Abstract
Aim:
In this prospective case–control study we aimed to compare diagnostic value of plasma PARK7 and NDKA in early diagnosis of acute stroke and evaluate the validated diagnostic values of PARK7 and NDKA in an independent patient cohort. We then assessed the quantitative relationship between the release of these markers: stroke severity and time. Blood samples were drawn upon hospital admission and 14 days later. PARK7 and NDKA concentrations were measured using an ELISA.
Results:
The expression of PARK7 (area under the curve [AUC] = 0.897) in acute stroke patients was more significant than in controls, relative to the NDKA expression (AUC = 0.462); p < 0.05. Their expressions were not related to the clinical characteristics of both groups; p > 0.05.
Conclusion:
Even though both markers cannot differentiate stroke etiologies (ischemic or hemorrhagic), plasma PARK7 has better diagnostic value than NDKA for early diagnosis of stroke. 72 plasma samples obtained from acute stroke patients and 78 plasma samples collected from non-stroke patients were analyzed in this study.
Keywords: : acute stroke, biomarkers, correlation, diagnostic value, hemorrhagic stroke, ischemic stroke, NDKA, PARK7, plasma, subarachnoid hemorrhage
Lay abstract
The gold standard diagnostic tool for stroke remains to be neuroimaging (CT or MRI), but it can be performed only in sophisticated hospitals. Biomarkers, including PARK7 and NDKA, have been suggested for early diagnosis of patients with stroke. The aim of this study was to determine which one has more diagnostic value for acute stroke. Two groups were included: one contained patients with stroke and the other patients without. After comparing the diagnostic values of both biomarkers in both groups, PARK7 seemed more reliable than NDKA.
According to recent surveys, the incidence rate of stroke is up to 119 patients per 100,000 person-years in high-risk regions like New Zealand, and reaches 345.1/100,000 person-years in PR China. In all epidemiologic stroke studies, the age-standardized mortality is significant, around 114.8/100,000 person-years in PR China, making stroke the second leading cause of mortality worldwide [1,2].
Although there has been considerable advancement in the understanding of its pathophysiology, efficient therapies are still needed to reduce the mortality and disability rate due to stroke. Stroke biomarkers may potentially aid in diagnosis, identification of intracerebral injury, evaluation of severity and prognosis of treatment [3–5].
Biomarkers are biological molecules identified and measured by biochemical techniques using blood, urine, cells or tissues that assist in the diagnosis, prognosis and physiology of a disease or the risk factor for this disease [6]. Timely diagnosis of stroke is important in clinical practice for efficient treatment. The measurements of circulating biomarkers may facilitate early diagnosis of stroke. Many molecules were studied to suggest such prediction for stroke [7–11], but most of them have shown some weakness, as their release occurs at a late stage after stroke onset [11].
The newly identified biomarkers NDKA and PARK7 are brain-specific and easily detectable upon exceeding normal values at the onset of stroke, suggesting that they could be reliable biomarkers for early diagnosis of stroke [11,12]. A recent study has shown the role of PARK7 and NDKA in stroke pathophysiology: PARK7 acting as an antioxidant, chaperoning molecules and transcriptional regulator in stroke, protecting from neuronal cell deaths. As an enzyme, NDKA has also been shown to catalyze stroke-associated reactions [12]. The objective of this study was to compare the diagnostic values of circulating PARK7 and NDKA in plasma of stroke patients, and evaluate their use and limitations as biomarkers for early stroke diagnosis.
Patients & methods
Clinical specimens
72 stroke patients (admitted to the emergency department of Shengjing Hospital affiliated to China Medical University, Shenyang, PR China) and 78 patients without stroke (patients with other illness, as controls) were enrolled in this study. The protocol was approved by the Medical Research and New Technology Ethics Committee of Shengjing Hospital affiliated to China Medical University.
Selection criteria
Stroke patients were recruited in accordance with the guidelines: patients with obvious manifestations of stroke, positive stroke neuroimaging and clinical confirmation by a neurologist were diagnosed as stroke cases. Patients with transient ischemic attack were excluded due the lack of proper characterization of the disease at admission. In order to avoid bias, we excluded patients with mimic stroke and neurologic disorders.
Plasma collection & storage
4 ml of venous blood samples were collected from all individuals using sodium citrate-coated tubes. The median time of first collection from stroke onset was 17 h (interquantile range [IQR] = 22; mean of 17.92 ± 17.717). The second time point collection was on day 14. Depending on the progression of the disease and management of patients, we collected blood from 20 patients on day 14 after admission. Plasma was obtained by centrifugation of whole blood at 1500xg for 15 min at 4°C in EP tubes and stored at -80 until laboratory analysis.
PARK7 & NDKA ELISA
The plasma levels of NDKA and PARK7 were measured by ELISA at the Shengjing Hospital's medical and pharmaceutical research laboratory in Benxi. Abcam (London, UK) ab215535 Human PARK7 SimpleStep ELISA kit and Mlbio (Good ELISA kit producers, Shanghai, PR China) Human NDKA kit were procured and used for the assay of PARK7 and NDKA, respectively. The experiments were conducted following manufacturers’ instructions.
Succinctly, 50 μl of diluted plasma was used for assays and the dilution ratios were twofold and fivefold for PARK7 and NDKA, respectively. Biomarker concentrations were calculated from their respective absorbance values determined at 450 nm. The results were expressed as ng/ml.
Statistical analysis
Statistical Package for Social Sciences (IBM SPSS version 20) was used for statistical analysis and graph drawing. Data are presented as mean ± standard deviation (x ± SD), unless otherwise noted. The significance of difference in clinical characteristics between stroke patients and controls was tested using Chi Square test. Both PARK7 and NDKA levels were measured at two time points (day 1 at admission and day 14) and compared using Wilcoxon's Signed Rank Test. The Mann-Whitney U test was used to compare the expression of both biomarkers between stroke patients and controls. Kruskal-Wallis test and Dunn's multiple comparison were used to discriminate the expression of these biomarkers in ischemic stroke (IS), hemorrhagic stroke (HS) and subarachnoid hemorrhage (SAH). Receiver-operating characteristic (ROC) curves were established to discriminate between stroke patients and controls. The association of biomarker levels with National Institute for Health Stroke Scale (NIHSS) was elucidated using Spearman correlation. Our attempt at comparing the diagnostic values of plasma PARK7 and NDKA was completed with ROC comparison using MedCalc 17.9.7 (Hanley & McNeil). All p-values were two-tailed and p < 0.05 was statistically significant.
Results
Clinical characteristics
Our study enrolled 72 stroke patients and 78 controls (nonstroke patients). The clinical characteristics of our sample individuals are listed in Table 1. There was no significant difference in the characteristics between stroke and control groups (p > 0.05). The mean age of stroke patients was 59.4 ± 14.1 years and the mean age of controls 53.7 ± 19.7 years old (p = 0.109). Both groups were predominantly male (46/26 in stroke group and 45/33 in control group; p = 0.121).
Table 1. . Clinical characteristics of the study population.
| Population characteristics | Stroke patients, n = 72 | Control, n = 78 | p-value | ||
|---|---|---|---|---|---|
| Age | Years | Mean ± SD | 59.4 ± 14.1 | 53.7 ± 19.7 | 0.109 |
| Min–max | 25–89 | 20–91 | |||
| Sex | Female | n (%) | 26 (36%) | 33 (42%) | 0.121 |
| Male | n (%) | 46 (64%) | 45 (58%) | ||
| Diabetes | n (%) | 25 (34.7%) | 34 (43.6%) | 0.267 | |
| Hyperlipidemia | n (%) | 10 (13.9%) | 12 (15.4%) | 0.796 | |
| Smokers | n (%) | 35 (48.6%) | 33 (42.3%) | 0.438 | |
| Hypertension | n (%) | 37 (51.4%) | 29 (43.9%) | 0.08 | |
| Systolic blood pressure | mmHg | Mean ± SD | 150.40 ± 29.72 | 140.33 ± 30.97 | 0.106 |
| Distolic blood pressure | mmHg | Mean ± SD | 88.50 ± 15.67 | 85.23 ± 16.16 | 0.328 |
| Types of stroke | Hemorrhagic | n (%) | 26 (36.1%) | ||
| Ischemic | n (%) | 39 (54.1%) | |||
| Subarachnoid hemorrhage | n (%) | 7 (9.8%) | |||
Without mention of biomarker levels, there were no significant differences in all clinical characteristics between the stroke (72) and control (78) groups (p > 0.05).
SD: Standard deviation.
Diagnostic values of NDKA & PARK7 in stroke
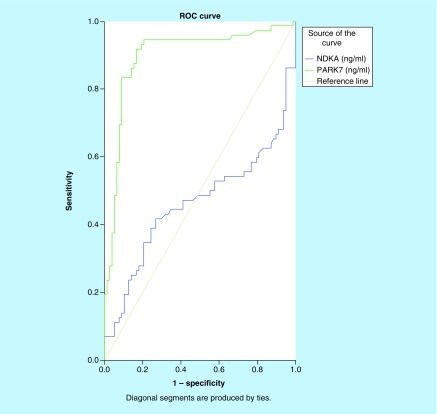
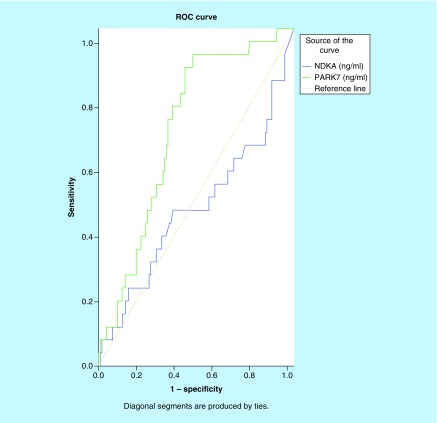
We investigated the differences in plasma PARK7 and NDKA levels between stroke patients and controls. As shown in Figure 1A & B, there was an increase in levels of both molecules in patients with stroke compared with the control patients. PARK7 was markedly elevated in stroke patients relative to controls (p < 0.001), while NDKA did not show such significance (p > 0.05). To evaluate the predictive power of circulating PARK7 and NDKA for stroke diagnosis, we performed ROC analysis for 72 patients with stroke. As displayed in Figure 2, we found that PARK7 had a higher diagnostic potential with an area under the curve (AUC) of 0.897 (95% CI: 0.841–0.954; p < 0.001) and an OR (odds ratio) of 1.087 (95% CI: 1.049–1.126; p < 0.001), while NDKA gave an AUC of 0.462 (95% CI: 0.365–0.560, p > 0.05) that corresponded to an OR of 0.882 (95% CI: 0.623–1.248).
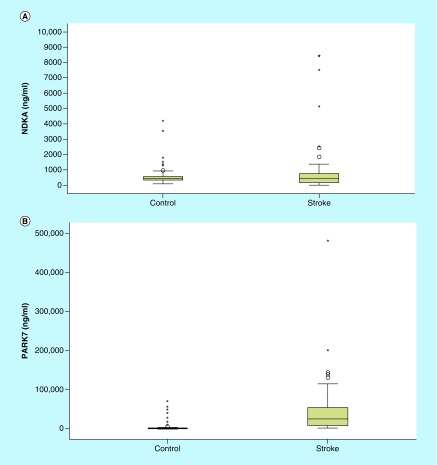
Figure 1. . Plasma levels of NDKA & PARK7 in stroke versus controls.
(A) Change in NDKA and PARK7 levels from day 1 to day 14 in stroke patients versus controls. Histogram of plasma NDKA level in stroke group and control group. Level of NDKA in stroke patients at admission time in emergency department. The plasma levels of NDKA were not significantly increased in stroke patients (n = 72) compared with controls (78); p > 0.05. (B) Histogram of plasma PARK7 level in stroke group and control group. Level of PARK7 in stroke patients at admission time at emergency department. The plasma levels of PARK7 were significantly increased in stroke patients (n = 72) compared with controls (78); *p = 0.000.
Figure 2. . Receiver-operating characteristic curves of plasma NDKA and PARK7 control versus stroke.
Comparison of the sensitivity and specificity for the diagnosis by plasma PARK7 and NDKA in stroke patients. Receiver-operating characteristic curves were drawn to evaluate the diagnostic values of PARK7 in comparison with NDKA. Following the area under the receiver-operating characteristic curve, PARK7 has greater diagnostic value than NDKA.
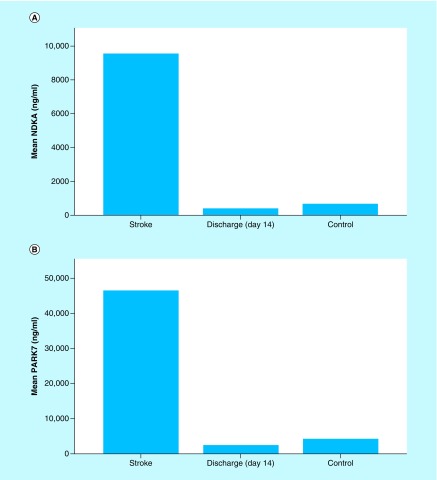
On day 14, the plasma levels of both biomarkers were decreased to the baseline levels (p < 0.05). Especially for PARK7, the level at day 14 was not statistically different from controls level (p > 0.05) as shown in Figure 3A & B.
Figure 3. . Histograms of biomarker means in stroke patients at admission vs 14 days after admission vs controls.
(A) Mean plasma NDKA levels in stroke patients at admission (day 1) versus at discharge (day 14) versus controls.Presentation of mean levels of NDKA in stroke patients at day 1 and on day 14 in comparison with controls. Alteration of Plasma NDKA levels in stroke patients. On day 14 (n = 20), the measurement of NDKA returns to the baseline levels with values less than controls (n = 78), p = 0.007 (discharge on day 14 vs controls). (B) Mean plasma PARK7 levels in stroke patients at admission (day 1) versus at discharge (day 14) versus controls. Presentation of mean levels of PARK7 in stroke patients at day 1 and on day 14 in comparison with controls. Alteration of plasma PARK7 levels in stroke patients. PARK7 is markedly increased in plasma samples gathered at admission after stroke onset (within 24 h). On day 14 (n = 20), the measurement of PARK7 returns to the baseline levels with values similar to controls (n = 78); p > 0.05 (discharge on day 14 vs control).
These results demonstrated that PARK7 may have more sensitivity and more specificity for stroke than NDKA.
Correlations between PARK7 & NDKA plasma levels & stroke severity
The severity of neuronal dysfunctions in patients with stroke was evaluated according to NIHSS performed by a trained neurologist on admission and day 14 after stroke onset. The pairwise comparison of NIHSS in two time points demonstrated significant difference (p = 0.003), suggesting the gradual improvement in neurological functions among the stroke patients due to the implementation of efficient therapies or improvement of patients conditions. Although there was a decrease in plasma PARK7 and NDKA levels from admission time to day 14 (p < 0.05), the correlation analysis between these biomarkers and NIHSS at each time point showed no significant association (p > 0.05).
Circulating PARK7 & NDKA to identify IS, HS & SAH
At admission, the level of each biomarker among patients with IS, HS and SAH was analyzed using nonparametric tests (Kruskal-Wallis H test followed by Dunn's multiple comparison p > 0.05/3). The results demonstrated that both molecules can similarly identify patients with the three types of stroke, there was no statistical difference in the capacity of PARK7 and NDKA to identify IS, HS or SAH (p > 0.0166).
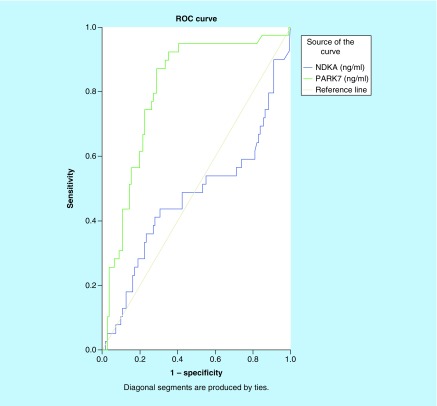
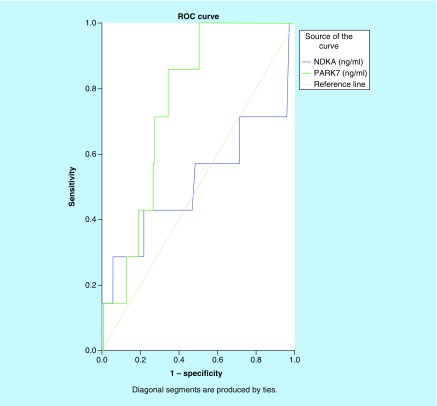
As shown in Figure 4, the ROC curve was used to compare the diagnostic capacity of both biomarkers for IS. With an AUC of 0.806 (95% CI: 0.727–0.844; p < 0.001), PARK7 presented a good diagnostic accuracy while NDKA showed no diagnostic capacity for IS, AUC = 0.473 (95% CI: 0.355–0.590; p > 0.05). Other ROC curves were drawn to compare the diagnostic values of PARK7 and NDKA for HS. As shown in Figure 5, the results suggested PARK7 as better than NDKA with an AUC of 0.702 (95% CI: 0.605–0.800; p = 0.001). Plasma NDKA level showed no diagnostic accuracy for HS: AUC of 0.466 (95% CI: 0.330–0.603; p > 0.05).
Figure 4. . Receiver-operating characteristic curves of plasma NDKA and PARK7 control versus ischemic stroke.
Comparison of the sensitivity and specificity for the diagnosis by plasma PARK7 and NDKA in ischemic stroke. Receiver-operating characteristic curves were drawn to evaluate the diagnostic values of PARK7 in comparison with NDKA. The PARK7 has greater area under the curve than NDKA.
Figure 5. . Receiver-operating characteristic curves of plasma NDKA and PARK7 control versus hemorrhagic stroke.
Comparison of the sensitivity and specificity of the diagnosis by plasma PARK7 and NDKA in hemorrhagic stroke. Receiver-operating characteristic curves were drawn to evaluate the diagnostic values of PARK7 in comparison with NDKA. The PARK7 has greater area under the curve than NDKA.
Finally, for the comparison of diagnostic capacity of both markers for SAH, we drew another ROC curve (Figure 6). The results also suggested PARK7 as better than NDKA (AUC: 0.756 vs 0.515; p = 0.001 vs p > 0.05).
Figure 6. . Receiver-operating characteristic curves of plasma NDKA and PARK7 control versus subarachnoid hemorrhage.
Comparison of the sensitivity and specificity of the diagnosis by plasma PARK7 and NDKA in subarachnoid hemorrhage. Receiver-operating characteristic curves were drawn to evaluate the diagnostic values of PARK7 in comparison with NDKA. The PARK7 has greater area under the curve than NDKA.
Discussion
The aim of our study was to compare the diagnostic value of plasma PARK7 and NDKA. PARK7 and NDKA were suggested as potential biomarkers for stroke diagnosis in recent studies [12]. PARK7 plays multiple important roles in stroke; it acts as an antioxidant, a transcription regulator, a chaperone molecule and a degradation protein. PARK7, also called DJ-1, is sensitive to oxidation because of its cysteine residue (C106) that picks up the hydrogen peroxide. Its degree of oxidation determines the activity of PARK7. It is thus involved in protection of neurons. It also has a therapeutic potency in neurodegenerative disorders including IS in which it helps to reduce the size of an infarct [12–18]. However, PARK7 impairs bacterial clearance in patients with sepsis because of its counteractive role to the reactive oxygen species, a killer of inflammation and bacterial affection. It also acts in the IL-1/TNF-α pathway involved in many inflammations: pancreatitis, gastrointestinal disease and celiac disease [19–21]. In addition to being involved in the fertilization, PARK7 has also been demonstrated to play a role in the repair of nucleotides and nucleic acids [18,22,23].
The detection of NDKA in victims of brain injuries suggested plasma NDKA as a diagnostic marker of head trauma, IS, HS, SAH, transient ischemic attack and mild traumatic brain injury [24,25].
The findings of this study suggest that PARK7 is more reliable than NDKA both in sensitivity and specificity. PARK7 especially, having increased early on in stroke patients, remained high up to 3 days after stroke onset and decreased to normal baseline values on day 14. These findings are consistent with previous studies [11,26–29].
As shown in Table 2, the pairwise comparison of ROC curves for both plasma markers in each type of stroke (IS, HS, SAH) demonstrated that the diagnostic value of PARK7 is greater than that of NDKA.
Table 2. . Pairwise comparison of receiver-operating characteristic curves of PARK7 and NDKA.
| Statistical variables | Statistical values |
|---|---|
| PARK7 ∼ NDKA for stroke | |
| Difference between areas | 0.360 |
| Standard error | 0.0564 |
| 95% CI | 0.249–0.470 |
| z statistic | 6.381 |
| Significance level | p < 0.0001 |
| PARK7 ∼ NDKA for ischemic stroke | |
| Difference between areas | 0.168 |
| Standard error | 0.0842 |
| 95% CI | 0.00331–0.334 |
| z statistic | 1.999 |
| Significance level | p = 0.0456 |
| PARK7 ∼ NDKA for hemorrhagic stroke | |
| Difference between areas | 0.279 |
| Standard error | 0.0699 |
| 95% CI | 0.142–0.416 |
| z statistic | 3.983 |
| Significance level | p = 0.0001 |
| PARK7 ∼ NDKA for subarachnoid hemorrhage | |
| Difference between areas | 0.241 |
| Standard error | 0.153 |
| 95% CI | – 0.0594 to 0.541 |
| z statistic | 1.572 |
| Significance level | p = 0.1159 |
Pairwise comparison of receiver-operating characteristic curve of both plasma markers for stroke and each type of stroke (IS, HS, SAH), we see that PARK7 has more diagnostic value at the admission time than NDKA with p < 0.0001, p = 0.0456 and p = 0.0001 for all stroke diseases, IS and HS, respectively. Unfortunately, we did not find statistical difference between both molecules for SAH, p = 0.1159; more studies with larger population of SAH patients are needed.
CI: Confidence interval; HS: Hemorrhagic stroke; IS: Ischemic stroke; SAH: Subarachnoid hemorrhage.
PARK7 is then more reliable for early diagnosis of stroke; it can be useful for sorting out stroke patients in emergency medical services, helping for early correct triage of stroke patients. It may also help identify individuals at risk of early neurologic deterioration [30,31].
The identification of suitable stroke biomarkers is a great challenge nowadays; however, the results suggest some limitations for their application in stroke management [6]. Although PARK7 and NDKA play known roles in stroke pathophysiology [12], there are some problems facing their use in clinical routine:
PARK7 and NDKA levels are similarly expressed in all stroke types, so they could not differentiate IS and HS. This result complements previous findings [11,26].
At the disease level and at a given time point, both molecules lacked value for assessing the neuronal dysfunction severity (NIHSS) in acute stroke patients. This finding correlates to those from recent studies [26,29].
NDKA levels may be different according to the laboratory technique used and the sampling time [11]. Since the median time in our study was 17 h after stroke onset, this might justify the slight different in reliability of NDKA compared with PARK7. With standard analytic kits, it remains less reliable for diagnosis of acute stroke [26,29].
We are aware of the limitations of our study. Our sample size might be small, but has some significance: it clarifies the diagnostic value of PARK7 over NDKA. The results obtained regarding PARK7 and NDKA reliability for acute stroke are complementary to latest findings [12,26,29] and our study suggests the superiority of PARK7 in diagnosis of acute stroke compared with NDKA. This is a new viewpoint, an important insight for more development in the biomarkers field. Nevertheless, the higher diagnostic value of PARK7 over NDKA will be improved upon in further large studies that are still ongoing in that field.
The median time that characterizes our population was about 17 h, the mean was 17.92 ± 17.717 h. Given that the treatment of stroke for better outcome (IS or HS) is time-dependent, such time is out of the traditional therapeutic window. However, this distribution is quite similar to the mean time of previous studies [11,26,33,34]. It might suggest that stroke patients do not reach hospital once the symptoms occur (wake-up strokes, for example); that critical stroke patients arrived at the emergency department late after being admitted to other hospitals or centers of lower levels; and that there are many factors putting stroke patients outside the window for time-dependent therapies, leading to a high rate of disability and mortality [32–34].
Conclusion
Although the ultimate purpose of this study was to elucidate which biomarker of PARK7 and NDKA is more reliable for diagnosis of acute stroke, we recognize its limitation: small size of sample. In conclusion, PARK7 seems to be the most accurate biomarker for stroke diagnosis and might be involved in triage of patients with acute stroke. However, more studies including healthy volunteers or patients with other diagnoses initially suspected as stroke are needed to specify the usefulness of both biomarkers.
Future perspective
We anticipate that PARK7 is usefulness in stroke diagnosis; its advantages over NDKA in prognosis, even in therapy of stroke patients, will be improved with further clinical research and animal experiments. In the future, we anticipate that more research in that direction will finally lead to the determination of a stroke biomarker as gold standard for diagnosis of stroke. Given that a significant proportion (25%) of patients who had not had stroke are referred to stroke services and belatedly diagnosed as having no stroke, the development of early diagnostic tools as well as blood biomarkers for stroke, which might help distinguish stroke from mimics, faces tremendous challenges; we anticipate that more research including stroke mimic subjects are already being run and will be conducted to compare blood marker levels between stroke patients and stroke mimic patients. Stroke mimics are diseases initially suspected as stroke at admission, diseases that imitate signs and symptoms of stroke (toxic-metabolic pathologies, seizure disorders, degenerative neurologic conditions and peripheral neuropathies): we anticipate that a biomarker that could accurately discriminate both groups (stroke vs stroke mimics or stroke vs patients initially suspected as stroke) would greatly impact stroke evaluation. The development of accurate analytic kits will also be a field of interest, focusing on the most reliable biomarkers [31,32,35–37].
Summary points.
Background
PARK7 and NDKA have been identified as playing role in stroke pathophysiology and as biomarkers for early diagnosis of stroke.
Our aim was to compare the diagnostic value of PARK7 and NDKA in acute stroke.
We aimed to answer to the question: is PARK7 more reliable than NDKA in acute stroke?
Methods
72 patients with stroke have been compared with 78 patients without stroke.
Plasma was withdrawn from each patient through centrifugation of 1500 g of blood samples.
The measurement of PARK7 and NDKA was performed by ELISA.
Nonparametric tests were conducted to discriminate both biomarkers.
Results
PARK7 and NDKA are expressed early in patients with stroke and decrease to baseline value on day 14 after admission.
The receiver-operating characteristic curves determined a great area under the curve for PARK7 than NDKA in stroke group but also in groups of different types of stroke (ischemic stroke, hemorrhagic stroke or subarachnoid hemorrhage).
Their expression are respectively similar in either ischemic stroke, hemorrhagic stroke and subarachnoid hemorrhage.
At a given time point, PARK7 and NDKA levels do not correlate with National Institute for Health Stroke Scale score value.
Discussion
Results of this study are complementary to latest findings: PARK7 has better diagnostic value for acute stroke than NDKA.
PARK7 may be more able to identify stroke patients that are more likely to benefit from early therapies.
Both markers have limitations; they are similarly expressed in ischemic stroke and hemorrhagic stroke, so they are not able to specify stroke type.
The limitation of this study may be its small sample size but its insight announces the superiority of PARK7 over NDKA.
Conclusion & future perspective
PARK7 is more accurate for diagnosis of patients in acute stroke and its usefulness will be improved in the future research.
Biomarkers in stroke are a developing field. With further studies, biomarkers may improve the ability to diagnose stroke, predict cause and certain complications, and stratify patients to treatment.
The development of more accurate biomarkers (such as PARK7) will help early triage of stroke patients requiring timely treatment.
Acknowledgments
The authors are grateful to Akankwasa Gilbert who helped in laboratory analysis and to all the team of Emergency Department and Benxi Lab for their support.
Footnotes
Author contributions
All the authors contributed significantly to this work from proposal conception, data collection (WX Feng), laboratory analysis, statistical analysis and manuscript edition. DSM Tulantched directed all steps under the supervision of Z Min.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
This study was approved by Institutional review board of Shengjing Hospital of CMU: The Medical Research and New Technology Ethics Committee of Shengjing Hospital affiliated to China Medical University (No: 2017PS048K). Accordingly with the Institutional review board, the need for consent to participate was waived because the samples used were the among other blood tubes collected from the selected patients admitted in the Hospital whose identification information had been removed. This study exploited only data filled in the Hospital record system without involvement from participants. Consent for publication: This study received the consent from The Scientific Medical Research Department of China Medical University. Each coauthors approved the final version.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Thrift AG, Thayabaranathan T, Howard G, et al. Global stroke statistics. Intern. J. Stroke. 2017;12(1):13–32. doi: 10.1177/1747493016676285. [DOI] [PubMed] [Google Scholar]
- 2.Wenzhi W, Bin J, Haixin S, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 3.Yiwang G, Pengyue L, Qingli G, et al. Pathophysiology and biomarkers in acute ischemic stroke – a review. Trop. J. Pharm. Res. 2013;12(6):1097–1105. [Google Scholar]; • Provides an overview of stroke biomarkers.
- 4.Ng GJL, Quek AML, Cheung C, Arumugam TV, Seet RCS. Stroke biomarkers in clinical practice: a critical appraisal. Neurochem. Inter. 2017;107:11–22. doi: 10.1016/j.neuint.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo R. Research for biomarkers in ischemic stroke. Cerebral. Blood. Flow. Met. 2015;26(2):225–232. [Google Scholar]
- 6.Kim SJ, Moon GJ, Bang OY. Biomarkers of stroke. J. Stroke. 2013;15(1):27–37. doi: 10.5853/jos.2013.15.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Defines the use of biomarkers in stroke, and how they can improve diagnosis of stroke.
- 7.Simats A, Garcia-Berrocoso T, Montaner J. Neuroinflammatory biomarkers: from stroke diagnosis and prognosis to therapy. Biochim. Biophys. Acta. 2016;1862(3):411–424. doi: 10.1016/j.bbadis.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Laborde CM, Mourino-Alvarez L, Akerstrom F, et al. Potential blood biomarkers for strokes. Expert Rev. Proteomics. 2012;9(4):437–449. doi: 10.1586/epr.12.33. [DOI] [PubMed] [Google Scholar]
- 9.Bustamante A, Lopez Cancio E, Pich S, et al. Blood biomarkers for early diagnosis of stroke: the stroke-chip study. Stroke. 2017;48(9):2419–2425. doi: 10.1161/STROKEAHA.117.017076. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds MA, Kirchick HJ, Dahlen JR, et al. Early biomarkers of stroke. Clin. Chem. 2003;49(10):1733–1739. doi: 10.1373/49.10.1733. [DOI] [PubMed] [Google Scholar]; • One of the first articles that raised the importance of biomarkers for early diagnosis of stroke.
- 11.Allard L, Burkhard PR, Lescuyer P, et al. PARK7 and nucleoside diphosphate kinase A as plasma markers for early diagnosis of stroke. Clin. Chem. 2005;51(11):2043–2051. doi: 10.1373/clinchem.2005.053942. [DOI] [PubMed] [Google Scholar]; • The first to determine the potential diagnostic value of PARK7 and NDKA for early diagnosis of stroke.
- 12.Mingina T, Zhao M. Role of PARK7 and NDKA in stroke management: a review of PARK7 and NDKA as stroke biomarkers. Biomark. Med. 2018;12(5):419–425. doi: 10.2217/bmm-2018-0013. [DOI] [PubMed] [Google Scholar]; • Reviewed the implication of PARK7 and NDKA in the management of stroke. It detailed different roles of these biomarkers in diagnosis and prognosis, as well as possible therapy of stroke.
- 13.Hiyoka M, Indien M, Yaganisawa D, Kitamura Y. DJ-1/PARK7: a new therapeutic target for neurodegenerative disorders. Biol. Pharm. Bull. 2017;40(5):548–552. doi: 10.1248/bpb.b16-01006. [DOI] [PubMed] [Google Scholar]
- 14.Antipova D, Bandopadhyay . Expression of DJ-1 in neurodegenerative disorders. In: Ariga H, Ariga SMM, editors. DJ-1/PARK7 Protein: Parkinson's Disease, Cancer and Oxidative Stress-Induced Diseases, in Advances in Experimental Medicine and Biology Series. Springer, Singapore; 2017. pp. 25–43. [DOI] [PubMed] [Google Scholar]
- 15.Ariga H, Takahashi-Nikki K, Kato I, Maita H, Niki T, Iguchi-Ariga SMM. Neuroprotective function of DJ-1 in Parkinson's disease. Oxid. Med. Cell. Longev. 2013;2013:683920. doi: 10.1155/2013/683920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid. Redox. Signal. 2011;15(1):111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaganida T, Kitamura Y, Yamane K, et al. Protection against oxidative stress-induced neurodegeneration by a modulator for DJ-1, the wild-type of familial Parkinson's disease linked PARK7. J. Pharmacol. Sci. 2009;109(3):463–469. doi: 10.1254/jphs.08323sc. [DOI] [PubMed] [Google Scholar]
- 18.Ariga H, Ariga SMM, editors. DJ-1/PARK7 Protein: Parkinson's Disease, Cancer and Oxidative Stress-Induced Diseases. Advances in Experimental Medicine and Biology Series: Springer, Singapore; 2017. VIII, 222. [Google Scholar]; • The book established all aspects of PARK7 in different diseases.
- 19.Amatullah H, Shan Y, Beauchamp BL, et al. DJ-1/PARK7 impairs bacterial clearance in sepsis. Am. J. Respir. Crit. Care Med. 2017;195(7):889–905. doi: 10.1164/rccm.201604-0730OC. [DOI] [PubMed] [Google Scholar]
- 20.Liu XW, Ma T, Cai Q, Wang L, Song HW, Liu Z. Elevation of serum PARK7 and IL-8 levels is associated with acute lung injury in patients with severe sepsis/septic shock. J. Intens. Care Med. 2017;23 doi: 10.1177/0885066617709689. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Voros P, Sziksz E, Himer L, et al. Expression of PARK7 is increased in celiac disease. Virchows Arch. 2013;463(3):401–408. doi: 10.1007/s00428-013-1443-z. [DOI] [PubMed] [Google Scholar]
- 22.Richarme G, Liu C, Mihoub M, et al. Guanine glycation repair by DJ- 1/PARK7 and its bacterial homologs. Science. 2017;357(6347):208–211. doi: 10.1126/science.aag1095. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Zhang WJ, Zhao X, et al. PARK7 protein translocating into spermatozoa mitochondria in Chinese Asthenozoospermia. Reproduction. 2014;148(3):249–257. doi: 10.1530/REP-14-0222. [DOI] [PubMed] [Google Scholar]
- 24.Sanafzadeh A, Lagerstedt L, Turck N, et al. Detection of cerebral lesions in mild traumatic brain injury-plasma Nucleotide Diphosphate Kinase (NDKA) as plasma biomarker. J. Neurol. Surg. 2015;75:S02. [Google Scholar]
- 25.Hoschstrasser DF, Sanchez JC, Lescuyer P, et al. Diagnostic method for brain damage-related disorders based on the detection of NDKA. European Patent Application. 2011 EP 2357477 A1. [Google Scholar]
- 26.Han X, Gao YH, Ma B, et al. The clinical relevance of serum NDKA, NMDA, PARK7 and UFDP levels with Phlegm-heat syndrome and treatment efficacy evaluation of traditional Chinese medicine in acute ischemic stroke. Evid. Based. Complement. Alternat. Med. 2015;2015:270498. doi: 10.1155/2015/270498. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Evaluates the significance of PARK7 and NDKA in a Chinese clinical set of ischemic stroke.
- 27.Lescuyer P, Allard L, Zimmerman-Ivol CG, et al. Identification of post-mortem cerebrospinal fluid as potential biomarkers of ischemia and neurodegeneration. Proteomics. 2004;4(8):2234–2241. doi: 10.1002/pmic.200300822. [DOI] [PubMed] [Google Scholar]
- 28.Allard L, Turck N, et Burkhard PR, et al. Ubiquitin fusion degradation protein 1 as a blood marker for the early diagnosis of ischemic stroke. Biomark. Insights. 2007;2(1):155–164. [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Ma B, Han X, et al. Correlation between the NDKA and PARK7 levels and acute ischemic stroke with qi deficiency syndrome as well as the NIHSS score. China J. Tradit. Chinese Med. Pharm. 2017;32(8):3785–3788. [Google Scholar]; • Among the articles that studied the association of PARK7 and NDKA levels with stroke severity through National Institute for Health Stroke Scale.
- 30.Hill MD. Diagnostic biomarkers for stroke: a stroke neurologist's perspective. Clin. Chem. 2005;51(11):2001–2002. doi: 10.1373/clinchem.2005.056382. [DOI] [PubMed] [Google Scholar]
- 31.Rothstein L, Jickling GC. Ischemic stroke biomarkers in blood. Biomark. Med. 2013;7(1):31–47. doi: 10.2217/bmm.12.104. [DOI] [PubMed] [Google Scholar]
- 32.Peacock WF. Where are the stroke markers? Clin. Chem. 2017;63(1):252–254. doi: 10.1373/clinchem.2016.255091. [DOI] [PubMed] [Google Scholar]
- 33.Barber PA, Zhang J, Demchuck M, et al. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015–1020. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- 34.Ashraf VV, Maneesh M, Praveenkumar R, Saifudheen K, Girijia AS. Factors delaying hospital arrival of patients with acute stroke. Ann. Indian Acad. Neurol. 2015;18(2):162–166. doi: 10.4103/0972-2327.150627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magauran BG, Jr, Nitka M. Stroke mimics. Emerg. Med. Clin. North Am. 2012;30(3):795–804. doi: 10.1016/j.emc.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Gibson LM, Whiteley W. The differential diagnosis of suspected stroke: a systematic review. J. R. Coll. Physicians Edinb. 2013;43:114–118. doi: 10.4997/JRCPE.2013.205. [DOI] [PubMed] [Google Scholar]
- 37.Saenger AK, Christenson RH. Stroke biomarkers: progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin. Chem. 2010;56(1):21–23. doi: 10.1373/clinchem.2009.133801. [DOI] [PubMed] [Google Scholar]