Abstract
Lentiviral vectors (LVs) are used for delivery of genes into hematopoietic stem and progenitor cells (HSPCs) in clinical trials worldwide. LVs, in contrast to retroviral vectors, are not associated with insertion site-associated malignant clonal expansions and, thus, are considered safer. Here, however, we present a case of markedly abnormal dysplastic clonal hematopoiesis affecting the erythroid, myeloid, and megakaryocytic lineages in a rhesus macaque transplanted with HSPCs that were transduced with a LV containing a strong retroviral murine stem cell virus (MSCV) constitutive promoter-enhancer in the LTR. Nine insertions were mapped in the abnormal clone, resulting in overexpression and aberrant splicing of several genes of interest, including the cytokine stem cell factor and the transcription factor PLAG1. This case represents the first clear link between lentiviral insertion-induced clonal expansion and a clinically abnormal transformed phenotype following transduction of normal primate or human HSPCs, which is concerning, and suggests that strong constitutive promoters should not be included in LVs.
Keywords: gene therapy, genotoxicity, lentiviral vector, non-human primate
Espinoza et al. present a case of markedly abnormal dysplastic clonal hematopoiesis affecting the erythroid, myeloid, and megakaryocytic lineages in a rhesus macaque transplanted with HSPCs that were transduced with a LV containing a strong retroviral murine stem cell virus (MSCV) constitutive promoter-enhancer in the LTR. Nine insertions were mapped in the abnormal clone, resulting in overexpression and aberrant splicing of several genes of interest, including the cytokine stem cell factor and the transcription factor PLAG1. This case represents the first clear link between lentiviral insertion-induced clonal expansion and a clinically abnormal transformed phenotype following transduction of normal primate or human HSPCs, which is concerning, and suggests that strong constitutive promoters should not be included in LVs.
Introduction
Lentiviral vectors (LVs) are successfully used for delivery of genetic material into hematopoietic stem and progenitor cells (HSPCs) in clinical trials for inherited non-malignant diseases.1, 2, 3, 4, 5, 6, 7, 8 In contrast to γ-retroviral vectors, which have been shown to induce insertion-associated leukemias in both clinical trials9 and non-human primate models,10 LVs have not been associated with malignant clonal expansions in clinical trials or large animal models.11 LVs integrate preferentially within active genes rather than near transcriptional start sites12, 13 and have thus been considered less likely to activate nearby proto-oncogenes. However, reports from recent clinical trials suggest that LVs are capable of inducing insertion-site associated clonal (albeit non-malignant) expansions.8, 14 Long-term monitoring of transduced HSPC clones in humans and non-human primates remains critical for assessing the safety of LV gene therapies.
Our laboratory has used a rhesus macaque (RM) genetic barcoding model to study the output of lentivirally transduced transplanted HSPCs at a clonal level over time;15, 16, 17, 18 such a setting closely resembles that of lentiviral HSPC gene therapy trials in which autologous HSPCs are collected, transduced, and reinfused into the patient. In our published and unpublished studies, we tracked over 101,000 individual LV insertions in 10 transplanted macaques followed for up to 6 years and observed no clonal expansions associated with a transformed or aberrant hematologic phenotype. We now report the development of aberrant, markedly dysplastic fatal clonal hematopoiesis in one animal, affecting the erythroid, myeloid, and megakaryocytic lineages and originating from a single transduced HSPC clone containing 9 integrated LVs.
Results
Lentiviral Transduction and Transplantation
We developed a lentiviral barcoding model to quantitatively study the output of thousands of individual HSPCs following autologous transplantation.15, 16, 17, 18 As part of our ongoing studies, we transplanted animal ZL34 with autologous CD34+ HSPCs transduced with a lentiviral barcoding vector driving GFP expression via the strong murine retroviral murine stem cell virus (MSCV) promoter-enhancer inserted in the lentiviral long terminal repeat (LTR) (Figure 1A). The transduction or transplantation protocols utilized were unchanged from our prior experience,15, 16, 17, 18 and the MOI, CD34+ transplanted dose (cells per kilogram), and GFP percentage (GFP%) of the infused cells were within the ranges utilized for prior animals (Table S1; n = 10). ZL34, along with another monkey, ZL40 (Table S1), received cytomegalovirus (CMV)-suppressing cidofovir for 4 months to study the effect of CMV reactivation on immune reconstitution. With the exception of ZL34, all other animals, including ZL40, also receiving cidofovir on the same schedule continued to show stable, highly polyclonal output from transduced HSPCs with no evidence of genotoxicity or malignant clonal expansions at the latest follow-up (4–70 months; median, 25 months), regardless of having received HSPCs transduced with vectors containing either an internal EF1α promoter (n = 3) or the LTR-embedded MSCV promoter (n = 7), as published previously.15, 16, 17
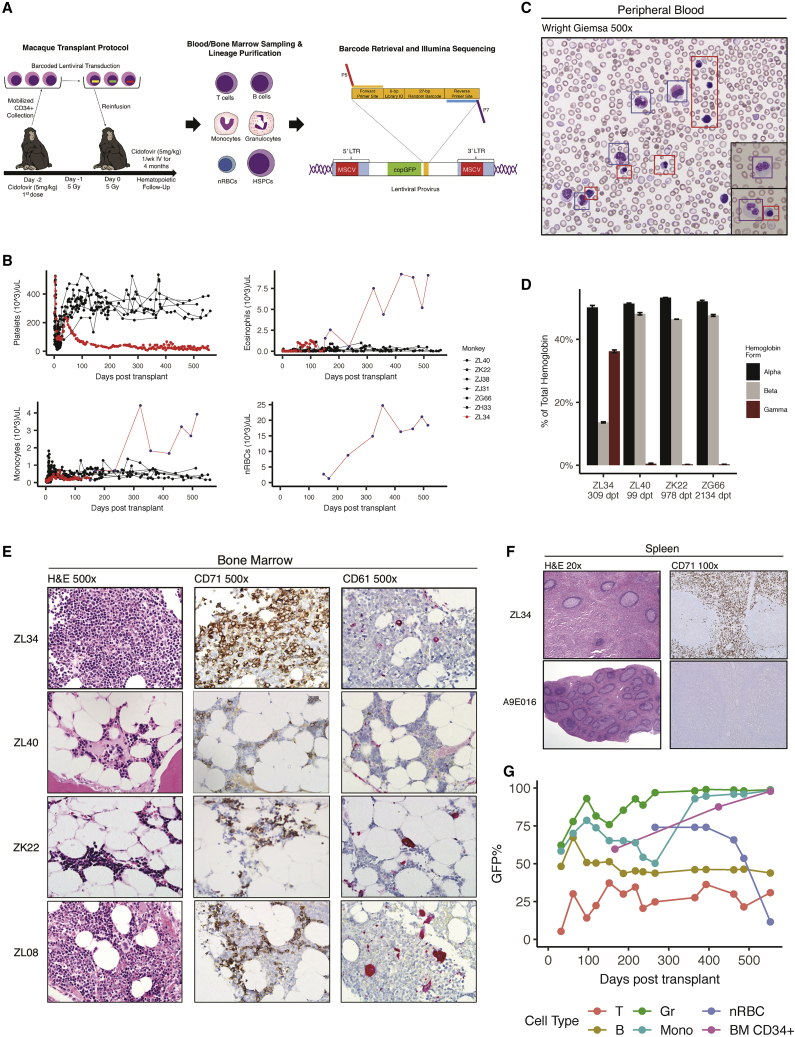
Figure 1.
Rhesus Macaque Autologous Transplantation Model and Development of Aberrant Hematopoiesis in Rhesus Macaque ZL34
(A) Transplantation timeline for ZL34. HSPCs were mobilized with G-CSF and AMD3100, collected via apheresis, enriched for CD34+ HSPCs, and transduced with a barcoded lentiviral vector. Following total body irradiation, transduced autologous CD34+ HSPCs were reinfused, and cidofovir was administered. The integrated form of the provirus is shown, detailing the position of the MSCV promoter and/or enhancer(s), copGFP marker gene, and high-diversity barcode library consisting of a 6-bp library ID and 27-bp random nucleotides, flanked by sequencing primer binding sites. (B) Platelet, eosinophil, monocyte, and nRBC concentrations in the blood over time in seven macaques receiving cells transduced with the barcoded LV containing the MSCV promoter and/or enhancer, including ZL34. Counts were determined by Coulter counter (red or black circles) or by automated PB smear scanning with Cellavision DM (blue dots, ZL34). (C) Wright’s-stained ZL34 PB smear from day 424 post-transplantation, showing frequent eosinophils (blue squares), nucleated erythroid cells (red squares), which are not found in normal rhesus macaque blood, and hypogranular neutrophils with dysplastic nuclei (black insets, purple squares). (D) HPLC assay of hemoglobin chain composition in the blood partitioned into α-, β-, and γ-globin molecules for ZL34 309 days post-transplantation (dpt) and three barcoded macaques without clonal expansions (ZL40, ZK22, and ZG66 99–2134 days post-transplantation). ZL34 values correspond to a predicted fetal hemoglobin of 70%. Error bars denote SD for three technical replicates. (E) BM biopsies from ZL34 (day 424), two healthy transplanted macaques (ZK22 and ZL40), and one healthy non-transplanted macaque (ZL08). CD71, immunohistochemistry for the erythroid precursor-specific transferrin receptor (CD71); CD61, immunohistochemistry for the megakaryocyte-specific glycoprotein IIIa (CD61). Note that ZL34’s megakaryocytes are small and uninuclear. (F) Spleen from ZL34 and a control-transplanted macaque (A9E016). (G) GFP%+ cells by flow cytometry over time in ZL34 PB T cells, B cells, granulocytes, monocytes, and nRBCs and BM CD34+ cells.
Development of Abnormal Hematopoiesis
ZL34 engrafted normally post-transplantation compared with other animals, promptly recovering neutrophils to more than 500/μL, platelets to more than 200,000/μL, and hemoglobin to more than 9 g/dL (Figures 1B, S1A, and S1B). However, ZL34’s platelet count declined beginning 49 days post-transplantation to levels far below the range maintained in other transplanted macaques (Figure 1B), requiring platelet transfusions to treat bleeding. Waxing and waning eosinophilia without identified infectious, drug, or allergic etiology emerged 50 days post-transplantation and became persistent by day 300 (Figure 1B). Eosinophilia did not develop in other macaques on the same transplant regimen. The morphology of neutrophils became markedly dysplastic, with hypogranularity and bilobulated and/or hypolobulated nuclei, despite normal neutrophil counts and lack of circulating blasts (Figures 1C and S1A). Monocytes and basophils also rose to well above normal range by day 300 (Figures 1B and S1C). In contrast, lymphocyte counts remained within expected post-transplant ranges (Figure S1D).
Nucleated red blood cells (nRBCs) are normally found only in the bone marrow (BM) of non-human primates and humans, with nuclear extrusion occurring prior to release into the blood. Strikingly, large numbers of nRBCs were prematurely released into the blood in ZL34, first noted on day 151 and reaching concentrations of more than 25,000/μL (Figures 1B and 1C). No other transplanted macaques had detectable circulating nRBCs. ZL34’s hemoglobin levels and RBC counts remained within the normal range until 1 year post-transplantation (Figures S1B and S1E). Analysis of globin chain ratios showed marked upregulation of γ-globin chains (and, thus, fetal hemoglobin) in ZL34’s RBCs long-term, in contrast to the normal dominance of adult β chains by day 100 or later in other transplanted macaques (Figure 1D).19
Compared with healthy transplanted (ZL40 and ZK22) and non-transplanted (ZL08) RMs, ZL34’s BM on day 424 was hypercellular, with increased eosinophils and marked erythroid dominance and markedly dysplastic uninuclear micro-megakaryocytes (Figure 1E). Blasts were not increased. The day 543 BM karyotype was normal in 20 of 20 metaphases. Splenic red pulp was markedly expanded because of extramedullary hematopoiesis, with infiltration of immature CD71+ erythroid cells (Figure 1F), an abnormal finding in macaques or humans. Pathologically and clinically, this hematologic disorder would be classified by World Health Organization (WHO) criteria as an overlap myelodysplastic/myeloproliferative neoplasm with extramedullary hematopoiesis. Bleeding necessitated euthanasia of ZL34 on day 553.
Inclusion of GFP from the copepod Pontellina plumata (copGFP) in the vector allowed analysis of the level of engraftment with transduced cells. The percent of GFP+ T and B cells remained generally stable over time and within the upper range of values observed in other barcoded RMs (Figures 1G and S1F).15, 16, 17, 18 In contrast, GFP%+ granulocytes (including neutrophils and eosinophils) and monocytes approached 100%, coincident with development of markedly abnormal blood counts, suggesting expansion of transduced cells within these lineages. GFP expression in the circulating nRBCs was also very high but dropped precipitously before euthanasia, potentially because of transgene silencing. Other barcoded animals did not show expansion of GFP+ cells of any lineage over time, with similar levels in all lineages other than delayed reconstitution of GFP+ T cells because of total body irradiation (TBI) effects on the thymus (Figure S1F).15, 16, 17
The percent of GFP+ CD34+ BM HSPCs also increased over time, reaching over 90%. Flow cytometric analysis of CD34+ subsets based on prior validation in macaques20 showed that the most primitive CD34+CD90+CD45RA− population, highly enriched for multipotent long-lived HSCs, was most markedly GFP+, with lower percentages in the CD34+CD90−CD45RA+ population containing lymphoid precursors (Figures S2A–S2C).20 Of note, the CD34+CD90+CD45RA− population was also markedly expanded in ZL34 BM compared with normal BM and present in the spleen, suggesting a differentiation block as well as extramedullary hematopoiesis (Figures S2A and S2B). These findings are consistent with development of a myeloproliferative neoplasm (MPN)/myelodysplastic syndromes (MDS) neoplastic syndrome resulting from abnormalities of transduced primitive HSPCs.
A Single Expanded Transduced Clone Was Responsible for Abnormal Hematopoiesis
We performed quantitative barcode retrieval from T cells, B cells, neutrophils, eosinophils, monocytes, nRBCs, and CD34+ cells using primers flanking the barcode region (Figures 2A–2G).15, 16, 17, 18 Six barcodes contributed equally and accounted for 100% of barcodes from nRBCs at all time points. The same 6 clones appeared and expanded in neutrophils and monocytes beginning on day 187, replacing earlier highly polyclonal contributions. Eosinophils on day 494 contained only the same 6 dominant barcodes. In contrast, T and B cells maintained polyclonal diversity over time, with intermittent detection of much lower levels of the 6 barcodes, likely because of contamination with clonally expanded myeloid or erythroid cells during sorting and/or aberrant marker expression on dysplastic myeloid cells.
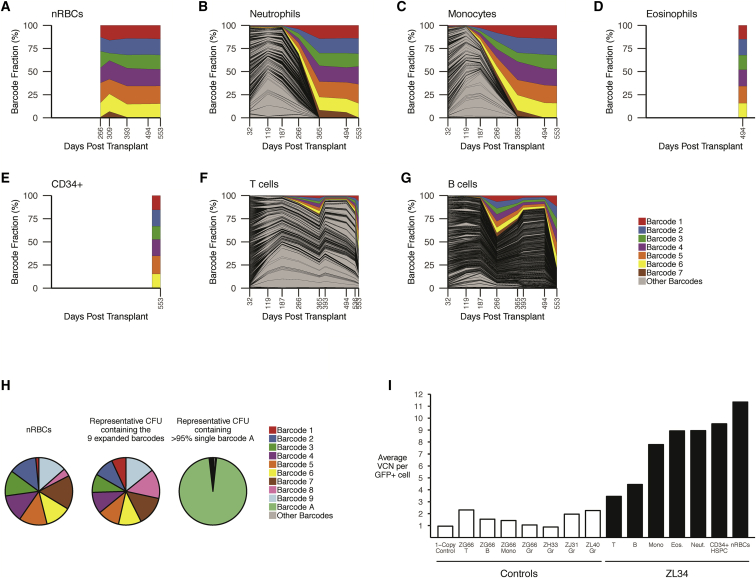
Figure 2.
Clonal Tracking via Barcode Retrieval in ZL34
(A–G) Longitudinal clonal tracking of barcodes retrieved from nRBCs (A), neutrophils (B), monocytes (C), eosinophils (D), BM CD34+ cells (E), T cells (F), and B cells (G). Each graph shows the fractional contributions from individual barcodes over time, with each colored ribbon representing the expanded barcodes detected via standard barcode recovery and all other barcodes shaded in gray. (H) Modified barcode recovery using the alternative forward primer on ZL34 nRBCs (393 days post-transplant), showing retrieval of 9 expanded barcodes. Modified barcode recovery using the same primers was performed on single-cell-derived GFP+ CFUs from ZL34 BM CD34+ cells (266 days post-transplant) plated at low density. Representative barcode analysis on a myeloid CFU containing all 9 barcodes is shown, matching those found in the circulating nRBC, confirming integration of the 9 barcodes in one original HSPC. For comparison, barcode analysis on a myeloid CFU from the same plate found to contain a single barcode, barcode A, is shown, confirming lack of cellular contamination across the plate that would complicate analysis. The small sector shown in gray represents multiple other barcodes with very low read counts, likely resulting from residual single cells not forming CFUs. (I) Vector copy number per GFP+ cell quantified via qPCR on Gr (granulocytes), Mono (monocytes), T cells, B cells, Eos (eosinophils), nRBCs, and CD34+ BM HSPCs from ZL34 (black bars, days 494–553 post-transplantation) and additional barcoded transplanted macaques (white bars, days 278–1,085 post-transplantation).
A seventh barcode was intermittently detected within the abnormal lineages because of a single mutation-related mismatch at the 3′ end of the primer annealing site (Figures 2A–2G), prompting redesign of an upstream primer to retrieve any additional insertions. Using this strategy, we recovered an additional 2 barcodes from circulating nRBCs for a total of 9 (Figure S3).
Because these expanded barcodes contributed roughly equally to every sample, we suspected multiple vector insertions within a single originating clonal HPSC. We plated ZL34 and control BM CD34+ cells in semi-solid medium, allowing barcode retrieval from individual colony-forming units (CFUs). Of note, despite the erythroid predominance in the BM, a reduced ratio of erythroid and/or myeloid colonies was observed in ZL34 compared with normal BM (Figure S4). We plucked individual GFP+ myeloid CFUs. All 9 barcodes were retrieved together from the majority of individual CFUs (Figure 2H). Rare CFUs lacked the 9 barcodes of interest, but each instead contained 1–4 other barcodes.
Vector copy numbers (VCNs) in samples from ZL34 and other lentivirally barcoded RMs was determined by qPCR. The normalized VCN per GFP+ cell for nRBCs, eosinophils, monocytes, neutrophils, and CD34+ HSPCs from late time points in ZL34 averaged 8.5 copies/cell (Figure 2I), which, along with the CFU barcode analysis, supports the conclusion that expansion from a single HSPC clone with 9 vector insertions was responsible for the myelodysplastic/myeloproliferative neoplasm in ZL34. The uninvolved B and T cell lineages in ZL34 had much lower VCNs per GFP+ cell, as did myeloid and lymphoid lineages in other barcoded macaques (Figure 2I).
Retrieval of LV Insertion Sites for the 9 Dominant Barcodes
We recovered 9 LV insertions from circulating nRBCs using standard methodologies (Figure 3A; Table S2). In contrast, insertions retrieved from neutrophils on day 96 were polyclonal (Table S2). We were able to link each of the 9 dominant barcodes to one of the 9 nRBC insertions via PCR amplification and sequencing (Figure 3A). 4 insertions localized to introns within the NCAM2, EIF3E, PLAG1, and IMMP2L genes, with PLAG1, NCAM2, and EIF3E proviral insertions in the same orientation as endogenous transcripts and the IMMP2L insertion in the opposite orientation. Additional genes were located within 1 Mb windows surrounding each of the 9 insertions (Figures 3B and S5). One insertion was less than 20 kb downstream of KITLG, encoding stem cell factor (SCF), a cytokine of importance in hematopoiesis. None of the insertions localized to known CCCTC-binding factor (CTCF) binding sites or RM sequences homologous to open chromatin regions mapped in human hematopoietic cells (Figure S6).
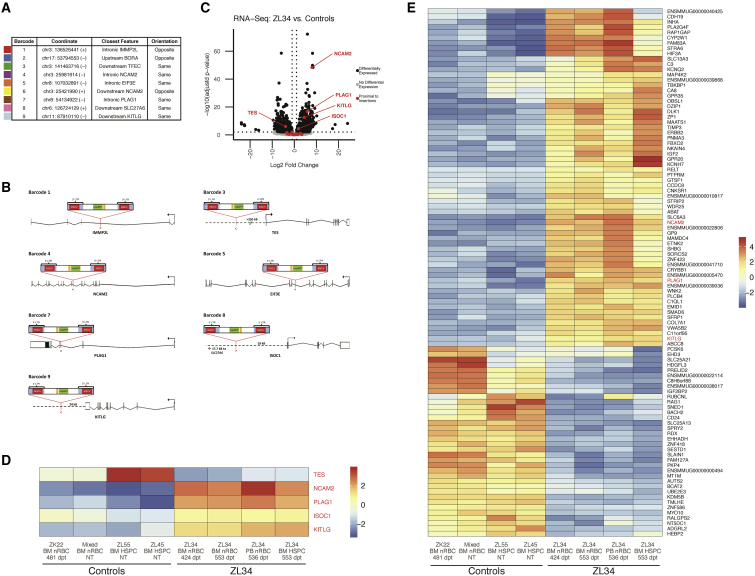
Figure 3.
Identification of Insertion Sites and Effect on Gene Expression in Hematopoietic Cells
(A) Localization in the rhesus genome, closest gene, and proviral orientation relative to the closest gene for the 9 insertion sites corresponding to the 9 expanded barcodes. (B) Orientation and location of the provirus relative to the 5 proximally differentially expressed genes and 2 genes with intronic disruptions. (C) Volcano plot of RNA-seq data from normal control versus ZL34 nRBCs and CD34+ cells showing all differentially expressed genes (black) as defined by adj. p < 0.01 and log2(fold change) > 1 or log2(fold change) < −1. Non-differentially expressed genes are shown in gray. Differentially expressed genes within 1 Mb upstream to 1 Mb downstream of any of the 9 insertion sites are colored in red and labeled. Two control BM nRBC samples (one from transplanted macaque ZK22 and one pooled from two non-transplanted macaques) and two control BM CD34+ samples (from two different non-transplanted macaques) were analyzed along with two independent BM nRBC samples, one PB nRBC sample, and one CD34+ sample from ZL34. (D) Mean-centered gene expression levels of NCAM2, KITLG, PLAG1, ISOC1, and TES. Expression levels are regularized log transformation (rlog) values from DESeq2. NT, non-transplanted. (E) Mean-centered gene expression levels of the top 100 differentially expressed genes (by adj. p value) in ZL34 compared with control nRBC and CD34+ HSPC samples. Expression levels are rlog values from DESeq2. A complete list of differentially expressed genes is given in Table S3.
Effect of Insertions on Gene Expression
We performed bulk RNA sequencing (RNA-seq) on ZL34 peripheral blood (PB) nRBCs, BM nRBCs, and BM CD34+ HSPCs obtained on days 424–553 post-transplantation as well as on control RM BM nRBCs and BM CD34+ HSPCs. No control RM PB nRBCs were obtained because nRBCs are not found in normal PB. We asked whether expression of genes interrupted by or within 1 Mb of any of the 9 insertions (Figures S5 and S7) were differentially expressed in ZL34 compared with controls. We found 5 genes within these windows to be significantly differentially expressed, combining nRBC and CD34 data and regressing out the source (PB or BM) and cell type (HSPCs or nRBCs) so that only significant differences attributed to condition (ZL34 cells versus controls) were identified (log2 fold change more than 1 or less than −1; adjusted p < 0.01) (Figures 3C and 3D). NCAM2 and PLAG1 with intronic insertions in the same orientation were overexpressed, KITLG with an insertion 24 kB downstream was overexpressed, ISOC1 with an insertion 18 kB upstream was overexpressed, and TES with an insertion 250 kB upstream was underexpressed. When restricting analysis to nRBCs, 4 genes were differentially expressed: NCAM2, PLAG1, KITLG, and ISOC1 (Figures S8A and S8B).
When considering all recovered transcripts, adjacent to insertions or not, a number of additional genes were differentially expressed. KITLG, NCAM2, and PLAG1 were among the top 100 in combined HSPC and nRBC analysis (Figure 3E; Table S3) and were either within the top 100 or differentially expressed within nRBCs alone (Figure S8C; Table S3). As expected from the hemoglobin analysis (Figure 1D), HBG2 transcripts encoding the γ-globin chain were significantly upregulated in ZL34 nRBCs (Figure S8C).
SCF binds to the c-kit tyrosine kinase receptor on HSPCs and stimulates survival and proliferation.21, 22 SCF is normally produced by BM stromal elements, not HSPCs. We measured soluble SCF levels in ZL34 and control blood and in media conditioned by cultured ZL34 and normal PB mononuclear cells (MNCs) (Figure 4A). The secreted isoform of SCF was not detected above background in cultured samples. An increase in SCF was detected in ZL34 serum. Using an antibody that reacts with the transmembrane isoform of SCF, we found clear increased expression of cell-associated SCF in hematopoietic cells, as shown by immunohistochemistry on marrow sections and via fluorescence-activated cell sorting (FACS) (Figures 4B, 4C, and S9B).
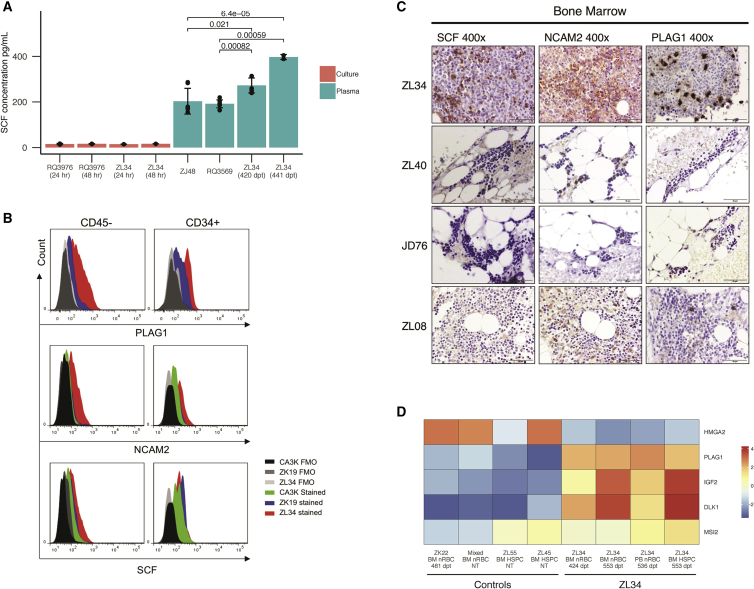
Figure 4.
Analysis of Dysregulated Expression of KITLG, NCAM2, and PLAG1
(A) Stem cell factor (SCF) concentrations in serum and in culture media from high-density cultured PB MNCs for ZL34 and controls, as determined by ELISA. Error bars are shown for technical replicates. p values are shown and were determined by Welch’s two-sample t test. (B) Expression of PLAG1, NCAM2, and SCF, assessed by flow cytometry in ZL34 and control non-transplanted normal macaque (ZK19) and a macaque recovering from malarial anemia (CA3K). Gating of BM CD45− cells was used as an alternative marker for nRBCs in these studies because of destruction of the CD71 epitope during permeabilization for intracellular staining. More than 85% of CD45− cells are nRBCs (Figure S9A). ZK19 is a non-transplanted normal monkey. (C) ZL34 and control BM samples (ZL08, non-transplanted; JD46 and ZL40, barcoded animals) stained via immunohistochemistry for PLAG1, SCF, and NCAM2. (D) Mean-centered gene expression levels for PLAG1, its putative upstream regulator HMGA2, and the PLAG1 downstream targets IGF2, DLK1, and MSI2 in ZL34 and control samples. Expression levels are rlog values from DESeq2.
PLAG1 is a zinc-finger transcription factor and proto-oncogene upregulated in salivary gland tumors23 and in murine and human myeloid leukemias.24, 25 At the protein level, PLAG1 was overexpressed on a per-cell basis in ZL34 CD45− nRBCs, HSPCs, and monocytes but not T or B cells, as shown via intracellular FACS and, most strikingly, immunohistochemistry, revealing numerous intensely PLAG1-positive BM cells compared with controls (Figures 4B 4C, and S9B). Increased activity of PLAG1 was confirmed by significant increases in expression of known downstream targets, including IGF2, which encodes insulin-like growth factor 2 (p = 3.66 × 10−17) and DLK1 (p = 7.70 × 10−31), both imprinted genes reported to be dysregulated in PLAG1-associated tumors (Figure 4D).26, 27, 28, 29 MSI2, a gene under direct regulation by PLAG130 whose product Musashi-2 is reported to promote HSPC expansion31 and self-renewal was also upregulated (p = 5.37 × 10−5) (Figure 4D). Of note, mRNA encoding HMGA2, a transcription factor functionally upstream of PLAG1,32 was expressed at a lower level in ZL34 nRBCs and HSPCs compared with control samples. Insertional overexpression of HMGA2 has been previously linked to clonal expansion in a lentiviral gene therapy clinical trial,8 suggesting that HMGA2 may stimulate HSPC clonal expansion via its effect on PLAG1 expression and activation of downstream targets active on HSPCs, such as MSI2, and that tonic low-level HMGA2 expression may be involved in normal HSPC homeostasis, controlled by feedback from downstream targets.
NCAM2 is an adhesion molecule required for cell-cell or cell-matrix interactions during nervous system development but is not normally expressed in hematopoietic cells, and dysregulation has not been linked to hematologic disease. Both FACS and immunohistochemistry confirmed overexpression of NCAM2 at a protein level in CD34+ cells, nRBCs, and monocytes (Figures 4B, 4C, and S9B). Decreased expression of the tumor suppressor TES has not been previously linked to hematologic malignancies. The TES gene itself was not directly disrupted by an insertion; thus, downregulation of this gene must have been indirect via disruption of a regulatory element. There is little information about the gene product of ISOC1. It is not expressed in hematopoietic cells and has no known link to hematologic disease.
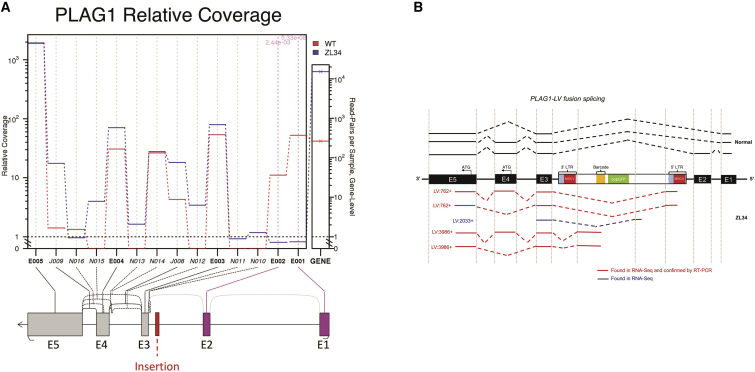
Insertion-Related Aberrant Splicing
LVs have been reported to disrupt splicing via formation of hybrid vector-gene transcripts expressed from viral promoters, resulting in dysregulated expression, alterations in isoform, and/or loss of transcript regulatory sequences.33, 34 We first interrogated RNA-seq data for any differentially spliced genes and found that ZL34 PLAG1 transcripts contained significantly lower levels of exons E1 and E2 compared with controls (Figure 5A). We did not detect significant variable splicing for any other gene with intronic insertions (Table S4). However, several other genes of hematopoietic interest, including HIF3A, demonstrated both changes in isoform abundance (Table S4) and overall upregulation (Figure 3E). We next mined RNA-seq data for fusion transcripts. We detected fusions between vector and PLAG1 sequences in ZL34 nRBCs and abundant hybrid forms containing vector sequences spliced to E3 before the translation start site,. These hybrid transcripts included isoforms with and without E4 (Figure 5B; Table S5), predicted to encode the two major isoforms of PLAG1 protein,35 but no mutant fusion proteins. We also detected fusion EIF3E and NCAM2 transcripts (Figure S10; Table S5); however, EIF3E was not overall upregulated in ZL34 (Figure S7). A subset of these hybrid transcripts was confirmed by RT-PCR (Figures 5B and S10). We did not detect hybrid transcripts for the other genes with intronic insertions or any non-LV fusion transcripts.
Figure 5.
Abnormal Splicing Related to Lentiviral Insertions
(A) Differential expression of exons within ZL34 relative to control samples, as determined from RNA-seq data, showing significant downregulation of exons 1 and 2 within ZL34 PLAG1 transcripts relative to control macaque nRBCs and HSPCs. (B) Hybrid vector-PLAG1 mRNA species detected via RNA-seq fusion transcript analysis and/or confirmation by PCR and Sanger sequencing in ZL34 and control nRBCs. Lines denote transcripts detected in ZL34 (red) and normal controls (black). The results show the absence of exons 1 and 2 in ZL34 PLAG1 transcripts along with hybrid vector-PLAG1 transcripts.
Discussion
We describe the first case of lentiviral insertion-induced genotoxicity, resulting in hematopoietic clonal expansion and a neoplastic phenotype in a large animal or human. Following autologous transplantation of HSPCs transduced with a barcoded LV containing the MSCV promoter-enhancer within the LTR, one macaque developed a fatal myeloproliferative/myelodysplastic syndrome linked to clonal expansion from a single transduced HSPC.
The development of leukemias in clinical gene therapy trials and in primate models utilizing murine γ-retrovirus vectors (RVs) to transduce HSPCs stimulated a search for safer gene transfer systems.9, 10, 11 Evidence implicated RVs activating nearby cellular proto-oncogenes with strong viral enhancers, exacerbated by RV integration favoring transcription start sites (TSSs).13 LVs had endogenous enhancers removed and were shown to have an integration pattern favoring gene bodies rather than promoters, hypothesized to be less likely to activate proto-oncogenes.36, 37 Genotoxicity models, including in vitro immortalization of murine HSPCs and acceleration of leukemia in tumor-prone mice, predicted significantly lower genotoxicity from LVs versus RVs, even when strong viral promoters and/or enhancers derived from RVs were inserted within LV backbones, although inclusion of these RV elements within LVs, particularly within the viral LTR, did increase genotoxicity over LVs without these elements.38, 39, 40, 41, 42 The majority of HSPC clinical gene therapy trials over the past decade have utilized LVs to try and decrease the risk of genotoxicity and take advantage of the increased efficiency of HSPC transduction associated with this vector class.1, 43
In the majority of our barcoded macaques, including ZL34, we used a LV construct containing the MSCV viral promoter and/or enhancer inserted within the LTR. The original MSCV RV vector was derived from a mutant murine myeloproliferative sarcoma virus (MSPV) with high expression in hematopoietic cells, and the MSCV promoter and/or enhancer was later shown to drive consistently high stable transgene expression in human HSPCs when included in LV vectors.44, 45 In a previous study, we reported development of acute myeloid leukemia (AML) following transduction of macaque HSPCs using an RV vector containing the MSCV promoter and/or enhancer.10 However, in 8 macaques transplanted with HSPCs transduced with the same LV barcoding vector containing the MSCV sequences, we tracked over 65,000 individual clones for up to 5 years without clonal expansions. In addition, 3 macaques that received HSPCs transduced with a barcoded LV containing an internal EF1α promoter showed clonal patterns indistinguishable from the MSCV animals (Table S1).15
Because RV promoters and/or enhancers included in LVs promote high constitutive transgene expression, two clinical trials for adrenoleukodystrophy have included an RV promoter and/or enhancer similar to MSCV, termed MND, in the LV to drive high expression of the transgene.4, 5, 46, 47 In contrast to the vector utilized in ZL34, the adrenoleukodystrophy trial LV placed the RV promoter and/or enhancer internally instead of within the LV LTR.4, 5, 46, 47 No clinical trials to date have utilized an LV with an RV promoter and/or enhancer within the LTR. Tumor-prone mouse models have suggested that an internal strong promoter and/or enhancer is less genotoxic than the same promoter and/or enhancer placed within the LV LTR.39 So far, no clonal expansions or hematopoietic toxicity have been reported in the 23 patients enrolled in these trials, with many years of follow-up. Other clinical trials using LVs containing endogenous or weaker constitutive promoters have reported polyclonal hematopoiesis for years post-transplantation.6, 48, 49, 50, 51 However, a thalassemia patient developed a marked clonal expansion linked to insertion of an LV containing an internal erythroid-specific promoter, resulting in increased expression and aberrant splicing of HMGA2.8 In contrast to our macaque, the clonal expansion in this patient was transient and not associated with any documented hematologic abnormalities. Recently, persistence and massive clonal expansion of a chimeric antigen receptor (CAR)-T cell clone in a patient was linked to an LV insertion inactivating the epigenetic regulator TET2.14
The expanded neoplastic clone in our macaque had 9 insertions, with 4 of the 9 insertions located within introns. Ongoing clinical trials for disorders requiring high transgene expression, such as sickle cell disease, are targeting and achieving VCNs of 4 or greater in vivo long-term to achieve disease correction; thus, a fraction of HSPCs must have VCNs as high as 9. We believe that the most compelling candidate insertion driving the HSPC phenotype was within the gene encoding the zinc-finger protein PLAG1. PLAG1 has been identified as a cooperating neoplastic “hit” via a mutagenesis screen in Cbfb-MYH11 murine leukemias and is upregulated in 20% of human AML.24 Recent studies have reported a role for PLAG1 in stimulating expression of Musashi-2, a protein that stimulates HSPC expansion.30 Detection of abundant aberrant splicing and LV-PLAG1 mRNA fusions corroborates previous reports of LV-associated aberrant splicing.8, 33, 34, 52, 53 Of note, the LV-PLAG1 splicing abrogated expression of non-translated E1 and E2 while maintaining expression of exons 3, 4, and 5, encompassing all translated sequences. We detected transcripts both with and without E4, predicting translation of both the short and long isoforms of PLAG1 protein.26 Functional differences between the two PLAG1 isoforms are poorly understood.
We observed upregulation of expression of known downstream targets of PLAG1 in ZL34 cells, most notably IGF2 and MSI2.30 Imprinting-related downregulation of IGF2 at the IGF2/H19 locus maintains HSPC quiescence, with loss of imprinting resulting in murine HSPC proliferation and exhaustion.54 MSI2 encodes the Musashi-2 RNA-binding protein, which promotes the expansion of long-term repopulating HSCs31 and regulates self-renewal.55 It is interesting to note that HMGA2 was downregulated in ZL34 hematopoietic cells compared with those from normal controls. HMGA2 is a transcription factor that functions as a critical upstream regulator of PLAG1 expression.32 An LV insertion resulting in overexpression of HMGA2 was linked to marked clonal expansion in an LV gene therapy trial noted above,8 and, based on the HSPC expansion seen in our animal, it seems possible that dysregulation of PLAG1 and downstream targets may have contributed to the expansion, although, to our knowledge, no aberrant hematologic phenotype developed in that patient. The downregulation of HMGA2 in ZL34 HSPCs coincident with upregulation of PLAG1 and downstream targets suggests that HMGA2 may be involved in normal hematopoiesis and regulated by feedback. Finally, the elevated levels of IGF2 mRNA resulting from PLAG1 overexpression may also have played a role in the persistently high fetal hemoglobin (HbF) levels observed in ZL34.56
Although we suspect that genotoxicity linked to the PLAG1 insertion likely played a key role in ZL34’s phenotype, we cannot rule out roles for other insertions. Given the doses transplanted, HSPCs with LV insertions in PLAG1 have almost certainly been delivered to our other macaques without development of abnormal clonal hematopoiesis. An insertion just downstream of KITLG resulted in upregulation of SCF, with a small increase in serum SCF. Cell-associated SCF protein was increased in HSPCs also expressing the KIT receptor, suggesting autocrine signaling, shown previously for other cytokines to drive proliferation in hematopoietic cells.57 We administered pharmacologic doses of SCF to macaques and did not observe eosinophilia or release of nRBCs into the circulation; thus, overexpression of SCF was unlikely to be the sole driver of ZL34’s aberrant hematopoiesis. However, it is of interest to note that production of fetal hemoglobin increases in baboons administered SCF58 and in human erythroid cultures supplemented with SCF,59 suggesting that dysregulation of SCF may have contributed to elevated HbF in ZL34. Insertions upregulating expression or leading to aberrant splicing of NCAM2, ISOC1, or EIF3E seem less likely to be contributing to clonal expansion and the phenotype, given the lack of known roles for these genes in hematopoiesis or leukemogenesis. Expression of testin (TES) RNA was decreased, a gene with tumor suppressor activities, potentially via inhibition of cell migration and metastasis.60 It is intriguing to speculate that loss of TES expression might have contributed to abnormal release of nRBCs into the blood. The constraints of the primate model and our inability to derive immortalized lines from ZL34 precluded further direct investigations of the contribution of each specific insertion to the hematologic phenotype.
In conclusion, this fatal genotoxic clonal expansion adverse event linked to LV insertions is concerning. The implications of this event must be balanced against the encouraging safety and efficacy of LV HSPC gene therapy clinical trials in patients with life-threatening diseases. It is also important to note that the vector resulting in toxicity in our macaque model included a strong viral promoter and/or enhancer within the LTR, a design shown to be associated with increased genotoxicity in preclinical models and not utilized in any clinical trial to date. However, we believe our findings imply that strong viral promoters should be avoided in LVs whenever possible. In addition, approaches to decrease LV-related aberrant splicing should be pursued, given our evidence that upregulation of PLAG1 was driven primarily by hybrid transcripts. Targeted gene editing approaches avoid semi-random vector insertion and have the potential to be a less genotoxic technology, although these approaches are in their infancy and may be associated with a different set of off-target genotoxicities.61, 62, 63
Materials and Methods
Autologous Transplantation of Lentivirally Barcoded HSPCs
CD34+ HSPCs were collected from ZL34, a 4-year-old male RM, following mobilization with granulocyte colony-stimulating factor (G-CSF)/AMD1300, transduced (MOI = 25) with a barcoded lentivirus library, and reinfused into the macaque following TBI as described in a protocol approved by the National Heart, Lung, and Blood Institute (NHLBI) Animal Care and Use Committee, following all applicable animal care regulations.15, 16, 17, 18 ZL34 received weekly dosing with 5 mg/kg cidofovir to suppress CMV reactivation.
Barcode Retrieval
DNA was extracted using the DNeasy Blood & Tissue Kit (QIAGEN). 200 ng was used for barcode retrieval PCR with Phusion High-Fidelity DNA Polymerase (Thermo Fisher Scientific) via 28 cycles of 98°C for 10 s, 70°C for 30 s, 72°C for 30 s, and then 72°C for 10 min. Indexed or non-indexed forward primers and indexed reverse primers were added (Table S6) for multiplex sequencing. Following gel purification, 15–24 multiplexed samples were used to create a DNA library for sequencing on an Illumina HiSeq2500/3000. Custom Python code (https://github.com/d93espinoza/barcode_extracter) was used for extraction and processing of barcodes from FASTQ files as described previously.15, 16, 17, 18 For modified barcode recovery, “Starcode” software (https://github.com/gui11aume/starcode) was used for extraction and processing of barcodes. Visualization was performed using custom R code.
Cell Purification and FACS Analysis
PB and BM samples were separated into granulocyte and mononuclear cell fractions via centrifugation over lymphocyte separation medium (MP Biomedical) and stained with antibodies (Table S7) prior to flow cytometric analysis and sorting to purities of more than 98%. CD34+ cells were purified by magnetic bead immunoselection (Miltenyi Biotec) to more than 95% purity. Neutrophils and eosinophils within the granulocyte pellet were separated based on CD33 expression. CD45–CD71+ flow gating was used to purify nRBCs for all figures, with the exception of those requiring intracellular PLAG1/NCAM2/SCF FACS staining (Figures 4B and S9), in which solely CD45− gating (>85% nRBCs) was used.
Colony Formation Assays
100 CD34+ BM cells purified from ZL34 BM were plated in 1 mL methylcellulose medium (STEMCELL Technologies, catalog number H4435) and cultured for 14 days. Single widely separated colonies were plucked for DNA extraction in 20 μL DirectPCR lysis reagent buffer (Viagen Biotech, catalog number 401-E) with 1 μL proteinase K and 2 mg/mL RNase.
Vector Integration Site Retrieval
Vector integration sites were identified and retrieved as described previously.51 Briefly, genomic DNA was sheared (Covaris) to an average size of 300–500 bp, end-repaired, and dA-tailed. T-linkers (Oligoseq) were ligated to the resulting fragments. PCR was performed using one LTR-specific primer and one linker-specific primer, followed by nested primers containing Illumina sequencing adaptors. The resulting library was sequenced on an Illumina MiSeq. Integration site junctions were trimmed with custom Perl scripts and mapped to rheMac8 using Blat.
Analysis of Vector Integration Sites Relative to Known Regulatory Sequences
The locations of vector integration sites were compared with CTCF binding sites as determined by chromatin immunoprecipitation sequencing (ChIP-seq). FASTQ files from four ChIP-seq experiments using hepatocytes of three male macaques were obtained (ArrayExpress: E-MTAB-437).64 We used STAR65 to map the reads to the rheMac8/Mmu8_8.0.1 reference genome and MACS266 to predict the genomic locations of CTCF binding sites from the aligned reads. Finally, the Bioconductor package GenomicRanges67 was used to compute overlaps between vector integration sites and CTCF binding sites.
Locations of vector integration sites were also more broadly compared with the locations of other regulatory elements, as determined by an assay for transposase-accessible chromatin using sequencing (ATAC-seq) data. ATAC-seq data from 18 human hematopoietic lineages were downloaded (GEO: GSE96772).68 We translated the ATAC-seq peaks from human genomic coordinates (aligned to hg19) into macaque genomic coordinates (aligned to rheMac8) using the University of California, Southern California (UCSC) batch coordinate conversion program Liftover and the appropriate chain file from the USCS Genome Browser website (http://genome.ucsc.edu). Again we used the Bioconductor package GenomicRanges67 to compute overlaps between vector integration sites and ATAC-seq peaks.
Hemoglobin Chain Measurements
Whole-blood cell pellets were lysed in 100 μL high-pressure liquid chromatography (HPLC)-grade water by pulse-vortexing three times for 30 s, followed by one cycle of freeze-thawing and centrifugation at 16,000 × g for 15 min. 10 μL of 100 mM Tris(2-chloroethyl) phosphate (TCEP; Thermo Fisher Scientific) was added, and after 5 min of incubation at room temperature, 85 μL of 0.1% trifluoroacetic acid (TFA)/32% acetonitrile was added. 10-μL samples were analyzed at a 0.7 mL/min flow rate for 50 min using the Agilent 1100 HPLC (Agilent Technologies) equipped with the Aeris 3.6-μm Widepore C4 200 (250 × 4.6 mm, Phenomenex, Torrance, CA) reverse-phase column using solvent A (0.12% TFA in water) and solvent B (0.08% TFA in acetonitrile). A starting concentration of solvent B was designated as 35% for the separation of globin proteins and the percentage of solvent B was changed as follows: 3 min at up to 41.2%, 3 min at up to 41.6%, 5 min at up to 42%, 4 min at up to 42.4%, 6 min at up to 42.8%, 6 min at up to 44.4%, 6 min at up to 47%, 7 min at up to 75%, and re-equilibration for 10 minutes at 35%. The globin types were detected at 215 nm and confirmed by an Agilent HPLC-6224 mass spectrometer equipped with an electrospray ionization (ESI) interface and a time-of-flight (TOF) mass detector as described previously.69, 70
Soluble SCF Detection Using ELISA
Plasma was obtained by centrifuging whole blood at 2,000 × g for 15 min at 4°C. 1 × 107 PB MNCs were cultured for 24 and 48 h in RPMI10 medium supplemented with 10% fetal bovine serum. The stem cell factor human SimpleStep ELISA Kit (Abcam, Cambridge, MA; catalog number 176109) was used to measure SCF levels in plasma and conditioned culture medium.
RNA-Seq and Analysis
CD45−CD71+ nRBC and CD34+ cells from ZL34 (samples collected on days 410–540 post-transplantation) or control monkey PB or BM (4 age-matched untransplanted animals and 1 transplanted animal day 465 post-transplantation) were purified by FACS (nRBCs) or immunoselection (CD34+ cells). Total RNA was extracted with RNAzol RT (MRC, OH, USA). RNA libraries were prepared using the Illumina TruSeq Stranded Total RNA kit with Ribo-Zero and sequenced on an Illumina HiSeq 3000.
RNA-seq data analysis was performed using STAR,65 DESeq2,71 and custom R code. Genome assembly Mmul_8.0.1 was used. Macaque annotation from Ensembl release 92 was used. Analysis of nRBC and HSPC data together was performed in DESeq2 using the design formula ∼source + cell type + condition, where source was PB or BM, cell type was HPSC or nRBC, and condition was ZL34 or control. nRBC-only analysis was performed using the design formula ∼source + condition. Built-in Cook’s cutoff was used in DESeq2 to alleviate the effect of outliers on resulting differentially expressed genes.
Histologic and Immunohistochemical Analyses
BM biopsies were obtained from the posterior iliac crests. Following fixation and decalcification, sections were prepared. Spleen samples were obtained at the time of autopsy. Antibodies utilized for immunohistochemical staining are listed in Table S7.
Analysis of Differential Usage of Splice Variants
We implemented a pipeline to detect fusion genes in parallel with computing differential usage of gene features and detects. In brief, STAR v 2.6.0c65 was used to align reads to a combination Macaca mulatta, (ENSEMBL Mmul_8.0.1) plus custom vector reference genomes and a combination Macaca mulatta annotation (ENSEMBL, Mmul_8.0.1.92 general transfer format) plus custom annotation for the vector sequence. Then STAR-Fusion (https://github.com/STAR-Fusion/STAR-Fusion/wiki) was used to compute fusion genes using a custom genome resource library for the macaque and vector reference genomes, which was built using FusionFilter. Differential usage of gene features was computed using QoRTs72 and JunctionSeq.73 Our implementation of differential gene expression relied on DESeq2.71 All components of our pipeline were unified under custom shell and R scripts that were run on the NIH High Performance Computing cluster. All of our source code and scripts are available via BitBucket (https://bitbucket.org/DunbarLab_Releases/variant-splicing_fusion-genes).
Data Availability
All data and code used in this study (RNA-seq and barcode data) will be made available upon request from the corresponding authors.
Author Contributions
Conceptualization, C.E.D. and C.W.; Analytics, D.A.E., S.F.C., and L.L.T.; Investigation, D.A.E., X.F., D.Y., C.W., K.R.C., I.M.Y., S.D., X.W., and S.S.D.; Resources, R.L., H.L.M., N.U., J.F.T., and K.J.H.; Animal Support, S.G.H., A.K., M.M., and R.E.D.; Writing, C.E.D., D.A.E., and C.W.; Supervision, C.E.D., C.W., and J.F.T.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This research was supported by the NHLBI Division of Intramural Research. Di Yang was funded by the Scientific Research Training Program for Young Talents sponsored by Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, China. We thank Keyvan Keyvanfar, the NHLBI FACS and DNA Sequencing and Genomics Cores, the NIH Biowulf High-Performance Computing Resource, NIH veterinary pathology, and NHLBI animal care staff for assistance. We acknowledge Patrick Duffy and Amber Raja for supplying marrow from animal CA3K.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2019.04.003.
Contributor Information
Chuanfeng Wu, Email: wuc3@mail.nih.gov.
Cynthia E. Dunbar, Email: dunbarc@nhlbi.nih.gov.
Supplemental Information
Viral integration site retrieval (5’ and 3’-based) in ZL34 nRBC DNA at 309 and 393 days post-transplant, and in granulocyte DNA at 96 days post-transplant. Green denotes the integration site recovered has been matched to one of the originally recovered barcodes and is concluded to arise from the single expanded clone in ZL34.
Differentially expressed genes reported from DESeq2, identified as described in Bulk RNA-seq analysis. ens_id = Ensembl gene ID. baseMean = the average of the DESeq2 normalized count values for all samples, normalized for sequencing depth. log2FoldChange = DESeq2 estimated effect size in log2 scale, comparing ZL34 to controls. lfcSE = standard error of the log2FoldChange estimate. stat = DESeq2 Wald statistic. pvalue = Wald test p-value. adj. p = Benjamini-Hochberg adjusted p-value.
Differential expression of gene features (e.g. exons or exon junctions) obtained from the comparison of RNA-seq data 5 from (i) four samples from ZL-34 (1 sample of nucleated erythrocytes from peripheral blood, 2 samples of nucleated erythrocytes from bone marrow and 1 sample of CD34+ cells obtained from bone marrow) and (ii) 4 samples from a wild type macaque (2 samples of nucleated erythrocytes obtained from bone marrow and 2 samples of CD34+ HSPCs obtained from BM). Differential expression of features was computed with our pipeline and a custom index for the combined macaque and lentiviral as described in the supplemental methods. Tab 1 is a gene level overview of features (e.g. exons or junctions) that are differentially expressed with an adjusted p-value of less than 0.05. The meanings of the columns is described in comments added to each column and also tabulated below. Tab 2 is a more detailed presentation of the results at the level of individual gene features. Again, the meanings of each of the columns is described in comments added to each column and also tabulated below. Columns on Tab 1: Column 1 (ID): ENSEMBL gene ID.(Macaque ENSEMBL release 92) Column 2 (Gene Symbol): HGNC symbol corresponding to ENSEMBL ID, if known Column 3 (Description): Description of gene function, if known. Column 4 (Chr): Chromosome on which gene is located. Column 5 (Start): (1-based) position of the start of gene 6 Column 6 (End): (1-based) end of the gene. Column 7 (Strand): Strand on which gene is located. Column 8 (baseMean): The base mean normalized coverage counts for the locus across all conditions. Column 9 (geneWisePadj): The gene-level p-value that one or more features belonging to this gene are differentially used. This value will be the same for all features belonging to the same gene. Column 10 (mostSIgID): The sub-feature OD for the most significant exon or splice junction belonging to the gene. Column 11 (mostSIgPadj): The adjusted p-value for the most signifiance exon or splice-junction belonging to the gene. Column 12 (numExons): The number of known non-overlapping exonic regions belonging to the gene. Column 13 (numKnown): The number of known splice junctions belonging to the gene. Column 14 (numNovel): The number of novel splice junctions belonging to the gene. Column 15 (exonsSig): The number of statistically significant non-overlapping exonic regions belonging to the gene. Column 16 (knownSIg): The number of statistically significant known splice junctions belonging to the gene Column 17 (novelSig): The number of statistically significant novel splice junctions belonging to the gene. Column 18 (numFeatures): The columns numExons, numKnown, and numNovel, separated by slashes. Column 19 (numSig): The columns exonsSig, knownSIg, and novelSig, separated by slashes. Columns on Tab 2: Column 1 (ID): ENSEMBL gene ID.(Macaque ENSEMBL release 92) Column 2 (testable): Whether enough reads to enable statistical comparison. Column 3 (pvalue): P-value for differential expression of the gene of which this is feature Column 4 (padjust): Adjusted p-value of the gene of which this is feature. Column 5 (Chr): Chromosome on which gene is located. Column 6 (Start): (1-based) position of the start of gene. Column 7 (End): (1-based) end of the gene. Column 8 (Strand): Strand on which gene is located. Column 9 (transcripts): Known transcripts involving this feature. Column 10 (featureType): Type of feature. Column 11 (p-adj): Adjusted p-value for the test of differential usage. Column 12 (log2FC(ZL34/WT)): Log 2 fold change for ZL34 versus WT.
Table of lentiviral endogenous mRNA fusions found in RNA-seq data obtained from four samples from ZL-34 (1 sample of nucleated erythrocytes from peripheral blood, 2 samples of nucleated erythrocytes from bone marrow and 1 sample of CD34+ cells obtained from bone marrow using our pipeline as described in the supplemental methods). The first column tabulates the left break point which is located in the lentiviral insertion (LVI). The LVI act as a splice donor and five different (LVI) break points were observed in fusion genes with EIF3E; 3 of the same LVI breakpoints were also observed in fusions with PLAG1 and NCAM2; we highlight the fact that the other 2 breakpoints were not observed with rows with zero entries. The second column depicts the right break point. In each case these are starts of known exons. Columns 3 through 6 denote the fusion fragments per million as computed from our pipeline and described in the supplemental methods. Most of the fusion genes were confirmed by PCR as indicated in the 7th column of the table. As depicted in the 8th column two of the splice junctions were previously reported.33
References
- 1.Naldini L., Trono D., Verma I.M. Lentiviral vectors, two decades later. Science. 2016;353:1101–1102. doi: 10.1126/science.aah6192. [DOI] [PubMed] [Google Scholar]
- 2.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 4.Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Veres G., Schmidt M., Kutschera I., Vidaud M., Abel U., Dal-Cortivo L., Caccavelli L. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 5.Eichler F., Duncan C., Musolino P.L., Orchard P.J., De Oliveira S., Thrasher A.J., Armant M., Dansereau C., Lund T.C., Miller W.P. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N. Engl. J. Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson A.A., Walters M.C., Kwiatkowski J., Rasko J.E.J., Ribeil J.A., Hongeng S., Magrin E., Schiller G.J., Payen E., Semeraro M. Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia. N. Engl. J. Med. 2018;378:1479–1493. doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- 7.Ribeil J.A., Hacein-Bey-Abina S., Payen E., Magnani A., Semeraro M., Magrin E., Caccavelli L., Neven B., Bourget P., El Nemer W. Gene Therapy in a Patient with Sickle Cell Disease. N. Engl. J. Med. 2017;376:848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 8.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seggewiss R., Pittaluga S., Adler R.L., Guenaga F.J., Ferguson C., Pilz I.H., Ryu B., Sorrentino B.P., Young W.S., 3rd, Donahue R.E. Acute myeloid leukemia is associated with retroviral gene transfer to hematopoietic progenitor cells in a rhesus macaque. Blood. 2006;107:3865–3867. doi: 10.1182/blood-2005-10-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivière I., Dunbar C.E., Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119:1107–1116. doi: 10.1182/blood-2011-09-349993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schröder A.R., Shinn P., Chen H., Berry C., Ecker J.R., Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 13.Wu X., Li Y., Crise B., Burgess S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- 14.Fraietta J.A., Nobles C.L., Sammons M.A., Lundh S., Carty S.A., Reich T.J., Cogdill A.P., Morrissette J.J.D., DeNizio J.E., Reddy S. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koelle S.J., Espinoza D.A., Wu C., Xu J., Lu R., Li B., Donahue R.E., Dunbar C.E. Quantitative stability of hematopoietic stem and progenitor cell clonal output in rhesus macaques receiving transplants. Blood. 2017;129:1448–1457. doi: 10.1182/blood-2016-07-728691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Li B., Lu R., Koelle S.J., Yang Y., Jares A., Krouse A.E., Metzger M., Liang F., Loré K. Clonal tracking of rhesus macaque hematopoiesis highlights a distinct lineage origin for natural killer cells. Cell Stem Cell. 2014;14:486–499. doi: 10.1016/j.stem.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C., Espinoza D.A., Koelle S.J., Potter E.L., Lu R., Li B., Yang D., Fan X., Donahue R.E., Dunbar C.E. Geographic clonal tracking in macaques provides insights into HSPC migration and differentiation. J. Exp. Med. 2017;215:217–232. doi: 10.1084/jem.20171341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu K.-R., Espinoza D.A., Wu C., Truitt L., Shin T.-H., Chen S., Fan X., Yabe I.M., Panch S., Hong S.G. The impact of aging on primate hematopoiesis as interrogated by clonal tracking. Blood. 2018;131:1195–1205. doi: 10.1182/blood-2017-08-802033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humbert O., Peterson C.W., Norgaard Z.K., Radtke S., Kiem H.P. A Nonhuman Primate Transplantation Model to Evaluate Hematopoietic Stem Cell Gene Editing Strategies for β-Hemoglobinopathies. Mol. Ther. Methods Clin. Dev. 2017;8:75–86. doi: 10.1016/j.omtm.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radtke S., Adair J.E., Giese M.A., Chan Y.Y., Norgaard Z.K., Enstrom M., Haworth K.G., Schefter L.E., Kiem H.P. A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates. Sci. Transl. Med. 2017;9:9. doi: 10.1126/scitranslmed.aan1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C.L., Johnson G.R. Stem cell factor enhances the survival but not the self-renewal of murine hematopoietic long-term repopulating cells. Blood. 1994;84:408–414. [PubMed] [Google Scholar]
- 22.Leary A.G., Zeng H.Q., Clark S.C., Ogawa M. Growth factor requirements for survival in G0 and entry into the cell cycle of primitive human hemopoietic progenitors. Proc. Natl. Acad. Sci. USA. 1992;89:4013–4017. doi: 10.1073/pnas.89.9.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kas K., Voz M.L., Röijer E., Aström A.K., Meyen E., Stenman G., Van de Ven W.J. Promoter swapping between the genes for a novel zinc finger protein and beta-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat. Genet. 1997;15:170–174. doi: 10.1038/ng0297-170. [DOI] [PubMed] [Google Scholar]
- 24.Castilla L.H., Perrat P., Martinez N.J., Landrette S.F., Keys R., Oikemus S., Flanegan J., Heilman S., Garrett L., Dutra A. Identification of genes that synergize with Cbfb-MYH11 in the pathogenesis of acute myeloid leukemia. Proc. Natl. Acad. Sci. USA. 2004;101:4924–4929. doi: 10.1073/pnas.0400930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landrette S.F., Kuo Y.H., Hensen K., Barjesteh van Waalwijk van Doorn-Khosrovani S., Perrat P.N., Van de Ven W.J.M., Delwel R., Castilla L.H. Plag1 and Plagl2 are oncogenes that induce acute myeloid leukemia in cooperation with Cbfb-MYH11. Blood. 2005;105:2900–2907. doi: 10.1182/blood-2004-09-3630. [DOI] [PubMed] [Google Scholar]
- 26.Declercq J., Van Dyck F., Braem C.V., Van Valckenborgh I.C., Voz M., Wassef M., Schoonjans L., Van Damme B., Fiette L., Van de Ven W.J. Salivary gland tumors in transgenic mice with targeted PLAG1 proto-oncogene overexpression. Cancer Res. 2005;65:4544–4553. doi: 10.1158/0008-5472.CAN-04-4041. [DOI] [PubMed] [Google Scholar]
- 27.Declercq J., Skaland I., Van Dyck F., Janssen E.A., Baak J.P., Drijkoningen M., Van de Ven W.J. Adenomyoepitheliomatous lesions of the mammary glands in transgenic mice with targeted PLAG1 overexpression. Int. J. Cancer. 2008;123:1593–1600. doi: 10.1002/ijc.23586. [DOI] [PubMed] [Google Scholar]
- 28.Van Dyck F., Scroyen I., Declercq J., Sciot R., Kahn B., Lijnen R., Van de Ven W.J. aP2-Cre-mediated expression activation of an oncogenic PLAG1 transgene results in cavernous angiomatosis in mice. Int. J. Oncol. 2008;32:33–40. [PubMed] [Google Scholar]
- 29.Voz M.L., Agten N.S., Van de Ven W.J., Kas K. PLAG1, the main translocation target in pleomorphic adenoma of the salivary glands, is a positive regulator of IGF-II. Cancer Res. 2000;60:106–113. [PubMed] [Google Scholar]
- 30.Belew M.S., Bhatia S., Keyvani Chahi A., Rentas S., Draper J.S., Hope K.J. PLAG1 and USF2 Co-regulate Expression of Musashi-2 in Human Hematopoietic Stem and Progenitor Cells. Stem Cell Reports. 2018;10:1384–1397. doi: 10.1016/j.stemcr.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rentas S., Holzapfel N., Belew M.S., Pratt G., Voisin V., Wilhelm B.T., Bader G.D., Yeo G.W., Hope K.J. Musashi-2 attenuates AHR signalling to expand human haematopoietic stem cells. Nature. 2016;532:508–511. doi: 10.1038/nature17665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klemke M., Müller M.H., Wosniok W., Markowski D.N., Nimzyk R., Helmke B.M., Bullerdiek J. Correlated expression of HMGA2 and PLAG1 in thyroid tumors, uterine leiomyomas and experimental models. PLoS ONE. 2014;9:e88126. doi: 10.1371/journal.pone.0088126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cesana D., Sgualdino J., Rudilosso L., Merella S., Naldini L., Montini E. Whole transcriptome characterization of aberrant splicing events induced by lentiviral vector integrations. J. Clin. Invest. 2012;122:1667–1676. doi: 10.1172/JCI62189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moiani A., Paleari Y., Sartori D., Mezzadra R., Miccio A., Cattoglio C., Cocchiarella F., Lidonnici M.R., Ferrari G., Mavilio F. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. J. Clin. Invest. 2012;122:1653–1666. doi: 10.1172/JCI61852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debiec-Rychter M., Van Valckenborgh I., Van den Broeck C., Hagemeijer A., Van de Ven W.J., Kas K., Van Damme B., Voz M.L. Histologic localization of PLAG1 (pleomorphic adenoma gene 1) in pleomorphic adenoma of the salivary gland: cytogenetic evidence of common origin of phenotypically diverse cells. Lab. Invest. 2001;81:1289–1297. doi: 10.1038/labinvest.3780342. [DOI] [PubMed] [Google Scholar]
- 36.Hematti P., Hong B.K., Ferguson C., Adler R., Hanawa H., Sellers S., Holt I.E., Eckfeldt C.E., Sharma Y., Schmidt M. Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS Biol. 2004;2:e423. doi: 10.1371/journal.pbio.0020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell R.S., Beitzel B.F., Schroder A.R., Shinn P., Chen H., Berry C.C., Ecker J.R., Bushman F.D. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2:E234. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montini E., Cesana D., Schmidt M., Sanvito F., Ponzoni M., Bartholomae C., Sergi L., Benedicenti F., Ambrosi A., Di Serio C. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 39.Montini E., Cesana D., Schmidt M., Sanvito F., Bartholomae C.C., Ranzani M., Benedicenti F., Sergi L.S., Ambrosi A., Ponzoni M. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cesana D., Ranzani M., Volpin M., Bartholomae C., Duros C., Artus A., Merella S., Benedicenti F., Sergi L., Sanvito F. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther. 2014;22:774–785. doi: 10.1038/mt.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zychlinski D., Schambach A., Modlich U., Maetzig T., Meyer J., Grassman E., Mishra A., Baum C. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol. Ther. 2008;16:718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]
- 42.Modlich U., Navarro S., Zychlinski D., Maetzig T., Knoess S., Brugman M.H., Schambach A., Charrier S., Galy A., Thrasher A.J. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunbar C.E., High K.A., Joung J.K., Kohn D.B., Ozawa K., Sadelain M. Gene therapy comes of age. Science. 2018;359:359. doi: 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- 44.Grez M., Akgün E., Hilberg F., Ostertag W. Embryonic stem cell virus, a recombinant murine retrovirus with expression in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 1990;87:9202–9206. doi: 10.1073/pnas.87.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramezani A., Hawley T.S., Hawley R.G. Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Mol. Ther. 2000;2:458–469. doi: 10.1006/mthe.2000.0190. [DOI] [PubMed] [Google Scholar]
- 46.Challita P.M., Skelton D., el-Khoueiry A., Yu X.J., Weinberg K., Kohn D.B. Multiple modifications in cis elements of the long terminal repeat of retroviral vectors lead to increased expression and decreased DNA methylation in embryonic carcinoma cells. J. Virol. 1995;69:748–755. doi: 10.1128/jvi.69.2.748-755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robbins P.B., Skelton D.C., Yu X.J., Halene S., Leonard E.H., Kohn D.B. Consistent, persistent expression from modified retroviral vectors in murine hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 1998;95:10182–10187. doi: 10.1073/pnas.95.17.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. [DOI] [PubMed] [Google Scholar]
- 49.Biasco L., Pellin D., Scala S., Dionisio F., Basso-Ricci L., Leonardelli L., Scaramuzza S., Baricordi C., Ferrua F., Cicalese M.P. In Vivo Tracking of Human Hematopoiesis Reveals Patterns of Clonal Dynamics during Early and Steady-State Reconstitution Phases. Cell Stem Cell. 2016;19:107–119. doi: 10.1016/j.stem.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hacein-Bey Abina S., Gaspar H.B., Blondeau J., Caccavelli L., Charrier S., Buckland K., Picard C., Six E., Himoudi N., Gilmour K. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313:1550–1563. doi: 10.1001/jama.2015.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Ravin S.S., Wu X., Moir S., Anaya-O’Brien S., Kwatemaa N., Littel P., Theobald N., Choi U., Su L., Marquesen M. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2016;8:335ra57. doi: 10.1126/scitranslmed.aad8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scholz S.J., Fronza R., Bartholomä C.C., Cesana D., Montini E., von Kalle C., Gil-Farina I., Schmidt M. Lentiviral Vector Promoter is Decisive for Aberrant Transcript Formation. Hum. Gene Ther. 2017;28:875–885. doi: 10.1089/hum.2017.162. [DOI] [PubMed] [Google Scholar]
- 53.Heckl D., Schwarzer A., Haemmerle R., Steinemann D., Rudolph C., Skawran B., Knoess S., Krause J., Li Z., Schlegelberger B. Lentiviral vector induced insertional haploinsufficiency of Ebf1 causes murine leukemia. Mol. Ther. 2012;20:1187–1195. doi: 10.1038/mt.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venkatraman A., He X.C., Thorvaldsen J.L., Sugimura R., Perry J.M., Tao F., Zhao M., Christenson M.K., Sanchez R., Yu J.Y. Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature. 2013;500:345–349. doi: 10.1038/nature12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hope K.J., Cellot S., Ting S.B., MacRae T., Mayotte N., Iscove N.N., Sauvageau G. An RNAi screen identifies Msi2 and Prox1 as having opposite roles in the regulation of hematopoietic stem cell activity. Cell Stem Cell. 2010;7:101–113. doi: 10.1016/j.stem.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 56.de Vasconcellos J.F., Tumburu L., Byrnes C., Lee Y.T., Xu P.C., Li M., Rabel A., Clarke B.A., Guydosh N.R., Proia R.L., Miller J.L. IGF2BP1 overexpression causes fetal-like hemoglobin expression patterns in cultured human adult erythroblasts. Proc. Natl. Acad. Sci. USA. 2017;114:E5664–E5672. doi: 10.1073/pnas.1609552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunbar C.E., Browder T.M., Abrams J.S., Nienhuis A.W. COOH-terminal-modified interleukin-3 is retained intracellularly and stimulates autocrine growth. Science. 1989;245:1493–1496. doi: 10.1126/science.2789432. [DOI] [PubMed] [Google Scholar]
- 58.Lavelle D., Molokie R., Ducksworth J., DeSimone J. Effects of hydroxurea, stem cell factor, and erythropoietin in combination on fetal hemoglobin in the baboon. Exp. Hematol. 2001;29:156–162. doi: 10.1016/s0301-472x(00)00654-8. [DOI] [PubMed] [Google Scholar]
- 59.Aerbajinai W., Zhu J., Kumkhaek C., Chin K., Rodgers G.P. SCF induces gamma-globin gene expression by regulating downstream transcription factor COUP-TFII. Blood. 2009;114:187–194. doi: 10.1182/blood-2008-07-170712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X. Tes, a potential Mena-related cancer therapy target. Drug Discov. Ther. 2008;2:1. [PubMed] [Google Scholar]
- 61.Haapaniemi E., Botla S., Persson J., Schmierer B., Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat. Med. 2018;24:927–930. doi: 10.1038/s41591-018-0049-z. [DOI] [PubMed] [Google Scholar]
- 62.Ihry R.J., Worringer K.A., Salick M.R., Frias E., Ho D., Theriault K., Kommineni S., Chen J., Sondey M., Ye C. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat. Med. 2018;24:939–946. doi: 10.1038/s41591-018-0050-6. [DOI] [PubMed] [Google Scholar]
- 63.Kosicki M., Tomberg K., Bradley A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018;36:765–771. doi: 10.1038/nbt.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt D., Schwalie P.C., Wilson M.D., Ballester B., Gonçalves A., Kutter C., Brown G.D., Marshall A., Flicek P., Odom D.T. Waves of retrotransposon expansion remodel genome organization and CTCF binding in multiple mammalian lineages. Cell. 2012;148:335–348. doi: 10.1016/j.cell.2011.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., Liu X.S. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lawrence M., Huber W., Pagès H., Aboyoun P., Carlson M., Gentleman R., Morgan M.T., Carey V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013;9:e1003118. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buenrostro J.D., Corces M.R., Lareau C.A., Wu B., Schep A.N., Aryee M.J., Majeti R., Chang H.Y., Greenleaf W.J. Integrated Single-Cell Analysis Maps the Continuous Regulatory Landscape of Human Hematopoietic Differentiation. Cell. 2018;173:1535–1548.e16. doi: 10.1016/j.cell.2018.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taggart C., Cervantes-Laurean D., Kim G., McElvaney N.G., Wehr N., Moss J., Levine R.L. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J. Biol. Chem. 2000;275:27258–27265. doi: 10.1074/jbc.M004850200. [DOI] [PubMed] [Google Scholar]
- 70.Apffel A., Fischer S., Goldberg G., Goodley P.C., Kuhlmann F.E. Enhanced sensitivity for peptide mapping with electrospray liquid chromatography-mass spectrometry in the presence of signal suppression due to trifluoroacetic acid-containing mobile phases. J. Chromatogr. A. 1995;712:177–190. doi: 10.1016/0021-9673(95)00175-m. [DOI] [PubMed] [Google Scholar]
- 71.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hartley S.W., Mullikin J.C. QoRTs: a comprehensive toolset for quality control and data processing of RNA-Seq experiments. BMC Bioinformatics. 2015;16:224. doi: 10.1186/s12859-015-0670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartley S.W., Mullikin J.C. Detection and visualization of differential splicing in RNA-Seq data with JunctionSeq. Nucleic Acids Res. 2016;44:e127. doi: 10.1093/nar/gkw501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Viral integration site retrieval (5’ and 3’-based) in ZL34 nRBC DNA at 309 and 393 days post-transplant, and in granulocyte DNA at 96 days post-transplant. Green denotes the integration site recovered has been matched to one of the originally recovered barcodes and is concluded to arise from the single expanded clone in ZL34.
Differentially expressed genes reported from DESeq2, identified as described in Bulk RNA-seq analysis. ens_id = Ensembl gene ID. baseMean = the average of the DESeq2 normalized count values for all samples, normalized for sequencing depth. log2FoldChange = DESeq2 estimated effect size in log2 scale, comparing ZL34 to controls. lfcSE = standard error of the log2FoldChange estimate. stat = DESeq2 Wald statistic. pvalue = Wald test p-value. adj. p = Benjamini-Hochberg adjusted p-value.
Differential expression of gene features (e.g. exons or exon junctions) obtained from the comparison of RNA-seq data 5 from (i) four samples from ZL-34 (1 sample of nucleated erythrocytes from peripheral blood, 2 samples of nucleated erythrocytes from bone marrow and 1 sample of CD34+ cells obtained from bone marrow) and (ii) 4 samples from a wild type macaque (2 samples of nucleated erythrocytes obtained from bone marrow and 2 samples of CD34+ HSPCs obtained from BM). Differential expression of features was computed with our pipeline and a custom index for the combined macaque and lentiviral as described in the supplemental methods. Tab 1 is a gene level overview of features (e.g. exons or junctions) that are differentially expressed with an adjusted p-value of less than 0.05. The meanings of the columns is described in comments added to each column and also tabulated below. Tab 2 is a more detailed presentation of the results at the level of individual gene features. Again, the meanings of each of the columns is described in comments added to each column and also tabulated below. Columns on Tab 1: Column 1 (ID): ENSEMBL gene ID.(Macaque ENSEMBL release 92) Column 2 (Gene Symbol): HGNC symbol corresponding to ENSEMBL ID, if known Column 3 (Description): Description of gene function, if known. Column 4 (Chr): Chromosome on which gene is located. Column 5 (Start): (1-based) position of the start of gene 6 Column 6 (End): (1-based) end of the gene. Column 7 (Strand): Strand on which gene is located. Column 8 (baseMean): The base mean normalized coverage counts for the locus across all conditions. Column 9 (geneWisePadj): The gene-level p-value that one or more features belonging to this gene are differentially used. This value will be the same for all features belonging to the same gene. Column 10 (mostSIgID): The sub-feature OD for the most significant exon or splice junction belonging to the gene. Column 11 (mostSIgPadj): The adjusted p-value for the most signifiance exon or splice-junction belonging to the gene. Column 12 (numExons): The number of known non-overlapping exonic regions belonging to the gene. Column 13 (numKnown): The number of known splice junctions belonging to the gene. Column 14 (numNovel): The number of novel splice junctions belonging to the gene. Column 15 (exonsSig): The number of statistically significant non-overlapping exonic regions belonging to the gene. Column 16 (knownSIg): The number of statistically significant known splice junctions belonging to the gene Column 17 (novelSig): The number of statistically significant novel splice junctions belonging to the gene. Column 18 (numFeatures): The columns numExons, numKnown, and numNovel, separated by slashes. Column 19 (numSig): The columns exonsSig, knownSIg, and novelSig, separated by slashes. Columns on Tab 2: Column 1 (ID): ENSEMBL gene ID.(Macaque ENSEMBL release 92) Column 2 (testable): Whether enough reads to enable statistical comparison. Column 3 (pvalue): P-value for differential expression of the gene of which this is feature Column 4 (padjust): Adjusted p-value of the gene of which this is feature. Column 5 (Chr): Chromosome on which gene is located. Column 6 (Start): (1-based) position of the start of gene. Column 7 (End): (1-based) end of the gene. Column 8 (Strand): Strand on which gene is located. Column 9 (transcripts): Known transcripts involving this feature. Column 10 (featureType): Type of feature. Column 11 (p-adj): Adjusted p-value for the test of differential usage. Column 12 (log2FC(ZL34/WT)): Log 2 fold change for ZL34 versus WT.
Table of lentiviral endogenous mRNA fusions found in RNA-seq data obtained from four samples from ZL-34 (1 sample of nucleated erythrocytes from peripheral blood, 2 samples of nucleated erythrocytes from bone marrow and 1 sample of CD34+ cells obtained from bone marrow using our pipeline as described in the supplemental methods). The first column tabulates the left break point which is located in the lentiviral insertion (LVI). The LVI act as a splice donor and five different (LVI) break points were observed in fusion genes with EIF3E; 3 of the same LVI breakpoints were also observed in fusions with PLAG1 and NCAM2; we highlight the fact that the other 2 breakpoints were not observed with rows with zero entries. The second column depicts the right break point. In each case these are starts of known exons. Columns 3 through 6 denote the fusion fragments per million as computed from our pipeline and described in the supplemental methods. Most of the fusion genes were confirmed by PCR as indicated in the 7th column of the table. As depicted in the 8th column two of the splice junctions were previously reported.33
Data Availability Statement
All data and code used in this study (RNA-seq and barcode data) will be made available upon request from the corresponding authors.