Abstract
Cooling towers for heating, ventilation and air conditioning are ubiquitous in the built environment. Often located on rooftops, their semi-open water basins provide a suitable environment for microbial growth. They are recognized as a potential source of bacterial pathogens and have been associated with disease outbreaks such as Legionnaires’ disease. While measures to minimize public health risks are in place, the general microbial and protist community structure and dynamics in these systems remain largely elusive. In this study, we analysed the microbiome of the bulk water from the basins of three cooling towers by 16S and 18S rRNA gene amplicon sequencing over the course of one year. Bacterial diversity in all three towers was broadly comparable to other freshwater systems, yet less diverse than natural environments; the most abundant taxa are also frequently found in freshwater or drinking water. While each cooling tower had a pronounced site-specific microbial community, taxa shared among all locations mainly included groups generally associated with biofilm formation. We also detected several groups related to known opportunistic pathogens, such as Legionella, Mycobacterium, and Pseudomonas species, albeit at generally low abundance. Although cooling towers represent a rather stable environment, microbial community composition was highly dynamic and subject to seasonal change. Protists are important members of the cooling tower water microbiome and known reservoirs for bacterial pathogens. Co-occurrence analysis of bacteria and protist taxa successfully captured known interactions between amoeba-associated bacteria and their hosts, and predicted a large number of additional relationships involving ciliates and other protists. Together, this study provides an unbiased and comprehensive overview of microbial diversity of cooling tower water basins, establishing a framework for investigating and assessing public health risks associated with these man-made freshwater environments.
Keywords: Microbial diversity, Legionella, Mycobacteria, Chlamydia, Protists, Free-living amoeba, Built environment, HVAC cooling tower
Graphical abstract
Highlights
-
•
Highly dynamic bacterial and protist community structure in cooling towers.
-
•
Cooling tower water basin communities resemble other open freshwater environments.
-
•
Core bacterial microbiome of cooling towers consists of biofilm-forming taxa.
-
•
Co-occurrence network analysis suggests importance of inter-kingdom relationships.
1. Introduction
Most modern commercial, industrial, and residential buildings rely on cooling towers as cost-efficient measures to remove excess heat. Containing large semi-open water volumes at a rather constant temperature, cooling towers are suitable environments for microbial growth throughout the year and have been implicated in bacterial outbreaks (Kurtz et al., 1982; Pagnier et al., 2009; Torvinen et al., 2013; Yamamoto et al., 1992). One of the major public health risks associated with cooling towers is Legionnaires’ disease. This respiratory tract infection is caused by Legionella pneumophila, a gammaproteobacterial pathogen acquired through inhalation of aerosols and is potentially fatal for immunocompromised patients (Hamilton et al., 2018; Walser et al., 2014). Other opportunistic bacterial pathogens detected in cooling towers include Mycobacterium spp., Pseudomonas spp., Burkholderia spp., and Pantoea spp. (Ceyhan and Ozdemir, 2008).
Protists, such as free-living amoebae, are common members of microbial communities in cooling towers (Barbaree et al., 1986; Berk et al., 2006; Delafont et al., 2016; Pagnier et al., 2009). They are predators of other microbes in the system and may also be reservoirs for bacterial pathogens such as Legionella and Mycobacterium spp., Pseudomonas aeruginosa, Francisella tularensis, Coxiella burnetii, and Vibrio cholerae (Abd et al., 2003; La Scola and Raoult, 2001; Sandström et al., 2010; Thomas and McDonnell, 2007; Thom et al., 1992; Van der Henst et al., 2016). These microbes are able to escape the regular phagolysosomal pathway and transiently replicate within amoeba trophozoites. Moreover, many free-living amoebae can form cysts, a resistant life stage providing protection from unfavorable environmental conditions including biocides (Fouque et al., 2012; Miltner and Bermudez, 2000). Efficient disinfection procedures targeting not only bacteria but also protists have thus been recognized as important in reducing the public health risk associated with cooling towers (Critchley and Bentham, 2009; Scheikl et al., 2016).
Bacteria-protist relationships are manifold (Cirillo et al., 1997, 1994; Fritsche et al., 1998; Greub and Raoult, 2004; Hess, 2016; Hess, 2017; Molmeret et al., 2005). They also include obligate bacterial symbionts, such as Caedibacter and Holospora species in ciliates (Dziallas, 2012; Fokin, 2004; Goertz, 2001), or Protochlamydia and Parachlamydia species, also known as environmental chlamydiae (Horn, 2008; Taylor-Brown et al., 2015). Yet, microbial communities and their interactions in cooling tower water are likely far more complex than recognized. The presence of specific bacterial pathogens has been investigated (Inoue et al., 2015; Li et al., 2015; Liu et al., 2011, 2009; States et al., 1987; Torvinen et al., 2013; Ulleryd et al., 2012), but surveys on the overall microbial community composition are rare (Llewellyn et al., 2017; Wang et al., 2013). Studies specifically investigating community assembly and dynamics in these systems are largely lacking, with currently only one recent report (Pereira et al., 2017). Except for a few notable studies (Llewellyn et al., 2017; Pereira et al., 2017; Wang et al., 2013), work on the microbial community of water towers has been based on cultivation dependent methods or PCR assays targeting specific microbial groups, which are inherently limited in both taxonomic resolution and scope. High-throughput DNA sequencing of 16S/18S ribosomal RNA gene PCR products, termed amplicon sequencing, is able to circumvent these limitations and provides detailed data on both bacterial and protist communities (Thompson et al., 2017). After all, it is the wide range of interactions - from antagonistic to synergistic - that shape microbial assemblages and ultimately determine the chances for pathogen proliferation.
In this study, we used amplicon sequencing to investigate seasonal and geographic variation in the composition of bacterial and protist communities found in three cooling towers over a one-year period. We focused our analysis on the bulk water in the water basins, because it is the source of aerosols formed during cooling tower operation (Nhu Nguyen et al., 2005). Bacteria-protist relationships were inferred from co-occurrence network analysis. These approaches identified key microbial players and provided detailed insight into the microbial community dynamics in these systems including putative bacteria-protist interactions.
2. Materials and methods
2.1. Site description, sample collection, and processing
Three different heating, ventilation and air conditioning (HVAC) cooling towers located on the rooftop of different buildings in the city of Vienna, Austria, were studied. Two buildings, a hospital and a business complex, were located near the city center (1.7 km apart from each other) whereas a second hospital was located at Vienna's periphery (5 km from the other two locations). The two hospitals are hereafter referred to as (“cooling tower 1”) CT-1 and CT-2, the business complex as CT-3. For all three sites, the water temperature was recorded throughout the sampling campaign. The cooling towers were subjected to disinfection with chlorine/bromine-based disinfectant (1–3 g/m3) over an automated dosimeter. CT-1 was disinfected three times a week, CT-2 every 18 h and CT-3 every four days.
During the sampling period between September 2013 and September 2014, water samples were taken on a biweekly basis. CT-3 was not operating during the winter season, resulting in fewer samples. From the tank basin, 3 L of bulk water was sampled and stored at 4 °C for up to a day before further processing. The water was stirred first and then 2 L per sample were vacuum-filtered onto a cellulose nitrate filter (diameter 0.2 μm; 12.5 cm2; Sartorius stedim) in 500 ml steps. In case of rapid filter clogging, filters with a slightly larger pore size (diameter 0.45 μm) were used in addition for further filtration of the same sample. Up to four filters were used per sample. All filters were subsequently used for downstream processing.
2.2. DNA extraction, and 16S/18S rRNA gene amplicon sequencing
The PowerWater® DNA Isolation Kit (Qiagen, Hilden, Germany) was used for DNA extraction from the filters. All steps were performed according to the standard centrifuge-based protocol recommended by the manufacturer. Briefly, the filters were inserted in a lysis buffer-containing bead beating tube where the cells were mechanically and chemically lysed. The lysates were transferred to a DNA-retaining spin column; lysates from the same sample were loaded onto the same column to pool the DNA. After washing, DNA was eluted in the DNA elution buffer and used for PCR.
A two-step barcoded amplicon sequencing approach was used as described previously (Herbold et al., 2015). Briefly, fragments of the bacterial and eukaryotic small subunit rRNA gene were amplified, resulting in 26 libraries each for CT-1 and CT-2, and 14 libraries for CT-3. V3 and V4 regions of the bacterial 16S rRNA gene were amplified with the primers Bakt_341F (5′-CCTACGGGNGGCWGCAG-3′) and Bakt_805R (5′-GACTACHVGGGTATCTAATCC-3’; Herlemann et al., 2011). Archaea were not targeted by this primer set and were not analysed in this study. The eukaryotic 18S rRNA gene primer-pair EUK_1391F (5′-GTACACACCGCCCGTC-3′) and EUK_1510R (5′-CCTTCYGCAGGTTCACCTAC-3′) based on the Earth Microbiome Project (Version 5, 2012; Amaral-Zettler et al., 2009; Gilbert et al., 2014) were used to amplify the 18S—V9 region. For each primer set and sequencing run, negative controls without the addition of DNA were performed and sequenced.
Each PCR reaction included 1x DreamTaq Green Buffer (Fermentas, Thermo Fisher Scientific, Vienna, Austria), 2 mM MgCl2, 0.2 mM dNTP mix (Fermentas), 0.1 mg mL−1 bovine serum albumin, 1 μM of each of the forward and reverse primers, 0.025 U DreamTaq polymerase (Fermentas), and 1 μL of DNA template. This first PCR amplification (94 °C for 3 min; 25 cycles of 45 s at 94 °C, 30 s at 52 °C (16S rRNA gene) or 57 °C (18S rRNA gene), 90 s at 72 °C; and 72 °C for 10 min) was performed in triplicates; PCR products were pooled and served as the template for the second barcoding PCR (95 °C for 3 min; 10 cycles of 30 s at 95 °C, 30 s at 55 °C, 60 s at 72 °C; and 72 °C for 7 min). Purification and quantification of PCR products were done as described (Herbold et al., 2015). The TruSeq Nano DNA Library Prep Kit (Illumina) was used for adaptor ligation and PCR without the fragmentation step. Sequencing was performed by Microsynth AG (Balgach, Switzerland) on a MiSeq system (Illumina) using the MiSeq Reagent kit V3. Resulting sequence datasets were deposited in the NCBI Sequence Read Archive under study accession number PRJEB21563.
2.3. Data compilation, filtering, clustering, and classification
Paired-end reads were demultiplexed and trimmed as described (Herbold et al., 2015) and were assembled using fastq-join within Qiime (Caporaso et al., 2010). 18S rRNA reads were forced to be trimmed by at least 88 nucleotides prior to trimming and assembly because the expected amplicon length of 178 bp was shorter than the average read length. Clustering into operational taxonomic units (OTUs) and chimera-checking were performed using Uparse (Edgar, 2013), using a distance of 0.03 and disallowing singletons to serve as a centroid. Taxonomic classification of OTU centroids was carried out using the Bayesian classifier implemented in mothur (Schloss et al., 2009) and the Silva SSU database (Version 132; Quast et al., 2013) for 16S rRNA data sets, or the protist ribosomal reference database PR2 (Version 4.11.1; Guillou et al., 2013) for 18S rRNA data sets. OTUs with a classification confidence score lower than 80% for 16S rRNA data sets and 60% for 18S rRNA data sets were changed to ‘unclassified’. The class Betaproteobacteria has recently been proposed to be reclassified as the new order Betaproteobacteriales within the class Gammaproteobacteria (Parks et al., 2018). For clarity and consistency with previous studies, the Betaproteobacteria were still treated as a separate class, but both names are provided. 16S rRNA OTUs classified as mitochondria and chloroplasts were omitted from subsequent analysis; this did not change the overall estimates of alpha and beta diversity measures. 16S rRNA OTUs present only in the negative controls or strongly overrepresented (50 fold) in the negative controls compared to all samples were considered to be cross-contamination and thus removed from the dataset (Table S1).
All 18S rRNA OTUs only present in the negative control were removed from the dataset. OTUs present in both control and samples were only retained if their sample-abundance exceed the control-abundance by 1000 fold (Table S2). Taking into account the known relationships between protists and pathogenic bacteria in the water-borne environment, we restricted our analysis of 18S rRNA sequences to the groups Amoebozoa (Lobosa, Conosa), Excavata (Discoba, Metamonada), Rhizaria (Cercozoa, Foraminifera) and Alveolata (Apicomplexa, Ciliophora, Dinoflagellata). Both data sets were rarefied individually to standardize sequence numbers using the rarify_even_depth function in the R-package phyloseq (McMurdie and Holmes, 2013) with replacement option turned off. For the 16S rRNA data set a minimum of 1713 sequences was used, representing the 15% quantile of the data; for the 18S rRNA data set, which had a greater library size variation, a cutoff of 59 sequences equivalent to 45% quantile of the data was used. Samples not fulfilling these criteria were removed from the subsequent analysis.
2.4. Alpha and beta diversity measures and co-occurrence analysis
Alpha diversity was assessed for both data sets with the R-package phyloseq (McMurdie and Holmes, 2013) using the estimators Chao1 and the Shannon diversity index. Bacterial community composition was compared by non-metric multidimensional scaling (NMDS) analysis based on Bray-Curtis dissimilarity.
For the OTU co-occurrence analysis, only those samples for which both 16S and 18S rRNA sequence data were available after rarefying were included. In addition, only OTUs present in at least 3 samples (for each cooling tower) and with a total number of sequences greater than 10 were kept to remove the number of infrequent OTUs. For each cooling tower, co-occurrence analysis of 16S rRNA and 18S rRNA OTUs was performed using CoNet (Faust and Raes, 2016), a plug-in of the software Cytoscape (Shannon et al., 2003). To calculate consensus networks, a combination of different correlation and distance measures including Pearson and Spearman correlation, mutual information, Kullback-Leibler divergence, and Bray-Curtis dissimilarity was used. The maximum lag was set to 1 to include slightly shifted associations. Distribution of all pair-wise scores was generated, and the 7.5% top-scoring edges supported by at least four out of five (positive correlations) or three out of five (negative correlations) measures were kept. This process was performed with 1000 renormalized permutations, resampling by row-shuffling, and p-value merging with Brown's method. After applying Benjamini-Hochberg's false discovery rate correction, edges with a corrected p-value below 0.05 were kept.
The R-package ampvis (Albertsen et al., 2015) was used to generate heatmaps; MetacodeR (Foster et al., 2017) was used to generate heat trees.
3. Results and discussion
In this study, we monitored the microbial community in the water basins of three heating, ventilation and air conditioning (HVAC) cooling towers - in the following referred to as CT-1, CT-2, and CT-3 - over the course of one year. The temperature in the basins ranged between 20.1 °C and 32.3 °C for CT-1 (99 percentile of all temperature data points), and between 19.9 °C and 29 °C for CT-2 throughout the sampling period. The average temperature during the summer months was slightly higher than during winter; 27.8 °C vs. 26.4 °C for CT-1, and 26.6 °C vs. 23.1 °C for CT-2. Temperature data for CT-3 were only available after mid-April 2014; its temperature range was 10.6–27.4 °C, larger than that of the other two towers. Elevated temperature in the water basin is a characteristic feature of cooling towers and together with the semi-open design of these systems provide good conditions for microbial growth. Cooling tower water tanks, like other freshwater or drinking water systems, develop biofilms (Liu et al., 2009). Yet, for the purpose of this study we focused on the analysis of bulk water for two reasons: (i) bulk water represents the seed community for biofilm formation in the basin, and (ii) it is the source of aerosols formed during cooling tower operation (Nhu Nguyen et al., 2005).
3.1. Complex but reduced bacterial diversity compared to open freshwater environments
In total, we collected 26 samples each for CT-1 and CT-2, and 14 samples for CT-3. Amplicon sequencing of 16S and 18S rRNA genes revealed taxonomically diverse bacterial and protist communities for all three cooling towers. To study seasonal dynamics of microbial diversity in the three cooling towers, we first estimated species-level richness and evenness using a threshold of 97% sequence similarity for operational taxonomic unit (OTU) definition. Good's coverage estimator was 0.984 ± 0.007 (mean ± SEM) for 16S OTUs and 0.958 ± 0.018 (mean ± SEM) for 18S OTUs, indicating that sequencing depth was sufficient for reliable community composition analysis. After rarefying, our data set included 23 CT-1 samples, 21 CT-2 samples, and 12 CT-3 samples. In total 590 bacterial OTUs and 321 protist OTUs were considered for our analysis (Tables S3 and S4).
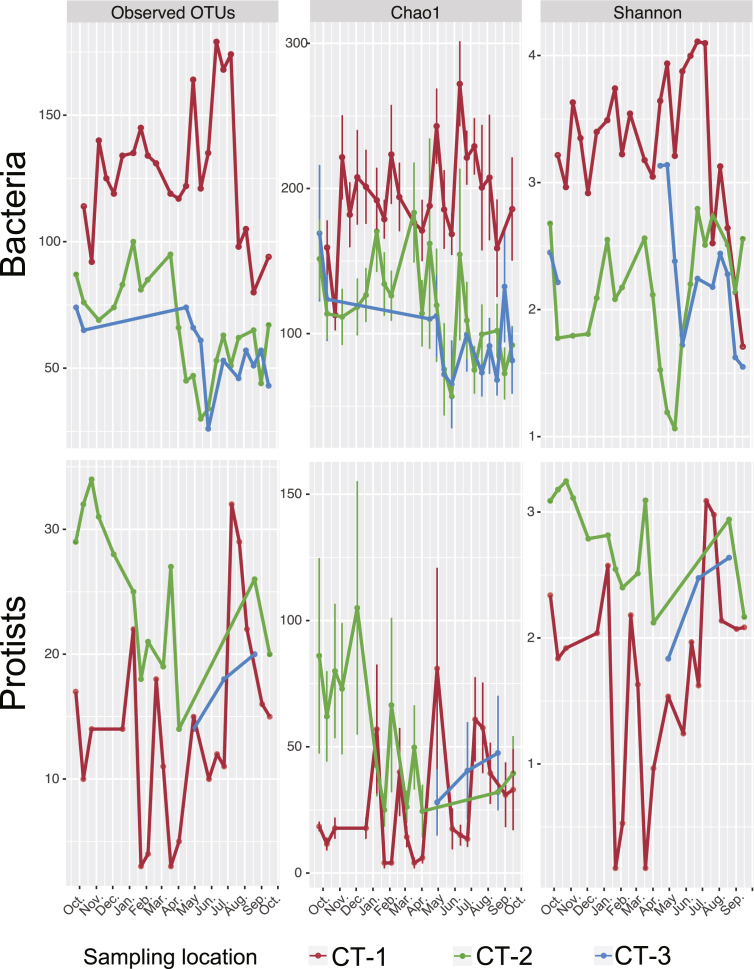
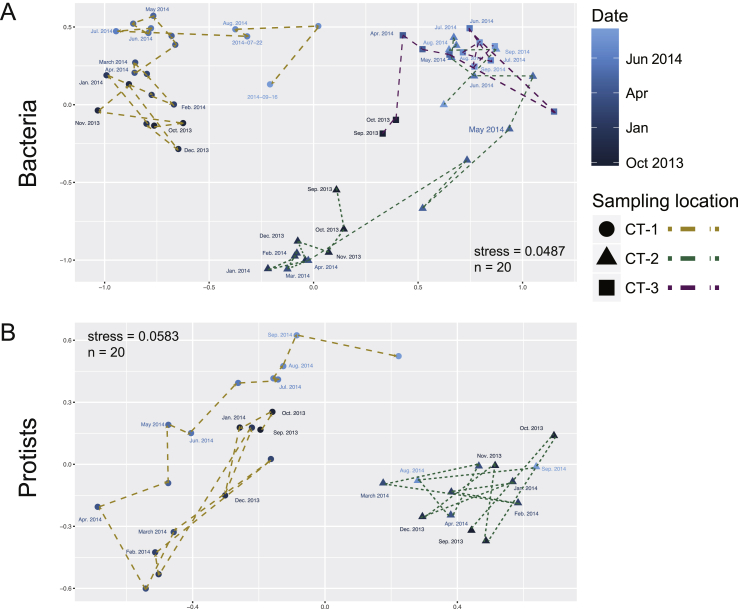
The three cooling towers showed clear differences in bacterial and protist species richness and evenness (Fig. 1). As a general pattern, protist diversity was much lower than bacterial diversity for all samples and sampling sites. Microbial diversity fluctuated strongly throughout the sampling period, and there was no clear common trend observed among the different cooling towers. It is interesting that while CT-1 had the highest bacterial diversity values (as inferred from Chao1 and Shannon estimators; Welch's t-test p-value < 0.0001), it also showed the lowest protist diversity and evenness compared to the other two locations, which were also more similar with each other (Fig. 1). This was also reflected in the NMDS-based beta-diversity analysis (Welch-corrected t-test of the average Bray-Curtis distance within one sampling site compared to other sites; p-value < 0.0001), revealing clear sample clusters according to sampling site and time point (Fig. 2). At all sampling sites, we observed gradual community shifts over time reflecting seasonal changes in bacterial and protist diversity. In CT-1, the high similarity between autumn, winter, and spring samples indicated a gradual rather than sudden change. In contrast, the community shift in CT-2 was more pronounced (Fig. 2).
Fig. 1.
Bacterial and protist diversity in three cooling tower water basins. The number of observed OTUs and diversity indices for estimation of species richness and evenness of bacterial and protist communities are indicated for cooling towers CT-1, CT-2, and CT-3 over a sampling period of one year (September 2013 to September 2014). Pronounced differences between sampling sites and time points in both bacterial and protist diversity can be observed.
Fig. 2.
Similarity of bacterial and protist community structure across time and space. Nonmetric multidimensional scaling (NMDS) analysis illustrating beta diversity among all samples based on the Bacterial or protist community composition (protist diversity was not included in CT-3 samples due to low sample number). For each cooling tower, the samples were sequentially connected with arrows. The first sample of each month are annotated with the date in the plot. Microbial communities of cooling tower CT-1 are clearly separated in this analysis, indicating a unique community composition. Shifts in bacterial community structure apparently follow a seasonal trend.
To better understand community structure we next classified all OTUs, grouped them at the genus level, and focused on the 20 most abundant genera (Fig. 3). CT-2 and CT-3 are both dominated by a few abundant genera, whereas in CT-1, abundances of the top genera are more evenly distributed. This analysis showed that the general bacterial community composition is different between the three cooling towers, yet Flavobacterium (Bacteroidetes), Hyphomicrobium, members of the Rhizobiales and Sphingomonadales (Alphaproteobacteria), Pseudomonas (Gammaproteobacteria), and Methyloversatilis (Betaproteobacteria/Betaproteobacteriales; Parks et al. 2018) are among the top abundant taxa in all three towers (Fig. 3). The most striking difference between the cooling towers is the extremely high Pseudomonas abundance in CT-2 (Fig. 3), which makes up almost 31.8% of all sequence reads (compared to up to 3% at the other two sampling sites) while other Gammaproteobacteria were generally not well represented in our data set. Our observations are consistent with findings from the only cooling tower for which a similar analysis is available so far (Pereira et al., 2017). In this study, members of the Rhizobiales, Burkholderiales, Methylophilales, and Cytophagales were also among the most abundant taxa. In contrast, we didn't detect Xanthomonadales among the top taxa, but found other groups to be well represented, including Corynebacteriales, Rhodocyclales, and Pseudomonadales (Fig. 3). It is noteworthy, that Betaproteobacteria (Betaproteobacteriales), commonly found in freshwater systems (Newton et al., 2011), are with two exceptions absent in the CT-1 top genera list, whereas they are abundant in CT-2 and CT-3.
Fig. 3.
The most abundant bacterial and protist genus at three cooling towers. For each sampling site, the average relative abundance of the 20 most frequently observed bacteria and protist taxa are listed at the genus level. In total 79% of all bacterial OTUs and 59% of all protist OTUs could be classified at the genus level (Tables S3 and S4). For clarity, the phylum as well as the order level are also indicated (the Proteobacteria were split into its representative classes). The Sphingomonadales, Pseudomonadales, Rhizobiales, and Burkholderiales including many biofilm associated taxa are among the top bacterial groups.
Two members of the recently identified Candidate Phyla Radiation (CPR) are among the most abundant taxa in CT-1: Parcubacteria (formerly OD1) and Saccharimonadia (formerly known as TM7), both now classified within the phylum Patescibacteria. Members of both groups show reduced genomes and only limited metabolic capabilities, likely endorsing a parasitic or symbiotic lifestyle (He et al., 2014; Nelson and Stegen, 2015). They are known to have small cell sizes and are able to pass through a 0.22 μm filter (Luef et al., 2015). CT-1 also included an unclassified member of the Rickettsiales (Alphaproteobacteria) among the top abundant taxa, again a group of microbes generally associated with an intracellular lifestyle.
Overall, the most abundant bacterial orders identified in the cooling tower samples are well in agreement with other reports on man-made water distribution systems, where orders belonging to Alpha-, Beta-, and Gammaproteobacteria are usually the prevalent taxa (Berry et al., 2006; Brown et al., 2015; Farenhorst et al., 2017; Kalmbach et al., 1997; Llewellyn et al., 2017; Martiny et al., 2005; McCoy and VanBriesen, 2012; Pereira et al., 2017; Revetta et al., 2010; Tokajian et al., 2005; Vaz-Moreira et al., 2012; Wang et al., 2013; Zhang et al., 2012). Bacterial communities in more natural settings such as lakes, rivers, or streams are often dominated by Betaproteobacteria (Betaproteobacteriales), but also include Actinobacteria, Bacteroidetes, Cyanobacteria, Verrucomicrobia, and Saccharimonadia (Boucher et al., 2006; Crump and Hobbie, 2005; Liu et al., 2012; Lozupone and Knight, 2005; Mueller-Spitz et al., 2009; Newton et al., 2011; Tang et al., 2015; Zwart et al., 2002). The dominance of these groups in the cooling tower microbial community structure is consistent with what has been reported for other freshwater systems. This suggests that community structure in cooling towers may be primarily influenced by the seed community in the incoming drinking water and then shaped by the specific conditions in the cooling tower water basin.
3.2. Seasonal microbial community dynamics
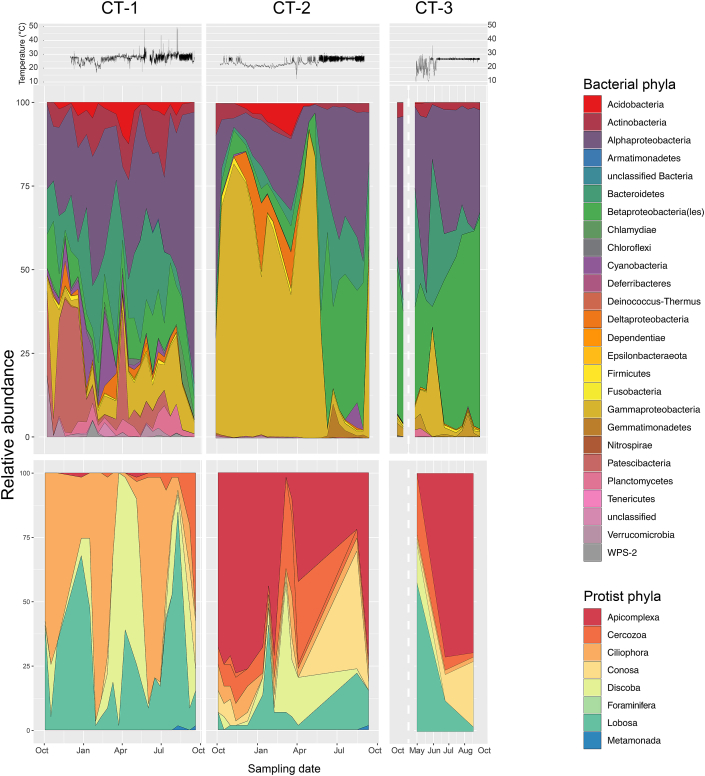
To further analyse temporal changes in community structure, we examined the relative abundance of phyla over time. Consistent with our previous analysis, we observed pronounced changes in bacterial community composition over time (Fig. 4). The most dramatic seasonal change occurred in cooling tower CT-2, where an abrupt decline of Gammaproteobacteria (Pseudomonas) was observed during June 2014 (Fig. 4). Pronounced variations were also observed in the more diverse community of CT-1. In CT-3, the overall bacterial community composition was rather stable compared to the other cooling towers (Fig. 4). Protistan community members of the Rhizaria, the Excavata, the Alveolata, and the Amoebozoa were found at all three sampling sites, with the latter two phyla being most abundant and showing pronounced temporal variations. Notably, changes in bacterial and protist diversity do not necessarily follow the same trend, indicating complex interactions and differences in response to environmental conditions (Fig. 3, Fig. 4).
Fig. 4.
Temporal dynamics of bacterial and protists diversity over a one year sampling period. Changes in relative abundance of bacteria and protists at the phylum level for cooling towers CT-1, CT-2, and CT-3 reveals temporal dynamics of community structure despite relatively constant environmental conditions. The average daily temperature data in the water basin is shown in the uppermost panel; lack of data for CT-3 is indicated by a white dashed line.
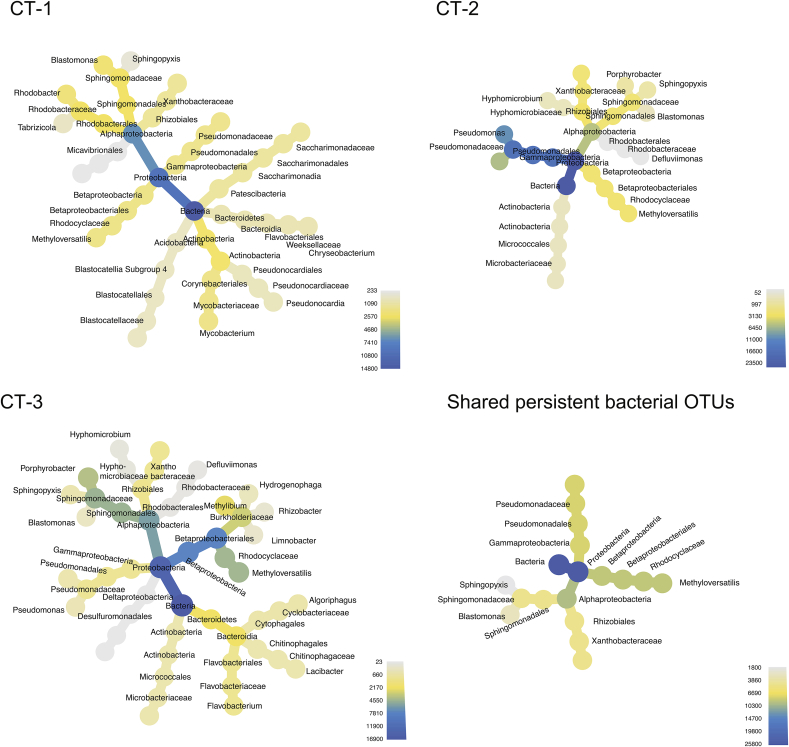
Next, we aimed to understand differences and similarities between bacterial and protist community composition and dynamics in the three cooling towers in more detail. To this end, we focused on various sets of OTUs: We defined a set of persistent OTUs as taxa that were detected at a given location at nearly all time points (taxa were allowed to be missing from only two samples, corresponding to at least 90% of all samples for CT-1 and CT-2, and 80% for CT-3). The second set was referred to as site-specific OTUs and included those taxa that were exclusively found at a single location. The third set comprised taxa detected at all cooling towers and was referred to as shared OTUs.
Consistent with our observed seasonal changes, there were few persistent OTUs, 13 in CT-1, 10 in CT-2, and 18 in CT-3 (Fig. 5). They included Actinobacteria (Mycobacterium sp., or members of the Microbacteriaceae), Proteobacteria (Sphingopyxis, Methyloversatilis, Blastomonas, Defluviimonas, Porphyrobacter, Xanthobacteraceae), Patescibacteria (Saccharimonadia, formerly TM7; Albertsen et al., 2013), Bacteroidetes, or Acidobacteria. Interestingly, most of the top five abundant taxa in each of the cooling towers also belonged to the set of persistent OTUs (Fig. 3, Fig. 5).
Fig. 5.
Persistent bacterial OTUs in three cooling tower water basins. For each sampling site, a heat tree depicts the persistent bacterial taxa at the genus level (unclassified taxa are unlabeled). Persistent taxa are defined as being present in nearly all samples of one sampling location. The color corresponds to the total number of sequences detected for each sampling site at the respective taxonomic level. Shared bacterial OTUs are persistent taxa found in all three sampling sites, all belonging to the Proteobacteria. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
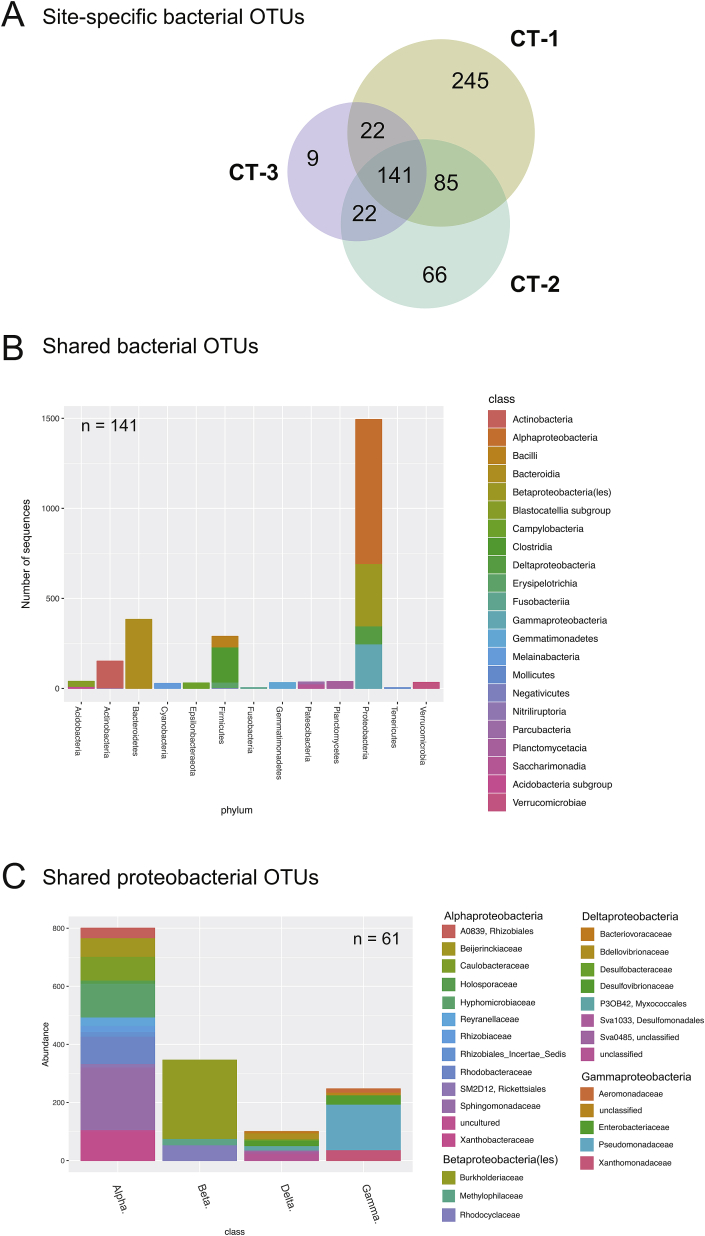
The differences between the cooling towers were further analysed by focusing on the set of site-specific taxa. Consistent with earlier observations (Fig. 1), CT-1 had by far the largest number of site-specific bacterial OTUs (245, 53.1.%), compared to CT-2 (66, 14.3%) and CT-3 (9, 1.9%; Fig. 6A). It is noteworthy, that there is no overlap between the sets of site-specific taxa and the persistent OTUs for any of the cooling towers. In direct comparison, the site-specific taxa comprised a wider range of diverse phyla, including members of the Patescibacteria, Chlamydiae, Chloroflexi, Planctomycetes, Verrucomicrobia, and Depedentiae (TM6; Delafont et al., 2015; Table S3). The transient character of the site-specific OTUs was further illustrated by most of them being detected at a maximum of nine time points. This indicated that there was a substantial fraction of taxa specific to individual cooling towers that failed to establish long-term colonization.
Fig. 6.
Site-specific and shared bacterial OTUs in three cooling towers. (A) The Venn diagram represents the proportion of shared and site-specific bacterial OTUs at the three sampling sites. Reflecting its higher species richness, CT-1 showed the greatest number of site-specific bacterial OTUs. The number of OTUs shared by all three sampling sites clearly exceeds the number of OTUs shared individually by two cooling towers. (B) The bar chart shows the taxonomic classification of the shared OTUs (occurring at least once at each sampling site). The majority of the OTUs belongs to the Proteobacteria, Bacteroidetes, Actinobacteria, and Firmicutes. (C) A more detailed view of the shared Proteobacteria on the family level. The most abundant groups are the Bradyrhizobiaceae, Caulobacteraceae, Hyphomicrobiaceae, Sphingomonadaceae, Comamonadaceae, and Pseudomonadaceae.
3.3. The core bacterial microbiome of cooling towers consists of biofilm-forming taxa
To better understand the common features of cooling tower microbial communities, we next focused on the set of shared OTUs, i.e. those detected at least once at more than one site. This set represents 30.6% of all bacterial OTUs in this study, and the fraction of shared OTUs varied between the individual cooling towers (28.6%, 44.9%, and 72.6%, respectively). The majority of shared OTUs belongs to the Alphaproteobacteria (Sphingomonadaceae and Hyphomicrobiaceae), Betaproteobacteria/Betaproteobacteriales (Burkholderiaceae), Actinobacteria, Bacteroidetes (Bacteroidia), and Gammaproteobacteria (Pseudomonadaceae) (Fig. 6B and C). From this large set of shared OTUs only six bacterial taxa were nearly always present, i. e. also belong to the set of persistent OTUs (Fig. 5). This included members of the genera Pseudomonas, Methyloversatilis, Blastomonas, Sphingopyxis, and Porphyrobacter, and the family Xanthobacteraceae (formerly classified as Bradyrhizobiaceae).
A common feature of these microbes is their ability to form or to be associated with microbial biofilms (Rickard et al., 2004; Soto-Giron et al., 2016; Zhang et al., 2012). Pseudomonas species are ubiquitous environmental bacteria well-known for their capability to form biofilms (Costerton, 1999; Jensen et al., 2010; Sauer et al., 2002). They are considered pioneers, specifically facilitating the initiation of biofilm formation (Douterelo et al., 2014). Members of the genus Methyloversatilis are found in various environments including drinking water and biofilms (Kalyuzhnaya et al., 2006; Liu et al., 2012; Shpigel et al., 2015; van der Kooij et al., 2017; Williams et al., 2004). Blastomonas species have the ability to coaggregate with various bacteria, an important feature for biofilm formation (Katharios-Lanwermeyer et al., 2014; Rickard et al., 2002). Additionally, other members of the order Sphingomonadales such as Sphingopyxis, Porphyrobacter, and the family Xanthobacteraceae (formerly Bradyrhizobiaceae) were repeatedly found to be associated with bacterial biofilms and are commonly present in drinking water environments (Bereschenko et al., 2010; Buse et al., 2014; Chao et al., 2015; Douterelo et al., 2014; Hong et al., 2010; Muchesa et al., 2017; Revetta et al., 2010; Rożej et al., 2015; Singh et al., 2003; Vaz-Moreira et al., 2011).
The occurrence and persistence of these microbes at all cooling towers suggest that biofilm formation is an important process in cooling tower microbial communities, likely contributing to maintenance and resistance against disinfection measures. Cooling tower basins offer a large surface area and are characterized by a slow water flow speed, a rather constant and elevated temperature, as well as increased concentrations of organic matter compared to closed drinking water systems. These conditions are well-suited for biofilm formation (Türetgen and Cotuk, 2007; Zacheus et al., 2000).
Biofilms are preferred niches of protists because of the high density of microbes serving as a potential food source, and grazing is a major factor modulating the structure and function of bacterial biofilms (Flemming et al., 2016; Matz et al., 2005; Parry, 2004). Biofilms are also a known reservoir and potential source for a number of bacterial pathogens (Delafont et al., 2014; Garcia et al., 2013; Greub and Raoult, 2004; Wingender and Flemming, 2011).
3.4. Opportunistic bacterial pathogens
Over the course of our one-year sampling campaign we detected few OTUs classified as genera comprising known (opportunistic) bacterial pathogens, as defined in a previous study (Pereira et al., 2017; Table S5). The abundance of these OTUs ranges from 0.01% to 28% depending on the sampling location, but the majority are below 1%. We observed a total of 29 OTUs belonging to three different phyla, Actinobacteria, Firmicutes, and Proteobacteria. This includes Bosea, Helicobacter, and Stenotrophobacter and several Arcobacter, Bacillus, Sphingomonas, Legionella, Mycobacterium, and Pseudomonas species (Table S5). In comparison to the cooling tower studied earlier, the cooling towers investigated here display a less diverse set of genera including potential pathogens (Pereira et al., 2017).
Of note, none of the Legionella sequences showed a particularly high similarity to the human pathogen Legionella pneumophila. This is consistent with no L. pneumophila outbreak having been reported in the vicinity of our sampling locations during this study. The Legionella species detected belong to a group commonly referred to as Legionella-like amoebal pathogens, a large number of species primarily found as parasites in free-living amoebae (Greub and Raoult, 2004; Marrie et al., 2001; Newsome et al., 1988). All Legionella OTUs belong to the set of site-specific taxa, and their relative abundances were below 0.1%, rendering them members of the rare biosphere in these systems.
We found four OTUs assigned to the genus Mycobacterium, two of which occurred in two cooling towers. One of those, M. wolinsky, showed a relatively high abundance in CT-1 (4%) and belonged to the persistent taxa in this cooling tower (Table S3, Fig. 5). M. wolinsky can cause bacteremia and, like the other mycobacteria detected in this study, is a member of the nontuberculous mycobacteria (NTM) group. This group includes opportunistic human pathogens found in a variety of habitats, ranging from soil, water, and man-made systems to aerosols (Hilborn et al., 2006; Parker et al., 1983; Vaerewijck et al., 2005). They are well-known colonizers of water distribution systems, and due to their unique cell wall composition may be involved in surface colonization and biofilm formation.
Six Pseudomonas OTUs were detected. P. stutzeri was the most abundant OTU in CT-2 (28.4%) and was also found in the other two cooling towers. P. toyotomiensis (15.7%) represented the second most abundant OTU in CT-2, and was among the persistent shared OTUs (Table S3, Fig. 5). Additional Pseudomonas species were seen, but at much lower frequencies. Not considered to be strict pathogens, members of this genus are ubiquitous and metabolically versatile; in addition to their prominent role in biofilm formation some are known as opportunistic human pathogens and cause various infections in e.g. the urinary tract, soft tissues, or the respiratory tract (Goetz et al., 1983; Keys et al., 1983; Potvliege et al., 1987).
Taken together, we couldn't find any evidence for the presence of sensu stricto human pathogens in the cooling tower microbial communities analysed in this study, not even among the rare biosphere.
3.5. A highly dynamic protist community
We detected the presence of four groups of protists, the Rhizaria, the Excavata, the Alveolata, and the Amoebozoa at all three sampling sites. Although the top taxa in all cooling towers belonged mainly to the Alveolata (Ciliophora and Apicomplexa) and Amoebozoa (Lobosa), we observed pronounced differences in relative abundances between the cooling towers (Fig. 3, Table S4). The most abundant protist groups in cooling tower CT-1 were classified into diverse taxa in the Ciliophora, like Ancistrum (9.3%) and Vorticella (7.1%), and Lobosa taxa including Vanella (8.1%), Cochliopodium (7.8%), and Vexillifera species (7.7%). In contrast, cooling towers CT-2 and CT-3 were dominated by diverse gregarines. These apicomplexan parasites are often associated with small invertebrates. Although the source of the gregarines in our cooling tower water system remains unknown, they were likely introduced together with their potential animal hosts. Still, there were also several well-known free-living amoebae among the most abundant taxa, such as Vexillifera, Stenamoeba, Vannella, Hartmannella, Echinamoebida, Cochliopodium, Acanthamoeba, and Allovahlkampfia (Fig. 3, Table S4). Taken together, the protist community detected in the three cooling towers was dominated by taxa frequently found in diverse freshwater habitats and able to persist in an environment characterized by steady water movement.
The protist community composition was more variable than the bacterial microbiome. No persistent protist taxa were observed, and the most frequently recurring OTUs were detected in less than 50% of the samples (Table S4). These included Stenamoeba, Ancistrum, and Vexillifera (CT-1) and gregarines (CT-2). The repeated occurrence of gregarines (often associated with small invertebrates) suggests that in addition to bacteria-protists interactions, small invertebrates (though not analysed in this study) may also affect the structure of cooling tower microbiomes.
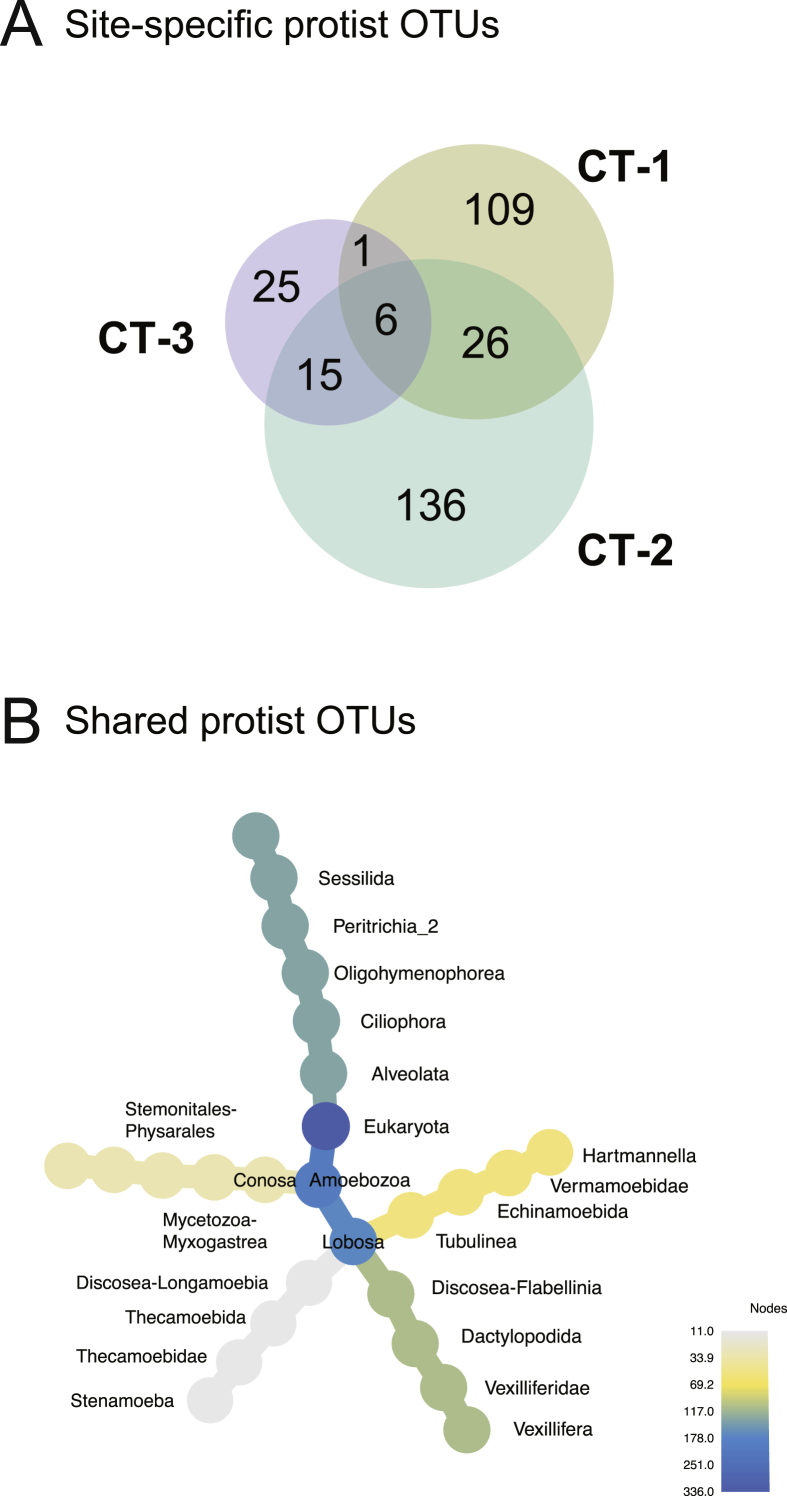
By far, the largest proportion of taxa was site-specific (Fig. 7A, Table S4). This included representatives of the genera Stenamoeba, Vannella, Echinamoeba, and Cochliopodium (Amoebozoa), Tetrahymena, Chilodonella, and gregarines (Alveolata), Neobodo and Vahlkampfiidae (Excavata), and Paracercomonas (Rhizaria). More than half of these site-specific OTUs do not occur at more than one time-point, further indicating that the protist community in the water basin is more variable than the bacterial community. Possible reasons for this may include (i) varying protist community composition of the source water, (ii) competition between protists, or (iii) random shearing of protists associated with bacterial biofilms.
Fig. 7.
Site-specific and shared protist OTUs in three cooling towers. The Venn diagram in (A) represents the proportion of shared and site-specific protist OTUs at the three sampling sites. In contrast to the less variable bacterial community composition, the majority of the protist OTUs are site-specific, and the number of OTUs shared by all three sampling sites constitutes only a very small fraction of all OTUs. (B) The heat tree indicates the taxonomic classification of the six shared OTUs, most of which are free-living amoebae (Hartmannella, Vexillifera, Stenamoeba). Unclassified taxa are unlabeled.
There were few OTUs found at all three cooling towers (6 corresponding to 1.7% of all protist OTUs). These included a member of the Sessilida, and free-living amoebae such as Hartmannella, Stenamoeba, Vexillifera, and a conosa member. (Fig. 7B). Taken together, the protist microbiome of cooling tower water is surprisingly diverse, with a species richness lower than the bacterial community but in the same order of magnitude. At the same time, it is highly dynamic and only showed a small core set of persistent and shared taxa.
3.6. Bacteria-protist interactions uncovered by co-occurrence networks
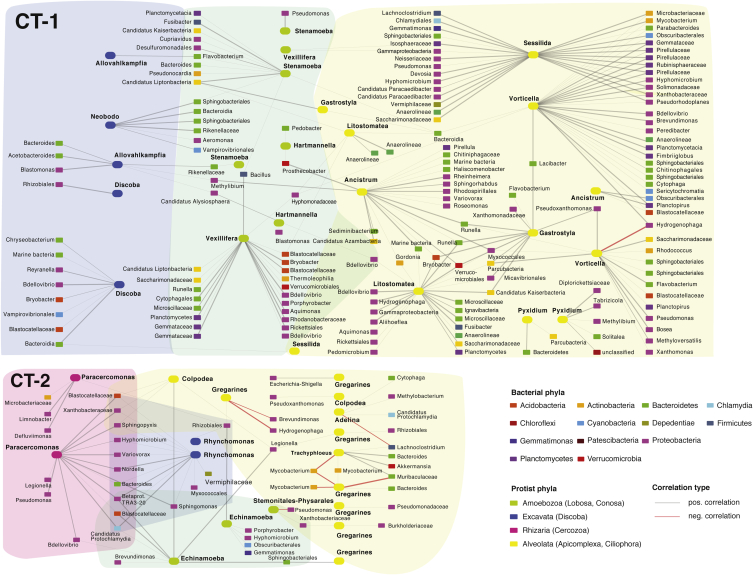
To gain insights into putative interactions between bacteria and protists in the cooling tower water microbiomes, we constructed and analysed co-occurrence networks for each cooling tower using all bacterial and protist OTUs, and a comprehensive approach for sequence abundance-based network inference (Faust and Raes, 2016). In our analysis, we only considered relationships between bacteria and protists (Fig. 8). The resulting co-occurrence networks were characterized by a high number of nodes (189 OTUs for CT-1, 61 for CT-2) and correlations (207 edges for CT-1, 78 for CT-2). Due to the low sample number, CT-3 was not included in this analysis. The networks are composed of mainly positive correlations, in which the abundances of bacterial and protist OTUs followed the same trend.
Fig. 8.
Co-occurrence network indicating bacteria-protist relationships in cooling tower water microbiomes. Co-occurrence networks including only interactions between bacterial and protist OTUs are shown for each sampling location; positive correlations are indicated by grey connections (edges), negative correlations are indicated by red edges. The number of negative correlations might be underestimated due to the conservative thresholds applied in this study. The width of the edges negatively correlates to the p-value of each predicted interaction. In CT-1 the majority of the protist nodes are members of the Alveolata, whereas all four protist group are represented in the CT-2 network. This analysis revealed interactions involving known protist-associated bacteria, constituting 15.6% (CT-1) and 24% (CT-2) of all predicted relationships. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Protists in the CT-1 network are mainly ciliates (Alveolata; Oligohymenophorea), followed by naked amoebae (Amoebozoa; Discosea). Most protists were correlated with a number of bacterial taxa, with Vorticella and an unclassified Sessilida showing highest centrality (i.e. number of edges) identifying them as key players (Fig. 8). Each protist node had its own individual set of bacterial interaction partners. Some bacterial nodes were shared between different protists but the majority (73%) of bacterial nodes were linked to only one protist, suggesting a certain degree of specificity of bacteria-protist interactions. In comparison, the CT-2 network had fewer protist nodes with high centrality. However, we could detect more bacterial taxa, who co-occurred with more than one protist (40%). The most abundant bacterial OTUs included in both networks were members of the Proteobacteria, the Planctomycetes, and the Bacteroidetes.
Remarkably, 15.6% of all predicted bacteria-protist relationships in the CT-1 network and 24% in the CT-2 network were comprised of bacterial genera with members known to associate with protists (Fig. 8). This included several well-known bacterial symbionts of free-living amoebae or ciliates, such as members of the genera Protochlamydia (Collingro et al., 2005; Fokin, 2004; Horn, 2008; Vannini et al., 2004) and the family Rickettsiaceae (Horn et al., 1999). Bacterial symbionts of protists are ubiquitous in ciliates and amoeba and in some cases represent ancient relationships originating millions of years ago (Görtz, 2001; Horn, 2008; Molmeret et al., 2005). These bacteria generally show reduced metabolic capabilities and are thus dependent on their protist hosts (Klein et al., 2012; McCutcheon and Moran, 2012; Schmitz-Esser et al., 2010). Some act as defensive symbionts or confer resistance to environmental stress (Dziallas et al., 2012; Fujishima et al., 2005; Grosser et al., 2018; Hori and Fujishima, 2003), but the relevance for the protist host is often unclear.
The networks also included several bacterial groups transiently thriving in protists, such as members of the genera Legionella, Mycobacterium, Pseudomonas, Nordella, Variovorax, Bosea, Acidovorax, Cupriavidus, Brevundimonas, and Flavobacterium (Corsaro et al., 2010; Drancourt, 2014; Evstigneeva et al., 2009; Fields et al., 2002; Greub and Raoult, 2004; La Scola et al., 2004; Michel et al., 1995; Thomas et al., 2008; Zeybek and Binay, 2014). Interestingly, our co-occurrence analysis also predicted members of the groups Parcubacteria (OD1; Corsaro et al., 2010) and Depedentiae (TM6) as interaction partner of protists. This observation further supports a symbiotic lifestyle for Parcubacteria as suggested by (meta-)genomic analysis (Gong et al., 2014; Nelson and Stegen, 2015) and provides additional support for the endosymbiotic lifestyle of Depedentiae members (Delafont et al., 2015). Our analysis underscores the potential of protists in cooling tower water microbiomes as reservoirs for intracellular bacteria, including relatives of opportunistic pathogens (Greub and Raoult, 2004).
Of note, the protist-associated bacteria in our network generally show interactions with several different protist OTUs. This indicates that in a natural setting, bacterial symbionts are more promiscuous and thrive in a wide range of different protists. Among the taxa included in the networks, there are several protists recognized previously as hosts for intracellular bacteria: The amoeba Vexillifera is a known host of the environmental chlamydia ‘Candidatus Neptunochlamydia vexilliferae’ (Pizzetti et al., 2016). Hartmannella (Vermamoeba) is the natural host of an intranuclear symbiont, ‘Candidatus Nucleicultrix amoebiphila‘ and the environmental chlamydia Rubidus massiliensis (Bou Khalil et al., 2016; Horn, 2008; Schulz et al., 2014). Echinamoeba is a known host for Pseudomonas aeruginosa (Michel et al., 1995). In addition, Allovahlkampfia which have been suggested to act as hosts for pathogenic bacteria (Mohamed and Huseein, 2016), were also represented in our networks. In this respect, we have recently isolated from the water sample of the tower CT-1 the first testate amoeba, Cochliopodium minus, containing a bacterial symbiont (Tsao et al., 2017). Taken together, our co-presence network analysis recovered a large number of known bacteria-protist interactions and suggested that such relationships are much more diverse than recognized currently.
4. Conclusions
-
●
Bacterial and protist communities in cooling towers are broadly similar at the class level to those in natural freshwater and drinking water systems. Although community structure can be highly dynamic, the presence of core taxa suggests that cooling tower basins select for biofilm-forming and biofilm-associated microbes.
-
●
Amplicon sequencing techniques that target ribosomal RNA genes is a useful tool to study microbial community dynamics in cooling tower water basins. Longer-term longitudinal studies including outbreak situations are required to better understand the role of the microbial community for propagation and spread of opportunistic bacterial pathogens that have been associated with disease outbreaks. The implementation of upcoming real-time sequencing technologies might facilitate online monitoring of cooling tower communities to predict biofilm formation and colonization with opportunistic pathogens.
-
●
Co-occurrence network analysis is a helpful approach to predict bacteria-protists interactions using amplicon sequencing data. The high number of nodes and degree of connectedness in the networks obtained here highlights the importance of inter-kingdom relationships in cooling tower water systems.
-
●
A design of cooling tower water basins and other measures that prevent biofilm formation should help to decrease the public health risk of cooling towers as a source for bacterial pathogens.
Author contributions
HFT, US, AI, JW, and MH conceived the study. HFT and US performed the sampling and DNA isolation; HFT performed PCR and prepared sequencing reactions. CH performed the demultiplexing, filtering and clustering of the reads. HFT performed the analysis of the data and prepared the figures; HFT and MH wrote the manuscript. All authors reviewed and edited the manuscript.
Declaration of interests
All authors declare that there is no conflict of interest.
Acknowledgement
This work was funded by Austrian Science Fund Project TRP209-B20. MH was supported by European Research Grant project EVOCHLAMY (ERC StG grant no. 281633). We thank Alexander Loy, Bela Hausmann, Claus Pelikan, Roey Angel, and Peter Hufnagl for their help and support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.watres.2019.04.028.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Table S1: Bacterial OTUs removed during the filtering process.
Supplementary Table S2: Protist OTUs removed during the filtering process.
Supplementary Table S3: Bacterial OTU table with sampling site specific relative abundance, occurrence, and the taxonomic classification.
Supplementary Table S4: Protist OTU table with sampling site specific relative abundance, occurrence, and the taxonomic classification.
Supplementary Table S5: List of opportunistic pathogens and relatives including best blast hits against NCBI database, Silva Living Tree Project database (Version 1.28), and taxonomic classification.
References
- Abd H., Johansson T., Golovliov I., Sandström G., Forsman M. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl. Environ. Microbiol. 2003;69:600–606. doi: 10.1128/AEM.69.1.600-606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsen M., Hugenholtz P., Skarshewski A., Nielsen K.L., Tyson G.W., Nielsen P.H. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotechnol. 2013;31:533–538. doi: 10.1038/nbt.2579. [DOI] [PubMed] [Google Scholar]
- Albertsen M., Karst S.M., Ziegler A.S., Kirkegaard R.H., Nielsen P.H. Back to basics - the influence of DNA extraction and primer choice on phylogenetic analysis of activated sludge communities. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral-Zettler L.A., McCliment E.A., Ducklow H.W., Huse S.M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA Genes. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaree J.M., Fields B.S., Feeley J.C., Gorman G.W., Martin W.T. Isolation of protozoa from water associated with legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl. Environ. Microbiol. 1986;51:422–424. doi: 10.1128/aem.51.2.422-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereschenko L.A., Stams A.J.M., Euverink G.J.W., van Loosdrecht M.C.M. Biofilm formation on reverse osmosis membranes is initiated and dominated by Sphingomonas spp. Appl. Environ. Microbiol. 2010;76:2623–2632. doi: 10.1128/AEM.01998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk S.G., Gunderson J.H., Newsome A.L., Farone A.L., Hayes B.J., Redding K.S., Uddin N., Williams E.L., Johnson R.A., Farsian M., Reid A., Skimmyhorn J., Farone M.B. Occurrence of infected amoebae in cooling towers compared with natural aquatic environments: implications for emerging pathogens. Environ. Sci. Technol. 2006;40:7440–7444. doi: 10.1021/es0604257. [DOI] [PubMed] [Google Scholar]
- Berry D., Xi C., Raskin L. Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 2006;17:297–302. doi: 10.1016/j.copbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Bou Khalil J.Y., Benamar S., Baudoin J.-P., Croce O., Blanc-Tailleur C., Pagnier I., Raoult D., La Scola B. Developmental cycle and genome analysis of “Rubidus massiliensis,” a new Vermamoeba vermiformis pathogen. Front. Cell. Infect. Microbiol. 2016;6 doi: 10.3389/fcimb.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher D., Jardillier L., Debroas D. Succession of bacterial community composition over two consecutive years in two aquatic systems: a natural lake and a lake-reservoir. FEMS Microbiol. Ecol. 2006;55:79–97. doi: 10.1111/j.1574-6941.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- Brown C.T., Hug L.A., Thomas B.C., Sharon I., Castelle C.J., Singh A., Wilkins M.J., Wrighton K.C., Williams K.H., Banfield J.F. Unusual biology across a group comprising more than 15% of domain Bacteria. Nature. 2015;523:208–211. doi: 10.1038/nature14486. [DOI] [PubMed] [Google Scholar]
- Buse H.Y., Lu J., Lu X., Mou X., Ashbolt N.J. Microbial diversities (16S and 18S rRNA gene pyrosequencing) and environmental pathogens within drinking water biofilms grown on the common premise plumbing materials unplasticized polyvinylchloride and copper. FEMS Microbiol. Ecol. 2014;88:280–295. doi: 10.1111/1574-6941.12294. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan N., Ozdemir G. Extracellular polysaccharides produced by cooling water tower biofilm bacteria and their possible degradation. Biofouling. 2008;24:129–135. doi: 10.1080/08927010801911316. [DOI] [PubMed] [Google Scholar]
- Chao Y., Mao Y., Wang Z., Zhang T. Diversity and functions of bacterial community in drinking water biofilms revealed by high-throughput sequencing. Sci. Rep. 2015;5 doi: 10.1038/srep10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J.D., Falkow S., Tompkins L.S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect. Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J.D., Falkow S., Tompkins L.S., Bermudez L.E. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingro A., Toenshoff E.R., Taylor M.W., Fritsche T.R., Wagner M., Horn M. “Candidatus Protochlamydia amoebophila”, an endosymbiont of Acanthamoeba spp. Int. J. Syst. Evol. Microbiol. 2005;55:1863–1866. doi: 10.1099/ijs.0.63572-0. [DOI] [PubMed] [Google Scholar]
- Corsaro D., Pages G.S., Catalan V., Loret J.-F., Greub G. Biodiversity of amoebae and amoeba-associated bacteria in water treatment plants. Int. J. Hyg Environ. Health. 2010;213:158–166. doi: 10.1016/j.ijheh.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999:80. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Critchley M., Bentham R. The efficacy of biocides and other chemical additives in cooling water systems in the control of amoebae. J. Appl. Microbiol. 2009;106:784–789. doi: 10.1111/j.1365-2672.2008.04044.x. [DOI] [PubMed] [Google Scholar]
- Crump B.C., Hobbie J.E. Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol. Oceanogr. 2005;50:1718–1729. [Google Scholar]
- Delafont V., Mougari F., Cambau E., Joyeux M., Bouchon D., Héchard Y., Moulin L. First evidence of amoebae-mycobacteria association in drinking water network. Environ. Sci. Technol. 2014;48:11872–11882. doi: 10.1021/es5036255. [DOI] [PubMed] [Google Scholar]
- Delafont V., Samba-Louaka A., Bouchon D., Moulin L., Héchard Y. Shedding light on microbial dark matter: a TM6 bacterium as natural endosymbiont of a free-living amoeba. Environ. Microbiol. Rep. 2015;7:970–978. doi: 10.1111/1758-2229.12343. [DOI] [PubMed] [Google Scholar]
- Delafont V., Bouchon D., Héchard Y., Moulin L. Environmental factors shaping cultured free-living amoebae and their associated bacterial community within drinking water network. Water Res. 2016;100:382–392. doi: 10.1016/j.watres.2016.05.044. [DOI] [PubMed] [Google Scholar]
- Douterelo I., Sharpe R., Boxall J. Bacterial community dynamics during the early stages of biofilm formation in a chlorinated experimental drinking water distribution system: implications for drinking water discolouration. J. Appl. Microbiol. 2014;117:286–301. doi: 10.1111/jam.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drancourt M. Looking in amoebae as a source of mycobacteria. Microb. Pathog. 2014;77:119–124. doi: 10.1016/j.micpath.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Dziallas C., Allgaier M., Monaghan M.T., Grossart H.-P. Act together—implications of symbioses in aquatic ciliates. Front. Microbiol. 2012;3 doi: 10.3389/fmicb.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Evstigneeva A., Raoult D., Karpachevskiy L., La Scola B. Amoeba co-culture of soil specimens recovered 33 different bacteria, including four new species and Streptococcus pneumoniae. Microbiology. 2009;155:657–664. doi: 10.1099/mic.0.022970-0. [DOI] [PubMed] [Google Scholar]
- Farenhorst A., Li R., Jahan M., Tun H.M., Mi R., Amarakoon I., Kumar A., Khafipour E. Bacteria in drinking water sources of a First Nation reserve in Canada. Sci. Total Environ. 2017;575:813–819. doi: 10.1016/j.scitotenv.2016.09.138. [DOI] [PubMed] [Google Scholar]
- Faust K., Raes J. CoNet app: inference of biological association networks using Cytoscape. F1000Research. 2016;5:1519. doi: 10.12688/f1000research.9050.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B.S., Benson R.F., Besser R.E. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming H.-C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- Fokin S.I. Bacterial endocytobionts of Ciliophora and their interactions with the host cell. Int. Rev. Cytol. 2004 doi: 10.1016/S0074-7696(04)36005-5. [DOI] [PubMed] [Google Scholar]
- Foster Z.S.L., Sharpton T.J., Grünwald N.J. Metacoder: an R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouque E., Trouilhé M.C., Thomas V., Hartemann P., Rodier M.H., Hécharda Y. Cellular, biochemical, and molecular changes during encystment of free-living amoebae. Eukaryot. Cell. 2012;11:382–387. doi: 10.1128/EC.05301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche T.R., Sobek D., Gautom R.K. Enhancement of in vitro cytopathogenicity by Acanthamoeba spp. following acquisition of bacterial endosymbionts. FEMS Microbiol. Lett. 1998;166:231–236. doi: 10.1111/j.1574-6968.1998.tb13895.x. [DOI] [PubMed] [Google Scholar]
- Fujishima M., Kawai M., Yamamoto R. Paramecium caudatum acquires heat-shock resistance in ciliary movement by infection with the endonuclear symbiotic bacterium Holospora obtusa. FEMS Microbiol. Lett. 2005;243:101–105. doi: 10.1016/j.femsle.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Garcia A., Goñi P., Cieloszyk J., Fernandez M.T., Calvo-Beguería L., Rubio E., Fillat M.F., Peleato M.L., Clavel A. Identification of free-living amoebae and amoeba-associated bacteria from reservoirs and water treatment plants by molecular techniques. Environ. Sci. Technol. 2013;47:3132–3140. doi: 10.1021/es400160k. [DOI] [PubMed] [Google Scholar]
- Gilbert J.A., Jansson J.K., Knight R. The Earth Microbiome project: successes and aspirations. BMC Biol. 2014;12:69. doi: 10.1186/s12915-014-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goertz H.D. Intracellular bacteria in ciliates. Int. Microbiol. 2001;4:143–150. doi: 10.1007/s10123-001-0029-9. [DOI] [PubMed] [Google Scholar]
- Goetz A., Yu V.L., Hanchett J.E., Rihs J.D. Pseudomonas stutzeri bacteremia associated with Hemodialysis. Arch. Intern. Med. 1983;143:1909–1912. [PubMed] [Google Scholar]
- Gong J., Qing Y., Guo X., Warren A. “Candidatus Sonnebornia yantaiensis”, a member of candidate division OD1, as intracellular bacteria of the ciliated protist Paramecium bursaria (Ciliophora, Oligohymenophorea) Syst. Appl. Microbiol. 2014;37:35–41. doi: 10.1016/j.syapm.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Greub G., Raoult D. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 2004;17:413–433. doi: 10.1128/CMR.17.2.413-433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser K., Ramasamy P., Amirabad A.D., Schulz M.H., Gasparoni G., Simon M., Schrallhammer M. More than the “killer trait”: infection with the bacterial endosymbiont Caedibacter taeniospiralis causes transcriptomic modulation in paramecium host. Genome Biol. Evol. 2018;10:646–656. doi: 10.1093/gbe/evy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou L., Bachar D., Audic S., Bass D., Berney C., Bittner L., Boutte C., Burgaud G., De Vargas C., Decelle J., Del Campo J., Dolan J.R., Dunthorn M., Edvardsen B., Holzmann M., Kooistra W.H.C.F., Lara E., Le Bescot N., Logares R., Mahé F., Massana R., Montresor M., Morard R., Not F., Pawlowski J., Probert I., Sauvadet A.L., Siano R., Stoeck T., Vaulot D., Zimmermann P., Christen R. The Protist Ribosomal Reference database (PR 2 ): A catalog of unicellular eukaryote Small Sub-Unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 2013;41:D597–D604. doi: 10.1093/nar/gks1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K.A., Hamilton M.T., Johnson W., Jjemba P., Bukhari Z., LeChevallier M., Haas C.N. Health risks from exposure to Legionella in reclaimed water aerosols: toilet flushing, spray irrigation, and cooling towers. Water Res. 2018;134:261–279. doi: 10.1016/j.watres.2017.12.022. [DOI] [PubMed] [Google Scholar]
- He X., McLean J.S., Edlund A., Yooseph S., Hall A.P., Liu S.-Y., Dorrestein P.C., Esquenazi E., Hunter R.C., Cheng G., Nelson K.E., Lux R., Shi W. Cultivation of a human-associated TM7 phylotype reveals a reduced genome and epibiotic parasitic lifestyle. Proc. Natl. Acad. Sci. Unit. States Am. 2014;112:244–249. doi: 10.1073/pnas.1419038112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbold C.W., Pelikan C., Kuzyk O., Hausmann B., Angel R., Berry D., Loy A. A flexible and economical barcoding approach for highly multiplexed amplicon sequencing of diverse target genes. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlemann D.P.R., Labrenz M., Jürgens K., Bertilsson S., Waniek J.J., Andersson A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011;5:1571–1579. doi: 10.1038/ismej.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess S. Description of Hyalodiscus flabellus sp. nov. (Vampyrellida, Rhizaria) and identification of its bacterial endosymbiont, “Candidatus megaira polyxenophila” (Rickettsiales, Alphaproteobacteria) Protist. 2017;168:109–133. doi: 10.1016/j.protis.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Hess S., Suthaus A., Melkonian M. “Candidatus Finniella” (Rickettsiales, Alphaproteobacteria), novel endosymbionts of viridiraptorid amoeboflagellates (Cercozoa, rhizaria) Appl. Environ. Microbiol. 2016;82:659–670. doi: 10.1128/AEM.02680-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn E.D., Covert T.C., Yakrus M.A., Harris S.I., Donnelly S.F., Rice E.W., Toney S., Bailey S.A., Stelma G.N. Persistence of nontuberculous Mycobacteria in a drinking water system after addition of filtration treatment. Appl. Environ. Microbiol. 2006;72:5864–5869. doi: 10.1128/AEM.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong P.Y., Hwang C., Ling F., Andersen G.L., LeChevallier M.W., Liu W.T. Pyrosequencing analysis of bacterial biofilm communities in water meters of a drinking water distribution system. Appl. Environ. Microbiol. 2010;76:5631–5635. doi: 10.1128/AEM.00281-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori M., Fujishima M. The endosymbiotic bacterium Holospora obtusa enhances heat-shock gene expression of the host Paramecium caudatum. J. Eukaryot. Microbiol. 2003;50:293–298. doi: 10.1111/j.1550-7408.2003.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Horn M. Chlamydiae as symbionts in eukaryotes. Annu. Rev. Microbiol. 2008;62:113–131. doi: 10.1146/annurev.micro.62.081307.162818. [DOI] [PubMed] [Google Scholar]
- Horn M., Fritsche T.R., Gautom R.K., Schleifer K.H., Wagner M. Novel bacterial endosymbionts of Acanthamoeba spp. related to the Paramecium caudatum symbiont Caedibacter caryophilus. Environ. Microbiol. 1999;1:357–367. doi: 10.1046/j.1462-2920.1999.00045.x. [DOI] [PubMed] [Google Scholar]
- Inoue H., Fujimura R., Agata K., Ohta H. Molecular Characterization of viable Legionella spp. in cooling tower water samples by combined use of ethidium monoazide and PCR. Microbes Environ. Environ. 2015;30:108–112. doi: 10.1264/jsme2.ME14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P.Ø., Givskov M., Bjarnsholt T., Moser C. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol. Med. Microbiol. 2010;59:292–305. doi: 10.1111/j.1574-695X.2010.00706.x. [DOI] [PubMed] [Google Scholar]
- Kalmbach S., Manz W., Szewzyk U. Isolation of new bacterial species from drinking water biofilms and proof of their in situ dominance with highly specific 16S rRNA probes. Appl. Environ. Microbiol. 1997;63:4164–4170. doi: 10.1128/aem.63.11.4164-4170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhnaya M.G., De Marco P., Bowerman S., Pacheco C.C., Lara J.C., Lidstrom M.E., Chistoserdova L. Methyloversatilis universalis gen. nov., sp. nov., a novel taxon within the Betaproteobacteria represented by three methylotrophic isolates. Int. J. Syst. Evol. Microbiol. 2006;56:2517–2522. doi: 10.1099/ijs.0.64422-0. [DOI] [PubMed] [Google Scholar]
- Katharios-Lanwermeyer S., Xi C., Jakubovics N.S., Rickard A.H. Mini-review: microbial coaggregation: ubiquity and implications for biofilm development. Biofouling. 2014;30:1235–1251. doi: 10.1080/08927014.2014.976206. [DOI] [PubMed] [Google Scholar]
- Keys T.F., Melton L.J., Maker M.D., Ilstrup D.M. A suspected hospital outbreak of pseudobacteremia due to Pseudomonas stutzeri. J. Infect. Dis. 1983;147:489–493. doi: 10.1093/infdis/147.3.489. [DOI] [PubMed] [Google Scholar]
- Klein C.C., Cottret L., Kielbassa J., Charles H., Gautier C., Ribeiro de Vasconcelos A.T., Lacroix V., Sagot M.F. Exploration of the core metabolism of symbiotic bacteria. BMC Genomics. 2012;13:438. doi: 10.1186/1471-2164-13-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J.B., Bartlett C.L.R., Newton U.A., White R.A., Jones N.L. Legionella pneumophila in cooling water systems: report of a survey of cooling towers in London and a pilot trial of selected biocides. J. Hyg. 1982;88:369–381. doi: 10.1017/s0022172400070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scola B., Raoult D. Survival of Coxiella burnetii within free-living amoeba Acanthamoeba castellanii. Clin. Microbiol. Infect. 2001;7:75–79. doi: 10.1046/j.1469-0691.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- La Scola B., Barrassi L., Raoult D. A novel alpha-Proteobacterium, Nordella oligomobilis gen. nov., sp. nov., isolated by using amoebal co-cultures. Res. Microbiol. 2004;155:47–51. doi: 10.1016/j.resmic.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Li L., Qin T., Li Y., Zhou H., Song H., Ren H., Li L., Li Y., Zhao D. Prevalence and molecular characteristics of waterborne pathogen Legionella in industrial cooling tower environments. Int. J. Environ. Res. Public Health. 2015;12:12605–12617. doi: 10.3390/ijerph121012605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang W., Sileika T., Warta R., Cianciotto N.P., Packman A. Role of bacterial adhesion in the microbial ecology of biofilms in cooling tower systems. Biofouling. 2009;25:241–253. doi: 10.1080/08927010802713414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang W., Sileika T., Warta R., Cianciotto N.P., Packman A.I. Disinfection of bacterial biofilms in pilot-scale cooling tower systems. Biofouling. 2011;27:393–402. doi: 10.1080/08927014.2011.577525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Yu Z., Guo H., Liu M., Zhang H., Yang M. Pyrosequencing analysis of eukaryotic and bacterial communities in faucet biofilms. Sci. Total Environ. 2012;435–436:124–131. doi: 10.1016/j.scitotenv.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Llewellyn A.C., Lucas C.E., Roberts S.E., Brown E.W., Nayak B.S., Raphael B.H., Winchell J.M. Distribution of Legionella and bacterial community composition among regionally diverse US cooling towers. PLoS One. 2017;12 doi: 10.1371/journal.pone.0189937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luef B., Frischkorn K.R., Wrighton K.C., Holman H.-Y.N., Birarda G., Thomas B.C., Singh A., Williams K.H., Siegerist C.E., Tringe S.G., Downing K.H., Comolli L.R., Banfield J.F. Diverse uncultivated ultra-small bacterial cells in groundwater. Nat. Commun. 2015;6:6372. doi: 10.1038/ncomms7372. [DOI] [PubMed] [Google Scholar]
- Marrie T.J., Raoult D., La Scola B., Birtles R.J., De Carolis E., Duperval R., Field S., Louie T., Houston S., Gribble M., Williams K., Nicolle L., Grossman R., Salit I., Saginur R., Gregson D., Laverdiere M., Joly J., Marrie T., Hutchinson J. Legionella-like and other amoebal pathogens as agents of community-acquired pneumonia. Emerg. Infect. Dis. 2001;7:1026–1029. doi: 10.3201/eid0706.010619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny A.C., Albrechtsen H.J., Arvin E., Molin S. Identification of bacteria in biofilm and bulk water samples from a nonchlorinated model drinking water distribution system: detection of a large nitrite-oxidizing population associated with Nitrospira spp. Appl. Environ. Microbiol. 2005;71:8611–8617. doi: 10.1128/AEM.71.12.8611-8617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz C., McDougald D., Moreno A.M., Yung P.Y., Yildiz F.H., Kjelleberg S. Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc. Natl. Acad. Sci. Unit. States Am. 2005;102:16819–16824. doi: 10.1073/pnas.0505350102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy S.T., VanBriesen J.M. Temporal variability of bacterial diversity in a chlorinated drinking water distribution system. J. Environ. Eng. 2012;138:786–795. [Google Scholar]
- McCutcheon J.P., Moran N.A. Extreme genome reduction in symbiotic bacteria. Nat. Rev. Microbiol. 2012;10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- McMurdie P.J., Holmes S. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome Census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel R., Burghardt H., Bergmann H. Acanthamoeba, naturally intracellularly infected with Pseudomonas aeruginosa, after their isolation from a microbiologically contaminated drinking water system in a hospital. Zentralbl. Hyg. Umweltmed. 1995;196:532–544. [PubMed] [Google Scholar]
- Miltner E.C., Bermudez L.E. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob. Agents Chemother. 2000;44:1990–1994. doi: 10.1128/aac.44.7.1990-1994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.E., Huseein E.A. Allovahlkampfia spelaea is a potential environmental host for pathogenic bacteria. J. Bacteriol. Parasitol. 2016;07 [Google Scholar]
- Molmeret M., Horn M., Wagner M., Santic M., Abu Kwaik Y. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 2005;71:20–28. doi: 10.1128/AEM.71.1.20-28.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchesa P., Leifels M., Jurzik L., Hoorzook K.B., Barnard T.G., Bartie C. Coexistence of free-living amoebae and bacteria in selected South African hospital water distribution systems. Parasitol. Res. 2017;116:155–165. doi: 10.1007/s00436-016-5271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Spitz S.R., Goetz G.W., McLellan S.L. Temporal and spatial variability in nearshore bacterioplankton communities of Lake Michigan. FEMS Microbiol. Ecol. 2009;67:511–522. doi: 10.1111/j.1574-6941.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- Nelson W.C., Stegen J.C. The reduced genomes of Parcubacteria (OD1) contain signatures of a symbiotic lifestyle. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome A.L., Scott T.M., Benson R.F., Fields B.S. Isolation of an Amoeba naturally harboring a distinctive Legionella species. Appl. Environ. Microbiol. 1988:1688–1693. doi: 10.1128/aem.64.5.1688-1693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R.J., Jones S.E., Eiler A., McMahon K.D., Bertilsson S. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 2011;75:14–49. doi: 10.1128/MMBR.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhu Nguyen T.M., Ilef D., Jarraud S., Rouil L., Campese C., Che D., Haeghebaert S., Ganiayre F., Marcel F., Etienne J., Desenclos J. A community-wide outbreak of legionnaires disease linked to industrial cooling towers—How far can contaminated aerosols spread? J. Infect. Dis. 2005;193:102–111. doi: 10.1086/498575. [DOI] [PubMed] [Google Scholar]
- Pagnier I., Merchat M., La Scola B. Potentially pathogenic amoeba-associated microorganisms in cooling towers and their control. Future Microbiol. 2009;4:615–629. doi: 10.2217/fmb.09.25. [DOI] [PubMed] [Google Scholar]
- Parker B.C., Ford M.A., Gruft H., Falkinham J.O. Epidemiology of infection by nontuberculous mycobacteria. IV. Preferential Aerosolization of Mycobacterium intracellulare from natural waters. Am. Rev. Respir. Dis. 1983;128:652–656. doi: 10.1164/arrd.1983.128.4.652. [DOI] [PubMed] [Google Scholar]
- Parks D.H., Chuvochina M., Waite D.W., Rinke C., Skarshewski A., Chaumeil P.-A., Hugenholtz P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 2018;36:996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- Parry J.D. Protozoan grazing of freshwater biofilms. Adv. Appl. Microbiol. 2004 doi: 10.1016/S0065-2164(04)54007-8. [DOI] [PubMed] [Google Scholar]
- Pereira R.P.A., Peplies J., Höfle M.G., Brettar I. Bacterial community dynamics in a cooling tower with emphasis on pathogenic bacteria and Legionella species using universal and genus-specific deep sequencing. Water Res. 2017;122:363–376. doi: 10.1016/j.watres.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Pizzetti I., Schulz F., Tyml T., Fuchs B.M., Amann R., Horn M., Fazi S. Chlamydial seasonal dynamics and isolation of “Candidatus Neptunochlamydia vexilliferae” from a Tyrrhenian coastal lake. Environ. Microbiol. 2016;18:2405–2417. doi: 10.1111/1462-2920.13111. [DOI] [PubMed] [Google Scholar]
- Potvliege C., Jonckheer J., Lenclud C., Hansen W. Pseudomonas stutzeri pneumonia and septicemia in a patient with multiple myeloma. J. Clin. Microbiol. 1987;25:458–459. doi: 10.1128/jcm.25.2.458-459.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revetta R.P., Pemberton A., Lamendella R., Iker B., Santo Domingo J.W. Identification of bacterial populations in drinking water using 16S rRNA-based sequence analyses. Water Res. 2010;44:1353–1360. doi: 10.1016/j.watres.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Rickard A.H., Leach S.A., Hall L.S., Buswell C.M., High N.J., Handley P.S. Phylogenetic relationships and coaggregation ability of freshwater biofilm bacteria. Appl. Environ. Microbiol. 2002;68:3644–3650. doi: 10.1128/AEM.68.7.3644-3650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard A.H., Gilbert P., Handley P.S. Influence of growth environment on coaggregation between freshwater biofilm bacteria. J. Appl. Microbiol. 2004;96:1367–1373. doi: 10.1111/j.1365-2672.2004.02297.x. [DOI] [PubMed] [Google Scholar]
- Rożej A., Cydzik-Kwiatkowska A., Kowalska B., Kowalski D. Structure and microbial diversity of biofilms on different pipe materials of a model drinking water distribution systems. World J. Microbiol. Biotechnol. 2015;31:37–47. doi: 10.1007/s11274-014-1761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström G., Saeed A., Abd H. Acanthamoeba polyphaga is a possible host for Vibrio cholerae in aquatic environments. Exp. Parasitol. 2010;126:65–68. doi: 10.1016/j.exppara.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Sauer K., Camper A.K., Ehrlich G.D., Costerton J.W., Davies D.G. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 2002;184:1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheikl U., Tsao H.F., Horn M., Indra A., Walochnik J. Free-living amoebae and their associated bacteria in Austrian cooling towers: a 1-year routine screening. Parasitol. Res. 2016;115:3365–3374. doi: 10.1007/s00436-016-5097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Esser S., Tischler P., Arnold R., Montanaro J., Wagner M., Rattei T., Horn M. The genome of the amoeba symbiont "Candidatus Amoebophilus asiaticus" reveals common mechanisms for host cell interaction among amoeba-associated bacteria. J. Bacteriol. 2010;192:1045–1057. doi: 10.1128/JB.01379-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz F., Lagkouvardos I., Wascher F., Aistleitner K., Kostanjšek R., Horn M. Life in an unusual intracellular niche: a bacterial symbiont infecting the nucleus of amoebae. ISME J. 2014;8:1634–1644. doi: 10.1038/ismej.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpigel N.Y., Pasternak Z., Factor G., Gottlieb Y. Diversity of bacterial biofilm communities on sprinklers from dairy farm cooling systems in Israel. PLoS One. 2015;10:e0139111. doi: 10.1371/journal.pone.0139111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Williams H.N., Spitznagel J.K., Smith D.L., Stine O.C., Labib M.E. Microbial diversity of biofilms in dental unit water systems. Appl. Environ. Microbiol. 2003;69:3412–3420. doi: 10.1128/AEM.69.6.3412-3420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Giron M.J., Rodriguez-R L.M., Luo C., Elk M., Ryu H., Hoelle J., Santo Domingo J.W., Konstantinidis K.T. Biofilms on hospital shower Hoses: Characterization and implications for nosocomial infections. Appl. Environ. Microbiol. 2016;82:2872–2883. doi: 10.1128/AEM.03529-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States S.J., Conley L.F., Towner S.G., Wolford R.S., Stephenson T.E., McNamara A.M., Wadowsky R.M., Yee R.B. An alkaline approach to treating cooling towers for control of Legionella pneumophila. Appl. Environ. Microbiol. 1987;53:1775–1779. doi: 10.1128/aem.53.8.1775-1779.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Xie G., Shao K., Dai J., Chen Y., Xu Q., Gao G. Bacterial community composition in oligosaline lake bosten: low overlap of betaproteobacteria and bacteroidetes with freshwater ecosystems. Microb. Environ. 2015;30:180–188. doi: 10.1264/jsme2.ME14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Brown A., Vaughan L., Greub G., Timms P., Polkinghorne A. Twenty years of research into Chlamydia-like organisms: a revolution in our understanding of the biology and pathogenicity of members of the phylum Chlamydiae. Pathog. Dis. 2014;73:1–15. doi: 10.1093/femspd/ftu009. [DOI] [PubMed] [Google Scholar]
- Thom S., Warhurst D., Drasar B.S. Association of Vibrio cholerae with fresh water amoebae. J. Med. Microbiol. 1992;36:303–306. doi: 10.1099/00222615-36-5-303. [DOI] [PubMed] [Google Scholar]
- Thomas V., McDonnell G. Relationship between mycobacteria and amoebae: ecological and epidemiological concerns. Lett. Appl. Microbiol. 2007;45:349–357. doi: 10.1111/j.1472-765X.2007.02206.x. [DOI] [PubMed] [Google Scholar]
- Thomas V., Loret J.-F., Jousset M., Greub G. Biodiversity of amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ. Microbiol. 2008;10:2728–2745. doi: 10.1111/j.1462-2920.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- Thompson L.R., Sanders J.G., McDonald D., Amir A., Ladau J., Locey K.J., Prill R.J., Tripathi A., Gibbons S.M., Ackermann G., Navas-Molina J.A., Janssen S., Kopylova E., Vázquez-Baeza Y., González A., Morton J.T., Mirarab S., Zech Xu Z., Jiang L., Haroon M.F., Kanbar J., Zhu Q., Jin Song S., Kosciolek T., Bokulich N.A., Lefler J., Brislawn C.J., Humphrey G., Owens S.M., Hampton-Marcell J., Berg-Lyons D., McKenzie V., Fierer N., Fuhrman J.A., Clauset A., Stevens R.L., Shade A., Pollard K.S., Goodwin K.D., Jansson J.K., Gilbert J.A., Knight R. A communal catalogue reveals Earth's multiscale microbial diversity. Nature. 2017;551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokajian S.T., Hashwa F.A., Hancock I.C., Zalloua P.A. Phylogenetic assessment of heterotrophic bacteria from a water distribution system using 16S rDNA sequencing. Can. J. Microbiol. 2005;51:325–335. doi: 10.1139/w05-007. [DOI] [PubMed] [Google Scholar]
- Torvinen E., Suomalainen S., Paulin L., Kusnetsov J. Mycobacteria in Finnish cooling tower waters. APMIS. 2013;122:353–358. doi: 10.1111/apm.12153. [DOI] [PubMed] [Google Scholar]
- Tsao H.-F., Scheikl U., Volland J.-M., Köhsler M., Bright M., Walochnik J., Horn M. ‘Candidatus Cochliophilus cryoturris’ (Coxiellaceae), a symbiont of the testate amoeba Cochliopodium minus. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-03642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türetgen I., Cotuk A. Monitoring of biofilm-associated Legionella pneumophila on different Substrata in model cooling tower system. Environ. Monit. Assess. 2007;125:271–279. doi: 10.1007/s10661-006-9519-8. [DOI] [PubMed] [Google Scholar]
- Ulleryd P., Hugosson A., Allestam G., Bernander S., Claesson B.E., Eilertz I., Hagaeus A.-C., Hjorth M., Johansson A., de Jong B., Lindqvist A., Nolskog P., Svensson N. Legionnaires' disease from a cooling tower in a community outbreak in Lidköping, Sweden- epidemiological, environmental and microbiological investigation supported by meteorological modelling. BMC Infect. Dis. 2012;12:313. doi: 10.1186/1471-2334-12-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaerewijck M.J.M., Huys G., Palomino J.C., Swings J., Portaels F. Mycobacteria in drinking water distribution systems: ecology and significance for human health. FEMS Microbiol. Rev. 2005;29:911–934. doi: 10.1016/j.femsre.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Van der Henst C., Scrignari T., Maclachlan C., Blokesch M. An intracellular replication niche for Vibrio cholerae in the amoeba Acanthamoeba castellanii. ISME J. 2016;10:897–910. doi: 10.1038/ismej.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij D., Bakker G.L., Italiaander R., Veenendaal H.R., Wullings B.A. Biofilm composition and threshold concentration for growth of Legionella pneumophila on surfaces exposed to flowing warm tap water without disinfectant. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.02737-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini C. Identification of the bacterial endosymbionts of the marine ciliate Euplotes magnicirratus (Ciliophora, Hypotrichia) and proposal of “Candidatus Devosia euplotis.”. Int. J. Syst. Evol. Microbiol. 2004;54:1151–1156. doi: 10.1099/ijs.0.02759-0. [DOI] [PubMed] [Google Scholar]
- Vaz-Moreira I., Nunes O.C., Manaia C.M. Diversity and Antibiotic resistance patterns of Sphingomonadaceae isolates from drinking water. Appl. Environ. Microbiol. 2011;77:5697–5706. doi: 10.1128/AEM.00579-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz-Moreira I., Egas C., Nunes O.C., Manaia C.M. Bacterial diversity from the source to the tap: a comparative study based on 16S rRNA gene-DGGE and culture-dependent methods. FEMS Microbiol. Ecol. 2012;83:361–374. doi: 10.1111/1574-6941.12002. [DOI] [PubMed] [Google Scholar]
- Wang J., Liu M., Xiao H., Wu W., Xie M., Sun M., Zhu C., Li P. Bacterial community structure in cooling water and biofilm in an industrial recirculating cooling water system. Water Sci. Technol. 2013;68:940–947. doi: 10.2166/wst.2013.334. [DOI] [PubMed] [Google Scholar]
- Walser S.M., Gerstner D.G., Brenner B., Höller C., Liebl B., Herr C.E.W. Assessing the environmental health relevance of cooling towers – A systematic review of legionellosis outbreaks. Int. J. Hyg. Environ. Health. 2014;217:145–154. doi: 10.1016/j.ijheh.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Williams M.M., Domingo J.W.S., Meckes M.C., Kelty C.A., Rochon H.S. Phylogenetic diversity of drinking water bacteria in a distribution system simulator. J. Appl. Microbiol. 2004;96:954–964. doi: 10.1111/j.1365-2672.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- Wingender J., Flemming H.-C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg Environ. Health. 2011;214:417–423. doi: 10.1016/j.ijheh.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Sugiura M., Kusunoki S., Ezaki T., Ikedo M., Yabuuchi E. Factors stimulating propagation of Legionellae in cooling tower water. Appl. Environ. Microbiol. 1992;58:1394–1397. doi: 10.1128/aem.58.4.1394-1397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacheus O.M., Iivanainen E.K., Nissinen T.K., Lehtola M.J., Martikainen P.J. Bacterial biofilm formation on polyvinyl chloride, polyethylene and stainless steel exposed to ozonated water. Water Res. 2000;34:63–70. [Google Scholar]
- Zeybek Z., Binay A.R. Growth ability of Gram negative bacteria in free-living amoebae. Exp. Parasitol. 2014;145:S121–S126. doi: 10.1016/j.exppara.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu W., Nie X., Li C., Gu J., Zhang C. Molecular analysis of bacterial communities in biofilms of a drinking water Clearwell. Microb. Environ. 2012;27:443–448. doi: 10.1264/jsme2.ME12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart G., Crump B., Kamst-van Agterveld M., Hagen F., Han S. Typical freshwater bacteria: an analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat. Microb. Ecol. 2002;28:141–155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Bacterial OTUs removed during the filtering process.
Supplementary Table S2: Protist OTUs removed during the filtering process.
Supplementary Table S3: Bacterial OTU table with sampling site specific relative abundance, occurrence, and the taxonomic classification.
Supplementary Table S4: Protist OTU table with sampling site specific relative abundance, occurrence, and the taxonomic classification.
Supplementary Table S5: List of opportunistic pathogens and relatives including best blast hits against NCBI database, Silva Living Tree Project database (Version 1.28), and taxonomic classification.