Abstract
Unexpected cardiac adverse effects are the leading causes of discontinuation of clinical trials and withdrawal of drugs from the market. Since the original observations in the mid-90s, it has been well established that cardiovascular risk factors and comorbidities (such as ageing, hyperlipidaemia, and diabetes) and their medications (e.g. nitrate tolerance, adenosine triphosphate-dependent potassium inhibitor antidiabetic drugs, statins, etc.) may interfere with cardiac ischaemic tolerance and endogenous cardioprotective signalling pathways. Indeed drugs may exert unwanted effects on the diseased and treated heart that is hidden in the healthy myocardium. Hidden cardiotoxic effects may be due to (i) drug-induced enhancement of deleterious signalling due to ischaemia/reperfusion injury and/or the presence of risk factors and/or (ii) inhibition of cardioprotective survival signalling pathways, both of which may lead to ischaemia-related cell death and/or pro-arrhythmic effects. This led to a novel concept of ‘hidden cardiotoxicity’, defined as cardiotoxity of a drug that manifests only in the diseased heart with e.g. ischaemia/reperfusion injury and/or in the presence of its major comorbidities. Little is known on the mechanism of hidden cardiotoxocity, moreover, hidden cardiotoxicity cannot be revealed by the routinely used non-clinical cardiac safety testing methods on healthy animals or tissues. Therefore, here, we emphasize the need for development of novel cardiac safety testing platform involving combined experimental models of cardiac diseases (especially myocardial ischaemia/reperfusion and ischaemic conditioning) in the presence and absence of major cardiovascular comorbidities and/or cotreatments.
Keywords: Toxicity, Safety, Cardiac, Heart, Ischaemia, Conditioning, Pre-conditioning, Post-conditioning , Comorbidity, Comedication, Remote conditioning
‘Hidden cardiotoxicity’: definition of term
Over the last 60 years, 462 medicinal products were withdrawn from the market for toxicity reasons, either worldwide or in one country only.1 Deaths, hepatic, cardiac, and nervous system toxicity accounted for most of the drug withdrawals.2 While among the withdrawn drugs are many analgesics, controversy still surrounds the use of some approved analgesics for pain management,3 since they might induce cardiotoxicity at higher concentrations.4 Thus drug-induced cardiotoxicity is a major problem, even occurring after introduction of the drug on the market. One explanation for these unwanted drug actions relates to the fact that current cardiac safety testing platforms focus on investigations of the unwanted actions of drug candidates on cardiac electrophysiology including some ion channels only in healthy animals/tissue (‘direct toxicity’), while the effects of drugs on the heart (tissue), however, may be altered in the presence of comorbidities/cotreatments since they affect ion channel expression and/or activity, mitochondrial function, electro-mechanical coupling, and modification of extracellular matrix composition favouring the induction of arrhythmias, contractile dysfunction, and potentially cardiomyocyte death. Thus, toxic drug effects can be ‘hidden’ when safety testing is only done in healthy heart (tissue) but may become obvious in the diseased state (‘hidden toxicity’). Thus, we define ‘hidden cardiotoxicity’ as toxicity that manifests only in the diseased state, e.g. in the heart during ischaemia/reperfusion injury and/or in the presence of major comorbidities leading to cardiovascular disease(s).
The major clinical importance of the novel concept of hidden cardiotoxicity is that it may lead to development of safety testing platforms that can detect hidden cardiotoxicity at the early pre-clinical stage, thereby preventing clinical trials and marketing of potentially cardiotoxic drugs, decrease the overall cost of development via increasing the success rate of drug development.
Drug-induced arrhythmias
Anti-arrhythmic drugs have been associated with relatively frequent pro-arrhythmic adverse effects for a long time. They may prolong the duration of repolarization and induce Torsades de Pointes (TdP) ventricular tachycardia that can degenerate into ventricular fibrillation,5 or they may impair impulse conduction. On the other hand, there has been growing concern regarding the very rare provocation of TdP and sudden cardiac death by several non-cardiovascular drugs,6 although the prevalence of arrhythmias associated with these non-cardiac drugs is very low (0.01–0.001%).
Unexpected pro-arrhythmic events associated with drug administration following myocardial infarction are best illustrated by the historical CAST and SWORD clinical trials that studied the effects of sodium and potassium channel inhibitor anti-arrhythmic drugs in post-myocardial infarction patients with impaired left ventricular function.7,8 Both trials were discontinued before completion due to increased all-cause mortality in patients assigned to treatment. In addition to the well-known acute ventricular arrhythmias occurring within a few minutes to hours following myocardial infarction, arrhythmogenic structural, and electric remodelling of the heart develops in the course of days to weeks favouring arrhythmogenesis (for review, see ref.9) The remodelling process in the surviving border zone tissue causes slowed impulse conduction, abnormal cell-to-cell coupling, and generation of early after-depolarizations [due to fibrosis, reduced connexin expression, ion channel (sodium, calcium, potassium) down-regulation], all promoting the induction and maintenance of re-entry type arrhythmias.10 It is conceivable, therefore, that cardiovascular and non-cardiovascular drugs with sodium channel blocking properties will further exacerbate these abnormalities (i.e. they induce unidirectional conduction block in tissue previously exhibiting slowed conduction) and can precipitate arrhythmias during ischaemia and following myocardial infarction. In this regard, some non-steroidal anti-inflammatory drugs (NSAIDs) and selective cyclooxygenase 2 (COX2) inhibitors were found to block cardiac ionic currents.11,12 A meta-analysis by Trelle et al.13 showed that most NSAIDs administered chronically increased morbidity and mortality in patients with cardiovascular disease. Clinically relevant cardiotoxicity is associated with the anti-emetics domperidone and metoclopramide due to their rather potent and local anaesthetic-like inhibition of cardiac sodium channels, leading to cardiovascular side effects such as malignant arrhythmias.14 Inhibition of the hERG (human Ether-a-go-go Related Gene) channel by clozapine also results in clinically overt cardiotoxicity.15,16
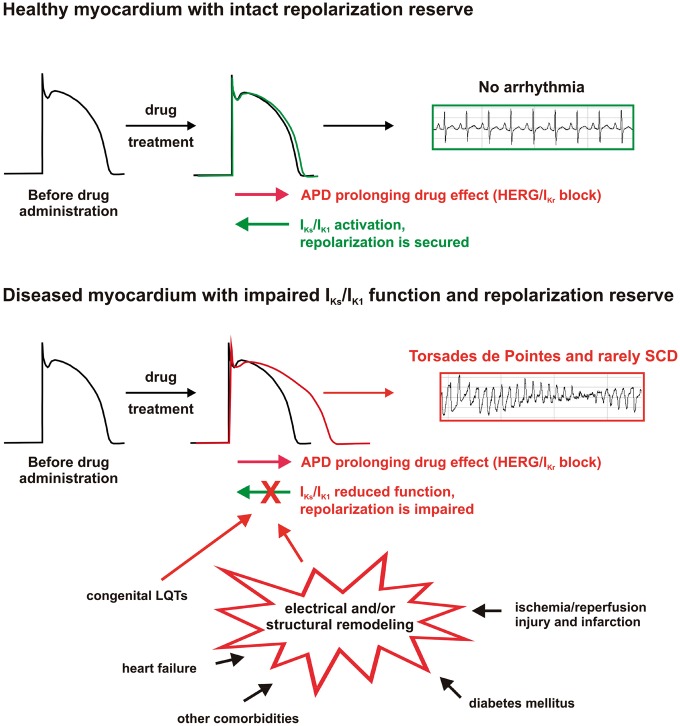
‘Hidden’ cardiac electrophysiological toxic effects of drugs can be also based on impairment of the repolarization process, which contributes to the weakening of repolarization reserve and enhancement of the arrhythmia substrate. The concept of repolarization reserve suggests that myocardial repolarization is redundant, and congenital or acquired loss of function of a repolarizing current and/or gain of function of a depolarizing current may not manifest as marked QT-interval prolongation on the electrocardiogram because other repolarizing currents can compensate.17,18 The repolarizing IKs potassium current was found to play a key role in repolarization reserve.19,20 Importantly, as part of electrical remodelling in myocardial infarction, chronic heart failure, cardiac hypertrophy, diabetes mellitus, the down-regulation of various potassium currents was observed.21–23 The possible combination of down-regulation, acute pharmacological block, or congenital loss of function of potassium channels—as multiple hits on repolarization—leads to impaired repolarization reserve and a consequent increase in susceptibility to ventricular arrhythmias.18,24–26 In the presence of proper triggers, otherwise harmless non-cardiovascular drugs even with mild potassium channel blocking effects can provoke unexpected but serious ventricular arrhythmias and sudden cardiac death, as illustrated on Figure 1.
Figure 1.
Schematic illustration of the role of impaired repolarization reserve in drug-induced arrhythmias in healthy and diseased cardiac tissue (hidden cardiotoxicity). In healthy myocardium (upper panel), the slow delayed rectifier (IKs), and the inward rectifier (IK1) potassium currents, key components of repolarization reserve, counteract the mild repolarization prolonging (mostly due to hERG/IKr blocking) effect of drugs. Therefore, repolarization (action potential duration) is only slightly prolonged and no arrhythmias occur. The proarrhythmic side effect of the drug remains hidden in normal conditions. However, in the diseased heart (lower panel), a number of congenital, and acquired pathological conditions lead to electrical and/or structural remodelling featuring impaired function and/or down-regulation of repolarizing currents, consequently, leading to reduced repolarization reserve and increased arrhythmia susceptibility. Without the compensating effect of IKs/IK1 activation, drug administration can lead to lethal ventricular arrhythmias. The hidden cardiotoxicity of the drug is revealed.
Diseases such as heart failure, hypertrophic cardiomyopathy, and ion channelopathies can provide arrhythmia trigger mechanisms as well. The expressions of sodium-calcium exchanger and the funny channel are enhanced in the failing myocardium.27,28 Delayed after-depolarizations can develop and cause triggered activity in congestive heart failure due to spontaneous calcium leak from the sarcoplasmic reticulum.29,30 Catecholaminergic polymorphic ventricular tachycardia triggers arrhythmias by abnormally increasing calcium release from the sarcoplasmic reticulum following beta-adrenergic stimulation as a consequence of mutations in the ryanodine receptor or calsequestrin.31,32 Athlete’s heart may represent a special example, where increased physical demand leads to compensatory electrical and structural remodelling manifested by cardiac hypertrophy,33 interstitial myocardial fibrosis,34 bradycardia,35,36 and increased repolarization heterogeneity making these hearts more susceptible to arrhythmias following additional challenges such as non-cardiovascular drugs, dietary ingredients, or certain doping agents.37
Thus, the reliable assessment of pro-arrhythmic potential during drug development is essential. Current pre-clinical and clinical guidelines on cardiac electrophysiological safety testing advocate pro-arrhythmic potential studies in cell lines, healthy tissues, isolated hearts, animals, and healthy human volunteers, and mainly concentrate on hERG channel inhibition and repolarization prolonging effects of drug candidates,38,39 not representing patients who exhibit increased arrhythmia susceptibility. There is an unmet need for more reliable models representing vulnerable patients for arrhythmias, with structural heart disease,24 reduced repolarization reserve,18 and/or other comorbidities. In addition, species dependent cardiac electrophysiological differences in pro-arrhythmia studies need to be considered when extrapolating results to humans.40,41
A selection of drugs found to cause unexpected serious ventricular arrhythmias and/or sudden cardiac death as ‘hidden cardiotoxicity’ is presented in Table 1.
Table 1.
Selected examples of drugs associated with possible hidden cardiotoxicity based on adverse electrophysiological actions
| Drug class | Compound | Possible arrhythmogenic mechanism(s) |
|---|---|---|
| Antibiotics | Erythromycin, clarithromycin | hERG inhibition |
| Grepafloxacine, sparfloxacine | hERG inhibition | |
| Antidepressants | Imipramine | I Na, hERG inhibition |
| Fluoxetine | I Na, ICa, L, hERG current and trafficking block | |
| Citalopram | hERG current and trafficking inhibition | |
| Antiepileptics | Retigabine | hERG, INa inhibition |
| Lacosamide | I Na inhibition | |
| Antifungal agents | Fluconazole | hERG current and trafficking inhibition |
| Antihistamines | Astemizole | hERG inhibition |
| Terfenadine | I Na, hERG inhibition | |
| Antimuscarinics | Terodiline | hERG inhibition |
| Antipsychotics | Haloperidol | hERG inhibition |
| Risperidone | hERG inhibition | |
| Clozapine | hERG inhibition | |
| ß2-agonists | Salbutamol | hERG inhibition |
| NSAIDs | Diclofenac | I Na, hERG, IKs inhibition |
| Celecoxib | I Na, hERG, IKs inhibition | |
| Opioid analgesics | Methadone | I Na, hERG inhibition |
| PDE inhibitors | Milrinone (PDE3 inhibitor) | cAMP dependent SR Ca2+ release, If activation |
| Vardenafil (PDE5 inhibitor) | hERG inhibition | |
| Prokinetics | Cisapride | hERG inhibition |
| Vasodilators | Bepridil | hERG, INa inhibition |
hERG, human ether-a-go-go-related gene potassium current; If, hyperpolarization-activated cyclic nucleotide gated pacemaker ‘funny’ current; IKs, slow component of the delayed rectifier potassium current; INa, voltage-gated sodium current; NSAIDs, non-steroidal anti-inflammatory drugs; PDE, phosphodiesterase; SR, sarcoplasmic reticulum.
Drug-induced cardiac dysfunction and/or irreversible myocardial injury
Cardiac dysfunction might occur either by (i) directly affecting cardiomyocyte function through modification of excitation-contraction coupling and/or intracellular calcium homeostasis and/or mitochondrial function42 or (ii) alterations of loading conditions (pre-load reserve/afterload mismatch)43 or heart rate (force-frequency relation)44 or (iii) alterations of the extracellular matrix composition.45
Irreversible myocardial injury may develop via different types of cell death mechanisms such as necrosis, apoptosis, necroptosis, and possibly altered autophagy. Necrosis is an energy-independent process that results in the disintegration of cells in living tissue, which could be exacerbated in the presence of compounds with ‘hidden cardiotoxicity’. The point of no return in necrosis is when the sufficient amount of energy for the maintenance of membrane potential and integrity is no longer available. The extent of necrotic tissue can be described either by histology,46 magnetic resonance imaging,47 or by measuring release of cellular components (e.g. lactate dehydrogenase, troponin I or T48). Apoptosis is an adenosine triphosphate-dependent, regulated process in which activation of effector caspases occur due to loss of mitochondrial membrane potential (intrinsic pathway) or activation of tumour necrosis factor receptors (exstrinsic pathway).49,50 Apoptosis can be characterized by e.g. caspase 3 activation,51 annexin-V externalization,52 or the TUNEL assay.53 Necroptosis, is a recently described form of caspase-independent programmed cell death,54 which could also be assessed to further explore details of cell death mechanisms.49,54 Autophagy is a pro-survival mechanism, which provides energy for cells via consuming their own components.55 However, in a number of studies, excessive activation of autophagic processes resulted in apoptotic- or necrotic cell death.56 Therefore, the autophagy should be determined as dynamic process, by assessing autophagic flux.57 Drugs that may exacerbate these cell death signalling pathways in different conditions of comorbidities may potentially show hidden cardiotoxic effects, however, current pre-clinical safety testing does not require testing these pathways.
Direct vascular and/or cardiotoxicity
Apart from their arrhythmogenic potential, analysis of various pre-clinical data, meta-analysis and observational studies showed that COX2 inhibitors and NSAIDs increase the risk of vascular and cardiotoxicity.
Although COX2 is regarded an inducible enzyme, experimental and clinical studies suggest that COX2 is constitutively expressed in some tissues, among them in the vascular endothelium, where it contributes to the maintenance of vascular homeostasis and integrity.58 Selective depletion of COX2 in vascular smooth muscle cells and endothelial cells depresses biosynthesis of prostaglandins and accelerates atherogenesis in low-density lipoprotein receptor knockout mice59 and suppression of COX2 activity increases leucocyte adherence to endothelial cells of normo- and hypertensive rats60 and increases smooth muscle cell calcification in mice with impaired kidney function.61 Impairment of endothelial cell prostaglandin synthesis by COX2 inhibition elevates blood pressure59,60 and diminishing COX2 expression or activity in hematopoietic cells can result in a predisposition to salt-sensitive hypertension.62 Together with increased platelet reactivity following COX2 inhibition (for review, see ref.63) these effects might lead to an increase in vascular toxicity and cardiovascular risk (for review, see ref.64)
The vascular and/or cardiotoxic risk depends on the dose, duration, and frequency of NSAID administration.65 For example, the NSAID diclofenac induces proteasome and mitochondrial dysfunction in murine cardiomyocytes and hearts leading to an increase in reactive oxygen species (ROS) formation and altered protein turnover.66 The reduction of the dose of NSAIDs may mitigate, but not avoid, the risk of cardiovascular adverse effects.67
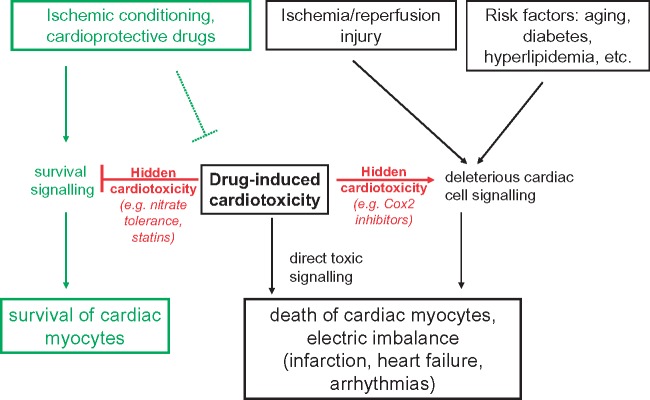
Numerous commonly used drugs such as certain anticancer medications [anthracyclines—(Doxorubicin/Adriamycin), cisplatin (Platinol), trastuzumab (Herceptin), imatinib (Gleevec), mitoxantrone (Novantrone), arsenic trioxide (Trisenox), bevacizumab (Avastin), sunitinib (Sutent), and sorafenib (Nevaxar)], the antiretroviral compound azidothymidine (AZT, Zidovudine), and several oral antidiabetics [e.g. rosiglitazone (Avandia)], likewise various substances of abuse [e.g. alcohol, methamphetamine, ecstasy, cocaine, and synthetic cannabinoids (K2, spice)] may induce direct cardiotoxicity.68 This cardiotoxicity is sometimes dose- and time-dependent, but may also develop unpredictably years after the initial drug exposure, more frequently in patients with cardiovascular comorbidities (Figure 2).
Figure 2.
Influence of ischemia/reperfusion injury and cardiovascular risk factors on cardiotoxic effects of drugs. Hidden cardiotoxicity of a drug is revealed if the drug inhibits cell survival signalling or activates deleterious cell signalling induced by cardiac diseases especially ischemia/reperfusion injury and/or its major risk factors including their comedications. APD, action potential duration; HERG, human ether-a-go-go-related gene potassium channel; LQTs, long QT syndromes; SCD, sudden cardiac death.
Multiple lines of evidence suggest that direct or indirect mitochondria-related toxicity is an important common effector mechanism of drug-induced direct cardiotoxicity. Mitochondrial toxicity may develop as a consequence of interference with the mitochondrial respiratory chain (e.g. uncoupling) or due to inhibition of the important mitochondrial enzymes (oxidative phosphorylation, Szent-Györgyi–Krebs cycle, mitochondrial DNA replication) among others. All these may facilitate increased generation of mitochondrial ROS, calcium overload, depletion of cellular nicotinamide-adenine-dinucleotide (NAD+) and adenosine triphosphate, and opening of the mitochondrial permeability transition pore with consequent triggering of apoptotic and/or necrotic cell death pathways.68
Doxorubicin is still a commonly used effective and broad spectrum antineoplastic agent despite its dose limiting cumulative cardiotoxicity. Among all cardiotoxic agents the mechanisms of doxorubicin-induced cardiotoxicity are among the best characterized, yet very complex and not completely understood. These will be briefly discussed in the following paragraphs, while for the discussion of the mechanisms of other direct cardiotoxic drugs, we would like to refer readers to recent overviews on the subject.68–70
Cardiomyocytes and endothelial cells are particularly sensitive to the direct toxic effects of doxorubicin. In the mitochondria of these cells doxorubicin via non-enzymatic redox cycling71–74 or iron-dependent75–77 processes triggers increased generation of ROS (e.g. superoxide anion). Mitochondrial iron accumulation due to defective function of ABCB8, a mitochondrial protein that facilitates iron export, may also contribute to the deleterious effects of doxorubicin in cardiomyocytes.77 Superoxide anion can be converted to hydrogen peroxide by mitochondrial superoxide dismutase or via diffusion-limited reaction it can rapidly react with nitric oxide to form peroxynitrite,78 a potent oxidant and cytotoxic reactive nitrogen species (RNS) that promotes mitochondrial protein oxidation/nitration and initiation of cell death pathways.79,80 Doxorubicin can also directly bind to mitochondrial abundant phospholipid, cardiolipin and can form adducts with mitochondrial DNA,81 and activate matrix metalloproteinases.82 Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 has also been proposed to contribute to doxorubicin-induced ROS generation in the heart.79,83,84
The doxorubicin-induced cardiotoxicity also involves disruption of key antioxidant mechanisms. Conversely, interventions aimed to enhance the key antioxidant defence systems (e.g. manganese superoxide dismutase85; catalase86; metallothionein,87 thioredoxin-188; glutaredoxin 289; and glutathione levels90) and to neutralize the mitochondrial ROS/RNS by mitochondrialy targeted antioxidants91 have demonstrated cardioprotective effects in rodent models of doxorubicin-induced cardiomyopathy, the latter without interference with its antitumour activity. The doxorubicin induced increased ROS/RNS generation coupled with impaired antioxidant defence eventualy leads to oxidative DNA injury and consequent activation of the nuclear enzyme poly(ADP)-ribose polymerase 1 (PARP-1) resulting in cellular depletion of NAD+ and adenosine triphosphate triggering cell death (both apoptotic or necrotic).92 Poly(ADP)-ribose polymerase 1 genetic deletion and inhibition is protective against doxorubicin-induced cardiotoxicity in mice92,93 Logically PARP inhibitors (e.g. the Federal Drug Administration approved anticancer drug olaparib to treat specific forms of ovarian cancer), could be combined with doxorubicin or cisplatin, due to potentially increased chemotherapeutic efficacy and decreased cardiotoxicity.68,92–94
Cardiomyocytes as non-dividing cells are considerably less sensitive to the topoisomerase inhibiting adverse effect of doxorubicin. However, the topoisomerase isoenzyme IIβ is essential in maintaining normal transcriptional activity in cardiomyocytes, and it has specific function in the maintenance of mitochondrial DNA, allowing mitochondrial transcription and replication.95 Accordingly, this enzyme is critically involved in cardiomyocyte-specific toxicity of doxorubicin.96
The above mentioned examples of doxorubicin-induced dose-dependent cumulative cardiotoxicity illustrate the complexity and the need for better understanding the common mechanisms of drug-induced direct cardiotoxicity to develop more effective screening strategies97 and models98,99 both in the clinical100,101 as well as in the pre-clinical settings. These efforts should also focus on identification of toxicity biomarkers,102 patient at risk,103 development of more efficient targeted drug delivery systems104–106 allowing reduction of the dose, and use of personalized prophylactic cardioprotective therapies.107
‘Hidden toxicity’
Since the original observations in the mid-90s, it has been well established that cardiovascular risk factors and comorbidities and their medications may interfere with cardiac ischaemic tolerance and endogenous cardioprotective signalling pathways by several cellular mechanisms including robust changes in cardiac gene expression profile at the transcript level [coding and non-coding ribonucleic acid (RNA)s] including transcripts of ion channels, enzymes involved in mitochondrial energy metabolism, transcription factors, etc. (for extensive reviews, see refs108–112) Therefore, drugs may exert ‘hidden’ cardiotoxic actions on the diseased heart via interfering with cell death and cardioprotective signalling.109,110 Some examples of drugs that show(ed) ‘hidden cardiotoxic’ effects that can be evidenced only in the comorbid, ischaemic heart are provided below.
The hidden cardiotoxic effect of a compound has been proven for the first time by an elegant study by Golomb et al.113 They showed that that subtoxic dosage of a known ‘direct’ cardiotoxic agent bis(2-chloroethoxy)methane may cause ‘hidden’ cardiotoxicity as revealed by impaired mitochondrial function only under ischaemic conditions.
Nitrate tolerance developing due to long-term use of nitrates, long-term use of statins, ATP-dependent potassium channel blocker anti-diabetic drugs, and COX2 inhibitors have been shown to interfere with ischaemia/reperfusion injury and the effect of endogenous cardioprotection (for reviews, see refs109–111) High-dose glyceryl trinitrate-induced nitrate tolerance blocked both pre- and post-conditioning114,115 and long-term use of statins antagonized the cardioprotective effect of ischaemic post-conditioning.116 Several studies demonstrated that ATP-dependent potassium channel blockers increase ischaemia/reperfusion injury and block the cardioprotective effect of ischaemic conditioning. Thus, it might not be surprising that ATP-dependent potassium channel blockers increase the risk of major adverse cardiac events and cardiovascular death in diabetic patients (especially with concomitant heart disease).117 Angiotensin converting enzyme (ACE) inhibitors reduce irreversible ischaemia/reperfusion injury, delay heart failure progression and are additive to or restore endogenous cardioprotection.118,119 Angiotensin converting enzyme transforms angiotensin I to angiotensin II, and also promotes the degradation of bradykinin into inactive metabolites. Bradykinin stimulates nitric oxide synthesis and synthesis of vasodilator prostaglandin via a COX pathway. Moreover, COX2 activation is also involved in endogenous cardioprotective signalling.120 COX inhibitors may therefore be deleterious in cardiovascular disease by counteracting part of ACE inhibitor efficacy. This has been clearly demonstrated with NSAIDs in hypertension, coronary artery disease, and chronic heart failure and most guidelines recommend avoiding their use in such patients.121
Apart from its direct cardiotoxic effects (as outlined above), doxorubicin depletes GATA-4, which in turn causes cardiomyocyte apoptosis.122 Endogenous cardioprotection increased GATA-4 expression and activity in the heart, thereby increasing affecting cardiomyocyte survival.123 Thus, depletion of GATA-4 by doxorubicin might interfere with endogenous cardioprotection and thus add a component of ‘hidden toxicity’ to the well-established direct toxicity of doxorubicin.
Need for novel assays to predict cardiotoxicity thereby increasing drug safety
Novel assays for early pre-clinical detection of cardiotoxicity of drugs are of great importance to increase success rate of drug development and patient safety. Using three-dimensional cardiac tissues derived from human-induced pluripotent stem cells (3D-hiPSC-CT) a doxorubicin-sensitive cytotoxicity and hERG channel blocker-sensitive change in electrical activity was detected, indicating its potential usefulness as drug screening system for drug discovery124 (for review, see ref.125) Similarly, using hiPSC-cardiomyocytes, drug effects on ROS production, intracellular calcium concentration, formation of DNA double strand breaks, gene or micro RNA expression, and electrophysiological properties can be quantified102,126,127 and together with parallel assessment of motion field imaging-derived contractile properties thus allow a better risk estimation of cardiotoxic drug effects.128,129 In hiPSC-cardiomyocytes exposed to doxorubicin changes in microRNA expression occurred before the occurrence of cytotoxicity markers such as lactate dehydrogenase, and the affected microRNAs also demonstrated a significant involvement in heart failure in patients and animal models.102 Thus, early changes in microRNA expression might also allow to predict cardiotoxicity in patients.130–132
However, all of these detection assays fail to address the issue of the importance of comorbidities and cotreatments and thus do not detect ‘hidden’ cardiotoxicity of drugs.
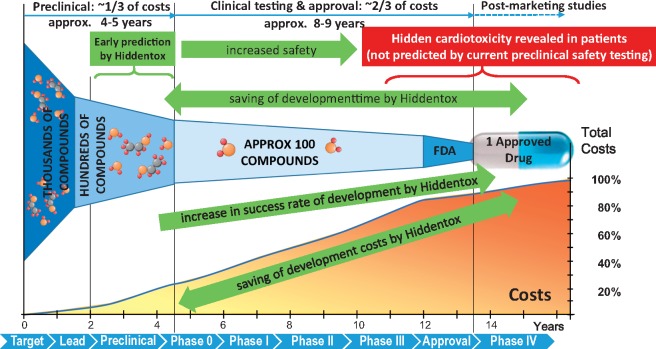
Therefore, we urge the need for development of novel cardiac safety testing platforms involving combined experimental models of various cardiac diseases, especially myocardial ischaemia/reperfusion and ischaemic conditioning in the presence and absence of major cardiovascular risk factors and comorbidities such as e.g. ageing, hyperlipidaemia, and diabetes and their major cotreatments. Although these additional tests will definitely increase the time and cost for pre-clinical safety testing, via the early detection of hidden cardiotoxicity of drugs it will ultimately lead to (Figure 3):
overall saving of time and cost of drug development for the pharmaceutical industry by early pre-clinical termination of the development of potentially cardiotoxic compounds;
increasing success rate of clinical drug development by more rational design of clinical trials to enroll patients that are not prone to manifest certain cardiotoxic side effects of a drug with potential hidden cardiotoxity in a disease condition;
increased patient safety by preventing the clinical testing and clinical use of potentially cardiotoxic drugs in patient populations that are prone to manifest hidden cardiotoxicity.
Figure 3.
Benefits of pre-clinical prediction of hidden cardiotoxicity by pre-clinical testing platforms (Hiddentox) in drug development. Hiddentox may lead to potential savings of drug development cost and development time by timely pre-clinical termination of compounds that show hidden cardiotoxic properties in diseased experimental models. Moreover, Hiddentox may increase success rate of drug development by pre-clinical determination of certain comorbidities in the presence of which hidden cardiotoxicity may manifest, thereby, improving knowledge for rational design of clinical trials on targeted patient populations. Finally, Hiddentox will increase patient safety during clinical trials and clinical use of drugs in the market by preventing potentially cardiotoxic drugs entering into clinical trials or to market.
As an example, in case the potential cardiotoxic effect of rofecoxib (Vioxx) were detected by assays for hidden cardiotoxicity in the early pre-clinical phase of its development, the manufacturer company could have saved significant amount of resources burnt for the development of rofecoxib and for the still ongoing legal issues related to its withdrawal from the market in 2004.133,134 Early prediction of hidden cardiotoxicity of rofecoxib could have prevented the unexpected manifestation of myocardial infarction of some patients taking Vioxx. However, more than a decade after its withdrawal, the mechanism of hidden cardiotoxicity of rofecoxib is still a question of debate. However, to increase the productivity of drug development, we definitely need to increase knowledge on mechanisms and early prediction of drug toxicity (Figure 3).135,136
Conclusion and outline
Cardiotoxicity seen only in the diseased heart with e.g. ischaemia/reperfusion injury and/or in the presence of its major comorbidities is termed as ‘hidden cardiotoxicity’. Little is known on the mechanism of hidden cardiotoxicity and ‘hidden cardiotoxicity’ cannot be revealed by the routinely used cardiac safety testing methods on healthy animals or tissues. Therefore, here, we emphasize the need for development of novel cardiac safety testing platforms involving combined experimental models of cardiac diseases, especially myocardial ischaemia/reperfusion and ischaemic conditioning in the presence and absence of major cardiovascular risk factors and comorbidities such as e.g. ageing, hyperlipidaemia, and diabetes and their cotreatments.
Funding
P.F. is the vice chair and R.S. is a working group leader of the European Co-operation in Science and Technology [COST action CA16225, EU-Cardioprotection]. P.F. holds grants from the Hungarian National Research, Development, and Innovation Office [OTKA K 109737, OTKA KH_17 125570, NVKP 16-1-2016-0017, and VEKOP-2.3.2-16-2016-00002]. R.S. holds grants from the German Research Foundation [CRC 1213, B05]. P.F. was supported by the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary, within the framework of the Therapeutic Development thematic programme of the Semmelweis University. Z.V.V. was supported by the Premium Postdoctoral Fellowship Program of the Hungarian Academy of Sciences.
Conflict of interest: P.F. is the founder and CEO of Pharmahungary Group, a group of R&D companies. R.S. received honoraria for lecturing from Sanofi.
References
- 1. Onakpoya IJ, Heneghan CJ, Aronson JK.. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med 2016;14:10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Onakpoya IJ, Heneghan CJ, Aronson JK.. Worldwide withdrawal of medicinal products because of adverse drug reactions: a systematic review and analysis. Crit Rev Toxicol 2016;46:477–489. [DOI] [PubMed] [Google Scholar]
- 3. Onakpoya IJ, Heneghan CJ, Aronson JK.. Post-marketing withdrawal of analgesic medications because of adverse drug reactions: a systematic review. Expert Opin Drug Saf 2018;17:63–72. [DOI] [PubMed] [Google Scholar]
- 4. Faria J, Barbosa J, Leal S, Afonso LP, Lobo J, Moreira R, Queiros O, Carvalho F, Dinis-Oliveira RJ.. Effective analgesic doses of tramadol or tapentadol induce brain, lung and heart toxicity in Wistar rats. Toxicology 2017;385:38–47. [DOI] [PubMed] [Google Scholar]
- 5. Fenichel RR, Malik M, Antzelevitch C, Sanguinetti M, Roden DM, Priori SG, Ruskin JN, Lipicky RJ, Cantilena LR; Independent Academic Task Force . Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol 2004;15:475–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, Escande D, Franz M, Malik M, Moss A, Shah R.. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications. Report on a Policy Conference of the European Society of Cardiology. Cardiovasc Res 2000;47:219–233. [DOI] [PubMed] [Google Scholar]
- 7.Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989;321:406–412. [DOI] [PubMed] [Google Scholar]
- 8. Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP.. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet 1996;348:7–12. [DOI] [PubMed] [Google Scholar]
- 9. Nattel S, Maguy A, Le Bouter S, Yeh YH.. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 2007;87:425–456. [DOI] [PubMed] [Google Scholar]
- 10. Baba S, Dun W, Cabo C, Boyden PA.. Remodeling in cells from different regions of the reentrant circuit during ventricular tachycardia. Circulation 2005;112:2386–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kristof A, Husti Z, Koncz I, Kohajda Z, Szel T, Juhasz V, Biliczki P, Jost N, Baczko I, Papp JG, Varro A, Virag L.. Diclofenac prolongs repolarization in ventricular muscle with impaired repolarization reserve. PloS One 2012;7:e53255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frolov RV, Singh S.. Celecoxib and ion channels: a story of unexpected discoveries. Eur J Pharmacol 2014;730:61–71. [DOI] [PubMed] [Google Scholar]
- 13. Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Juni P.. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stoetzer C, Voelker M, Doll T, Heineke J, Wegner F, Leffler A.. Cardiotoxic antiemetics metoclopramide and domperidone block cardiac voltage-gated Na+ channels. Anesth Analg 2017;124:52–60. [DOI] [PubMed] [Google Scholar]
- 15. Curto M, Girardi N, Lionetto L, Ciavarella GM, Ferracuti S, Baldessarini RJ.. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep 2016;18:68.. [DOI] [PubMed] [Google Scholar]
- 16. Lee SY, Kim YJ, Kim KT, Choe H, Jo SH.. Blockade of hERG human K+ channels and IKr of guinea-pig cardiomyocytes by the antipsychotic drug clozapine. Br J Pharmacol 2006;148:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol 1998;21:1029–1034. [DOI] [PubMed] [Google Scholar]
- 18. Varro A, Baczko I.. Cardiac ventricular repolarization reserve: a principle for understanding drug-related proarrhythmic risk. Br J Pharmacol 2011;164:14–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Varro A, Balati B, Iost N, Takacs J, Virag L, Lathrop DA, Csaba L, Talosi L, Papp JG.. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol 2000;523(Pt 1):67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, Nagy Z, Bogats G, Lathrop DA, Papp JG, Varro A.. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation 2005;112:1392–1399. [DOI] [PubMed] [Google Scholar]
- 21. Li GR, Lau CP, Leung TK, Nattel S.. Ionic current abnormalities associated with prolonged action potentials in cardiomyocytes from diseased human right ventricles. Heart Rhythm 2004;1:460–468. [DOI] [PubMed] [Google Scholar]
- 22. Volders PG, Sipido KR, Vos MA, Spatjens RL, Leunissen JD, Carmeliet E, Wellens HJ.. Downregulation of delayed rectifier K(+) currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation 1999;100:2455–2461. [DOI] [PubMed] [Google Scholar]
- 23. Lengyel C, Virag L, Biro T, Jost N, Magyar J, Biliczki P, Kocsis E, Skoumal R, Nanasi PP, Toth M, Kecskemeti V, Papp JG, Varro A.. Diabetes mellitus attenuates the repolarization reserve in mammalian heart. Cardiovasc Res 2007;73:512–520. [DOI] [PubMed] [Google Scholar]
- 24. Vos MA, de Groot SH, Verduyn SC, van der Zande J, Leunissen HD, Cleutjens JP, van Bilsen M, Daemen MJ, Schreuder JJ, Allessie MA, Wellens HJ.. Enhanced susceptibility for acquired torsade de pointes arrhythmias in the dog with chronic, complete AV block is related to cardiac hypertrophy and electrical remodeling. Circulation 1998;98:1125–1135. [DOI] [PubMed] [Google Scholar]
- 25. Lengyel C, Varro A, Tabori K, Papp JG, Baczko I.. Combined pharmacological block of I(Kr) and I(Ks) increases short-term QT interval variability and provokes torsades de pointes. Br J Pharmacol 2007;151:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Major P, Baczkó I, Hiripi L, Odening KE, Juhász V, Kohajda Z, Horváth A, Seprényi G, Kovács M, Virág L, Jost N, Prorok J, Ördög B, Doleschall Z, Nattel S, Varró A, Bősze Z.. A novel transgenic rabbit model with reduced repolarization reserve: long QT syndrome caused by a dominant-negative mutation of the KCNE1 gene. Br J Pharmacol 2016;173:2046–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J, Drexler H.. Gene expression of the cardiac Na(+)-Ca2+ exchanger in end-stage human heart failure. Circ Res 1994;75:443–453. [DOI] [PubMed] [Google Scholar]
- 28. Cerbai E, Pino R, Porciatti F, Sani G, Toscano M, Maccherini M, Giunti G, Mugelli A.. Characterization of the hyperpolarization-activated current, I(f), in ventricular myocytes from human failing heart. Circulation 1997;95:568–571. [DOI] [PubMed] [Google Scholar]
- 29. Vermeulen JT, McGuire MA, Opthof T, Coronel R, de Bakker JM, Klopping C, Janse MJ.. Triggered activity and automaticity in ventricular trabeculae of failing human and rabbit hearts. Cardiovasc Res 1994;28:1547–1554. [DOI] [PubMed] [Google Scholar]
- 30. Shannon TR, Pogwizd SM, Bers DM.. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 2003;93:592–594. [DOI] [PubMed] [Google Scholar]
- 31. Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A.. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002;106:69–74. [DOI] [PubMed] [Google Scholar]
- 32. Eldar M, Pras E, Lahat H.. A missense mutation in the CASQ2 gene is associated with autosomal-recessive catecholamine-induced polymorphic ventricular tachycardia. Trends Cardiovasc Med 2003;13:148–151. [DOI] [PubMed] [Google Scholar]
- 33. Atchley AE Jr, Douglas PS.. Left ventricular hypertrophy in athletes: morphologic features and clinical correlates. Cardiol Clin 2007;25:371–382, v. [DOI] [PubMed] [Google Scholar]
- 34. Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, Brugada J, Nattel S, Mont L.. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011;123:13–22. [DOI] [PubMed] [Google Scholar]
- 35. D’Souza A, Bucchi A, Johnsen AB, Logantha SJRJ, Monfredi O, Yanni J, Prehar S, Hart G, Cartwright E, Wisloff U, Dobryznski H, DiFrancesco D, Morris GM, Boyett MR.. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat Commun 2014;5:3775.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D’Souza A, Pearman CM, Wang Y, Nakao S, Logantha SJRJ, Cox C, Bennett H, Zhang Y, Johnsen AB, Linscheid N, Poulsen PC, Elliott J, Coulson J, McPhee J, Robertson A, da Costa Martins PA, Kitmitto A, Wisløff U, Cartwright EJ, Monfredi O, Lundby A, Dobrzynski H, Oceandy D, Morris GM, Boyett MR.. Targeting miR-423-5p reverses exercise training-induced HCN4 channel remodeling and sinus bradycardia. Circ Res 2017;121:1058–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varro A, Baczko I.. Possible mechanisms of sudden cardiac death in top athletes: a basic cardiac electrophysiological point of view. Pflugers Arch 2010;460:31–40. [DOI] [PubMed] [Google Scholar]
- 38.Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on S7B Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals; availability. Notice. Fed Regist 2005;70:61133–61134. [PubMed] [Google Scholar]
- 39.Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed Regist 2005;70:61134–61135. [PubMed] [Google Scholar]
- 40. Jost N, Virag L, Comtois P, Ordog B, Szuts V, Seprenyi G, Bitay M, Kohajda Z, Koncz I, Nagy N, Szel T, Magyar J, Kovacs M, Puskas LG, Lengyel C, Wettwer E, Ravens U, Nanasi PP, Papp JG, Varro A, Nattel S.. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J Physiol 2013;591:4189–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zicha S, Moss I, Allen B, Varro A, Papp J, Dumaine R, Antzelevich C, Nattel S.. Molecular basis of species-specific expression of repolarizing K+ currents in the heart. Am J Physiol Heart Circ Physiol 2003;285:H1641–H1649. [DOI] [PubMed] [Google Scholar]
- 42. Schlüter KD. Cardiomyocytes—Active Players in Cardiac Disease. Switzerland: Springer International Publishing AG; 2016. [Google Scholar]
- 43. Ross J., Jr. Mechanisms of cardiac contraction. What roles for preload, afterload and inotropic state in heart failure? Eur Heart J 1983;4:19–28. [DOI] [PubMed] [Google Scholar]
- 44. Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol 2004;500:73–86. [DOI] [PubMed] [Google Scholar]
- 45. Villars PS, Hamlin SK, Shaw AD, Kanusky JT.. Role of diastole in left ventricular function, I: biochemical and biomechanical events. Am J Crit Care 2004;13:394–403; quiz 404–5. [PubMed] [Google Scholar]
- 46. Giricz Z, Varga ZV, Koncsos G, Nagy CT, Gorbe A, Mentzer RM Jr, Gottlieb RA, Ferdinandy P.. Autophagosome formation is required for cardioprotection by chloramphenicol. Life Sci 2017;186:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baranyai T, Giricz Z, Varga ZV, Koncsos G, Lukovic D, Makkos A, Sarkozy M, Pavo N, Jakab A, Czimbalmos C, Vago H, Ruzsa Z, Toth L, Garamvolgyi R, Merkely B, Schulz R, Gyongyosi M, Ferdinandy P.. In vivo MRI and ex vivo histological assessment of the cardioprotection induced by ischemic preconditioning, postconditioning and remote conditioning in a closed-chest porcine model of reperfused acute myocardial infarction: importance of microvasculature. J Transl Med 2017;15:67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gorbe A, Eder A, Varga ZV, Paloczi J, Hansen A, Ferdinandy P, Eschenhagen T.. Protection by the NO-Donor SNAP and BNP against Hypoxia/Reoxygenation in Rat Engineered Heart Tissue. PloS One 2015;10:e0132186.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giricz Z, Koncsos G, Rajtik T, Varga ZV, Baranyai T, Csonka C, Szobi A, Adameova A, Gottlieb RA, Ferdinandy P.. Hypercholesterolemia downregulates autophagy in the rat heart. Lipids Health Dis 2017;16:60.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bartekova M, Simoncikova P, Fogarassyova M, Ivanova M, Okruhlicova L, Tribulova N, Dovinova I, Barancik M.. Quercetin improves postischemic recovery of heart function in doxorubicin-treated rats and prevents doxorubicin-induced matrix metalloproteinase-2 activation and apoptosis induction. Int J Mol Sci 2015;16:8168–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barlaka E, Gorbe A, Gaspar R, Paloczi J, Ferdinandy P, Lazou A.. Activation of PPARbeta/delta protects cardiac myocytes from oxidative stress-induced apoptosis by suppressing generation of reactive oxygen/nitrogen species and expression of matrix metalloproteinases. Pharmacol Res 2015;95–96:102–110. [DOI] [PubMed] [Google Scholar]
- 52. Belliard A, Sottejeau Y, Duan Q, Karabin JL, Pierre SV.. Modulation of cardiac Na+, K+-ATPase cell surface abundance by simulated ischemia-reperfusion and ouabain preconditioning. Am J Physiol Heart Circ Physiol 2013;304:H94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Valen G. The basic biology of apoptosis and its implications for cardiac function and viability. Ann Thorac Surg 2003;75:S656–S660. [DOI] [PubMed] [Google Scholar]
- 54. Adameova A, Goncalvesova E, Szobi A, Dhalla NS.. Necroptotic cell death in failing heart: relevance and proposed mechanisms. Heart Fail Rev 2016;21:213–221. [DOI] [PubMed] [Google Scholar]
- 55. Huang C, Yitzhaki S, Perry CN, Liu W, Giricz Z, Mentzer RM Jr, Gottlieb RA.. Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Transl Res 2010;3:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Biala AK, Kirshenbaum LA.. The interplay between cell death signaling pathways in the heart. Trends Cardiovasc Med 2014;24:325–331. [DOI] [PubMed] [Google Scholar]
- 57. Gottlieb RA, Andres AM, Sin J, Taylor DP.. Untangling autophagy measurements: all fluxed up. Circ Res 2015;116:504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martinez-Gonzalez J, Badimon L.. Mechanisms underlying the cardiovascular effects of COX-inhibition: benefits and risks. Curr Pharm Des 2007;13:2215–2227. [DOI] [PubMed] [Google Scholar]
- 59. Tang SY, Monslow J, Todd L, Lawson J, Pure E, FitzGerald GA.. Cyclooxygenase-2 in endothelial and vascular smooth muscle cells restrains atherogenesis in hyperlipidemic mice. Circulation 2014;129:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Muscara MN, Vergnolle N, Lovren F, Triggle CR, Elliott SN, Asfaha S, Wallace JL.. Selective cyclo-oxygenase-2 inhibition with celecoxib elevates blood pressure and promotes leukocyte adherence. Br J Pharmacol 2000;129:1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gao C, Fu Y, Li Y, Zhang X, Zhang L, Yu F, Xu SS, Xu Q, Zhu Y, Guan Y, Wang X, Kong W.. Microsomal prostaglandin E synthase-1-derived PGE2 inhibits vascular smooth muscle cell calcification. Arterioscler Thromb Vasc Biol 2016;36:108–121. [DOI] [PubMed] [Google Scholar]
- 62. Zhang MZ, Yao B, Wang Y, Yang S, Wang S, Fan X, Harris RC.. Inhibition of cyclooxygenase-2 in hematopoietic cells results in salt-sensitive hypertension. J Clin Invest 2015;125:4281–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Funk CD, FitzGerald GA.. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol 2007;50:470–479. [DOI] [PubMed] [Google Scholar]
- 64. Walker C, Biasucci LM.. Cardiovascular safety of non-steroidal anti-inflammatory drugs revisited. Postgrad Med 2018;130:55–71. [DOI] [PubMed] [Google Scholar]
- 65. Singh BK, Haque SE, Pillai KK.. Assessment of nonsteroidal anti-inflammatory drug-induced cardiotoxicity. Expert Opin Drug Metab Toxicol 2014;10:143–156. [DOI] [PubMed] [Google Scholar]
- 66. Ghosh R, Goswami SK, Feitoza LF, Hammock B, Gomes AV.. Diclofenac induces proteasome and mitochondrial dysfunction in murine cardiomyocytes and hearts. Int J Cardiol 2016;223:923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tacconelli S, Bruno A, Grande R, Ballerini P, Patrignani P.. Nonsteroidal anti-inflammatory drugs and cardiovascular safety—translating pharmacological data into clinical readouts. Expert Opin Drug Saf 2017;16:1–807. [DOI] [PubMed] [Google Scholar]
- 68. Varga ZV, Ferdinandy P, Liaudet L, Pacher P.. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol 2015;309:H1453–H1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nemeth BT, Varga ZV, Wu WJ, Pacher P.. Trastuzumab cardiotoxicity: from clinical trials to experimental studies. Br J Pharmacol 2017;174:3727–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cappetta D, Rossi F, Piegari E, Quaini F, Berrino L, Urbanek K, De Angelis A.. Doxorubicin targets multiple players: a new view of an old problem. Pharmacol Res 2018;127:4–14. [DOI] [PubMed] [Google Scholar]
- 71. Davies KJ, Doroshow JH.. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 1986;261:3060–3067. [PubMed] [Google Scholar]
- 72. Doroshow JH, Davies KJ.. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 1986;261:3068–3074. [PubMed] [Google Scholar]
- 73. Kumar D, Lou H, Singal PK.. Oxidative stress and apoptosis in heart dysfunction. Herz 2002;27:662–668. [DOI] [PubMed] [Google Scholar]
- 74. Singal PK, Iliskovic N.. Doxorubicin-induced cardiomyopathy. N Engl J Med 1998;339:900–905. [DOI] [PubMed] [Google Scholar]
- 75. Berthiaume JM, Wallace KB.. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol 2007;23:15–25. [DOI] [PubMed] [Google Scholar]
- 76. Myers C. The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol 1998;25:10–14. [PubMed] [Google Scholar]
- 77. Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H.. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 2014;124:617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pacher P, Beckman JS, Liaudet L.. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C.. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation 2003;107:896–904. [DOI] [PubMed] [Google Scholar]
- 80. Mukhopadhyay P, Batkai S, Rajesh M, Czifra N, Harvey-White J, Hasko G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P.. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol 2007;50:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pereira GC, Pereira SP, Tavares LC, Carvalho FS, Magalhaes-Novais S, Barbosa IA, Santos MS, Bjork J, Moreno AJ, Wallace KB, Oliveira PJ.. Cardiac cytochrome c and cardiolipin depletion during anthracycline-induced chronic depression of mitochondrial function. Mitochondrion 2016;30:95–104. [DOI] [PubMed] [Google Scholar]
- 82. Bai P, Mabley JG, Liaudet L, Virag L, Szabo C, Pacher P.. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Oncol Rep 2004;11:505–508. [PubMed] [Google Scholar]
- 83. Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM, McDermott BJ, Grieve DJ.. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res 2010;70:9287–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shimauchi T, Numaga-Tomita T, Ito T, Nishimura A, Matsukane R, Oda S, Hoka S, Ide T, Koitabashi N, Uchida K, Sumimoto H, Mori Y, Nishida M.. TRPC3-Nox2 complex mediates doxorubicin-induced myocardial atrophy. JCI Insight 2017;2:93358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK.. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest 1996;98:1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kang YJ, Chen Y, Epstein PN.. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem 1996;271:12610–12616. [DOI] [PubMed] [Google Scholar]
- 87. Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN.. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest 1997;100:1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shioji K, Kishimoto C, Nakamura H, Masutani H, Yuan Z, Oka S, Yodoi J.. Overexpression of thioredoxin-1 in transgenic mice attenuates adriamycin-induced cardiotoxicity. Circulation 2002;106:1403–1409. [DOI] [PubMed] [Google Scholar]
- 89. Diotte NM, Xiong Y, Gao J, Chua BH, Ho YS.. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta 2009;1793:427–438. [DOI] [PubMed] [Google Scholar]
- 90. Mohamed HE, El-Swefy SE, Hagar HH.. The protective effect of glutathione administration on adriamycin-induced acute cardiac toxicity in rats. Pharmacol Res 2000;42:115–121. [DOI] [PubMed] [Google Scholar]
- 91. Dickey JS, Gonzalez Y, Aryal B, Mog S, Nakamura AJ, Redon CE, Baxa U, Rosen E, Cheng G, Zielonka J, Parekh P, Mason KP, Joseph J, Kalyanaraman B, Bonner W, Herman E, Shacter E, Rao VA.. Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model. PloS One 2013;8:e70575.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, Szabo C.. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther 2002;300:862–867. [DOI] [PubMed] [Google Scholar]
- 93. Pacher P, Liaudet L, Mabley JG, Cziraki A, Hasko G, Szabo C.. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int J Mol Med 2006;17:369–375. [PMC free article] [PubMed] [Google Scholar]
- 94. Berger NA, Besson VC, Boulares AH, Burkle A, Chiarugi A, Clark RS, Curtin NJ, Cuzzocrea S, Dawson TM, Dawson VL, Hasko G, Liaudet L, Moroni F, Pacher P, Radermacher P, Salzman AL, Snyder SH, Soriano FG, Strosznajder RP, Sumegi B, Swanson RA, Szabo C.. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br J Pharmacol 2018;175:192–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sobek S, Boege F.. DNA topoisomerases in mtDNA maintenance and ageing. Exp Gerontol 2014;56:135–141. [DOI] [PubMed] [Google Scholar]
- 96. Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET.. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012;18:1639–1642. [DOI] [PubMed] [Google Scholar]
- 97. Khouri MG, Ky B, Dunn G, Plappert T, Englefield V, Rabineau D, Yow E, Barnhart HX, St John Sutton M, Douglas PS.. Echocardiography core laboratory reproducibility of cardiac safety assessments in cardio-oncology. J Am Soc Echocardiogr 2018;31:361–371.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P.. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol 2009;296:H1466–H1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Maillet A, Tan K, Chai X, Sadananda SN, Mehta A, Ooi J, Hayden MR, Pouladi MA, Ghosh S, Shim W, Brunham LR.. Modeling doxorubicin-induced cardiotoxicity in human pluripotent stem cell derived-cardiomyocytes. Sci Rep 2016;6:25333.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Marwick TH. Cancer therapy-related cardiac dysfunction: unresolved issues. Can J Cardiol 2016;32:842–846. [DOI] [PubMed] [Google Scholar]
- 101. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM.. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 102. Chaudhari U, Nemade H, Gaspar JA, Hescheler J, Hengstler JG, Sachinidis A.. MicroRNAs as early toxicity signatures of doxorubicin in human-induced pluripotent stem cell-derived cardiomyocytes. Arch Toxicol 2016;90:3087–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Burridge PW, Li YF, Matsa E, Wu H, Ong SG, Sharma A, Holmstrom A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC.. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 2016;22:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fukuda A, Tahara K, Hane Y, Matsui T, Sasaoka S, Hatahira H, Motooka Y, Hasegawa S, Naganuma M, Abe J, Nakao S, Takeuchi H, Nakamura M.. Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. PloS One 2017;12:e0185654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kanwal U, Irfan Bukhari N, Ovais M, Abass N, Hussain K, Raza A.. Advances in nano-delivery systems for doxorubicin: an updated insight. J Drug Target 2018;26:296–310. [DOI] [PubMed] [Google Scholar]
- 106. Qu C, Li J, Zhou Y, Yang S, Chen W, Li F, You B, Liu Y, Zhang X.. Targeted delivery of doxorubicin via CD147-mediated ROS/pH dual-sensitive nanomicelles for the efficient therapy of hepatocellular carcinoma. AAPS J 2018;20:34.. [DOI] [PubMed] [Google Scholar]
- 107. Abdel-Qadir H, Nolan MT, Thavendiranathan P.. Routine prophylactic cardioprotective therapy should be given to all recipients at risk of cardiotoxicity from cancer chemotherapy. Can J Cardiol 2016;32:921–925. [DOI] [PubMed] [Google Scholar]
- 108. Ferdinandy P, Szilvassy Z, Baxter GF.. Adaptation to myocardial stress in disease states: is preconditioning a healthy heart phenomenon? Trends Pharmacol Sci 1998;19:223–229. [DOI] [PubMed] [Google Scholar]
- 109. Ferdinandy P, Schulz R, Baxter GF.. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 2007;59:418–458. [DOI] [PubMed] [Google Scholar]
- 110. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R.. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142–1174. [DOI] [PubMed] [Google Scholar]
- 111. Hausenloy DJ, Garcia-Dorado D, Botker HE, Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J, Madonna R, Ovize M, Perrino C, Prunier F, Schulz R, Sluijter JPG, Van Laake LW, Vinten-Johansen J, Yellon DM, Ytrehus K, Heusch G, Ferdinandy P.. Novel targets and future strategies for acute cardioprotection: position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:564–585. [DOI] [PubMed] [Google Scholar]
- 112. Perrino C, Barabasi AL, Condorelli G, Davidson SM, De Windt L, Dimmeler S, Engel FB, Hausenloy DJ, Hill JA, Van Laake LW, Lecour S, Leor J, Madonna R, Mayr M, Prunier F, Sluijter JPG, Schulz R, Thum T, Ytrehus K, Ferdinandy P.. Epigenomic and transcriptomic approaches in the post-genomic era: path to novel targets for diagnosis and therapy of the ischaemic heart? Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Golomb E, Schneider A, Houminer E, Dunnick J, Kissling G, Borman JB, Nyska A, Schwalb H.. Occult cardiotoxicity: subtoxic dosage of Bis(2-chloroethoxy)methane impairs cardiac response to simulated ischemic injury. Toxicol Pathol 2007;35:383–387. [DOI] [PubMed] [Google Scholar]
- 114. Szilvassy Z, Ferdinandy P, Nagy I, Jakab I, Koltai M.. The effect of continuous versus intermittent treatment with transdermal nitroglycerin on pacing-induced preconditioning in conscious rabbits. Br J Pharmacol 1997;121:491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fekete V, Murlasits Z, Aypar E, Bencsik P, Sarkozy M, Szenasi G, Ferdinandy P, Csont T.. Myocardial postconditioning is lost in vascular nitrate tolerance. J Cardiovasc Pharmacol 2013;62:298–303. [DOI] [PubMed] [Google Scholar]
- 116. Kocsis GF, Pipis J, Fekete V, Kovacs-Simon A, Odendaal L, Molnar E, Giricz Z, Janaky T, van Rooyen J, Csont T, Ferdinandy P.. Lovastatin interferes with the infarct size-limiting effect of ischemic preconditioning and postconditioning in rat hearts. Am J Physiol Heart Circ Physiol 2008;294:H2406–H2409. [DOI] [PubMed] [Google Scholar]
- 117. Li Y, Hu Y, Ley SH, Rajpathak S, Hu FB.. Sulfonylurea use and incident cardiovascular disease among patients with type 2 diabetes: prospective cohort study among women. Diabetes Care 2014;37:3106–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tian Y, Li H, Liu P, Xu JM, Irwin MG, Xia Z, Tian G.. Captopril pretreatment produces an additive cardioprotection to isoflurane preconditioning in attenuating myocardial ischemia reperfusion injury in rabbits and in humans. Mediators Inflamm 2015;2015:819232.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Van der Mieren G, Nevelsteen I, Vanderper A, Oosterlinck W, Flameng W, Herijgers P.. Angiotensin-converting enzyme inhibition and food restriction restore delayed preconditioning in diabetic mice. Cardiovasc Diabetol 2013;12:36.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hausenloy DJ, Yellon DM.. The second window of preconditioning (SWOP) where are we now? Cardiovasc Drugs Ther 2010;24:235–254. [DOI] [PubMed] [Google Scholar]
- 121. Meune C, Mourad JJ, Bergmann JF, Spaulding C.. Interaction between cyclooxygenase and the renin-angiotensin-aldosterone system: rationale and clinical relevance. J Renin Angiotensin Aldosterone Syst 2003;4:149–154. [DOI] [PubMed] [Google Scholar]
- 122. Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M.. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci USA 2004;101:6975–6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Suzuki YJ, Nagase H, Day RM, Das DK.. GATA-4 regulation of myocardial survival in the preconditioned heart. J Mol Cell Cardiol 2004;37:1195–1203. [DOI] [PubMed] [Google Scholar]
- 124. Takeda M, Miyagawa S, Fukushima S, Saito A, Ito E, Harada A, Matsuura R, Iseoka H, Sougawa N, Mochizuki-Oda N, Matsusaki M, Akashi M, Sawa Y.. Development of in vitro drug-induced cardiotoxicity assay by using three-dimensional cardiac tissues derived from human induced pluripotent stem cells. Tissue Eng Part C Methods 2017;24:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Conant G, Lai BFL, Lu RXZ, Korolj A, Wang EY, Radisic M.. High-content assessment of cardiac function using heart-on-a-chip devices as drug screening model. Stem Cell Rev 2017;13:335–346. [DOI] [PubMed] [Google Scholar]
- 126. Maillet A, Tan KP, Brunham LR.. Use of human pluripotent stem cell derived-cardiomyocytes to study drug-induced cardiotoxicity. Curr Protoc Toxicol 2017;73:22.5.1–22.5.22. [DOI] [PubMed] [Google Scholar]
- 127. Chaudhari U, Nemade H, Wagh V, Gaspar JA, Ellis JK, Srinivasan SP, Spitkovski D, Nguemo F, Louisse J, Bremer S, Hescheler J, Keun HC, Hengstler JG, Sachinidis A.. Identification of genomic biomarkers for anthracycline-induced cardiotoxicity in human iPSC-derived cardiomyocytes: an in vitro repeated exposure toxicity approach for safety assessment. Arch Toxicol 2016;90:2763–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kopljar I, De Bondt A, Vinken P, Teisman A, Damiano B, Goeminne N, Van den Wyngaert I, Gallacher DJ, Lu HR.. Chronic drug-induced effects on contractile motion properties and cardiac biomarkers in human induced pluripotent stem cell-derived cardiomyocytes. Br J Pharmacol 2017;174:3766–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang L, Xu MX, Yin QS, Zhu CY, Cheng XL, Ren YR, Zhuang PW, Zhang YJ.. Screening, verification, and analysis of biomarkers for drug-induced cardiac toxicity in vitro based on RTCA coupled with PCR Array technology. Toxicol Lett 2017;268:17–25. [DOI] [PubMed] [Google Scholar]
- 130. Nishimura Y, Kondo C, Morikawa Y, Tonomura Y, Torii M, Yamate J, Uehara T.. Plasma miR-208 as a useful biomarker for drug-induced cardiotoxicity in rats. J Appl Toxicol 2015;35:173–180. [DOI] [PubMed] [Google Scholar]
- 131. Sandhu H, Maddock H.. Molecular basis of cancer-therapy-induced cardiotoxicity: introducing microRNA biomarkers for early assessment of subclinical myocardial injury. Clin Sci 2014;126:377–400. [DOI] [PubMed] [Google Scholar]
- 132. Wang W, Shi Q, Mattes WB, Mendrick DL, Yang X.. Translating extracellular microRNA into clinical biomarkers for drug-induced toxicity: from high-throughput profiling to validation. Biomark Med 2015;9:1177–1188. [DOI] [PubMed] [Google Scholar]
- 133. Faunce T, Townsend R, McEwan A.. The Vioxx pharmaceutical scandal: Peterson v Merke Sharpe & Dohme (Aust) Pty Ltd (2010) 184 FCR 1. J Law Med 2010;18:38–49. [PubMed] [Google Scholar]
- 134. Mayer TV. Merck's Careful Study of Vioxx. Am J Cardiol 2008;101:1068–1069; author reply 1069. [DOI] [PubMed] [Google Scholar]
- 135.Associations. IFoPM. The pharmaceutical industry and global health, facts and figures. 2012. https://www.ifpma.org/wp-content/uploads/2016/01/IFPMA_-_Facts_And_Figures_2012_LowResSinglePage.pdf (accessed 1 April 2018).
- 136. Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL.. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov 2010;9:203–214. [DOI] [PubMed] [Google Scholar]