Abstract
Aims
To compare the occurrence of cerebral, cardiovascular, and renal events in patients with hyperuricaemia treated with febuxostat and those treated with conventional therapy with lifestyle modification.
Methods and results
This multicentre, prospective, randomized open-label, blinded endpoint study was done in 141 hospitals in Japan. A total of 1070 patients were included in the intention-to-treat population. Elderly patients with hyperuricaemia (serum uric acid >7.0 to ≤9.0 mg/dL) at risk for cerebral, cardiovascular, or renal disease, defined by the presence of hypertension, Type 2 diabetes, renal disease, or history of cerebral or cardiovascular disease, were randomized to febuxostat and non-febuxostat groups and were observed for 36 months. Cerebral, cardiovascular, and renal events and all deaths were defined as the primary composite event. The serum uric acid level at endpoint (withdrawal or completion of the study) in the febuxostat (n = 537) and non-febuxostat groups (n = 533) was 4.50 ± 1.52 and 6.76 ± 1.45 mg/dL, respectively (P < 0.001). The primary composite event rate was significantly lower in the febuxostat group than in non-febuxostat treatment [hazard ratio (HR) 0.750, 95% confidence interval (CI) 0.592–0.950; P = 0.017] and the most frequent event was renal impairment (febuxostat group: 16.2%, non-febuxostat group: 20.5%; HR 0.745, 95% CI 0.562–0.987; P = 0.041).
Conclusion
Febuxostat lowers uric acid and delays the progression of renal dysfunction.
Registration
ClinicalTrials.gov (NCT01984749).
Keywords: Febuxostat , Hyperuricaemia , Elderly patient , Cerebral disease , Cardiovascular disease , Renal disease
See page 1787 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz199)
Introduction
Hyperuricaemia, an abnormally high serum uric acid level, is the cause of gout and is associated with arthritis and tophus.1,2 Uric acid-lowering agents can prevent the recurrence of urate deposition-related diseases.3 Previous studies revealed that hyperuricaemia may contribute to the development and progression of chronic kidney disease (CKD), cerebral and cardiovascular diseases, and mortality.4–8 The metabolism of purine bases generates hypoxanthine, which is converted to uric acid in a two-step process catalysed by xanthine oxidoreductase, leading to a production of reactive oxygen species, which may be deeply associated with the development of cardiovascular events. Febuxostat, a nonpurine xanthine oxidoreductase inhibitor (XOI), was approved in 2011 in Japan, and clinical evaluation showed that febuxostat has a more potent serum uric acid-lowering action compared with allopurinol.9,10 However, the superiority of XOI for better cardiovascular outcomes is controversial.11,12 A recent cohort study revealed that there was no difference in the risk of all cause death and cardiovascular events between patients with febuxostat compared with allopurinol.12 The Febuxostat vs. Placebo Randomized Controlled Trial Regarding Reduced Renal Function in Patients With Hyperuricaemia Complicated by Chronic Kidney Disease (CKD) Stage 3 (FEATHER) study demonstrated that febuxostat did not show a suppressing effect on the estimated glomerular filtration rate (eGFR) decline compared with placebo in patients with Stage 3 CKD and asymptomatic hyperuricaemia.13 The Food and Drug Administration required the comparison of febuxostat and allopurinol for risk of serious adverse cardiovascular events; thus, a randomized controlled trial, the Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) trial, was performed. It clarified that all-cause mortality and cardiovascular mortality were higher with febuxostat treatment than with allopurinol treatment in gout patients with cardiovascular disease.14 However, it remains to be elucidated whether the mortality results of the CARES trial are due to beneficial effects of allopurinol or deleterious effects of febuxostat.
In the present randomized controlled trial, Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy (FREED), we aimed to compare the occurrence of cerebral, cardiovascular, and renal events in elderly patients with hyperuricaemia at risk for cerebral or cardiorenovascular disease treated with febuxostat and those treated with conventional therapy with lifestyle modification.
Methods
Study design
The study design and rationale have been reported previously.15 Briefly, this study was a multicentre, prospective, randomized open-label, blinded endpoint, two-arm parallel treatment groups study conducted as an investigator-initiated study in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies issued by the Ministry of Health, Labour and Welfare in Japan. This study protocol was reviewed by the central institutional review board prior to approval by the institutional review board of each participating study site, and all of the patients registered to this study gave written informed consent. A steering committee created the protocol of the FREED study, observed the progress and made decisions about the management of the study. The members of the Independent Data Monitoring Committee and Events Evaluation Committee, who were unaware of the treatment assignments, objectively assessed the safety and adjudicated all suspected endpoint events. This study was funded by a grant from Teijin Pharma Limited that was paid to the Kumamoto Circulation Society according to a support contract, but the sponsor had no involvement in the planning, implementation, analysis, or interpretation of study results. This study was registered at ClinicalTrials.gov (identification number NCT01984749).
Study population
Elderly patients aged 65 years or older with hyperuricaemia (serum uric acid >7.0 to ≤9.0 mg/dL) who had one or more risks for cerebral, cardiovascular, or renal disease were enrolled in this study before randomization (detailed inclusion and exclusion criteria are provided in Supplementary material online, Table S1). Established risks for cerebral, cardiovascular, or renal disease were defined as a history of or active hypertension, a history of or active Type 2 diabetes mellitus, renal disease (eGFR ≥30 to <60 mL/min/1.73 m2 within 3 months prior to enrolment), and a history of cerebral or cardiovascular disease occurring >3 months prior to enrolment. The patients enrolled were followed up for 36 months. All participants provided written informed consent.
Randomization, dose adjustment, and procedure
Patients were randomly assigned in a 1:1 ratio to either febuxostat or non-febuxostat group. Randomization was stratified in accordance with sex, serum uric acid (<7.5 or ≥7.5 mg/dL), Type 2 diabetes mellitus, cerebrovascular or cardiovascular disease, eGFR (<45 or ≥45 mL/min/1.73 m2), and each institution.
The treatment protocol of the study is shown in Supplementary material online, Figure S1. Outpatient visits were scheduled at screening, at enrolment, at randomization, at 4, 8, 12, 24 weeks after randomization and every 6 months during subsequent years of the study. In the febuxostat group, the investigators prescribed the febuxostat preparation (Feburic® tablets; Teijin Pharma Limited, Tokyo, Japan). Febuxostat has been orally administered once daily during the 36-month study period starting from the time of enrolment. Dose increase was performed as follows: (i) the starting febuxostat dose was 10 mg/day; (ii) at week 4, the dose was increased to 20 mg/day; (iii) at week 8, the dose was increased to the target dose of 40 mg/day. In the non-febuxostat group, administration of 100 mg of oral allopurinol was considered if serum uric acid was elevated during the study period starting from the time of enrolment. The dose of both febuxostat and allopurinol was adjusted to prevent serum uric acid from decreasing to <2.0 mg/dL. Additionally, all patients underwent lifestyle modification for the management of hyperuricaemia. Serial proportion of patients with serum uric acid level <6.0 mg/dL and serum uric acid at endpoint were assessed. Regarding concomitant therapies during the study period, concurrent diseases and adverse events were appropriately treated at the discretion of the physicians in charge of this study. Therapies that already started at the time of enrolment in this study were continued during the study period without any change as much as possible. The following medications were not started or discontinued and their dosage was not changed as much as possible: antiplatelet agents, antihypertensive agents, antidiabetic agents, and antidyslipidaemic agents. Data on concomitant therapies were collected from the time of enrolment until study completion or withdrawal from the study.
Study endpoint
Fatal and non-fatal cerebral, cardiovascular and renal events, and death other than cerebral or cardiorenal vascular disease during the study period were defined as the primary composite endpoint in the study, which consisted of the following: (i) death due to cerebral, cardiovascular, or renal disease; (ii) new or recurring cerebrovascular disease [stroke (cerebral haemorrhage, cerebral infarction, subarachnoid haemorrhage, stroke of unknown type), transient ischaemic attack]; (iii) new or recurring non-fatal coronary artery disease (myocardial infarction, unstable angina); (iv) cardiac failure requiring hospitalization; (v) arteriosclerotic disease requiring treatment (aortic aneurysm, aortic dissection, and arteriosclerosis obliterans); (vi) renal impairment [development of microalbuminuria (≥30 to <300 mg/g⋅creatinine (Cr))/mild proteinuria (≥0.15 to <0.50 g/g⋅Cr), progression to overt albuminuria (≥300 mg/g⋅Cr)/severe proteinuria (≥0.50 g/g⋅Cr), or worsening of overt albuminuria confirmed by two consecutive laboratory tests performed after the initiation of study treatment; doubling of serum Cr level; progression to end-stage renal disease]; (vii) new atrial fibrillation (including paroxysmal atrial fibrillation); (viii) death due to other cause (Supplementary material online, Table S2). The secondary endpoint consisted of each component of cerebral, cardiovascular, and renal vascular events, and a hard endpoint was defined as a composite of death due to any cause, cerebrovascular disease or non-fatal coronary artery disease. Estimated glomerular filtration rate slopes per year were compared between the febuxostat and non-febuxostat groups. The relationship between serum uric acid at 12 weeks after randomization and primary composite endpoint was also assessed. The following parameters were assessed as the exploratory endpoint: (i) absolute values and changes in high-sensitivity C-reactive protein (hs-CRP), N-terminal pro-brain natriuretic peptide (NT-proBNP), and haemoglobin A1c (HbA1c); (ii) occurrence of malignant tumours; (iii) occurrence of venous thrombosis requiring treatment.
Statistical analysis
Approximately 500 patients were in each group to detect a difference in the occurrence of the primary composite endpoint between two groups, with 80% power at a two-sided 5% significant level.15 Data were analysed using the intention-to-treat (ITT) population and expressed as mean ± standard deviation and percentage unless otherwise stated. Continuous variables that did not show a normal distribution are expressed as medians (25th to 75th percentile ranges). A safety analysis was also performed in the ITT population. The repeated-measure analysis of variance was used to compare the time course difference of uric acid levels between the two groups. The time from randomization to occurrence of any cerebral, cardiovascular, and renal events or all deaths was analysed. The Kaplan–Meier method was used to estimate the event rate based on the time of onset of the events and Greenwood’s method was used to calculate the two-sided 95% confidence interval (CI). Intergroup comparisons were performed using the Cox proportional hazards model including stratification factors for randomization as a covariate. Secondary endpoints were analysed using Fine and Gray’s subdistribution hazard model. The eGFR slope of each patient was calculated from a regression line of a series of eGFR values. Between-group difference of eGFR slopes and its 95% CI were calculated and examined by Wilcoxon rank sum test. The statistical significance level was set at P < 0.05. Statistical analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC, USA) by persons in charge of statistical analysis, as instructed by the responsible biostatistician. The Independent Data Monitoring Committee had the responsibility to decide the continuation of this study in accordance with the assessment of the results of the interim analysis scheduled in advance.15
Results
Study patients
A total of 1184 patients (men and women) from 141 institutions throughout Japan from November 2013 to October 2014 were enrolled; 100 who declined to participate were subsequently excluded. Residual 1084 patients were randomly assigned, but 14 patients were excluded from the randomized population as a result of consent withdrawal (seven patients), inclusion ineligibility or exclusion criteria (five patients), loss at follow-up (one patient), and investigator’s discretion (one patient) prior to data collection at baseline. Thus, 1070 patients were included in the ITT population, with 537 assigned to the febuxostat group and 533 assigned to the non-febuxostat group (Figure 1). Baseline patient characteristics were well balanced between the two groups (Table 1 and Supplementary material online, Table S3). The maximum dose during the study period and the dose at endpoint (withdrawal or completion of the study) of the febuxostat group are shown in Supplementary material online, Table S4. The mean febuxostat dose per day was 29.1 ± 12.3 mg at endpoint, and 67.4% of the patients received 40 mg in the febuxostat group, whereas 27.2% of the patients received 100 mg allopurinol in the non-febuxostat group (Figure 1). Supplementary material online, Figure S2 shows the serum uric acid level at the initiation of allopurinol administration (mean ± standard deviation 8.18 ± 1.05 mg/dL).
Figure 1.
Patient distribution. CCVR, cerebral, cardiovascular and renal; SUA, serum uric acid.
Table 1.
Baseline characteristics of the study patients
| Total (n = 1070) | Febuxostat group (n = 537) | Non-febuxostat group (n = 533) | P-value (febuxostat vs. non-febuxostat) | |
|---|---|---|---|---|
| Male | 739 (69.1) | 371 (69.1) | 368 (69.0) | 1.000 |
| Age (years) | 75.7 ± 6.6 | 75.4 ± 6.7 | 76.0 ± 6.5 | 0.137 |
| Body mass index (kg/m2) | 24.67 ± 3.68 | 24.74 ± 3.71 | 24.61 ± 3.65 | 0.325 |
| Haemoglobin (g/dL) | 13.51 ± 1.63 | 13.55 ± 1.60 | 13.46 ± 1.65 | 0.424 |
| Total protein (g/dL) | 7.20 ± 0.46 | 7.20 ± 0.45 | 7.19 ± 0.46 | 0.928 |
| Total bilirubin (mg/dL) | 0.60 ± 0.28 | 0.62 ± 0.30 | 0.59 ± 0.28 | 0.299 |
| Hypertension | 1007 (94.1) | 506 (94.2) | 501 (94.0) | 0.897 |
| Systolic blood pressure (mmHg) | 132.6 ± 14.4 | 132.9 ± 14.8 | 132.3 ± 14.0 | 0.426 |
| Diastolic blood pressure (mmHg) | 73.5 ± 10.2 | 73.5 ± 10.2 | 73.6 ± 10.2 | 0.716 |
| Type 2 diabetes | 396 (37.0) | 197 (36.7) | 199 (37.3) | 0.849 |
| Haemoglobin A1c (%) | 5.87 ± 0.62 | 5.87 ± 0.63 | 5.87 ± 0.60 | 0.815 |
| Hyperlipidaemia | 622 (58.1) | 317 (59.0) | 305 (57.2) | 0.577 |
| LDL cholesterol (mg/dL) | 107.3 ± 29.7 | 108.3 ± 31.2 | 106.3 ± 28.1 | 0.421 |
| HDL cholesterol (mg/dL) | 54.3 ± 14.9 | 54.2 ± 14.9 | 54.4 ± 15.0 | 0.812 |
| Triglyceride (mg/dL) | 137.0 (96.0–191.0) | 135.0 (96.0–193.5) | 138.0 (94.0–189.0) | 0.757 |
| Renal diseasea | 707 (66.1) | 357 (66.5) | 350 (65.7) | 0.796 |
| eGFR (mL/min/1.73 m2) | 54.98 ± 14.64 | 54.62 ± 14.11 | 55.35 ± 15.16 | 0.608 |
| Alcohol habit | 477 (44.6) | 239 (44.5) | 238 (44.7) | 1.000 |
| Active smoking | 461 (43.1) | 222 (41.3) | 239 (44.8) | 0.267 |
| Coronary artery disease | 90 (8.4) | 45 (8.4) | 45 (8.4) | 1.000 |
| Chronic heart failure | 74 (6.9) | 41 (7.6) | 33 (6.2) | 0.393 |
| Stroke | 86 (8.0) | 39 (7.3) | 47 (8.8) | 0.370 |
| Vascular disease | 25 (2.3) | 9 (1.7) | 16 (3.0) | 0.162 |
| Malignant tumour | 32 (3.0) | 15 (2.8) | 17 (3.2) | 0.724 |
| hs-CRP (mg/dL) | 0.080 (0.040–0.170) | 0.082 (0.040–0.172) | 0.078 (0.039–0.167) | 0.520 |
| NT-proBNP (pg/mL) | 119.0 (59.0–264.0) | 114.0 (58.0–268.0) | 124.0 (62.0–263.0) | 0.328 |
| Serum uric acid (mg/dL) | 7.52 ± 1.05 | 7.54 ± 1.06 | 7.50 ± 1.03 | 0.324 |
| Urinary albumin (mg/g⋅Cr) | 17.8 (7.8–64.3) | 17.4 (7.5–54.8) | 19.5 (8.3–67.45) | 0.278 |
| Urinary protein (g/g⋅Cr) | 0.084 (0.044–0.165) | 0.082 (0.043–0.163) | 0.086 (0.044–0.170) | 0.558 |
Values are presented as n (%), mean ± standard deviation, or median (25th–75th percentile ranges).
Cr, creatinine; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Renal disease defined as eGFR <60 mL/min/1.73 m2.
The median follow-up duration (from randomization to endpoint of the study) in the febuxostat and non-febuxostat groups was 35.5 and 35.1 months, respectively. The overall withdraw ratio for reasons other than the primary composite endpoint during the study was 17.0% (16.8% in the febuxostat group and 17.3% in the non-febuxostat group). Patient reason and agreement withdrawal was 9.3% and 8.5%, respectively. There were no patients with continuous levels of serum uric acid >11.0 mg/dL in either group (Figure 1).
Serum uric acid
Changes in mean serum uric acid level during the study are shown in Figure 2. Serum uric acid levels were comparable at baseline between the febuxostat and non-febuxostat groups. However, the levels continued to be significantly lower in the febuxostat group than in the non-febuxostat group after randomization (P < 0.001). At endpoint, the serum uric acid level in the febuxostat group was significantly lower than that in the non-febuxostat group (4.50 ± 1.52 vs. 6.76 ± 1.45 mg/dL, P < 0.001).
Figure 2.
Serial changes in serum uric acid level in the febuxostat and non-febuxostat groups. Analysis of variance and Holm method as a post hoc analysis were used. Closed circle, febuxostat group; open circle, non-febuxostat group. Values are presented as mean ± standard deviation. *P < 0.001 (Holm method).
The proportion of patients with serum uric acid levels <6.0 mg/dL are shown in Supplementary material online, Table S5. More than 85% of the patients in the febuxostat group achieved uric acid levels <6.0 mg/dL within 12 weeks, whereas less than 30% of the patients in the non-febuxostat group achieved uric acid levels <6.0 mg/dL at endpoint (Supplementary material online, Figure S3).
Primary endpoint
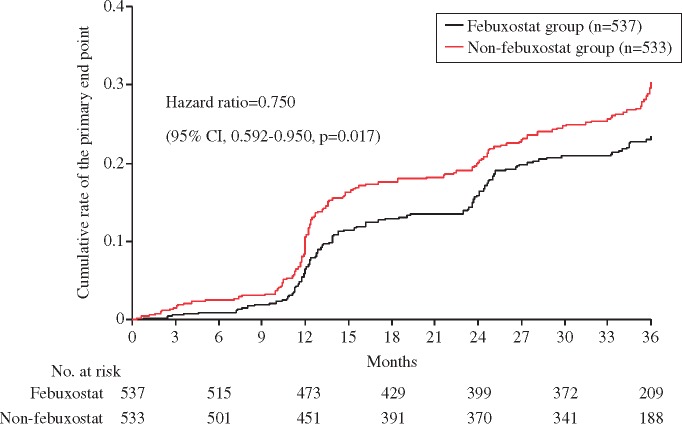
During the study period, the primary composite endpoint was observed in 125 patients (23.3%) in the febuxostat group and in 153 patients (28.7%) in the non-febuxostat group (Table 2). The Kaplan–Meier curves for the primary composite endpoint are shown in Take home figure. There was significant difference in the primary composite endpoint between the two groups after adjustment with stratification factors for randomization.
Table 2.
Hazard ratio and 95% CIs for component of the primary and secondary endpoints
| Febuxostat group (n = 537) | Non-febuxostat group (n = 533) | Hazard ratio (95% confidence interval) | P-value | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Composite of death due to any cause, cerebrovascular disease, non-fatal coronary artery disease, heart failure requiring hospitalization, arteriosclerotic disease requiring treatment, renal impairment, and atrial fibrillation | 125 (23.3) | 153 (28.7) | 0.750 (0.592–0.950) | 0.017 |
| Secondary endpoints | ||||
| Death due to cerebral, cardiovascular, or renal disease | 6 (1.1) | 6 (1.1) | 0.958 (0.314–2.926) | 0.940 |
| Cerebrovascular disease | 9 (1.7) | 7 (1.3) | 1.271 (0.479–3.371) | 0.630 |
| Non-fatal coronary artery disease | 4 (0.7) | 7 (1.3) | 0.559 (0.167–1.869) | 0.345 |
| Heart failure requiring hospitalization | 9 (1.7) | 12 (2.3) | 0.699 (0.290–1.689) | 0.427 |
| Arteriosclerotic disease requiring treatment | 2 (0.4) | 3 (0.6) | 0.644 (0.107–3.873) | 0.631 |
| Renal impairment | 87 (16.2) | 109 (20.5) | 0.745 (0.562–0.987) | 0.041 |
| Atrial fibrillation | 4 (0.7) | 3 (0.6) | 1.320 (0.292–5.968) | 0.719 |
| Death due to other causes | 4 (0.7) | 6 (1.1) | 0.635 (0.179–2.253) | 0.482 |
| Hard endpoint: composite of death due to any cause, cerebrovascular disease, or non-fatal coronary artery disease | 23 (4.3) | 26 (4.9) | 0.861 (0.492–1.506) | 0.600 |
Values are presented as n (%).
CI, confidence interval.
Figure 3.
The 95% confidence interval and adjusted hazard ratios for the primary composite event. *P-value less than 5% indicates significant heterogeneity of hazard ratios between the two groups. CI, confidence interval; Cr, creatinine; hs-CRP, high-sensitivity C-reactive protein; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Secondary endpoint
In the individual component of the primary composite endpoint, the most frequent event was renal impairment (Table 2). Amongst renal impairment, the development of microalbuminuria or mild proteinuria was common in the febuxostat and non-febuxostat groups (Supplementary material online, Table S6). Regarding a hard endpoint, there was a no significant difference between the groups (Table 2 and Supplementary material online, Figure S4).
The hazard risk of the primary endpoint in each prespecified subgroup by baseline variables is shown in Figure 3. Significant heterogeneity for possible interactions between febuxostat treatment and baseline variable was not observed in subgroup. Supplementary material online, Figure S5 shows the serial changes in eGFR during the study period. Estimated glomerular filtration rate slopes per year revealed no significant difference in the mean eGFR slope between the febuxostat and non-febuxostat groups [−0.37 (−2.32 to 1.44) vs. −0.69 (−2.63 to 1.39) mL/min/1.73 m2, P = 0.606]. No significant relationship was observed between serum uric acid at 12 weeks after randomization and primary composite endpoint (n = 980, P = 0.121) (Supplementary material online, Figure S6A). However, serum uric acid level >7 mg/dL was a strong risk factor compared with >5 to ≤6 mg/dL after adjustment with stratification factors for randomization (Supplementary material online, Figure S6B).
Take home figure.
The Kaplan–Meier curves for the primary composite event. Black line, febuxostat group; red line, non-febuxostat group.
Exploratory endpoint
The hs-CRP and NT-proBNP levels at each measured point and at endpoint were comparable between febuxostat and non-febuxostat groups. However, the HbA1c levels at 30 months (P = 0.024), 36 months (P = 0.021) and at endpoint (P = 0.035) were significantly lower in the febuxostat group than in the non-febuxostat group (Supplementary material online, Figure S7).
The occurrence of malignant tumours in the febuxostat [n = 21 (3.9%)] and non-febuxostat groups [n = 25 (4.7%)] was comparable (P = 0.529). Venous thrombosis requiring treatment was not observed in either group, but the development of gout flares was lesser in the febuxostat group [n = 6 (1.1%)] than in the non-febuxostat group [n = 14 (2.6%), P = 0.069) during the study period.
Discussion
The present FREED study demonstrated that febuxostat significantly decreased serum uric acid levels, and its effect was associated with reduction of cerebral, cardiovascular, and renal events as the primary composite endpoint in patients aged 65 years or older with hyperuricaemia compared with conventional therapy with lifestyle modification. In a primary composite endpoint, renal events were clearly reduced by febuxostat treatment. Our results are consistent with and expanded those of previous studies.16,17 The FREED study showed a large difference in the lowering of the uric acid level in the febuxostat and non-febuxostat (conventional therapy) groups. Oxidative stress generated by the metabolic converting step from xanthine to uric acid may enhance the progression of atherosclerosis through induction of endothelial injury.18,19 Thus, it is a reasonable therapy for hyperuricaemia to control serum uric acid level with strong uric acid-lowering effect of XOI, which may lead to better cardiovascular outcomes.
In the CARES trial, all-cause mortality and cardiovascular mortality were higher with febuxostat than those with allopurinol, but these two XOIs yielded similar result with respect to rates of adverse cerebral and cardiovascular events.14 The FREED study demonstrated that lowering of uric acid with febuxostat may contribute to better prognosis than conventional therapy in our primary composite outcome, although fatal and non-fatal cerebral and cardiovascular events were similar. Differences in these results can be attributed to the presence of gout. For example, in the CARES trial, there was an interaction between febuxostat and allopurinol groups in terms of non-steroidal anti-inflammatory drugs use and absence of low-dose aspirin use, which could lead to increased cardiovascular events.20 Moreover, approximately half of the patients discontinued treatment during the trial. According to Choi et al.,21 the use of non-XOI or placebo group is needed to determine whether the results of the CARES trial were due to the beneficial effects of allopurinol or the deleterious effects of febuxostat. Since it is unethical to compare the treatment with XOI with placebo, our findings might address this question, but our results do not solve this problem directly. In our comparison of the use of febuxostat and conventional therapy with allopurinol 100 mg as low as 27% of the patients, we had a low number of patient dropouts. Our results showed that major cerebrocardiovascular events and mortality were similar between the febuxostat and non-febuxostat groups. The FEATHER study did not find an increased number of cardiovascular events with febuxostat in comparison to placebo,13 which can lead to the conclusion that, in the CARES trial, allopurinol may have a beneficial impact on mortality rather than febuxostat having deleterious effects.
There was a large difference in the incidence of renal impairment, assessed by the development of albuminuria or proteinuria, which would lead to the progression of CKD. Patients with CKD have increased morbidity and mortality as a result of cardiovascular events. Therefore, albuminuria is not only a risk factor for adverse cardiovascular outcomes but may also be a therapeutic target or an indicator of therapeutic response.22,23 Febuxostat decreased the exacerbation of albuminuria or proteinuria in the FREED study, and it has been suggested that febuxostat has better renoprotective effect than allopurinol.17 However, febuxostat could not improve the serial change of eGFR, similar to the result of the FEATHER study. Compared with the renal protection from XOIs, febuxostat may not aggravate kidney function, but no cardiovascular protection may be expected. Based on the results of the CARES trial, the FEATHER study and the present study, treatment with febuxostat did not reduce major cerebrocardiovascular events. Febuxostat decreased the development of gout attacks in the present study. However, no significant reduction in hs-CRP was demonstrated in the febuxostat group compared with the non-febuxostat group during the study period. Secondary explanatory analysis of the CANTOS trial, showing a reduction of inflammation and a lower rate of recurrent cardiovascular events, recently disclosed that canakinumab administration was associated with reduced risk for gout attacks without any change in serum uric acid levels.24 Hybrid treatment with febuxostat and inhibitors of interleukin-1β may be useful not only in preventing gout attacks but also in yielding cerebral, cardiovascular, and renal benefits.
Our study should be interpreted with caution. First, we included patients with asymptomatic hyperuricaemia without gout, which was different from previous studies, including the CARES trial. Although comparison of our results with those of previous studies would be difficult, our findings are an important for hyperuricaemic patients without gout in the primary care setting.
Second, our sample size was limited, and we employed not only hard endpoints but also relatively soft endpoints, such as development of albuminuria/proteinuria. Additionally, observation period was relatively short. However, the deterioration of renal function as a part of our primary composite outcome is an important marker for CKD,25 because the prevalence of patients with CKD was predicted as 13% of the Japanese adult population.26 Our findings have possible important applications in the preventive therapy of asymptomatic hyperuricaemic patients with high renal risk.
High serum uric acid level has a clear relationship with development of renal disease.27,28 Febuxostat was also reported to be more suitable than allopurinol for patients with moderate to severe renal dysfunction.29 These findings, as well as the result of our study, shows that evaluation of soft endpoints may be clinically important for patients with hyperuricaemia treated with febuxostat.
In conclusion, febuxostat lowers uric acid and delays the progression of renal dysfunction.
Supplementary Material
Acknowledgements
The authors thank Yuki Sakurada, Komaki Yamaguchi, Kazuyo Nasu, and Aya Furusato for secretarial assistance.
Funding
Teijin Pharma Limited, Japan.
Conflict of interest: S. K reports other from Teijin Home Healthcare, Limited, grants from Philips Respironics Goudou Kaisha, grants from Teijin Pharma, Limited, grants from Daiichi Sankyo Company, Limited, grants from Chugai Pharmaceutical Company, Limited, outside the submitted work. I.H. reports grants and personal fees from Sanwa Kagaku Kenkyusho Co. Ltd, personal fees from Feizer Co. Ltd, grants and personal fees from Fuji Yakuhin Co. Ltd, grants from Dainippon Sumitomo Pharmaco. Co., grants from Teijin Pharma, outside the submitted work. K. K. reports grants from Teijin Pharma, personal fees from Tanabe-mitsubishi, outside the submitted work. Y. S. reports grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, grants from the Ministry of Health, Labour and Welfare Scientific Research, grants from the Japan Agency for Medical Research and Development, grants from the Ministry of Health, Labour and Welfare Scientific Research, grants from the Ministry of Health, Labour and Welfare Scientific Research, grants from the Japan Agency for Medical Research and Development, grants from the Japan Agency for Medical Research and Development, during the conduct of the study; grants and personal fees from Astellas Pharma Inc., grants from Boston Scientific Japan K.K., grants from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Daiichi Sankyo Co., Ltd., grants and personal fees from Dainippon Sumitomo Pharma Co., Ltd., grants from Eisai Co., Ltd., grants from Fuji Yakuhin Co., Ltd., grants from Kyowa Hakko Kirin Co., Ltd., grants from Medtronic, Inc., grants and personal fees from Mitsubishi Tanabe Pharma Corporation, grants, personal fees and other from MSD K.K., grants from Nihon Medi-Physics Co.,Ltd., grants and personal fees from Ono Pharmatical Co., Ltd., grants and personal fees from Otsuka Pharmaceutical Co., Ltd., grants and personal fees from Pfizer Japan Inc., grants and personal fees from Sanofi K.K., grants from Shionogi & Co., Ltd, grants and personal fees from Takeda Pharmaceutical Co., Ltd., grants from Teijin Pharma Ltd., grants from ZERIA Pharmaceutical Co., Ltd., personal fees from Asahi Kasei Pharma Corporation, personal fees from Bayer Holding Ltd, personal fees from Kowa Pharmaceutical Co., Ltd, grants and personal fees from Nippon Boehringer Ingelheim Co., Ltd., personal fees from Novartis Pharma K.K., personal fees from Taisho Toyama Pharmaceutical Co., Ltd., personal fees from Toa Eiyo LTD., grants from St. Jude Medical Japan Co., Ltd., outside the submitted work. Other authors have nothing to disclose.
References
- 1. Campion EW, Glynn RJ, DeLabry LO.. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med 1987;82:421–426. [DOI] [PubMed] [Google Scholar]
- 2. Yü T, Yu TF.. Milestones in the treatment of gout. Am J Med 1974;56:676–685. [DOI] [PubMed] [Google Scholar]
- 3. Perez-Ruiz F, Calabozo M, Pijoan JI, Herrero-Beites AM, Ruibal A.. Effect of urate-lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum 2002;47:356–360. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 5. Li M, Hu X, Fan Y, Li K, Zhang X, Hou W, Tang Z.. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep 2016;6:19520.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA.. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2010;62:170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kojima S, Sakamoto T, Ishihara M, Kimura K, Miyazaki S, Yamagishi M, Tei C, Hiraoka H, Sonoda M, Tsuchihashi K, Shimoyama N, Honda T, Ogata Y, Matsui K, Ogawa H; Japanese Acute Coronary Syndrome Study (JACSS) Investigators. Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study). Am J Cardiol 2005;96:489–495. [DOI] [PubMed] [Google Scholar]
- 8. Bose B, Badve SV, Hiremath SS, Boudville N, Brown FG, Cass A, de Zoysa JR, Fassett RG, Faull R, Harris DC, Hawley CM, Kanellis J, Palmer SC, Perkovic V, Pascoe EM, Rangan GK, Walker RJ, Walters G, Johnson DW.. Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant 2014;29:406–413. [DOI] [PubMed] [Google Scholar]
- 9. Takano Y, Hase-Aoki K, Horiuchi H, Zhao L, Kasahara Y, Kondo S, Becker MA.. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci 2005;76:1835–1847. [DOI] [PubMed] [Google Scholar]
- 10. Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, Ueda T, Yamamoto T, Yamanaka H, Matsuzawa Y.. An allopurinol-controlled, multicenter, randomized, open-label, parallel between-group, comparative study of febuxostat (TMX-67), a non-purine-selective inhibitor of xanthine oxidase, in patients with hyperuricemia including those with gout in Japan: phase 2 exploratory clinical study. J Clin Rheumatol 2011;17(Suppl 2):S44–S49. [DOI] [PubMed] [Google Scholar]
- 11. Foody J, Turpin RS, Tidwell BA, Lawrence D, Schulman KL.. Major cardiovascular events in patients with gout and associated cardiovascular disease or heart failure and chronic kidney disease initiating a xanthine oxidase inhibitor. Am Health Drug Benefits 2017;10:393–401. [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang M, Solomon DH, Desai RJ, Kang EH, Liu J, Neogi T, Kim SC.. Assessment of cardiovascular risk in older patients with gout initiating febuxostat versus allopurinol. Circulation 2018;138:1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, Ito S, Yamamoto T, Tomino Y, Ohno I, Shibagaki Y, Iimuro S, Imai N, Kuwabara M, Hayakawa H, Ohtsu H, Ohashi Y; FEATHER Study Investigators. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis 2018;72:798–810. [DOI] [PubMed] [Google Scholar]
- 14. White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, Hunt B, Castillo M, Gunawardhana L; CARES Investigators. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018;378:1200–1210. [DOI] [PubMed] [Google Scholar]
- 15. Kojima S, Matsui K, Ogawa H, Jinnouchi H, Hiramitsu S, Hayashi T, Yokota N, Kawai N, Tokutake E, Uchiyama K, Sugawara M, Kakuda H, Wakasa Y, Mori H, Hisatome I, Waki M, Ohya Y, Kimura K, Saito Y; Febuxostat for Cerebral and Cardiorenovascular Events Prevention Study (FREED) investigators. Rationale, design, and baseline characteristics of a study to evaluate the effect of febuxostat in preventing cerebral, cardiovascular, and renal events in patients with hyperuricemia. J Cardiol 2017;69:169–175. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka K, Nakayama M, Kanno M, Kimura H, Watanabe K, Tani Y, Hayashi Y, Asahi K, Terawaki H, Watanabe T.. Renoprotective effects of febuxostat in hyperuricemic patients with chronic kidney disease: a parallel-group, randomized, controlled trial. Clin Exp Nephrol 2015;19:1044–1053. [DOI] [PubMed] [Google Scholar]
- 17. Sezai A, Soma M, Nakata K, Hata M, Yoshitake I, Wakui S, Hata H, Shiono M.. Comparison of febuxostat and allopurinol for hyperuricemia in cardiac surgery patients (NU-FLASH Trial). Circ J 2013;77:2043–2049. [DOI] [PubMed] [Google Scholar]
- 18. Heinig M, Johnson RJ.. Role of uric acid in hypertension, renal disease, and metabolic syndrome. Cleve Clin J Med 2006;73:1059–1064. [DOI] [PubMed] [Google Scholar]
- 19. Zoppini G, Targher G, Bonora E.. The role of serum uric acid in cardiovascular disease in type 2 diabetic and non-diabetic subjects: a narrative review. J Endocrinol Invest 2011;34:881–886. [DOI] [PubMed] [Google Scholar]
- 20. Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology (Oxford) 2010;49:1229–1238. [DOI] [PubMed] [Google Scholar]
- 21. Choi H, Neogi T, Stamp L, Dalbeth N, Terkeltaub R.. New Perspectives in Rheumatology: Implications of the Cardiovascular Safety of Febuxostat and Allopurinol in Patients With Gout and Cardiovascular Morbidities Trial and the Associated Food and Drug Administration Public Safety Alert. Arthritis Rheumatol 2018;70:1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bouchi R, Babazono T, Yoshida N, Nyumura I, Toya K, Hayashi T, Hanai K, Tanaka N, Ishii A, Iwamoto Y.. Association of albuminuria and reduced estimated glomerular filtration rate with incident stroke and coronary artery disease in patients with type 2 diabetes. Hypertens Res 2010;33:1298–1304. [DOI] [PubMed] [Google Scholar]
- 23. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM.. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004;110:921–927. [DOI] [PubMed] [Google Scholar]
- 24. Solomon DH, Glynn RJ, MacFadyen JG, Libby P, Thuren T, Everett BM, Ridker PM.. Relationship of interleukin-1β blockade with incident gout and serum uric acid levels: exploratory analysis of a randomized controlled trial. Ann Intern Med 2018;169:535–542. [DOI] [PubMed] [Google Scholar]
- 25. Chapter 1: definition and classification of CKD. Kidney Int Suppl 2013;3:19–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S.. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 2009;13:621–630. [DOI] [PubMed] [Google Scholar]
- 27. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS.. Uric acid and incident kidney disease in the community. J Am Soc Nephrol 2008;19:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R.. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 2008;19:2407–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shibagaki Y, Ohno I, Hosoya T, Kimura K.. Safety, efficacy and renal effect of febuxostat in patients with moderate-to-severe kidney dysfunction. Hypertens Res 2014;37:919–925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.