Abstract
Cardiotoxic effects from cancer therapy are a major cause of morbidity during cancer treatment. Unexpected toxicity can occur during treatment and/or after completion of therapy, into the time of cancer survivorship. While older drugs such as anthracyclines have well-known cardiotoxic effects, newer drugs such as tyrosine kinase inhibitors, proteasome inhibitors, and immunotherapies also can cause diverse cardiovascular and metabolic complications. Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) are increasingly being used as instruments for disease modelling, drug discovery, and mechanistic toxicity studies. Promising results with hiPSC-CM chemotherapy studies are raising hopes for improving cancer therapies through personalized medicine and safer drug development. Here, we review the cardiotoxicity profiles of common chemotherapeutic agents as well as efforts to model them in vitro using hiPSC-CMs.
Keywords: Cardio-oncology , Chemotherapy , Induced pluripotent stem cells , Genomics , Precision medicine
Introduction
Cancer continues to be a leading cause of morbidity and mortality. In the USA, it is projected that ∼40% of the population will eventually be diagnosed with cancer, and that 20% of the population will die from cancer.1 These grim statistics have engendered a massive scientific effort seeking to improve the diagnosis, treatment, and prevention of various types of cancers, including the pursuit of therapies tailored to individual patients that can both maximize therapeutic benefits and minimize adverse effects.
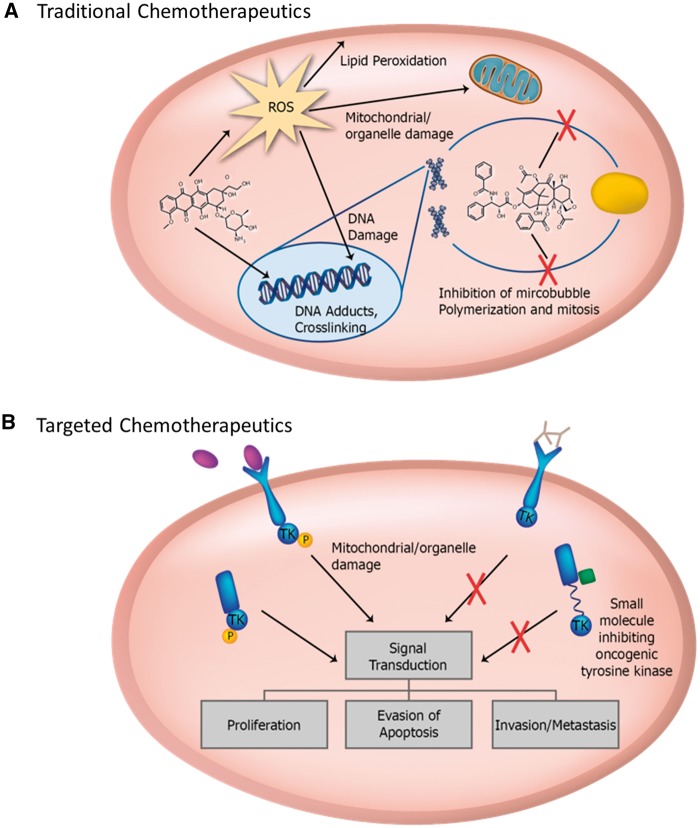
While many of the approved drugs are effective cancer treatments, unpredictable subsets of patients persistently experience deleterious side effects. Cardiotoxicity has been observed for decades in traditional chemotherapeutics such as anthracyclines and radiation therapy (Figure 1).2–5 More recently, targeted chemotherapeutics such as kinase inhibitors (KIs), which include antibody-based drugs like trastuzumab and small molecule inhibitors,3,6 have been developed to more precisely target malignant cells. Nevertheless, cardiovascular toxicities continue to be reported even with these drugs.
Figure 1.
(A) Traditional chemotherapeutics non-specifically target malignant cells, causing impairment of mitotic processes such as microtubule formation, cellular damage, or direct DNA damage and ROS generation. (B) Targeted cancer therapies target pathways or proteins unique to cancer cells or essential for their survival. ROS, reactive oxygen species.
The advent of human induced pluripotent stem cells (hiPSCs)7 has opened the door to investigating fundamental biomedical questions using human patient cell models instead of animal models. This allows experiments to directly compare the responses of clinically affected patients with unaffected patients as well as mechanistic studies of drug toxicities using renewable sources of human cells. This review discusses the major classes of chemotherapeutics and their mechanisms of action and toxicity and provides an update on current hiPSC-based investigations of cardiovascular toxicity.
Traditional chemotherapeutics
Anthracyclines
The anthracyclines, which include the drug doxorubicin (DOX), are one of the most effective classes of chemotherapeutics. However, DOX is associated with cardiotoxicity, which may not emerge for years or even decades following therapy, bringing significant implications for survivors of childhood cancers.8 Chronic toxicity has an overall incidence of 9%, and while 71% of patients experienced partial recovery of left ventricular ejection fraction (LVEF), only 11% experienced full recovery.9
Anthracyclines form DNA adducts and generate reactive oxygen species (ROS), resulting in DNA damage, mitochondrial damage, and lipid membrane peroxidation.10 It is now known that topoisomerase IIß (TOP2ß) is a major player in the cardiotoxic effects of anthracyclines. Deletion of TOP2ß in mice prevented the development of heart failure, as well as many of the cellular changes that have been implicated in toxicity, including ROS formation and double-strand DNA breaks.11 Studies in DOX cardiotoxicity are ongoing and will likely reveal additional mechanisms of toxicity.
Cardiotoxicity is also associated with other traditional chemotherapeutics, albeit to a significantly lesser extent than anthracyclines. As these reactions are rare and the mechanisms unclear, they will not be covered in this review.
Modelling toxicity of anthracyclines with human induced pluripotent stem cell-derived cardiomyocytes
The development of hiPSCs has opened new avenues of research and of developing experimental models. Human induced pluripotent stem cells permit the use of human cells that can be passaged indefinitely in vitro and scaled up exponentially, allowing the study of unique individual patients without reliance on isogenic strains of animals. Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) have been particularly useful for cardiovascular research because the alternative options require invasive biopsy of human cardiac tissue samples, which also cannot be maintained in prolonged culture. In addition to overcoming these limitations, hiPSC-CMs can recapitulate the physiology of in vivo cardiomyocytes, express most cardiac-specific ion channels and currents, and have a functional contractile apparatus. They also can be characterized into atrial, nodal, and ventricular subtypes through patch-clamp analysis of action potential morphologies.12
However, there are some limitations to the use of hiPSC-CMs. For instance, they are known to be immature, more akin to foetal cardiomyocytes in structure, electrophysiology, gene programs, and metabolism than adult cardiomyocytes.13,14 They have disorganized sarcomeric structures and the cells lack T-tubules.15,16 The differences between the in vivo environment and in vitro culture conditions make it unlikely the cells will ever fully resemble adult cardiomyocytes, which is a serious limitation of these cells. These shortcomings can complicate the interpretation of drug responses as well as making it difficult to predict the translational impact in mature cells. Much work remains to be done in discovering the factors and cellular programs that lead to mature phenotypes. New advancements in 3D culture and organ-on-a-chip technologies may provide additional avenues to overcome these limitations by generating more physiologic experimental conditions and promoting maturation of the cells. Additionally, these approaches allow the integration of cardiac fibroblasts and endothelial cells that form capillary-like networks into the model.17 Such a 3D model was used to test DOX drug-toxicity and was found to exhibit greater resistance to the drug compared to 2D cultures, generating physiologic responses such as changes in beating rate similar to those of living organs.18
In addition, investigators are expanding the application of hiPSCs to model chemotherapy-mediated cardiotoxicity. A recent study found that hiPSC-CMs derived from individuals who experienced clinical DOX cardiotoxicity showed increased sensitivity to DOX in vitro compared to hiPSC-CMs derived from patients who experienced no cardiotoxicity following DOX therapy.12 The documented abnormalities included sarcomeric disarray, increases in caspase 3/7 activity, increased ROS, and changes in calcium handling. Another study by Holmgren et al.19 found dose-dependent toxicity in hiPSC-CMs, including contractile dysfunction, troponin T release, and increased expression of genes related to senescence, stress, and DNA damage repair. Modelling of other agents such as alkylating agents, platinums, and anti-microtubule agents has been more limited.20 Given the lack of mechanistic insight into the cardiotoxicity of these other traditional drugs, hiPSCs are an attractive tool to study their action on individual patient and tissue levels.
Targeted chemotherapeutics
Kinase inhibitors
Early clinical successes with trastuzumab (Herceptin),21 a monoclonal antibody efficacious against a subset of breast cancers, and imatinib (Gleevec), the first FDA-approved small molecule tyrosine kinase inhibitor (TKI) for the treatment of chronic myelogenous leukaemia (CML) and gastrointestinal stromal tumour (GIST),22,23 have spurred the development of new ‘targeted’ approaches to cancer therapy. Trastuzumab targets human epidermal growth factor receptor 2 (HER2), a tyrosine kinase receptor and oncogene overexpressed in approximately 30% of breast cancers.21 Tyrosine kinase inhibitors have been developed against vascular endothelial growth factor receptors (VEGFR1, VEGFR2, and VEGFR3), platelet-derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR), mammalian target of rapamycin (mTOR), and serine/threonine protein kinase B-raf (BRAF), as well as other kinases.24
Human epidermal growth factor receptor 2 inhibitors
Trastuzumab cardiotoxicity has been well-documented, typically resulting in subclinical impairments in LVEF, but occasionally leading to clinical congestive heart failure (CHF).21 A retrospective analysis of breast cancer patients found that trastuzumab was associated with a four-fold increased risk of cardiomyopathy; however, when it is combined with an anthracycline, the risk is increased seven-fold compared to women who never received chemotherapy.25 For about 75% of treated patients, these effects are reversible upon discontinuation of the drug, but cardiomyopathy persists in other patients.21
Clues to the toxic effects have been recently identified in vitro using hiPSC-CMs. When treated with trastuzumab, hiPSC-CMs demonstrated alterations in metabolism, including down-regulation of glucose uptake and mitochondrial genes, and up-regulation of pyruvate dehydrogenase kinase 4 (PDK4), an inhibitor of glycolysis.26 Other studies using primary human cardiomyocytes suggest the toxicity of trastuzumab may be caused by impaired pro-survival signalling downstream of HER2, leading to collateral impairment of HER3 and HER4, and ultimately interfering with neuregulin (NRG) signalling. In a mechanistic study of trastuzumab toxicity, Eldridge et al. demonstrated that hiPSC-CMs express three of the four HER receptors (HER1, HER2, and HER4), and were able to reproduce earlier findings that NRG1 activates ErbB signalling in a protective manner against anthracycline and trastuzumab toxicity. This finding underscores the importance of using human cells, as trastuzumab is specific to the human HER2 receptor with no cross-reactivity to murine receptors,27 which significantly limits the usefulness of mice in antibody toxicity studies. The attenuation of the toxic effects through NRG1-mediated signalling suggests a prophylactic target that can be used in patients. Consequences of blocking this pathway include the accumulation of intracellular ROS, activation of pro-apoptotic proteins, and mitochondrial dysfunction.28 The blockade of cell-survival pathways and activation of pro-apoptotic pathways exacerbate concomitant anthracycline toxicity,10 leading to the development of CHF in 27% of patients.21
Small molecule tyrosine kinase and vascular endothelial growth factor pathway inhibitors
Several studies have found an increased risk of CHF with TKIs targeting the VEGF receptor kinase pathway.29 For example, up to 28% of patients receiving sunitinib experience >10% decrease in LVEF, and 8% of patients can develop clinical CHF.30 These studies likely underestimate the true incidence of myocardial dysfunction associated with these drugs due to factors such as ambiguous signs of CHF that occur commonly in cancer (such as peripheral oedema and fatigue), the exclusion of patients with pre-existing heart issues in oncology clinical trials, and the lack of a standard definition of cardiomyopathy.6 A summary of known cardiotoxic effects and associated drugs is found in Table 1. Interestingly, TKI-associated vascular toxicity may be more clinically significant than the direct cardiac effects, resulting in systemic and pulmonary arterial hypertension, cerebrovascular accident, peripheral arterial disease, and thrombo-embolic events. These effects have been extensively reviewed elsewhere and are not covered in detail in this review.31,32
Table 1.
Cardiotoxic effects of targeted therapies
| Target | Drugs | Class | Cardiovascular effects |
|---|---|---|---|
| VEGF receptors | Sunitinib, Sorafenib, Pazopanib, Axitinib, Regorafenib, Vendatinib, Lenvatinib, Cabozantinib | Small molecule TKIs | Cardiomyopathy, hypertension, vascular events |
| VEGF | Bevacizumab | Monoclonal antibody | Cardiomyopathy, hypertension, vascular events |
| VEGF | Aflibercept | Recombinant VEGFR domains (VEGF trap) | Cardiomyopathy |
| HER2 | Trastuzumab | Monoclonal antibody | Cardiomyopathy |
| Bruton’s kinase | Ibrutinib | Small molecule TKI | Arrhythmias |
| MEK | Trametinib, Cobimetinib | Small molecule TKIs | Cardiomyopathy |
| Proteasome | Carfilzomib | Proteasome inhibibtor | Cardiomyopathy, arrhythmias, hypertension, vascular events |
| CTLA-4 | Ipilmumab | Immune checkpoint blocker | Myocarditis, Takotsubo cardiomyopathy |
| PD-1 | Nivolumab, Pembrolizumab | Monoclonal antibody | Myocarditis, arrhythmias |
CHF, congestive heart failure; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; HER2, human epidermal growth factor receptor 2; MEK, mitogen-activated protein kinase kinase; PD, platelet-derived; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
The mechanisms of toxicity for VEGF inhibitors are less clear as many of the drugs have targets other than the VEGF pathway. However, there is evidence to suggest that a common mechanism of cardiac dysfunction may be the activation of hypoxia inducible factor-1-alpha (HIF-1α). Targeting the VEGF pathways results in decreased capillary density, inducing myocardial hypoxia and HIF-1α stability and activation.6 Conditional HIF-1α overexpression in mice leads to a reversible cardiomyopathy characterized by impaired calcium handling and ventricular dysfunction, as well as histologic and mitochondrial changes that appear similar to changes seen in TKI-induced cardiomyopathy.33
Recently, Sharma et al.34 demonstrated the potential utility of hiPSC-CMs in a high-throughput manner to screen for TKI cardiotoxicity, which may pave the way for future studies on TKIs using similar approaches. Although the mechanisms of toxicity were not specifically studied, the authors screened 21 TKIs and DOX. From this initial screening, seven compounds were found to exhibit significant in vitro toxicity. These compounds included the VEGF pathway inhibitors sorafenib, regorafenib, nilotinib, and vandetanib. Of these compounds, both nilotinib and vandetanib have FDA black-box warnings associated with cardiotoxicity. Other studies have also included TKIs in hiPSC-CM-based toxicity screening,35,36 but these studies were not aiming to directly study the mechanisms of their toxicity.
Proteasome inhibitors
Proteasome inhibitors are used commonly in the treatment of multiple myeloma, but also have associated cardiovascular toxicity.31 Proteasome inhibitors include bortezomib, carfilzomib, and ixazomib, which target the ubiquitin-proteasome degradation system, a critical cell maintenance pathway that allows malignant plasma cells to degrade misfolded immunoglobulins. While bortezomib has been clinically found to have minimal cardiovascular toxicity,37 carfilzomib has been found to be associated with a number of cardiovascular issues such as CHF, hypertension, and thrombo-embolic events.38 The mechanisms behind the toxicity are unclear; the vascular effects of carfilzomib suggest that the vascular toxicity may precede myocardial damage.38 Experiments have found dose-dependent decreases in hiPSC-CM contractility following exposure to either bortezomib and carfilzomib. Even after removal of the agents, contractility continued to decrease before recovering after several days.39
Immunotherapies
Immunotherapies, which unleash the immune system to attack cancer cells, have been revolutionary and particularly effective in certain cancer types. They include immune checkpoint blockers or adoptive cell transfer (ACT), both of which have been associated with immune-mediated myocarditis. Immune checkpoint blockers are monoclonal antibodies against cell-surface antigens that prevent targeting by the host immune system.40 Adoptive cell transfer utilizes autologous T-cells that express T-cell receptors (TCR) specific for tumour-associated antigens.41 Antibodies against two checkpoints, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and PD-1, have been associated with myocarditis, electrical conduction abnormalities, and ventricular dysfunction.42 The effects of these drugs seem to be exacerbated when used in combination, resulting in increased risk of life-threatening immune myocarditis.43
Similar reactions have occurred during ACT when T-cells expressing a TCR specific for melanoma-associated antigen 3 (MAGE-3) or melanoma antigen recognized by T-cells (MART-1).42 These antigens are typically restricted to germline and melanoma cells, but the first two patients treated with MAGE-3-targeting T-cells died after developing cardiogenic shock and myocardial damage similar to that seen in graft-vs.-host disease.44 Melanoma-associated antigen 3 has subsequently been shown to have cross-reactivity with a peptide derived from the cardiac protein titin.45 Given that hiPSC-CMs express many of the proteins found in in situ cardiomyocytes, they present an interesting if as yet unexplored option for screening many of these immune-based therapies.
Toxicity screening—a road to personalized medicine?
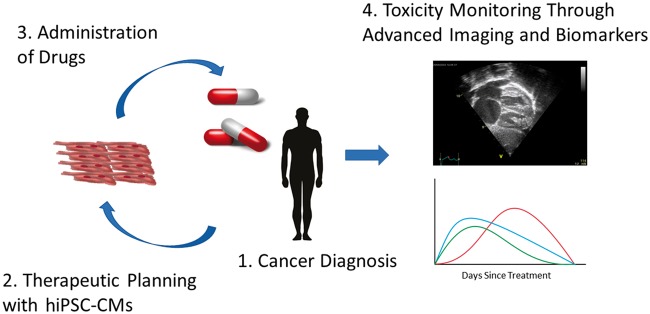
Much like anthracyclines, there has been great excitement for modelling TKI-induced cardiotoxicity using hiPSC-CMs. A future application of hiPSC-CMs may be their usefulness in predicting cardiotoxicity. A preliminary demonstration of this concept was achieved by Burridge et al.12 in their work with DOX-treated breast-cancer patients. To our knowledge, no additional investigations have been conducted utilizing chemotherapeutic agents in a patient-specific manner, though other studies have demonstrated the impact of patient variability in drug toxicities. For example, drug screening studies have shown an increased sensitivity of hiPSC-CMs derived from patients with DCM, HCM, or long QT syndrome to cisapride,46 a drug which was notably removed from the US market following numerous reports of sudden cardiac death.47 Similar studies on long QT patient-derived cells have shown an increased sensitivity to certain drugs compared control hiPSC-CM lines.48 Although much work remains to be done to validate the pre-clinical application of hiPSC-CMs, preliminary results are encouraging and may eventually change the way drugs are screened for population and individual use. The current approach to mitigating toxicity begins with the identification of known risk factors and co-morbidities combined with regular screening of patients through echocardiography, ECG, angiography, and serum biomarkers.49 It is in this area that hiPSC-CMs may prove the most useful by allowing a personalized medicine approach to pre-screen a patient’s own cardiomyocytes in ‘toxicity trials’ before taking a potentially cardiotoxic drug (Figure 2).
Figure 2.
Future chemotherapy protocols may involve a combination of rational drug selection based on human induced pluripotent stem cell-derived cardiomyocyte (hiPSC-CM) screening as well as monitoring for the earliest signs of toxicity through advanced imaging techniques and cardiac biomarkers.
Employing hiPSC-CMs creatively raises hopes of dramatically improving drug development by accurately assessing the potential cardiotoxicity of drugs early in the process, before they enter into the much more costly phases of pre-clinical and clinical trials.16 Aside from saving money and resources, significant patient morbidity and mortality can be prevented by reducing unnecessary clinical trials, and rare toxic reactions affecting patients can be caught before entering the market (Figure 3).
Figure 3.
For drug development, human induced pluripotent stem cell-derived cells or tissues can be used to screen for uncommon or rare toxic reactions before beginning clinical testing. Unsafe drug candidates can be removed from the testing pool instead of progressing to clinical trials.
Conclusion
Chemotherapy has a reputation of being difficult to endure, sometimes carrying lasting effects even after the cancer has gone into remission. Although clinical medicine has come a long way in improving treatment protocols, adverse reactions still occur unpredictably. Cardiovascular toxicity can be particularly damaging by causing permanent dysfunction, CHF, and sudden death. The advent of hiPSCs presents an unprecedented opportunity to improve our approach to cancer therapy on three fronts: a better understanding of the mechanisms of cardiotoxicity and how to mitigate them, the development of personalized medicine based on patients’ own cells and tissues, and a potentially revolutionary transformation in the way drugs are developed and brought to the clinic. However, major limitations to the use of hiPSC-CMs exist, particularly issues related to their immature phenotype. Nevertheless, these cells have already proven to be useful predictors of toxicity, showing dysfunction in the face of known cardiotoxins and correctly identifying them from screening libraries.
Acknowledgements
The authors gratefully acknowledge Blake Wu for critical reading of the manuscript and Amy Thomas for preparing the illustrations. Because of space constraints, the authors apologize in advance for not including all of the relevant citations on the subject matter.
Funding
American Heart Association (AHA) 17MERIT33610009, Burroughs Wellcome Fund 1015009, National Institutes of Health (NIH) R01 HL113006, NIH R01 HL123968, NIH R01 HL132875 and NIH R01 HL126527 (J.C.W.), NIH T32 OD011121 (J.P.S), NIH K01 HL135455, Stanford Translational Research and Applied Medicine (TRAM) scholar grant and AHA 13SDG17340025 (N.S.), in part.
Conflict of interest: J.M. has served as a consultant for Pfizer, Novartis, Bristol Myers Squibb, Regeneron, Takeda and Daiichi Sankyo. He has also received research funding from Pfizer and Bristol Myers Squibb. The remaining authors declare no conflicts of interest.
References
- 1. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2014, Bethesda, MD: National Cancer Institute. https://seer.cancer.gov/csr/1975_2014 (5 January 2018). [Google Scholar]
- 2. Rosa GM, Gigli L, Tagliasacchi MI, Di Iorio C, Carbone F, Nencioni A, Montecucco F, Brunelli C.. Update on cardiotoxicity of anti-cancer treatments. Eur J Clin Invest 2016;46:264–284. [DOI] [PubMed] [Google Scholar]
- 3. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 2016;375:1457–1467. 10.1056/NEJMra1100265 [DOI] [PubMed] [Google Scholar]
- 4. Chang H-M, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH.. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: Part 1. J Am Coll Cardiol 2017;70:2536–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang H-M, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH.. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: Part 2. J Am Coll Cardiol 2017;70:2552–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ky B, Vejpongsa P, Yeh ETH, Force T, Moslehi JJ.. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res 2013;113:754–764. 10.1161/CIRCRESAHA.113.300218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S.. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- 8. Von Hoff DD, Rozencweig M, Layard M, Slavik M, Muggia FM.. Daunomycin-induced cardiotoxicity in children and adults: a review of 110 cases. Am J Med 1977;62:200–208. [DOI] [PubMed] [Google Scholar]
- 9. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 10. Hahn VS, Lenihan DJ, Ky B.. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc 2014;3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang S, Liu X, Bawa-Khalfe T, Lu L-S, Lyu YL, Liu LF, Yeh ETH.. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012;18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 12. Burridge PW, Li YF, Matsa E, Wu H, Ong S-G, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC.. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 2016;22:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lundy SD, Zhu W-Z, Regnier M, Laflamme MA.. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev 2013;22:1991–2002. 10.1089/scd.2012.0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao F, Wagner RA, Wilson KD, Xie X, Fu J-D, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, Robbins RC, Wu JC, Csete M.. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One 2008;3:e3474.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karakikes I, Ameen M, Termglinchan V, Wu JC.. Human induced pluripotent stem cell-derived cardiomyocytes: Insights into molecular, cellular, and functional phenotypes. Circ Res 2015;117:80–88. 10.1161/CIRCRESAHA.117.305365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sayed N, Liu C, Wu JC.. Translation of human-induced pluripotent stem cells. J Am Coll Cardiol 2016;67:2161–2176. 10.1016/j.jacc.2016.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eder A, Vollert I, Hansen A, Eschenhagen T.. Human engineered heart tissue as a model system for drug testing. Adv Drug Deliv Rev 2016;96:214–224. 10.1016/j.addr.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 18. Amano Y, Nishiguchi A, Matsusaki M, Iseoka H, Miyagawa S, Sawa Y, Seo M, Yamaguchi T, Akashi M.. Development of vascularized iPSC derived 3D-cardiomyocyte tissues by filtration layer-by-layer technique and their application for pharmaceutical assays. Acta Biomater 2016;33:110–121. [DOI] [PubMed] [Google Scholar]
- 19. Holmgren G, Synnergren J, Bogestål Y, Améen C, Åkesson K, Holmgren S, Lindahl A, Sartipy P.. Identification of novel biomarkers for doxorubicin-induced toxicity in human cardiomyocytes derived from pluripotent stem cells. Toxicology 2015;328:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sirenko O, Crittenden C, Callamaras N, Hesley J, Chen Y-W, Funes C, Rusyn I, Anson B, Cromwell EF.. Multiparameter in vitro assessment of compound effects on cardiomyocyte physiology using iPSC cells. J Biomol Screen 2013;18:39–53. [DOI] [PubMed] [Google Scholar]
- 21. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L.. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 22. Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL.. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344:1031–1037. [DOI] [PubMed] [Google Scholar]
- 23. Demetri GD, Mehren M, von Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CDM, Joensuu H.. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–480. [DOI] [PubMed] [Google Scholar]
- 24. Wu P, Nielsen TE, Clausen MH.. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci 2015;36:422–439. 10.1016/j.tips.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 25. Bowles EJA, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KAB, Davis RL, Habel LA, Yood MU, Mccarty C, Magid DJ, Wagner EH.. Pharmacovigilance Study Team. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. JNCI J Natl Cancer Inst 2012;104:1293–1305. 10.1093/jnci/djs317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Necela BM, Axenfeld BC, Serie DJ, Kachergus JM, Perez EA, Thompson EA, Norton N.. The antineoplastic drug, trastuzumab, dysregulates metabolism in iPSC-derived cardiomyocytes. Clin Transl Med 2017;6:5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pegram M, Ngo D.. Application and potential limitations of animal models utilized in the development of trastuzumab (Herceptin®): a case study. Adv Drug Deliv Rev 2006;58:723–734. [DOI] [PubMed] [Google Scholar]
- 28. Jie B, Zhang X, Wu X, Xin Y, Liu Y, Guo Y.. Neuregulin-1 suppresses cardiomyocyte apoptosis by activating PI3K/Akt and inhibiting mitochondrial permeability transition pore. Mol Cell Biochem 2012;370:35–43. 10.1007/s11010-012-1395-7 [DOI] [PubMed] [Google Scholar]
- 29. Richards CJ, Je Y, Schutz FAB, Heng DYC, Dallabrida SM, Moslehi JJ, Choueiri TK.. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol 2011;29:3450–3456. [DOI] [PubMed] [Google Scholar]
- 30. Chu TF, Rupnick M. A, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan J. A, Harris DM, Ismail NS, Chen J-H, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH.. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370:2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li W, Garcia D, Cornell RF, Gailani D, Laubach J, Maglio ME, Richardson PG, Moslehi J, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J.. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol 2015;66:1160–1178. [DOI] [PubMed] [Google Scholar]
- 32. Moslehi JJ, Deininger M.. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol 2015;33:4210–4218. 10.1200/JCO.2015.62.4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moslehi J, Minamishima YA, Shi J, Neuberg D, Charytan DM, Padera RF, Signoretti S, Liao R, Kaelin WG.. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation 2010;122:1004–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmström A, Matsa E, Zhang Y, Kumar A, Fan AC, Álamo JC. D, Wu SM, Moslehi JJ, Mercola M, Wu JC.. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med 2017;9:eaaf2584.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, Abilez OJ, Hu S, Ebert AD, Navarrete EG, Simmons CS, Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley EA, Bers DM, Robbins RC, Longaker MT, Wu JC.. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013;12:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Devalla HD, Schwach V, Ford JW, Milnes JT, El-Haou S, Jackson C, Gkatzis K, Elliott DA, Chuva de Sousa Lopes SM, Mummery CL, Verkerk AO, Passier R.. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol Med 2015;7:394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laubach JP, Moslehi JJ, Francis SA, San Miguel JF, Sonneveld P, Orlowski RZ, Moreau P, Rosiñol L, Faber EA, Voorhees P, Mateos M-V, Marquez L, Feng H, Desai A, Velde H. v.d, Elliott J, Shi H, Dow E, Jobanputra N, Esseltine D-L, Niculescu L, Anderson KC, Lonial S, Richardson PG. A retrospective analysis of 3954 patients in phase 2/3 trials of bortezomib for the treatment of multiple myeloma: towards providing a benchmark for the cardiac safety profile of proteasome inhibition in multiple myeloma. Br J Haematol 2017;178:547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li W, Garcia D, Cornell RF, Gailani D, Laubach J, Maglio ME, Richardson PG, Moslehi J.. Cardiovascular and thrombotic complications of novel multiple myeloma therapies. JAMA Oncol 2016; 29:1934–1939. [DOI] [PubMed] [Google Scholar]
- 39. Judge LM, Perez-Bermejo JA, Truong A, Ribeiro AJ, Yoo JC, Jensen CL, Mandegar MA, Huebsch N, Kaake RM, So P-L, Srivastava D, Pruitt BL, Krogan NJ, Conklin BR.. A BAG3 chaperone complex maintains cardiomyocyte function during proteotoxic stress. JCI Insight 2017;2:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Postow MA, Callahan MK, Wolchok JD.. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–1982. 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosenberg SA, Restifo NP.. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62–68. 10.1126/science.aaa4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang DY, Okoye GD, Neilan TG, Johnson DB, Moslehi JJ.. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 2017;19:21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA, Anders RA, Sosman JA, Moslehi JJ.. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH.. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013;122:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK.. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 2013;5:197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC, Wu JC.. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation 2013;127:1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Quigley EM. Cisapride: what can we learn from the rise and fall of a prokinetic? J Dig Dis 2011;12:147–156. [DOI] [PubMed] [Google Scholar]
- 48. Braam SR, Tertoolen L, Casini S, Matsa E, Lu HR, Teisman A, Passier R, Denning C, Gallacher DJ, Towart R, Mummery CL.. Repolarization reserve determines drug responses in human pluripotent stem cell derived cardiomyocytes. Stem Cell Res 2013;10:48–56. [DOI] [PubMed] [Google Scholar]
- 49. Manrique C, Tiwari N, Park M, Carlos J, Garcia M.. Diagnostic strategies for early recognition of cancer therapeutics-related cardiac dysfunction. Clin Med Insights Cardiol 2017;11:1179546817697983. 10.1177/1179546817697983 [DOI] [PMC free article] [PubMed] [Google Scholar]