a photomultiplier tube (PMT) (PMTH-S1-1P28, Zolix Instruments, Beijing, China). In the additional experiment using both G-CaMP and mCherry, an additional excitation (590 nm) and emission (641 ± 75 nm) system was attached for detection of mCherry fluorescence, which is not affected by neuronal activity and thus can be used as an indicator of total stability of the fiber photometry system (Futatsuki et al., 2018). The resulting signal was digitized by an A/D converter (PowerLab8/35, ADInstruments, Inc., Dunedin, New Zealand) at 1 kHz and recorded with Labchart version-7 software (ADInstruments, Inc.).
2.6. Procedure of recoding of neuronal activity
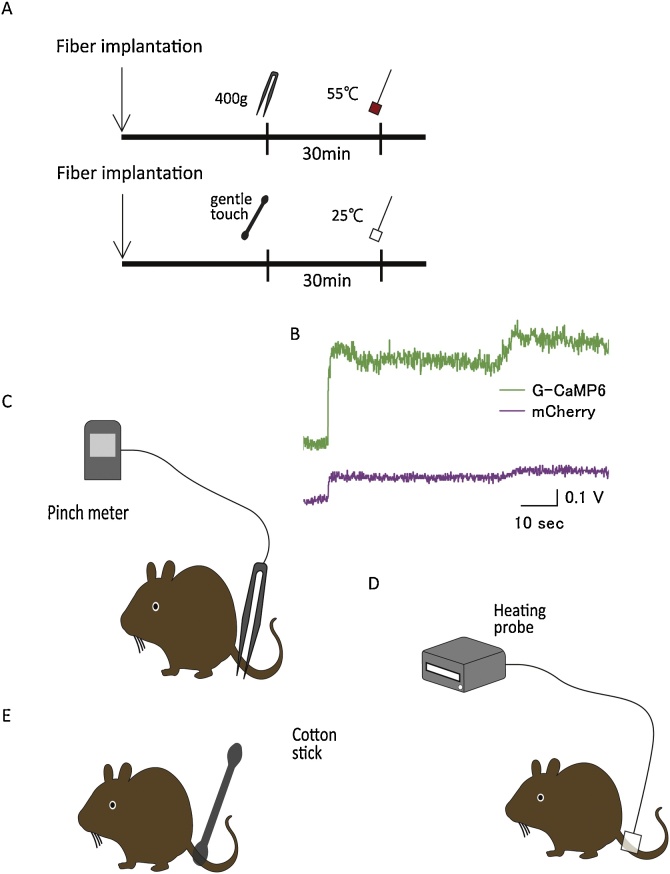
At least two weeks following viral injection, a silica fiber was surgically implanted into the mouse’s brain under 2–3% isoflurane anesthesia in order to record the activity of LC NA neurons or RVM/DR 5-HT neurons. The animal’s head was fixed in the stereotaxic instrument (ST-7, Narishige, Tokyo, Japan) with an aid of supportive ear bar (EB-6, Narishige) of which touching surfaces to the animal were covered with local anesthetic jelly (lidocain, 2% Xylocaine, AstraZeneca). The optic fiber was slowly moved to the place just above LC (from bregma -5.35 mm, lateral +1.28 mm, ventral -3.65 mm from the surface of the brain), RVM (from bregma -5.64 mm, lateral 0.0 mm, ventral -5.30 mm from the surface of the brain), and DR (from bregma -4.20 mm, lateral 0.0 mm, ventral -3.00 mm from the surface of the cranium). Fluorescence signal was continuously monitored during fiber implantation so that optimal position of the fiber tip was easily recognized by abrupt increase of the output signal (Fig. 2B). When output signal no further increased and was stable for more than 20 s, the tip was fixed at the position. After the fiber was fixed to the skull on its optimal position, inhalation anesthesia was discontinued and the animal was allowed to recover from anesthesia for three hours followed by recording session of less than one hour. The mice were split into experimental groups (Fig. 2A, upper) and control groups (Fig. 2A, lower) so that every area of interest (LC, RVM, and DR) was examined in 5 experimental mice and 5 control mice.
Fig. 2.
Recording Procedure.
(A) Fiber implantation was carried out under isoflurane anesthesia and recording started after mice awoke from anesthesia. The time interval between the two stimuli was set at 30 min to reduce the effects of previous stimulus (upper: Experimental group; lower: Control group). (B) Optimal location of the optic fiber was confirmed by abrupt increase of fluorescence intensity during implantation procedure. Typical trace in LC neurons was shown. We applied two acute nociceptive stimuli to mice in the experimental group (C, D) and two acute control stimuli to mice in the control group (D, E). (C) Mechanical tail pinch stimulus of with a force of 400 g was carried out for 3 s. (D) Heat stimulus at 55 °C or low temperature heat stimulus at 25 °C was carried out for 3 s. (E) Gentle touch test was carried out by touching the root of the tail with a cotton stick for 3 s.
In the experimental group, two nociceptive stimuli were applied in order: a mechanical tail pinch stimulus with a force of 400 g (Fig. 2C) and heat stimulus at a temperature of 55 °C (Fig. 2D). To reduce the effects of the previous stimulus, the inter-stimulus interval was set at 30 min. A pinch meter (PM-201, Soshin-Medic, Chiba, Japan) was used for the mechanical tail pinch stimulus. The apparatus was attached to the root of the tail for three seconds with a force of 400 g. A heating probe (5R7-570, Oven Industries, Inc., Mechanicsburg, PA, USA) was used and set at 55 °C for the heat stimulus. The apparatus was attached to the root of the tail for three seconds. In the control group, two noninvasive stimuli were applied in order: gentle touching (Fig. 2E) and low temperature heat stimulus set at 25 °C (Fig.2D). The inter-stimulus interval was again set at 30 min in order to match the experimental group. A cotton stick was used for the gentle touch test and the same heating probe (5R7-570) was used for the low temperature heat stimulus.
In the additional experiment using both G-CaMP and mCherry, we also examined LC, RVM, and DR in 3 mice per site. In this case, each mouse received four stimulations in the following order: gentle touching, low temperature heat, tail pinch, and high temperature heat.
The neuronal activity characteristic index in this experiment was defined as follows: F: averaged fluorescent signal intensity during the three seconds before stimulus and defined as 100%; ΔF: maximum value of fluorescent signal intensity during each stimulus subtracted by F; onset latency: time duration from start of stimulus to the time when fluorescence signal intensity exceeded the maximum value during the baseline period; peak latency: time duration from start of stimulus to the maximum signal intensity resulting from the stimulus; return to base line time: time duration for peak signal to return to the base line value.
2.7. Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Cell numbers in the immunohistochemical examination were counted in two coronal sections from one mouse and average value was used as the value for the animal. Statistical analysis was performed using GraphPad Prism version 7 (GraphPad software, San Diego, CA, USA). In the fiber photometry study, maximum fluorescence signal intensity during the observation period of 30-s was compared with those during the baseline period of 3-s preceding the start of stimulation using paired t-test. Time characteristics of the neuronal responses (onset latency, etc., see chapter 2.6) are compared between pinch trial and high heat trial using paired t-test. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Restricted expression of DBH-tTA
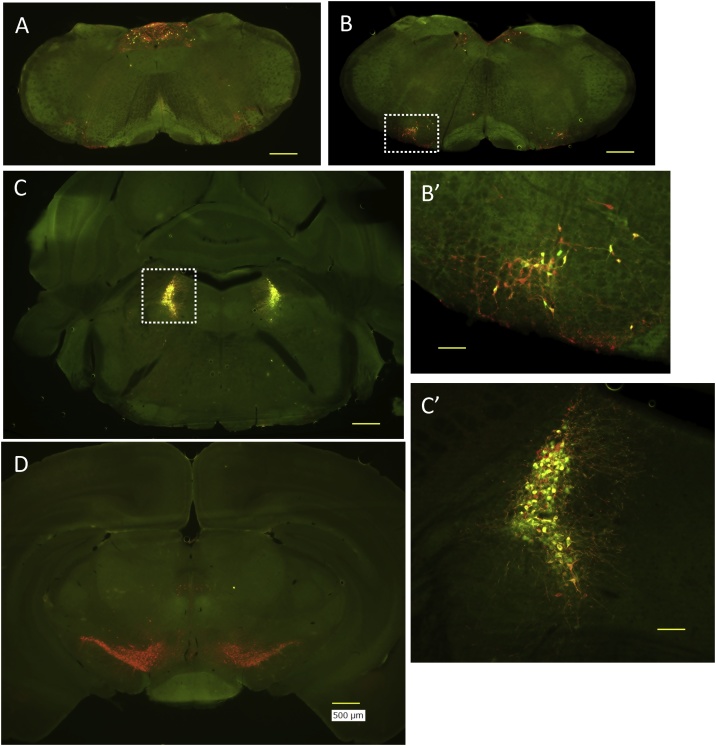
DBH promoter regulated t-TA expression in the brain was examined by crossing DBH-tTA mouse with a reporter mouse, tetO-GCaMP6 knockin moue (Fig. 3). Anti TH staining revealed noradrenergic neurons in the nucleus of solitary tract (Fig. 3A) and in the locus coeruleus (Fig. 3C and C’), adrenergic neurons in the rostral ventrolateral medulla (Fig. 3B and B’), and dopaminergic neurons in the ventral tegmental area and the substantia nigra (Fig. 3D). Of these, only noradrenergic and adrenergic neurons but not dopaminergic neurons showed co-expression of Tet system-driven green fluorescence. Therefore, restricted expression of DBH-tTA was confirmed as expected.
Fig. 3.
Restricted expression of DBH-tTA.
Dopamine β-hydroxylase (DBH) promoter regulated t-TA expression in the brain was examined by crossing DBH-tTA mouse with a reporter mouse, tetO-GCaMP6 knockin moue. Anti tyrosine hydroxylase staining (red) revealed noradrenergic (A, nucleus of solitary tract and C, locus coeruleus), adrenergic (B, rostral ventrolateral medulla), and dopaminergic neurons (D, ventral tegmental area and substantia nigra). Of these, only noradrenergic and adrenergic neurons but not dopaminergic neurons showed co-expression of Tet system-driven green fluorescence. B’ and C’ are higher magnification of the areas designated in rectangles in B and C, respectively. Photographs indicate the representative results from a mouse. Similar results were obtained in three mice. Calibration bars are 500 μm in A–D and 100 μm in B’ and C’.
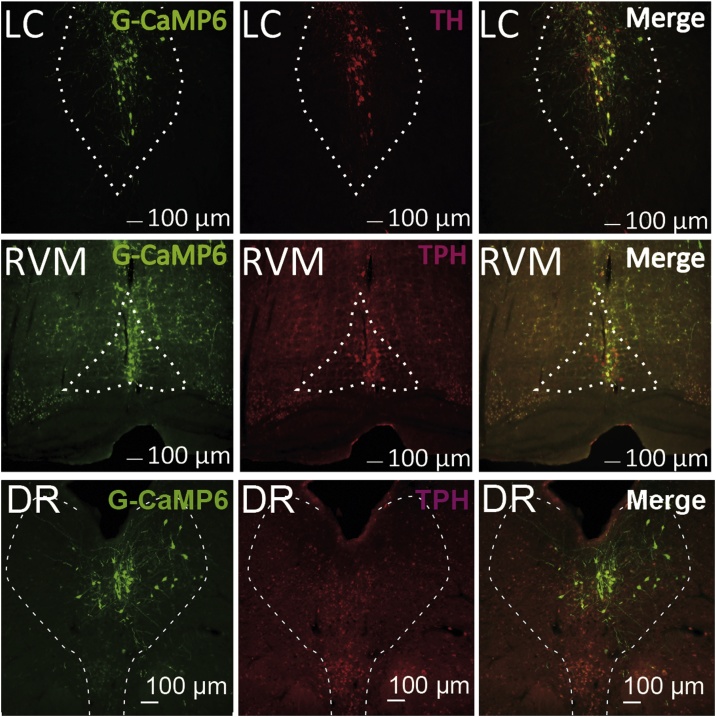
3.2. Restricted expression of AAV vector driven G-CaMP6
Restricted expression of G-CaMP6 was confirmed in LC NA neurons and RVM/DR 5-HT neurons (Fig. 4). Namely, most of the GFP-positive cells in LC (47.1 ± 3.9, n = 8 animals) were also TH-positive (43.6 ± 3.9, 93%), most of the GFP-positive cells in RVM (26.5 ± 2.6, n = 6 animals) were also TPH-positive (25.4 ± 2.3, 96%), and most of the GFP-positive cells in DR (43.9 ± 4.6, n = 6 animals) were also TPH-positive (39.7 ± 3.7, 90%). The penetration rate (GFP and TH/TPH double positive cells out of all TH/TPH cells) in each nucleus was moderate (60˜80%) but ectopic expression of GFP outside of the nucleus was few (4˜6%). Thus, fluorescence signal from G-CaMP6 seemed to reflect the activity of the majority of NA neurons in the LC and the majority of 5-HT neurons in the RVM/DR.
Fig. 4.
Confirmation of G-CaMP6 expression in LC and RVM/DR neurons.
Specific expression of G-CaMP6 was confirmed in LC NA neurons and RVM/DR 5-HT neurons. Almost all of the G-CaMP6 expression overlapped with DBH immunoreactivity or TPH immunoreactivity, and ectopic expression was rarely observed.
3.3. Effect of each stimulus on G-CaMP6 fluorescence
Fluctuation of G-CaMP6 fluorescence intensity during the baseline period was 2.09 ± 0.07% (LC), 2.26 ± 0.04% (RVM), and 2.61 ± 0.16% (DR) (values were calculated from 20 tests in 10 mice per group).
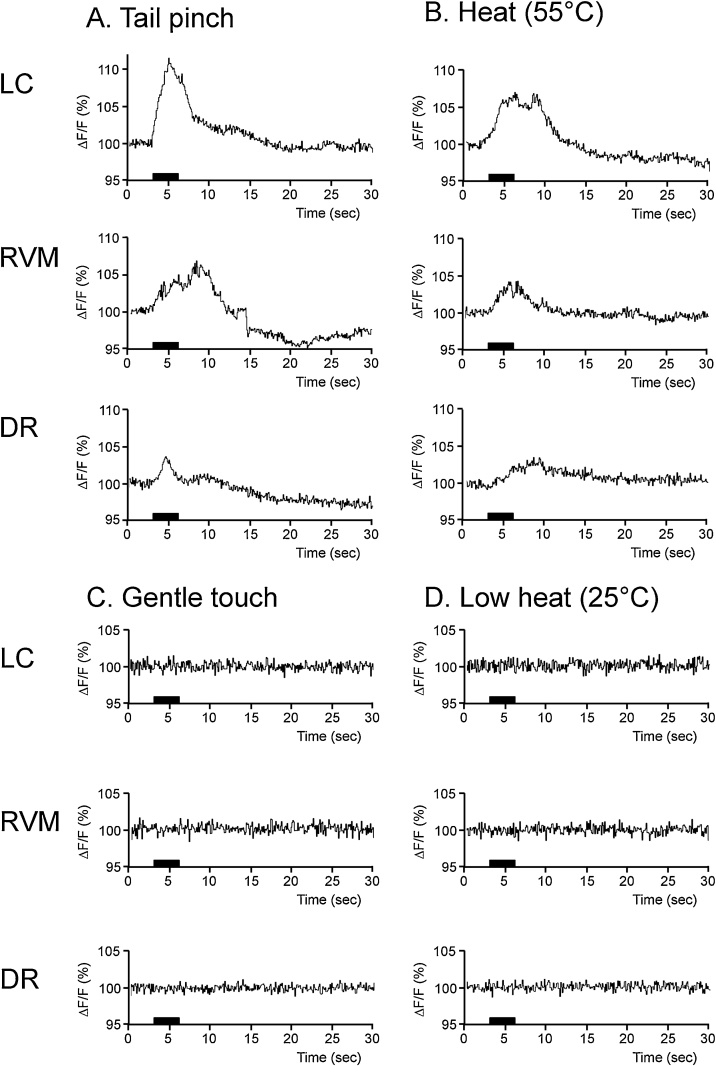
G-CaMP6 fluorescence intensity in LC NA neurons increased above the range of basal fluctuation as soon as acute nociceptive stimuli were applied (Fig.5), reached a peak point, and then rapidly returned to the baseline. In the tail pinch test, maximum signal intensity (ΔF/F) during the observation period was 12.4 ± 2.8%, which was significantly larger (p = 0.01, paired t-test in 5 mice) than that during the preceding baseline period (2.1 ± 0.2%). In the heat test, in a similar manner, the maximum signal intensity (10.6 ± 2.5%) was significantly larger than that during the preceding baseline period (1.9 ± 0.3%, p = 0.02). On the other hand, gentle touch (2.8 ± 0.2%) or low heat stimulus (2.6 ± 0.2%) did not cause significant change in the fluorescence intensity (Fig. 6A). These values were close to those of spontaneous fluctuation during the baseline period (2.1–2.6%, see above), indicating neuronal activation occurred due to the nociceptive stimuli.
Fig. 5.
G-CaMP6 fluorescent signals in LC and RVM/DR neurons in mice exposed to acute nociceptive stimuli.
G-CaMP6 signals increased soon after acute nociceptive stimuli (A: tail pinch stimulus with a force of 400 g; B: heat stimulus at 55 °C). In contrast, G-CaMP6 signals were mostly unchanged by control stimuli (C: gentle touching; D: low temperature heat stimulus at 25 °C). Graphs are averaged traces of fluorescent signals (n = 5).
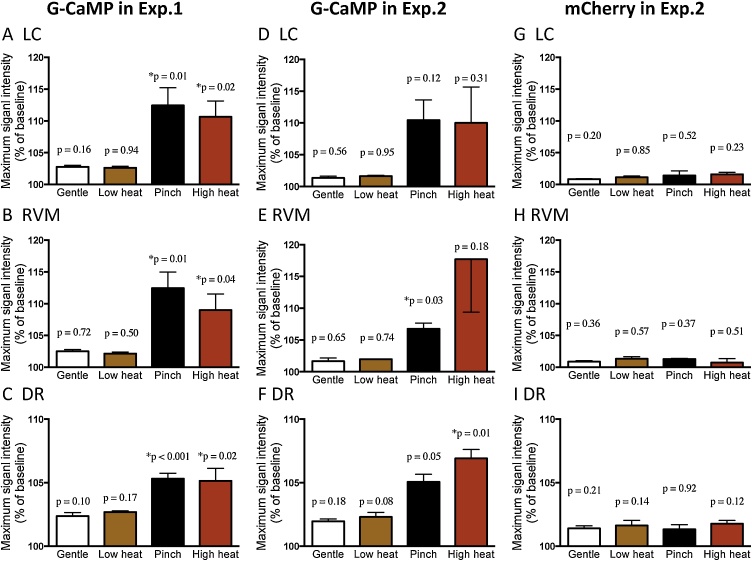
Fig. 6.
The changes of GCaMP6 and mCherry fluorescence signals in LC and RVM/DR neurons.
Response magnitude in the GCaMP6 fluorescence was summarized for LC neurons (A, D), RVM neurons (B, E), and DR neurons (C, F) in experiment-1 (only G-CaMP experiment, A–C) and experiment-2 (both G-CaMP6 and mCherry were used, D–F). Nociceptive stimuli of tail pinch (Pinch) and high heat (55 °C) increased G-CaMP6 fluorescence in both experiments while gentle touch to the tail (Gentle) and low heat (25 °C) stimuli did not induce significant changes in fluorescence signal intensity. Fluorescence of mCherry did not change in response to nociceptive nor control stimuli (G–I). P values were calculated using paired t-test between maximum signal intensity after the stimulation and that during baseline period. * indicates p < 0.05. Values are mean ± SEM for 5 animals in experiment-1 and 3 animals in experiment-2.
G-CaMP6 fluorescence intensity in RVM/DR 5-HT neurons increased over the range of basal fluctuation directly after acute nociceptive stimulation (Fig. 5 and 6B, C), reached a peak point (12.4 ± 2.5%, p = 0.01 in RVM-pinch; 9.1 ± 2.5%, p = 0.04 RVM-heat; 5.3 ± 0.4%, p < 0.001 in DR-pinch; and 5.1 ± 1.0%, p = 0.02 in DR-heat) and then rapidly returned to baseline, similar to LC NA neurons. Maximum signal intensities during the two acute nociceptive stimulations were significantly greater than those of the baseline fluctuation and those during control stimuli were not (Fig. 6B, C), indicating neuronal activation was induced due to the stimuli.
In the additional experiment using G-CaMP6 and mCherry (experiment-2), basically similar results were obtained (Fig. 6D-I). Namely, G-CaMP6 fluorescence increased only after tail pinch (5.1–10.4%) or high heat (6.9–17.7%) and not after gentle touch or low heat (1.3–2.3%) in LC, RVM, and DR (Fig. 6D–F). In addition, mCherry fluorescence did not change even after tail pinch or high heat (0.7–1.6%, Fig. 6G-I), indicating that observed change in G-CaMP6 fluorescence was actually reflected a change in neuronal activity but not from artifacts.
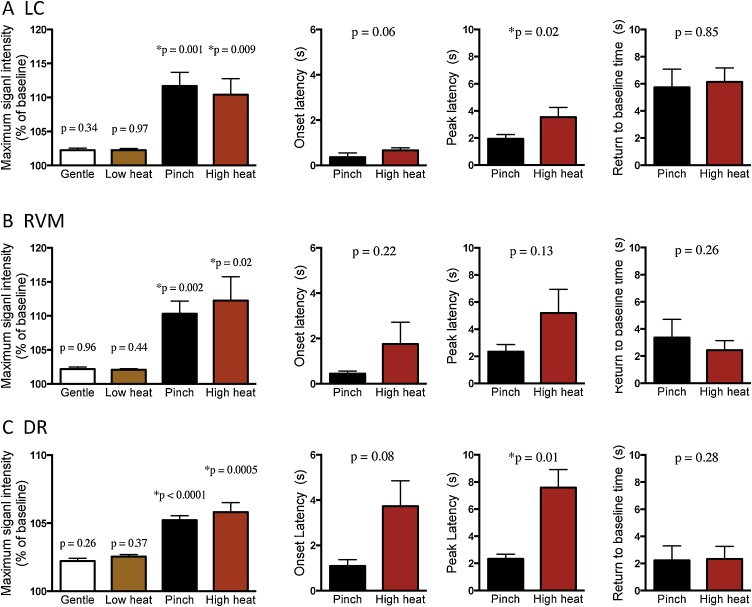
Since we had similar results in experiment-1 (G-CaMP6 only) and experiment-2 (G-CaMP6 and mCherry), we mixed up the data to improve statistical power for analyzing signal intensity and timing (onset latency, peak latency, and return to baseline time) (Fig. 7). In all the three nuclei tested, nociceptive stimuli of tail pinch (5.2–11.7%) and high heat (5.8–12.3%) but not control stimuli of gentle touching (2.0–2.2%) and low heat (2.1–2.5%) significantly increased G-CaMP6 fluorescence (Fig. 7A–C). Onset latency to heat stimulus (0.7 ± 0.1 s in LC, 1.8 ± 1.0 s in RVM, and 3.7 ± 1.1 s in DR) tended to be longer than that to pinch stimulus (0.4 ± 0.2 s in LC, 0.5 ± 0.1 s in RVM, and 1.1 ± 0.3 s in DR) (Fig. 7, middle left column). In addition, peak latency to heat stimulus was significantly longer in LC (3.5 ± 0.7 s in heat and 1.9 ± 0.3 s in pinch, p = 0.02) and DR (7.6 ± 1.3 s in heat and 2.3 ± 0.4 s in pinch, p = 0.01) and tended to be longer in RVM (5.2 ± 1.8 s in heat and 2.4 ± 0.5 s in pinch, p = 0.13) (Fig. 7, second to right column). The result indicated that the heat stimulus caused a relatively slow response when compared to the pinch stimulus. The return to baseline time was similar between heat stimulus and pinch stimulus (Fig. 7, right column), showing clear contrast to the difference in rising phase. Thus the temporal response pattern seemed to be dependent on the stimulus used (pinch vs. heat).
Fig. 7.
The changes of the activity of LC NA neurons and RVM/DR 5-HT neurons by acute nociceptive stimuli.
Response magnitude (leftmost column) and time-related characteristics (2nd column: onset latency; 3rd column: peak latency; rightmost column: return to baseline time) in the responses of LC NA neurons (A), RVM 5-HT neurons (B), and DR 5-HT neurons (C). Gentle touch to the tail (Gentle) and low heat (25 °C) stimuli did not induce significant changes in fluorescence signal intensity. Therefore, time-related characteristics in the responses were calculated only for tail pinch and high heat (55 °C) stimuli. P values in the figures in the leftmost column were calculated using paired t-test between maximum signal intensity after the stimulation and that during baseline period. P values in the figures in the right three columns were calculated using paired t-test between pinch stimulus and high heat stimulus. * indicates p < 0.05. Values are mean ± SEM in 8 animals.
4. Discussion
In this study, we examined whether acute nociceptive stimuli affected the activity of LC NA neurons, RVM 5-HT neurons, and DR 5-HT neurons in awake mice using a fiber photometry system. The present results indicate that acute nociceptive stimuli rapidly increase the activity of all of the examined neuronal groups.
Activation of LC NA neurons in response to nociceptive stimuli has already been shown through studies using microdialysis (Sajedianfard et al., 2005; Voisin et al., 2005), a study performing histology with the cellular activation marker c-Fos (Singewald and Philippu, 1998) and, studies using electrophysiological recording combined with cell-type identification by physiological characteristics of the neuron such as spike shape and spontaneous firing rate in anesthetized animals (Alba-Delgado et al., 2012; Chiang and Aston-Jones, 1993). Through our use of fiber photometry, we have provided the first evidence showing the activation is immediate and occurs in a time range of seconds in awake mice.
Immediate activation of RVM 5-HT neurons in response to nociceptive stimuli has also already been shown, but only in anesthetized animals (Wessendorf and Anderson, 1983; Chiang and Gao, 1986). It has also been shown that nociceptive stimuli increase 5-HT concentration in the spinal dorsal horn (Taguchi and Suzuki, 1992; Weil-Fugazza et al., 1984). Our results in awake mice are in line with these observations.
The DR is one of the main origins of the nuclei that make up the serotonergic system. It is located in the ventral periaqueductal gray matter, and mainly forms ascending serotonergic pathways. As mentioned above, there are many studies examining the nociceptive system in regards to LC NA neurons and RVM 5-HT neurons, but it remains unclear how DR 5-HT neurons responded to acute nociceptive stimuli. Our results show, for the first time, direct evidence of the immediate activation of DR 5-HT neurons in response to nociceptive stimuli. Although most DR 5-HT neurons project to supr A-D R structures, some of them project to the RVM (Cho and Basbaum, 1991; Kwiat and Basbaum, 1990). Our results give potential evidence of a possible activation of a DR-RVM 5-HT neuronal pathway, and may merit further investigation in the future.
In the clinical field, serotonin noradrenalin reuptake inhibitor (SNRI) drugs are FDA-approved drugs that are used to target the DAS in order to treat pain symptoms (Finnerup et al., 2015; Watson et al., 2011). SNRI drugs have a mechanism of action that increases synaptic NA and 5-HT concentrations. Some researchers have indicated that the RVM is related to neuropathic pain (Porreca et al., 2002) and chronic pain (Kwon et al., 2014; Ossipov et al., 2014). Unfortunately, there is scarce evidence available regarding possible DR involvement in pain modulation induced by SNRI treatment.
Although we have successfully shown that acute nociceptive stimuli causes an increase in the activities of LC NA neurons and RVM/DR 5-HT neurons, there are some limitations to this study. First, we focused on the nuclei (the LC and RVM/DR) in the brainstem, but not on areas to which they project, such as amygdala, hippocampus, prefrontal cortex and spinal dorsal horn (target site of the DAS) (McDevitt et al., 2014; Vertes, 1991). Measurement of activity in the target sites may need to be addressed in the future. Second, this study only explored acute stimuli. In the future, a study also examining chronic stimuli may be of interest. Third, time-resolution of fiber photometry system (˜sec) is not so high as conventional electrophysiological method (˜msec). Fiber photometry measures activity of ensemble average of the labeled neurons but not single unit activity. We should extend our research using combination of advantages of multiple methodologies.
In conclusion, acute nociceptive stimuli rapidly increased the activity of LC NA neurons and RVM/DR 5-HT neurons. These findings indicate that LC NA neurons and RVM/DR 5-HT neurons may be a therapeutic target for pain treatment. The fiber photometry system could prove to be a useful tool for evaluating the real time activity of neurons in response to nociceptive stimuli and also how that activity is modulated by certain drugs.
Author contributions
S.M. and A.Yamashita designed the study; S.M., A.Yamashita, R.N., Y.I., A.Yamanaka and T.K. conducted the study and analyzed the data; and S.M. and T.K. wrote the manuscript. All authors approved the final version of the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
We thank Jordan L. Pauli for English editing, Miki Sakoda for her excellent technical assistance, and all the members of the department of Physiology for their support. We also thank all the staff members of Institute of Laboratory Animal Sciences at Kagoshima University for keeping the animals in good condition. We acknowledge the Joint Research Laboratory, Kagoshima University Graduate School of Medical and Dental Sciences, for the use of their facilities. This work was supported by JSPS KAKENHI Grants (17K16387 to S.M.; 17K14936 to A. Yamashita; 16H05130 to T.K.) and CREST JST (JPMJCR1656 to A. Yamanaka).
References
- Alba-Delgado C., Mico J.A., Sanchez-Blazquez P., Berrocoso E. Analgesic antidepressants promote the responsiveness of locus coeruleus neurons to noxious stimulation: Implications for neuropathic pain. Pain. 2012;153:1438–1449. doi: 10.1016/j.pain.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Alba-Delgado C., Llorca-Torralba M., Horrillo I., Ortega J.E., Mico J.A., Sanchez-Blazquez P. Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol. Psychiatry. 2013;73(1):54–62. doi: 10.1016/j.biopsych.2012.06.033. [DOI] [PubMed] [Google Scholar]
- Andersen E., Dafny N. An ascending serotonergic pain modulation pathway from the dorsal raphe nucleus to the parafascicularis nucleus of the thalamus. Brain Res. 1983;269(1):57–67. doi: 10.1016/0006-8993(83)90962-9. [DOI] [PubMed] [Google Scholar]
- Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011;22(5-6):390–404. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- Borges G., Neto F., Mico J.A., Berrocoso E. Reversal of monoarthritis-induced affective disorders by diclofenac in rats. Anesthesiology. 2014;120(6):1476–1490. doi: 10.1097/ALN.0000000000000177. [DOI] [PubMed] [Google Scholar]
- Carlsson A., Magnusson T., Rosengren E. 5-hydroxytryptamine of the spinal cord normally and after transection. Experientia. 1963;19:359. doi: 10.1007/BF02152316. [DOI] [PubMed] [Google Scholar]
- Chiang C., Aston-Jones G. Response of locus coeruleus neurons to footshock stimulation is mediated by neurons in the rostral ventral medulla. Neuroscience. 1993;53(3):705–715. doi: 10.1016/0306-4522(93)90618-p. [DOI] [PubMed] [Google Scholar]
- Chiang C.Y., Gao B. The modification by systemic morphine of the responses of serotonergic and non-serotonergic neurons in nucleus raphe magnus to heating the tail. Pain. 1986;26(2):245–257. doi: 10.1016/0304-3959(86)90079-5. [DOI] [PubMed] [Google Scholar]
- Cho H.J., Basbaum A.I. GABAergic circuitry in the rostral ventral medulla of the rat and its relationship to descending antinociceptive controls. J. Comp. Neurol. 1991;303(2):316–328. doi: 10.1002/cne.903030212. [DOI] [PubMed] [Google Scholar]
- Commons K.G. Ascending serotonin neuron diversity under two umbrellas. Brain Struct. Funct. 2016;221(7):3347–3360. doi: 10.1007/s00429-015-1176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardin V., Oliveras J.L., Besson J.M. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. II. Differential characteristics of the analgesia induced by ventral and dorsal PAG stimulation. Brain Res. 1984;306(1-2):125–139. doi: 10.1016/0006-8993(84)90361-5. [DOI] [PubMed] [Google Scholar]
- Finnerup N.B., Attal N., Haroutounian S., McNicol E., Baron R., Dworkin R.H. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futatsuki T., Yamashita A., Ikbar K.N., Yamanaka A., Arita K., Kakihana Y. Involvement of orexin neurons in fasting- and central adenosine-induced hypothermia. Sci. Rep. 2018;8(1):2717. doi: 10.1038/s41598-018-21252-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentall I.D., Mesigil R., Pinzon A., Noga B.R. Temporal and spatial profiles of pontine-evoked monoamine release in the rat’s spinal cord. J. Neurophys. 2003;89(6):2943–2951. doi: 10.1152/jn.00608.2002. [DOI] [PubMed] [Google Scholar]
- Hickey L., Li Y., Fyson S.J., Watson T.C., Perrins R., Hewinson J. Optoactivation of locus ceruleus neurons evokes bidirectional changes in thermal nociception in rats. J. Neurosci. 2014;34(12):4148–4160. doi: 10.1523/JNEUROSCI.4835-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma Y., Kusumoto-Yoshida I., Yamanaka A., Ootsuka Y., Kuwaki T. Inactivation of serotonergic neurons in the rostral medullary raphé attenuates stress-induced tachypnea and tachycardia in mice. Front. Physiol. 2018;9:982. doi: 10.3389/fphys.2018.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N., Sugio S., Macklin W.B., Tomita K., Tanaka K.F., Ikenaka K. Gene induction in mature oligodendrocytes with a PLP-tTA mouse line. Genesis. 2012;50:424–428. doi: 10.1002/dvg.20808. [DOI] [PubMed] [Google Scholar]
- Inutsuka A., Yamashita A., Chowdhury S., Nakai J., Ohkura M., Taguchi T. The integrative role of orexin/hypocretin neurons in nociceptive perception and analgesic regulation. Sci. Rep. 2016;6:29480. doi: 10.1038/srep29480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B.L., Martin-Cora F.J., Fornal C.A. Activity of medullary serotonergic neurons in freely moving animals. Brain Res. Rev. 2002;40(1-3):45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Jordan L.M., Liu J., Hedlund P.B., Akay T., Pearson K.G. Descending command systems for the initiation of locomotion in mammals. Brain Res. Rev. 2008;57(1):183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kwiat G.C., Basbaum A.I. Organization of tyrosine hydroxylase- and serotonin-immunoreactive brainstem neurons with axon collaterals to the periaqueductal gray and the spinal cord in the rat. Brain Res. 1990;528(1):83–94. doi: 10.1016/0006-8993(90)90198-k. [DOI] [PubMed] [Google Scholar]
- Kwon M., Altin M., Duenas H., Alev L. The role of descending inhibitory pathways on chronic pain modulation and clinical implications. Pain Pract. 2014;14(7):656–667. doi: 10.1111/papr.12145. [DOI] [PubMed] [Google Scholar]
- Lechin F., van der Dijs B., Hernandez-Adrian G. Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Prog. NeuroPsychopharmacol. Biol. Psychitr. 2006;30(4):565–585. doi: 10.1016/j.pnpbp.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Lovick T.A., Wolstencroft J.H. Inhibitory effects of nucleus raphe magnus on neuronal responses in the spinal trigeminal nucleus to nociceptive compared with non-nociceptive inputs. Pain. 1979;7(2):135–145. doi: 10.1016/0304-3959(79)90005-8. [DOI] [PubMed] [Google Scholar]
- McDevitt R.A., Tiran-Cappello A., Shen H., Balderas I., Britt J.P., Marino R.A.M. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep. 2014;8(6):1857–1869. doi: 10.1016/j.celrep.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Millan M.J. Descending control of pain. Prog. Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Moriya S., Yamashita A., Kawashima S., Nishi R., Yamanaka A., Kuwaki T. Acute aversive stimuli rapidly increase the activity of ventral tegmental area dopamine neurons in awake mice. Neuroscience. 2018;386:16–23. doi: 10.1016/j.neuroscience.2018.06.027. [DOI] [PubMed] [Google Scholar]
- Ochi T., Goto T. The antinociceptive effect induced by FR140423 is mediated through spinal 5-HT2A and 5-HT3 receptors. Eur. J. Pharmacol. 2000;409(2):167–172. doi: 10.1016/s0014-2999(00)00832-3. [DOI] [PubMed] [Google Scholar]
- Ohkura M., Sasaki T., Sadakari J., Gengyo-Ando K., Kagawa-Nagamura Y., Kobayashi C. Genetically encoded green fluorescent Ca2+ indicators with improved detectability for neuronal Ca2+ signals. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura Y., Tanaka K.F., Tsunematsu T., Yamanaka A., Yoshioka M. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int. J. Neuropsychopharmacol. 2014;17:1777–1783. doi: 10.1017/S1461145714000637. [DOI] [PubMed] [Google Scholar]
- Ossipov M.H., Morimura K., Porreca F. Descending pain modulation and chronification of pain. Curr. Opin. Support Palli Care. 2014;8(2):143–151. doi: 10.1097/SPC.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F., Ossipov M.H., Gebhart G.F. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25(6):319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Rea K., Olango W.M., Okine B.N., Madasu M.K., McGuire I.C., Coyle K. Impaired endocannabinoid signalling in the rostral ventromedial medulla underpins genotype-dependent hyper-responsivity to noxious stimuli. Pain. 2014;155(1):69–79. doi: 10.1016/j.pain.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Ren K., Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr. Opin. Anaesthesiol. 2008;21(5):570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D.V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164(3878):444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Sajedianfard J., Khatami S., Semnanian S., Naghdi N., Jorjani M. In vivo measurement of noradrenaline in the locus coeruleus of rats during the formalin test: a microdialysis study. Eur. J. Pharmacol. 2005;512(2-3):153–156. doi: 10.1016/j.ejphar.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Seifert F., Maihofner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell. Mol. Life Sci. 2009;66(3):375–390. doi: 10.1007/s00018-008-8428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N., Philippu A. Release of neurotransmitters in the locus coeruleus. Prog. Neurobiol. 1998;56(2):237–267. doi: 10.1016/s0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Taguchi K., Suzuki Y. The response of the 5-hydroxyindole oxidation current to noxious stimuli in the spinal cord of anesthetized rats: modification by morphine. Brain Res. 1992;583(1-2):150–154. doi: 10.1016/s0006-8993(10)80018-6. [DOI] [PubMed] [Google Scholar]
- Tanaka K.F., Matsui K., Sasaki T., Sano H., Sugio S., Fan K. Expanding the repertoire of optogenetically targeted cells with an enhanced gene expression system. Cell Rep. 2012;2:397–406. doi: 10.1016/j.celrep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Tyce G.M., Yaksh T.L. Monoamine release from cat spinal cord by somatic stimuli: an intrinsic modulatory system. J. Physiol. (Paris) 1981;314:513–529. doi: 10.1113/jphysiol.1981.sp013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes R.P. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991;313(4):643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Voisin D.L., Guy N., Chalus M., Dallel R. Nociceptive stimulation activates locus coeruleus neurones projecting to the somatosensory thalamus in the rat. J. Physiol. (Paris) 2005;566(Pt 3):929–937. doi: 10.1113/jphysiol.2005.086520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C.P., Gilron I., Sawynok J., Lynch M.E. Nontricyclic antidepressant analgesics and pain: are serotonin norepinephrine reuptake inhibitors (SNRIs) any better? Pain. 2011;152(10):2206–2210. doi: 10.1016/j.pain.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Weil-Fugazza J., Godefroy F., Le Bars D. Increase in 5-HT synthesis in the dorsal part of the spinal cord, induced by a nociceptive stimulus: blockade by morphine. Brain Res. 1984;297(2):247–264. doi: 10.1016/0006-8993(84)90566-3. [DOI] [PubMed] [Google Scholar]
- Wessendorf M.W., Anderson E.G. Single unit studies of identified bulbospinal serotonergic units. Brain Res. 1983;279(1-2):93–103. doi: 10.1016/0006-8993(83)90166-x. [DOI] [PubMed] [Google Scholar]
- Yaksh T.L., Hammond D.L., Tyce G.M. Functional aspects of bulbospinal monoaminergic projections in modulating processing of somatosensory information. Fed. Proc. 1981;40(13):2786–2794. [PubMed] [Google Scholar]
- Yoshimura M., Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J. Pharmacol. Sci. 2006;101(2):107–117. doi: 10.1254/jphs.crj06008x. [DOI] [PubMed] [Google Scholar]
- Zhang F.E., Cao J.L., Zhang L.C., Zeng Y.M. Activation of p38 mitogen-activated protein kinase in spinal cord contributes to chronic constriction injury-induced neuropathic pain. Acta Physiol. Sin. 2005;57(5):545–551. [PubMed] [Google Scholar]