Abstract
The presence of mycotoxins in staple food can have adverse effect that result in ill health and associated socio-economic losses. Mycotoxins are naturally occurring toxins produced by certain fungi and can be found in staple food plants such as ginger. Ginger is a renowned medicinal plant that is extensively used for cooking and healing. However, this medicinal plant is with little information about its possible mycotoxins contamination. This study determined the occurrence and prevalence of Aflatoxin B1, B2, G1 and G2 and Ochratoxin A contamination in raw ginger sold around Mahikeng, North West Province, South Africa. Samples were collected purposively from various retailers over winter and summer. The analytical procedure optimized was based on immunoaffinity column cleanup (IAC), followed by High performance liquid chromatography with fluorescence (HPLC-FLC) detection. ELISA was also used for mycotoxin screening. On HPLC, the limits of detection and quantification for the four Aflatoxins were 3.9 × 10−7-1.4 × 10 -3 and 1.3 × 10-6 - 4.7 × 10-3 for samples collected in winter, and 3.7 × 10−7- 1.4 × 10-3, LOQ 1.2 × 10-6 – 4.6 × 10-3 for the summer samples. The average recoveries at three spiking levels ranged from 62 to 91% for the summer samples and 70–93% for those collected in winter. A linearity was observed for the analytes whose correlation coefficients were within the range of 0.9995 and 1.000 for the winter samples and 0.9995 and 1.000 for those collected in summer. The results showed that the contamination levels, especially for samples collected in summer were greater than the legally permissible limits. The t-test analysis shows that the mean and standard deviation of the four types of Aflatoxins considered were higher in summer than in winter. The findings of the study indicated that ginger, as for all agricultural commodities, are prone to mycotoxin contamination.
Chemical compounds studied in this article: Aflatoxins B1, B2, G1, G2, Ochratoxin A
Keywords: Column cleanup, Medicinal plant, Immunoaffinity, Human health, Season
1. Introduction
Mycotoxins are toxic compounds that are produced naturally by certain type of mould. They are produced in numerous food stuffs and have effects on the health of consumers as well as the wellbeing of human and animals. These secondary metabolites of moulds have numerous debilitating effects on humans and animals due to their carcinogenicity, teratogenicity and immunosuppression ability [1,2]. Approximately 400 types of mycotoxins exists, including Aflatoxins and Ochratoxin A [3] (Table 1), and are found almost everywhere in the environment including food crops, dairy products (milk) and orchards, [4,62], thereby increasing the risk of human consumption.
Table 1.
Properties of Aflatoxins B1, B2, G1, G2 and Ochratoxin A.
| Chemical Structures | IUPAC Name | Health effects | Target organ | References |
|---|---|---|---|---|
| Aflatoxin B1 | ||||
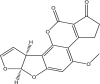 |
(3S,7R)-11-methoxy-6,8,19-trioxapentacyclo[10.7.0.02,9.03,7.013,17]nonadeca-1,4,9,11,13(17)-pentaene-16,18-dione | Hepatotoxic, Hepatocellular carcinoma, mutagenic, genotoxic, teratogenic and causes immunosuppression | Liver | [22,45,46,47,58] |
| Aflatoxin B2 | ||||
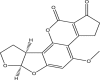 |
(3S,7R)-11-methoxy-6,8,19-trioxapentacyclo[10.7.0.02,9.03,7.013,17]nonadeca-1,9,11,13(17)-tetraene-16,18-dione | Hepatotoxic, immunosuppression, teratogenic, mutagenic | Liver | [22,46,45,47] |
| Aflatoxin G1 | ||||
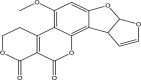 |
11-methoxy-6,8,16,20-tetraoxapentacyclo[10.8.0.02,9.03,7.013, 18]icosa-1,4,9,11,13(18)-pentaene-17,19-dione |
Hepatotoxic, immunosuppression, teratogenic, genotoxic, mutagenic | Liver | [22,46,45,47,58] |
| Aflatoxin G2 | ||||
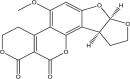 |
(3S,7R)-11-methoxy-6,8,16,20-tetraoxapentacyclo [10.8.0.02,9.03,7.013,18]icosa-1,9,11,13(18)-tetraene-17,19-dione | Hepatotoxic, immunosuppression, teratogenic, mutagenic | Liver | [22,45,46,47] |
| Ochratoxin A | ||||
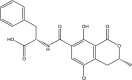 |
(2S)-2-[[(3R)-5-chloro-8-hydroxy-3-methyl-1-oxo-3,4-dihydroisochromene-7-carbonyl]amino]-3-phenylpropanoic acid | Carcinogenic, genotoxic, immuno-suppressive, nephrotoxic and upper urinary tract disease | Kidney, liver | [21,42,59,60] |
Ginger is regarded as having medicinal properties, including having antioxidant and antimicrobial effects against some bacterial, such as Escherichia coli, Vibrio cholera, Streptococcus faecalis, Klebsiella sp. and Salmonella sp. [5,7,8]. Recent studies have also reported that ginger has antidiabetic and anti-inflammatory properties [6], and is used to prevent and combat various types of ailments, such as diarrhoea and vomiting [10]. It belongs to the kingdom Plantae and since ancient times in many cultures, has been regarded as a ‘medicinal and edible’ herb/spice [2]. Despite its numerous health benefits, a few publications have reported that Aflatoxins and Ochratoxin A occur in ginger (Zingiber officinale) and its by-products at various contamination levels [2,[11], [12], [13]] (Table 2).
Table 2.
Typical crops contaminated by Aflatoxins and Ochratoxin A and their reported maximum tolerated levels.
| Crops | Mycotoxin | Maximum Tolerated Level EU (μg / kg) |
References |
|---|---|---|---|
| Rice Corn Wheat Peanuts Sorghum Spices |
Aflatoxin B1, B2, G1 and G2 (AFB1, AFB2, AFG1, and AFG2) | 2 – 12 Aflatoxin B1 4 – 15 for total Aflatoxin |
[48,49,50,51,52,53] |
| Cereals Dried vine fruit Wine Grapes Coffee Pistachio |
Ochratoxin A | 2 – 10 | [54,55,56,57,61] |
Extracts from some plants are reported to prevent and even cure completely some ailments due to their ability to produce certain chemical compounds that protect the body against germs and pathogens [14,64,65]. This has placed a considerable demand for medicinal plants, including ginger, as there is a gradual shift away from the use of orthodox medicine to local/medicinal plants, including in Africa, where many people already rely on plant remedies for a range of health issues [66]. This study will therefore investigate the mycotoxin contamination (Aflatoxins and Ochratoxin A) in ginger (Zingiber officinale) consumed in the North-West Province of South Africa. There is also little information about the seasonal variations in the prevalence of Aflatoxins in ginger during the winter and summer seasons, which this study will explore.
2. Research method
The study was conducted in Mahikeng, the capital city of North West Province, which borders with Botswana to the north and Namibia to the west. Its temperatures range from 17 −31 °C in summer and from 3 −21 °C in winter. The total annual rainfall is approximately 360 mm (about 14 in., with most of it falling during summer (October – April).
Using purposive sampling method, 100 samples of locally cultivated ginger (Zingiber officinale) were purchased from different shops/markets in Mahikeng during summer and winter. The first 50 were collected during summer and the second 50 during winter as follows: 10 samples were collected in 10 shops, with the procedure being repeated two weeks later from the same shops. This two weeks interval was to ensure that new samples of ginger were displayed and to ensure that fresh samples were collected. This procedure was repeated until 50 samples were collected during both winter and summer (Winter and summer are the chosen season of interest for this research because of the extreme temperatures of these two seasons). The condition and state of the ginger samples was taken into consideration at the point of collection to avoid getting mould/spores contaminated samples.
3. Sample processing
The ginger samples were washed with water, chopped and stored at 4 °C in a refrigerator prior to extraction and analysis to prevent the growth of mould and to avoid their putrefaction. All the samples were then extracted, screened for Aflatoxins and Ochratoxin A using Enzyme linked immuno-sorbent assay (ELISA), and quantified using the High Performance Liquid Chromatography (HPLC).
3.1. Extraction procedure for aflatoxins
Extraction of Aflatoxins was carried out according to Wen et al. [2] method with minor differences. Ten gram of each sample of ginger was weighed and placed in a 100 ml Erlenmeyer flask, 2 g of sodium chloride (NaCl) was added followed by 50 ml of methanol/water (80/20, v/v), after which the mixtures were vortexed for 20 min to obtain a proper mixture. The extract was filtered using whatmann filter paper, 10 ml of the filtrate was then diluted by 40 ml of 20% tween-20-PBS (20% phosphate buffer saline) in a 50 ml Erlenmeyer flask, after which it was filtered through the glass microfiber filter.
3.2. Immunoaffinity clean-up
Twenty five (25) ml of the final filtrate was passed through the Immunoaffinity columns (IACs), which was washed with 20 ml of Phosphate Buffer solution (pH 7.4), the toxins were finally eluted with 1 ml of 100% methanol. Thereafter, 1 ml of distilled water was passed through the column to wash. Screening and quantification of the aflatoxins were carried out using the ELISA and HPLC respectively.
3.3. Extraction procedure for ochratoxin A
This was done according to the standard method of Maryam and Jinap [15] with slight modifications. Fifty (50) ml milliliters of methanol/water (80/20, v/v) was added to 12.5 g of the blended ginger sample containing 2.0 g of sodium chloride and vortexed for 20 min then filtered using Whatmann filter paper. 10 ml of the filtrate was diluted with 40 ml of PBS and the diluent filtered once more through a glass microfiber filter, with 25 ml of the final filtrate being passed through the Immunoaffinity columns (IACs). The column was then washed with 20 ml of PBS solution (pH 7.4) and the toxins finally eluted with 1 ml of 100% methanol. 1 ml of distilled water was then passed through the column to wash the dilute, which was evaporated and dissolved in 200 μl methanol/water (50:50, v/v). Screening and quantification of Ochratoxin A was carried out through ELISA and HPLC respectively. Competitive ELISA procedures were carried out in order to screen for Aflatoxins B1, B2, G1, G2 and Ochratoxin A in the collected ginger samples according to the method described by the application note. Eighty (80) out of the 100 samples, were optimized.
4. Mycotoxins quantification using high performance liquid chromatography (HPLC)
To quantify the mycotoxins, analysis was carried out with the High Performance Liquid Chromatography machine (HPLC), with approximately 100 μl of the extract being utilised.
4.1. HPLC conditions
Analysis was carried out on KOBRA® CELL with an analytical column of Inertsil ODS-3 V (5 μm, 4.6 mm X 150 mm) being maintained with the guard at 40 °C. The mobile phase consisted of a water: methanol ratio of 60:40 v/v, with 119 mg of potassium bromide and 350 μl 4 M Nitric acid added to 1 L of mobile phase at a flow rate of 1.0 ml/minute. Standard concentrations used were 1 ng/ml, 5 ng/ml, 25 ng/m l, and 100 ng/ml. The injection volume was 100 μl, with the elution being monitored by a fluorescence detector in the order of G2, G1, B2 and B1. The fluorescence conditions were optimized at an excitation level of 362 nm wavelength, and emission level of 425 nm for aflatoxins B1 and B2, and 455 nm for Aflatoxins G1 and G2.
4.2. Linearity
The calibration curves were constructed with different standard analytes, each solution being injected in triplicate. Construction of the calibration curves were done by plotting the peak area (y) against each analytes concentration (x) obtained from the HPLC analysis. The linearity was determined and expressed as a correlation coefficient (R), with Table 1.1 showing that the linear relationship of the analytes were very good, with R ranging from 0.9995 to 1.0000 for the different standards used.
4.3. LOD and LOQ
The Limit of detection and Limit of quantification were calculated as signal–to–noise (S/N) ratio of 3 and 10, respectively.
4.4. Recovery
The recovery was used to evaluate the accuracy of the established method, the experiments being performed at three spiking levels (1.0, 5.0, 10.0 μg/kg for AFB1 and AFG1, and 0.25, 0.75 and 1.25 μg/kg for AFB2 and AFG2) by adding an appropriate quantity of AFs standard solutions to the blank ginger samples. The spiked samples were extracted, cleaned-up and analysed by HPLC, as previously described. The recovery was calculated using the formula:
| Recovery = (measured concentration for spiked sample / spiked concenration) x 100 |
5. Results and discussions
5.1. Enzyme linked immunosorbent assay (ELISA)
In this study, OTA and Aflatoxin were screened for in the samples using the ELISA, with the results being summarized in Table 3, which presents the percentage occurrence of Aflatoxins and Ochratoxin A. It indicates that during summer, all the screened samples contain Aflatoxins and Ochratoxin A within the range of 6.4–411.1 ppb and 0.0968–3.309 ppb respectively. It also shows that during winter, all samples contain both Aflatoxins and Ochratoxin A within the range of 3.625–105.7 ppb and 0.0960–3.395 ppb respectively. The mean values are 0.578 ppb, 0.566 ppb, 1.761 ppb and 1.758 ppb respectively for Aflatoxin summer and winter, Ochratoxin A summer and winter respectively.
Table 3.
Screened Mycotoxins on ELISA and their Contamination Level.
| Mycotoxin | Season | Contamination % | Range (PPB) | Mean ± SD (PPB) |
|---|---|---|---|---|
| Aflatoxin | Summer | 100 | 6.4 – 411.1 | 0.578 (±0.462) |
| Winter | 100 | 3.625 – 105.7 | 0.566 (±0.446) | |
| Ochratoxin A | Summer | 100 | 0.0968 - 3.309 | 1.761 (±1.185) |
| Winter | 100 | 0.0960 – 3.395 | 1.758 (±1.172) |
Analysis on the ELISA showed that Aflatoxin levels in the samples were high (Table 3), which was also confirmed from the analysis carried out on the HPLC. The Aflatoxin levels in the ginger samples were higher than those recommended by the European Union (EU), this being 15 μg/kg and a maximum of 12 μg/kg for Aflatoxin B1.
Ochratoxin A concentration ranges between 0.0960–3.395 μg/kg for the ginger collected in winter, and between 0.0968–3.309 μg/kg for those collected in summer, as screened through the ELISA. The concentrations were lower than the recommended EU limit of 10 μg/kg. The high concentration observed in winter may be due to the fungi being able to thrive in the temperatures, which range from 20 to 33 °C, leading to the production of Ochratoxin A according to Passamani et al. [18]. This means that the temperature does not need to be high for fungi to thrive and produce Ochratoxin A.
5.2. High performance liquid chromatography
High Performance Liquid Chromatography (HPLC) was done according to Wen et al. [2] method. In Table 4, the HPLC quality assurance parameters are well represented, with the experiments performed for recoveries at three spiking levels (1.0, 5.0, 10.0 μg/kg for AFB1 and AFG1 and 0.25, 0.75 and 1.25 μg/kg for AFB2 and AFG2) all being good. Details about linearity, limit of detection and limit of quantification are also shown in Table 4, and indicate good sensitivity of the method. The linear relationships of the analytes are very good, with R greater than 0.9995, and the LODs and LOQs for the four aflatoxins being within the range of 1.3 × 10-6 - 5.7 × 10-5 and 1.7 × 10-4-3.9 × 10 -6 respectively.
Table 4.
HPLC quality assurance parameters.
| Analytes | Spiking level (ng/ml) |
Recovery level (%) |
LOD (μg/kg) |
LOQ (μg/kg) |
Average recoveries (%) |
Mean recoveries |
R | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | ||||
| AflatoxinB1 | 1 | 1 | 0.70 | 0.75 | 5.7 × 10−5 | 70 | 75 | ||||
| 5 | 5 | 3.80 | 4.2 | 1.7 × 10−4 | 76 | 84 | 72.33 | 75.7 | 0.9997 | ||
| 10 | 10 | 7.1 | 6.8 | 71 | 68 | ||||||
| Aflatoxin B2 | 0.25 | 0.25 | 0.16 | 0.18 | 1.4 × 10−3 | 64 | 72 | ||||
| 0.75 | 0.75 | 0.45 | 0.55 | 4.2 × 10−3 | 60 | 73.3 | 71 | 79.9 | 1.0000 | ||
| 1.25 | 1.25 | 1.12 | 1.18 | 89 | 94.4 | ||||||
| Aflatoxin G1 | 1 | 1 | 0.92 | 0.9 | 92 | 90 | |||||
| 5 | 5 | 4.1 | 4.3 | 3.9 × 10 −7 | 1.2 × 10−6 | 82 | 86 | 89 | 89 | 0.9998 | |
| 10 | 10 | 9.3 | 9.1 | 93 | 91 | ||||||
| Aflatoxin G2 | 0.25 | 0.25 | 0.17 | 0.22 | 68 | 88 | |||||
| 0.75 | 0.75 | 0.65 | 0.69 | 1.3 × 10−6 | 3.9 × 10−6 | 87 | 92 | 75 | 89.3 | 0.9995 | |
| 1.25 | 1.25 | 0.87 | 1.1 | 70 | 88 | ||||||
Table 5 summarized the prevalence of the different Aflatoxins in the ginger samples during both seasons. During winter, 86% of the ginger samples collected contains AFB1, 56% contained AFB2, 38% contained AFG1 and 6% contained AFG2. The results obtained showed mean results of 0.165 μg/kg for AFB1, 0.389 μg/kg for AFB2, 0.017 μg/kg for AFG1and 0.009 μg/kg for AFG2. During summer, 98% of the samples were positive for AFB1, 96% for AFB2, 56% for AFG1 and 12% for AFG2. The results showed mean values of 1.21 μg/kg for AFB1, 12.10 μg/kg for AFB2, 9.78 μg/kg for AFG1 and 0.40 μg/kg for AFG2.
Table 5.
Summary of Aflatoxins in the ginger samples during both seasons.
| Occurrence (%) |
Range (μg/kg) |
Mean ± SD |
||||
|---|---|---|---|---|---|---|
| Analytes | Winter | Summer | Winter | Summer | Winter | Summer |
| Aflatoxin B1 | 86 | 98 | 0.02-0.74 | 0.01-6.04 | 0.165 (±0.115) | 1.21 (±1.82) |
| Aflatoxin B2 | 56 | 96 | 0.04-3.44 | 0.14-9.95 | 0.389 (±0.494) | 7.10 (±37.33) |
| Aflatoxin G1 | 38 | 56 | 0.002-0.17 | 0.01-6.59 | 0.017 (±0.025) | 5.78 (±27.24) |
| Aflatoxin G2 | 6 | 12 | 0.002-0.2 | 0.89-13.67 | 0.009 (±0.003) | 0.40 (±1.98) |
5.3. Statistical modelling of the Comparative Analysis of the prevalence of Aflatoxins contamination between summer and winter Ginger
To compare the effect of summer and winter on the Aflatoxin contamination of the ginger, the t-test was used to analyse the data, although conclusions could be drawn based on some conventional expectations [18,19]. The t-test analysis was the best fit for this objective, as Hussain et al. [20] contended that a t-test is an analysis of two population mean(s) using statistical examination. A t-test with two samples is used mostly for small sample sizes to test the difference between them when the variances of two normal distributions are not known. t-test analysis is usually used to assess whether the means of two groups are statistically different from each other. It was used in this study to establish if there were any significant differences between the prevalence of Aflatoxin contamination in the ginger between the two seasons (summer and winter).
The equation used was as follows:
As indicated by the analysis (Table 6), there was a clear difference between the contamination level of the ginger for AFG2 in summer (M = 0.399, SD = 1.981) and winter (M = 0.001, SD = 0.003), with a P<0.01. The samples collected in summer also showed a higher level of AFG1 than those collected in winter, with the summer mean and SD of AFG1 being 9.778 and 27.51, while that of winter being 0.68 and 0.025 respectively, with a P < 0.01. In the case of AFB2, its prevalence in summer (M = 1.206, SD = 1.83522) was much higher than that of winter (M = 0.142, SD = 0.114), as was the case for AFB1, with mean and SD of the summer samples (M = 12.097, SD = 37.713) being higher than that of the winter samples (M = 0.1418, SD = 0.114)
Table 6.
Independent Sample t-test to identify the differences in the prevalence of Aflatoxins contamination between summer and winter.
| Mycotoxins | Seasons | Mean | Std. dev | N | T | Df | Sig. |
|---|---|---|---|---|---|---|---|
| AFG2 | Summer Winter |
0.3987 0.0006 |
1.98113 0.00280 |
50 49 |
1.421 | 49.000 | 0.008 |
| AFG1 | Summer Winter |
9.7779 0.0068 |
27.51565 0.02526 |
50 49 |
2.511 | 49.000 | 0.000 |
| AFB1 | Summer Winter |
12.0969 0.1662 |
37.71280 0.49945 |
50 49 |
2.237 | 49.018 | 0.001 |
| AFB2 | Summer Winter |
1.2055 0.1418 |
1.83522 0.11421 |
50 49 |
4.090 | 49.387 | 0.000 |
This study aimed at screening ginger consumed locally in the North West Province of South Africa for Aflatoxins and Ochratoxin A. The evaluation and analysis on HPLC reveals the presence of AFB1, AFB2, AFG1 and AFG2, while Ochratoxin A was also identified when screened for on ELISA. These mycotoxins were identified in virtually all the samples of the ginger screened and, according to the literature, are the major mycotoxins commonly encountered in ginger [21,22].
The ginger in this study was likely to have been contaminated with Aflatoxigenic fungi, such as Aspergillus parasiticus or Aspergillus flavus [23,24]. According to the result from the analysis carried out on HPLC, the AFB1 level in the ginger collected during winter ranged from 0.02-0.74 μg/kg, for AFB2 from 0.04 to 3.44 μg/kg, for AFG1 from 0.002-0.17 μg/kg, while for AFG2 from 0.002 – 0.2 μg/kg. In the samples collected during summer, AFB1 ranged from 0.01 to 6.04 μg/kg, AFB2 from 0.14 to 9.95 μg/kg, AFG1 from 0.01 to 6.59 μg/kg, and AFG2 from 0.89 to 13.67 μg/kg (Table 5).
Moreover, the results indicated that there was a clear difference in the results between the seasons (Table 5), which were both above the European Unions recommended level of Aflatoxins and Ochratoxin A. These high levels may result in considerable histopathological changes, especially in the liver tissue, which is the main organ responsible for metabolising Aflatoxin [25]. This is also in line with Atanda et al. [24], Bjorn et al. [26], who emphasised that the intake of Aflatoxins, specifically at concentrations higher than the recommended levels, can result in cellular dissociation, infiltration, necrosis and fibrosis, as well as bile duct hyperplasia.
In addition, the occurrence of Aflatoxins and Ochratoxin A in the samples analysed in this study were in line with those report in China by Wen et al. [2], revealing that AFs and OTA were also found in ginger. The findings of AFB1, AFB2, AFG1 and AFG2 in South Africa spices in a study conducted by Bhat and Kumar et al. [23] corroborates the finding of this research. It can therefore be deduced [27,2] that ginger contains Aflatoxin in numerous regions of the work, is the effects of which are known to have various debilitating effects on human health. Furthermore, the concentration of mycotoxins in the ginger samples collected during winter were lesser than those collected in summer. This is in line with the findings of Zain et al. [28]; Milanovic et al, Ishaque et al [29,30] who also found a high prevalence of aflatoxins and ochratoxin A contamination in food during summer, which may be due to the higher temperatures.
The increased concentration of mycotoxins during summer could also be as a result of improper agricultural practices, such as storage facilities and unhygienic/unsanitised display facilities in the shop, where environmental factors (temperature, humidity and moisture) are not controlled and might influence the growth of fungi and the production of mycotoxins [31]. In addition, contamination of the ginger could have occurred in the field due to poor field sanitation, insect infestation control and early harvesting, these contributing factors having been identified by Trucksess and Koeltzow [32]. The variation in these results indicates that mycotoxins are present and generally thrive more in summer than in winter, as the weather condition during this time favours the growth of micro-organisms and fungi [33].
The contamination of ginger with Aflatoxin in this study represents a serious health challenge, as they are known to suppress the immune system and exert carcinogenic, mutagenic and teratogenic effects to human [34,32]. The high levels of Aflatoxin in this study indicated the prevalence of Aspergillus flavus and Aspergillus parasiticus in ginger that are consumed as staple in South Africa. The presence of ochratoxin A in the ginger samples further indicates that the ginger were majorly contaminated with Penicillium verrucosum, Penicillium nordicum or other species of Penicillium and Aspergillus, with Penicillium species being one of the major producers of Ochratoxin A [31]
Furthermore, higher concentrations of mycotoxin occurrence were recorded by the ELISA analysis than on HPLC. This could be due to cross-reactivity with related substances as well as matrix dependence, which often leads to overestimation. In addition, the antibodies have the advantage of high specificity and sensitivity, as the target compounds are not the antigens but mycotoxins, as compounds with similar chemical groups can also interact with the antibodies [33]. This can also be explained by the matrix effect or interference, which commonly occur in ELISA methods, resulting in under- or over-estimates in mycotoxin concentrations in commodity samples [32]. Furthermore, Tian et al. [33] noted that extraction solvents such as methanol may also affect screening on the ELISA, as they could lead to co-extraction of fatty materials, which could interfere in the assay and contribute to the high values detected.
In summary, a simple, rapid and sensitive method based on IAC clean-up coupled with HPLC was used to identify AFB1, AFB2, AFG1, and AFG2 in ginger in this study. The developed method was successfully used to analyse 100 ginger samples collected at different times during summer and winter in the North West Province of South Africa. The result show that all the samples contained either Aflatoxin or Ochratoxin A, with the validated method showing satisfactory linearity, precision and accuracy. The four Aflatoxins were separated and no interference peaks observed. All positive samples were verified and compared to the results from the ELISA, with no false-positive samples being identified, which confirms that the methods were sensitive and reliable. Regarding determining the prevalence of the mycotoxins during winter and summer seasons, it was observed that Alatoxins and Ochratoxin A had the ability to thrive better in summer than in winter due to the higher temperatures and dampness that enabled the fungi to thrive.
6. Recommendation
More attention needs to be paid to the cultivation, harvesting and storage conditions even to the consumption of ginger produced in South Africa. Prevention strategies (mechanical, physical, biological and chemical as described by Omotayo et al. [63] can be used to manage the Aflatoxins and Ochratoxin A contamination of ginger, as ginger screened in the study contains Aflatoxin greater than the recommended level by the European Union. There is therefore need to ensure that the public are protected from possible adverse effects associated with ginger consumption, a number of recommendations are made regarding its production. The farmer, must engage in good agricultural practices and tidiness, to good sorting of the produce in order to avoid selling fungi infected ginger produce to the retailers or the consumers. To the middle men or retailer, proper storage its storage through refrigeration, sorting e.t.c. is recommended by this study. Consumers are encouraged to buy from reliable ginger selling outlets or farms. To protect consumers from the negative effects of mycotoxins from food in general, and ginger in particular, policies need to be put in place to ensure that producers are informed and monitored about appropriate prevention and cleaning methods. Standard methods also need to be provided to retailers about cleaning it before it gets displayed for sale.
7. Conclusion
Ginger (Zingiber officinale), having been referred to as a medicinal plant [5] is being used all over the world for several health purposes. It is consumed in a variety of ways, ranging from infusing and drinking the extracts to using it as spice. In 2013, according to the Food and Agriculture Organization of the United Nations, India had the highest percentage of ginger production, followed by China, Nepal, Indonesia and Nigeria. Considering the high consumption rate of ginger in many countries, public health awareness needs to be increased around the risk involved with ginger use when it is not properly cleaned before consumption. Without affecting the ginger industry, there needs to be greater public awareness about the need to wash it before working or consuming it, due to the possible presence of AFs and OTAs that can affect immunosuppression negatively affect the nervous system [34,35]. Ginger is an important part of food flavouring globally, with many people also using it for health purposes. The home ginger grower and retail consumer need to be made aware of the risks associated with using ginger, and any root plant, due to the presence of fungi and other organisms in the soil that can have health consequences if not managed effectively. Putting effective measures in place throughout the retail chain to reduce the levels of mycotoxins and the likelihood of consumers’ health being affected.
Funding
The study was fully supported and funded by the North West University, South Africa.
Conflict of interest
None.
Acknowledgements
The authors are highly indebted to professor Mwanza for his skilful assistance, supervision and other laboratory procedures in the course of this research work. We also appreciate the Professional English language editing service rendered by Carrin Martin as well as the effort of Dr Adeyemi Jerry (Department of Chemistry, North West University) for the professional drawing of the chemical structures used.
Contributor Information
Oluwadara Pelumi Omotayo, Email: alamuoluwadara@gmail.com.
Abiodun Olusola Omotayo, Email: 25301284@nwu.ac.za.
Olubukola Oluranti Babalola, Email: olubukola.babalola@nwu.ac.za.
Mulunda Mwanza, Email: Mulunda.mwanza@gmail.com.
References
- 1.Klarić M.S., Rašić D., Peraica M. Deleterious effects of mycotoxin combinations involving Ochratoxin A A. Toxins. 2013;5:1965–1987. doi: 10.3390/toxins5111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen J.K., Hu W., Wang Y.J., Yang M. Multi-mycotoxins analysis in ginger and related products by UHPLC-FLR detection and LC-MS/MS confirmation. Food Control. 2014;43:82–87. [Google Scholar]
- 3.Ashiq S. Natural occurrence of mycotoxins in food and feed: pakistan Perspective. Compr. Rev. Food Sci. Food Saf. 2015;14:159–175. doi: 10.1111/1541-4337.12122. [DOI] [PubMed] [Google Scholar]
- 4.Hafizu I.K., Isa A.B., Farouk T.S. Modelling the dynamics of toxicity associated with aflatoxins in foods and feeds. Toxicol. Rep. 2017;4:358–363. doi: 10.1016/j.toxrep.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sah P., Al-Tamimi B., Al-Nassri N., Al-Mamari R. Effect of temperature on antibiotic properties of garlic (Allium sativum L.) and ginger (Zingiber officinale Rosc.) Afr. J. Biotechnol. 2012;11:16192–16195. [Google Scholar]
- 6.Jarfarnejad S., Keshavarz S.A., Mahbubi S., Saremi S., Arab A., Abbasi S., Djafarian K. J. Funct. Foods. 2017;29:127–134. [Google Scholar]
- 7.Bellik Y. Total antioxidant activity and antimicrobial potency of the essential oil and oleoresin of Zingiber officinale Roscoe. Asian Pac. J. Trop. Dis. 2014;4:40–44. [Google Scholar]
- 8.Riaz H., Begum A., Raza S.A., Khan Z.M., Yousaf H., Tariq A. Antimicrobial property and phytochemical study of ginger found in local area of Punjab, Pakistan. Int. Curr. Pharm. J. 2015;4:405–409. [Google Scholar]
- 10.Heba N.G.E., Abdel R.A.M., Samira R.M. Toxic eff ;ect of Moringa peregrina seeds on histological and biochemical analyses of adult male Albino rats. Toxicol. Rep. 2018;5:38–45. doi: 10.1016/j.toxrep.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trucksess M.W., Weaver C.M., Oles C.J., Fry F.S., Jr, Noonan G.O., Betz J.M., Rader J.I. Determination of aflatoxins B1, B2, G1, and G2 and Ochratoxin A A in ginseng and ginger by multitoxin immunoaffinity column cleanup and liquid chromatographic quantitation: collaborative study. J. AOAC Int. 2008;91:511–529. [PMC free article] [PubMed] [Google Scholar]
- 12.Whitaker T.B., Trucksess M.W., Weaver C.M., Slate A. Sampling and analytical variability associated with the determination of aflatoxins and Ochratoxin A A in bulk lots of powdered ginger marketed in 1-lb bags. Anal. Bioanal. Chem. 2009;395:1291–1299. doi: 10.1007/s00216-009-2880-z. [DOI] [PubMed] [Google Scholar]
- 13.Kolf-Clauw M., Castellote J., Joly B., Bourges-Abella N., Raymond-Letron I., Pinton P., Oswald I.P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: histopathological analysis. Toxicol. Vitr. 2009;23:1580–1584. doi: 10.1016/j.tiv.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Alamu O.P., Adeeyo O.A., Babalola O.O. Effect of mangifera indica extracts on the testes of adult male wistar rats. J. Hum. Ecol. 2016;56 [Google Scholar]
- 15.Maryam J., Jinap S. Optimization and validation of a method for extraction and quantification of ochratoxin A in black pepper. Int. Food Res. J. 2015;22(2):502–509. [Google Scholar]
- 18.Olorunfemi O.D., Adesiji G.B., Oladipo F.O., Adisa R.S., Daudu A.K., Suweba G. Determinants of value addition initiatives use by fish farmers in Kwara and Kogi States, north central Nigeria. Sci. Technol. Arts Res. J. 2015;4:316–323. [Google Scholar]
- 19.Leandra N.Z., Livia D.P., Roice E.R., Tania P., Marlei J.A., Deisy M.S., Fernando S.R., Carlos A.F. Aflatoxin B1 residues in human livers and their relationship with markers of hepatic carcinogenesis in Sao paulo, Brazil. Toxicol. Rep. 2018;5:778–7845. doi: 10.1016/j.toxrep.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain A.I., Anwar F., Chatha S.A., Latif S., Sherazi S.T., Ahmad A., Worthington J., Sarker S.D. Chemical composition and bioactivity studies of the essential oils from two Thymus species from the Pakistani flora. LWT-Food Sci. Technol. 2012;50:185–192. [Google Scholar]
- 21.Blanchard D.J., Manderville R.A. An internal charge transfer-DNA platform for fluorescence sensing of divalent metal ions. Chem. Commun. 2016;52:9586–9588. doi: 10.1039/c6cc04613d. [DOI] [PubMed] [Google Scholar]
- 22.Fatma C., Hasan H.D. Effects of aflatoxin on liver and protective effectiveness of esterified glucomannan in merino rams. Sci. World J. 2012;5:1–5. doi: 10.1100/2012/462925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhat Z.F., Kumar P. Effect of skin, enrobing and refrigerated storage on the quality characteristics of chicken meat balls. J. Food Sci. Technol. 2013;50:890–899. doi: 10.1007/s13197-011-0414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atanda O., Ogunrinu M., Olorunfemi F. A neutral red desiccated coconut agar for rapid detection of aflatoxigenic fungi and visual determination of aflatoxins. World Mycotoxin J. 2011;4:147–155. [Google Scholar]
- 25.D.R. Henke, S.I Thompson, Tool handle and method for making same. Google Patents. 9610679(2014).
- 26.Björn L.O., Bengtson S.A., Li S., Hecker C., Ullah S., Roos A., Nilsson A.M. Thermal emissivity of avian eggshells. J. Therm. Biol. 2016;57:1–5. doi: 10.1016/j.jtherbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Adegoke G.O., Letuma P. Mycotoxin and Food Safety in Developing Countries. 1st ed. InTech; Croatia: 2013. Strategies for the prevention and reduction of mycotoxins in developing countries; pp. 123–136. [Links] [Google Scholar]
- 28.Zain M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011;15:129–144. [Google Scholar]
- 29.Milanović Z., Pantelić S., Trajković N., Sporiš G., Kostić R., James N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging. 2013;8:549–556. doi: 10.2147/CIA.S44112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishaque K., Salam Z., Taheri H. Simple, fast and accurate two-diode model for photovoltaic modules. Sol. Energy Mater. Sol. Cells. 2011;95:586–594. [Google Scholar]
- 31.Han Z., Zheng Y., Luan L., Ren Y., Wu Y. Analysis of Ochratoxin A and Ochratoxin B in traditional Chinese medicines by ultra-high-performance liquid chromatography–tandem mass spectrometry using [13 C 20]-Ochratoxin A A as an internal standard. J. Chromatogr. A. 2010;1217:4365–4374. doi: 10.1016/j.chroma.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 32.Trucksess M.W., Koeltzow D.E. Evaluation and application of immunochemical methods for mycotoxins in food. ACS Publications. 1995;5:326–334. [Google Scholar]
- 33.Tian Y., Liu N., Liao Y., Sun C., Wang S., Wu A. Functional agents to biologically control deoxynivalenol contamination in cereal grains. Front. Microbiol. 2016;7:395. doi: 10.3389/fmicb.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed S., El-Shayeb N., Hashem A., Saleh S., Abdel-Fattah A. Biochemical studies on immobilized fungal β-glucosidase. Br. J. Chem. Eng. 2013;30:747–758. [Google Scholar]
- 35.Chilaka C.A., De Kock S., Phoku J.Z., Mwanza M., Egbuta M.A., Dutton M.F. Fungal and mycotoxin contamination of South African commercial maize. J. Food Agric. Environ. 2012;10:296–303. [Google Scholar]
- 42.Faucet-Marquis V., Pont F., Størmer F.C., Rizk T., Castegnaro M., Pfohl-Leszkowicz A. Evidence of a new dechlorinated Ochratoxin A A derivative formed in opossum kidney cell cultures after pretreatment by modulators of glutathione pathways: correlation with DNA-adduct formation. Mol. Nutr. Food Res. 2006;50:530–542. doi: 10.1002/mnfr.200500219. [DOI] [PubMed] [Google Scholar]
- 45.Ying-Chih L., Liang L., Alena V.M., Peter M.B., Michael P.S., Stephen L.R. Molecular basis of Aflatoxin-induced mutagenesis—role of the Aflatoxin B1-formamidopyrimidine adduct. Carcinogenesis. 2014;35:1461–1468. doi: 10.1093/carcin/bgu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laura E.S., Andrew J.P., Paul C.T., Jean H.H., Rebecca J.S. Aflatoxin exposure during pregnancy, maternal Anemia, and adverse birth outcomes. Am. J. Trop. Med. Hyg. 2017;96:770–776. doi: 10.4269/ajtmh.16-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pierron A., Imourana A.K., Isabelle P.O. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2017;2:63–68. doi: 10.1016/j.aninu.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao X., Hong-Gui Q., Xue-Mei Y., Yan-Yan W., Lu-Nan Q., Liang M., Bang-De X., Jian Hong Z., Le-Qun L. Expression of P62 in hepatocellular carcinoma involving hepatitis B virus infection and aflatoxin B1 exposure. Cancer Med. 2018;6:2357–2369. doi: 10.1002/cam4.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Modupeade C.A., Ogechi P.A., Ngozi P.A., Olusegun O.A., Mulunda M. Microbiological quality and risk assessment for aflatoxins in Groundnuts and roasted cashew nuts meant for human consumption. J. Toxicol. 2018;13:81–88. doi: 10.1155/2018/1308748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghada S.R.A., Ahmed M.A., Ferial A.I., Sherif R.M. Fungi and aflatoxins associated with wheat grains in Gaza governorates. Afr. J. Microbiol. Res. 2015;9:2275–2282. [Google Scholar]
- 51.Maryam J. Natural occurrence of aflatoxins contamination in commercial spices in Iran. Iran. J. Health, Saf. Environ. 2016;3:513–517. [Google Scholar]
- 52.Nikhil P.G., Sharad M. Analysis of aflatoxins B1, B2, G1 and G2 in peanuts: validation study. Anal. Chem. 2018;17:2. [Google Scholar]
- 53.Osman M.A., Salama A.N., Abdel-Wahhab M.A., Sherif S.R. Fungi and mycotoxins associated with Egyptian sorghum grains. Moj Toxicol. 2017;3:51–56. [Google Scholar]
- 54.Hyun J.L., Dojin R. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: public health perspectives of their Co-occurrence. J. Agric. Food Chem. 2017;65:7034–7051. doi: 10.1021/acs.jafc.6b04847. [DOI] [PubMed] [Google Scholar]
- 55.Abdulmumin A.N. Occurrence, harmful effects and analytical determination of ochratoxin a in coffee. J. Appl. Pharm. Sci. 2015;5:2231–3354. [Google Scholar]
- 56.Leonardo F.M., Ana Lucia S.M.F., Lucas C.R.M., Tassia C.P., Eliete S.B., Elisa Y.H. Aflatoxins and ochratoxin A in different cocoa clones (Theobroma cacao L.) developed in the southern region of Bahia, Brazil. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess. 2018;35:134–143. doi: 10.1080/19440049.2017.1397293. [DOI] [PubMed] [Google Scholar]
- 57.Amal S.H., Eman M.S., Weam S., Mona M.T., Heba M.K.S., Salwa F.H., Nevein N.F., Karima A.E. Comparative hepatotoxicity of aflatoxin B1 among workers exposed to different organic dust with emphasis on polymorphism role of glutathione S-Transferase gene. Open Acc. Maced. J. Med. Sci. 2016;4:312–318. doi: 10.3889/oamjms.2016.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Theumer M.G., Henneb Y., Khoury L., Sninis S.P., Tadrist C., Canlet O., Pueli P., Oswald M.A. Genotoxicity of aflatoxins and their precursors in human cells. Toxicol. Lett. 2018;287:100–107. doi: 10.1016/j.toxlet.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Marie-Christin S., Luise S., Ulrike R., Hans-Ulrich H., Michael G., Gerald S. Synergistic action of the nephrotoxic mycotoxins ochratoxin A and citrinin at nanomolar concentrations in human proximal tubule-derived cells. Toxicol. Lett. 2018;291:149–157. doi: 10.1016/j.toxlet.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Eriko T., Atsunori Y., Masahiro N., Shim M., Fumiyuki N.M., Makoto S. Ochratoxin A induces karyomegaly and cell cycle aberrations in renal tubular cells without relation to induction of oxidative stress responses in rats. Toxicol. Lett. 2014;224:64–72. doi: 10.1016/j.toxlet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Taghizadeh S.F., Rezaee R., Davarynejad G., Asili J., Nemati S.H., Goumenou M., Tsakiris I., Tsatsakis A.M., Shirani K., Karimi G. Risk assessment of exposure to aflatoxin B1 and ochratoxin A through consumption of different Pistachio (Pistacia vera L.) cultivars collected from four geographical regions of Iran. Environ. Toxicol. Pharmacol. 2018;61:61–66. doi: 10.1016/j.etap.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Tsakiris I.N., Tzatzarakis M.N., Alegakis A.K., Vlachou M.I., Renieri E.A., Tsatsakis A.M. Risk assessment scenarios of children’s exposure to aflatoxin M1 residues in different milk types from the Greek market. Food Chem. Toxicol. 2013;56:261–265. doi: 10.1016/j.fct.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 63.Omotayo O.P., Omotayo A.O., Mwanza M., Babalola O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019;35:1–7. doi: 10.5487/TR.2019.35.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Razgonova M.P., Veselov V.V., Zakharenko A.M., Golokhvast K.S., Nosyrev A.E., Cravotto G., Tsatsakis A.M., Spandidos D.A. Panax ginseng components and the pathogenesis of Alzheimer’s disease (Review) Mol. Med. Rep. 2019;19:2975–2998. doi: 10.3892/mmr.2019.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Razgonova M.P., Kalenik T.K., Veselov V.V., Zakharenko A.M., Taghizadehghalehjoughi Ali, Vita V., Barbuceanu F., Izotov B.N., Stratidakis A.K., Tsatsakis A.M., Golokhvast K.S. Supercritical green technologies for obtaining ginsenosides from Far-Eastern wild Panax ginseng C.A. Meyer using SFE for applying in drug, food and cosmetic industries. Farmacia. 2019;67:81–91. [Google Scholar]
- 66.Docea A.O., Vassilopoulou L., Fragou D., Arsene A.L., Fenga C., Kovatsi L., Petrakis D., Nosyrev V.N.A.E., Izotov B.N., Golokhvast K.S., Zakharenko A.M., Vakis A., Tsitsimpikou C., Drakoulis N. Cyp polymorphisms and pathological conditions related to chronic exposure to organochlorine pesticides. Toxicol. Rep. 2017;4:335–341. doi: 10.1016/j.toxrep.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


