Abstract
Here we report the first large‐scale synthesis of Fmoc‐(S)‐2‐amino‐6,6,6‐trifluorohexanoic acid via asymmetric alkylation of chiral Ni(II)‐complex of glycine Schiff base with CF3(CH2)3I. The synthesis was performed on over 100 g scale and can be recommended as the most advanced procedure for reliable preparation of large amounts of enantiomerically pure Fmoc‐(S)‐2‐amino‐6,6,6‐trifluorohexanoic acid for protein engineering and drug design. Chiral auxiliary used in this protocol can be >90 % recovered and reused.
Keywords: asymmetric synthesis, amino acids, fluorine, large-scale synthesis, stereochemical outcome
Current trends in the design of new pharmaceuticals and drug formulations prominently feature application of fluorinated moieties as well as residues of tailor‐made amino acdis (AAs).1 In particular, the selective fluorination paradigm is widely used to control oxidative metabolic stability of drug molecules,2, 3 while the incorporation of tailor‐made amino AAs leads to more precise representation of the natural peptide‐receptor interactions.4 One would agree, that fluorine‐containing AAs, possessing both of these structural traits, represent an exciting class of biologically important compounds increasingly attracting attention of many synthetic research groups.5, 6 Among structurally divers fluorinated AAs currently used in the drug design,7 derivatives of (S)‐2‐amino‐6,6,6‐trifluorohexanoic acid 1 (Scheme 1) showed a plethora of useful biological properties8 and have been extensively used in de novo peptide/protein engineering.9 In particular, fluoro‐AA 1 was recently used in the design of new generation of antibiotics (Figure 1) derived from natural peptide teixobactin.9h
Scheme 1.

Approach for preparation of Fmoc derivative (S)‐3 developed in this work.
Figure 1.

Structure of new peptide containing AA 1.
Structurally, AA 1 is relatively simple and can be prepared by variety of general synthetic approaches including transformation of functional groups,10 including biomimetic transamination,11 and alkylation of glycine equivalents. The latter approach was particularly well‐studied and known in asymmetric stoichiometric and catalytic versions.12 While the literature methods have apparent scientific interest, their application for economical large‐scale preparation of target AA 1 is rather problematic. As part of a larger project focused on the development of a potential pharmaceutical drug, we needed a practically sounding access to large quantities of the corresponding Fmoc derivative of AA 3. In this paper, we disclose our results on the development of >100 g ‐scale synthesis of our target product 3 via alkylation of a new generation of chiral nucleophilic glycine equivalent (S)‐2.
Our interest in tailor‐made AAs is rather broad, covering various structural motives,13 type of key functionalities14 and their chiroptical properties such as Self‐Disproportionation of Enantiomers.15 However, our major interest in the field is related to the applications of Ni(II) complexes of AA Schiff bases as a general methodology for asymmetric synthesis of tailor‐made AAs.16 Using the modular design for the chiral tridentate ligands,17 we explored various structural ideas, featuring N−H functionality18 as well as elements helical chirality.19 One of the most recent developments is a tri‐chloro‐substituted ligand 4 (Scheme 2) rationally optimized to increase its stereocontrolling properties.20 So far, this new ligand was quite successfully used for the dynamic kinetic resolution of unprotected α‐21 and β‐AAs.22 On the other hand its application for asymmetric synthesis of tailor‐made AAs via other approaches is virtually unstudied.23
Scheme 2.

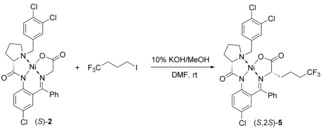
Large‐scale synthesis of Fmoc derivative (S)‐3 via alkylation of chiral nucleophilic glycine equivalent (S)‐2.
Proline‐derived compounds (S)‐ or (R)‐4 are commercially available or can be expediently prepared on kilogram scale.24 The reaction of tridentate ligands of type 4 with glycine in the presence of base and source of Ni(II) is well‐established procedure and was successfully used for preparation of glycine Schiff base complex (S)‐2.24, 25 In particularly, similar to the previously reported synthesis23 in this work we selected quite inexpensive NiCl2 and K2CO3, which were refluxed in MeOH along with (S)‐4 and glycine to afford complex (S)‐2 in excellent chemical yield. Taking advantage of our previous extensive experience with alkyl halide alkylations,26 we selected to use the homogeneous conditions, as versus to PTC,27 using DMSO or DMF as the reaction solvent.
Considering the goal of large‐scale synthesis, optimization of the alkyl halide alkylation of the glycine moiety in complexes (S)‐2 was focused on both chemical and economic issues. In particular, we pursued adequate stoichiometry and minimum volume of solvents. As shown, for example in Table 1, the use of just one equivalent of KOH and CF3(CH2)3I was quite sufficient to achieve over 99 % conversion of the starting Ni(II) complex (S)‐2. In general the alkylation reaction was robust, chemically rather clean and reliably reproducible. The undesirable diastereomer (S,2R)‐5 was observed in amounts not exceeding 1–2 %, confirming our high expectation for excellent stereocontrolling ability of tri‐chloro ligand (S)‐4. The major technical problem was found to be caused by oxidative decomposition of starting (S)‐2 and alkylated products 5 used strongly basic reaction conditions. These unwanted reactions were completely eliminated by using commercial deoxygenated (dry, oxygen‐free) DMF.28 The final optimized condition were found to as follows; the use of 1.05 equiv. of 1,1,1‐Trifluoro‐4‐iodobutane, 1.05 equiv. of 10 % KOH/MeOH, with dry deoxidized DMF as solvent at 0–5 °C for 2 h under argon.
Table 1.
Optimization of reaction conditions.[a]
| |||||
|---|---|---|---|---|---|
| Entry | Time (min) | Solvent | 2 (%)[b] | Product 5 (%)[b] | (S,2R)‐5 (%)[b] |
| 1 | 10 | DMF | 0.36 | 86.34 | 1.87 |
| 2 | 60 | DMF | 0.33 | 87.21 | 1.73 |
| 3 | 120 | DMF | 0.30 | 88.49 | 1.55 |
| 4 | 120 | dry DMF | 0.09 | 96.49 | 0.35 |
| 5 | 10 | deoxidized DMF | 0.14 | 95.53 | 2.00 |
| 6 | 60 | deoxidized DMF | 0.12 | 94.44 | 1.76 |
| 7 | 120 | deoxidized DMF | 0.12 | 94.34 | 1.72 |
| 8 | 120 | dry deoxidized DMF | 0.10 | 98.46 | 0.47 |
[a] Reaction conditions: Ni‐complex 2, CF3(CH2)I (1.05 equiv), 10 % KOH/MeOH (1.05 equiv), in DMF at room temperature under argon. [b] Determined by HPCL.
One of the important findings made in this work is that we were able to develop an economical procedure for isolation of virtually diastereomerically pure major product (S,2S)‐5. Thus, taking into account that for the large‐scale synthesis chromatographic purification is inacceptable and crystallization is undesirable, we focused our attention on a precipitation of major product (S,2S)‐5 directly from the reaction mixture. In particular, we found that two‐step addition of water at ambient temperature results in gradual precipitation of target product (S,2S)‐5 of excellent chemical and diastereomeric purity (>99 % de).
Usually, the disassembly procedure of Ni(II) complexes of this type is performed in MeOH under the action of HCl.16 However, developing the large‐scale synthesis, we found that this procedure would require detrimental amount of the solvent. Accordingly, we screened other reaction conditions and found that the use of dimethoxyethane (DME) allows to reach rather optimal solution. Despite incomplete solubility of (S,2S)‐5 on the intial stages of the process and partially biphasic system, the disassembly proceed quite well under heating at 50–60 °C. The red‐orange color of (S,2S)‐5 was gradually changed to green (NiCl2) within about 3 hrs of the reaction time. Upon cooling to the ambient temperature the hydrochloric salt of chiral ligand (S)‐4 was precipitated and conveniently isolated by filtration. It should be noted that chiral ligand (S)‐4 was recovered with total chemical yield of 98 % (see for details SI) and was shown to be stereochemically intact, underscoring economically attractive feature of this approach.
Upon evaporation of the aqueous solution, containing hydrochloric salt of (S)‐1 and NiCl2, the mixture was dissolved in acetonitrile and treated with ethylenediaminetetraacetic acid (EDTA) to chelate the Ni(II) ions. The resultant solution was treated with Fmoc‐OSu, using rather standard Fmoc‐protection reaction conditions. For the final purification of target product (S)‐3, we resorted to a crystallization procedure. It was found that toluene serve as the solvent of choice allowing precipitation of Fmoc derivative (S)‐3 of excellent purity.
Using this method product (S)‐3 was prepared on 105.9 gram scale with total yield of 83.2 % and of >99 % enantiomeric purity.
In summary, the results reported in this work convincingly demonstrate that Ni(II) complex of amino acid Schiff bases methodology can be successfully applied for over 100 g‐scale synthesis of pharmaceutically important Fmoc‐(S)‐2‐amino‐6,6,6‐trifluorohexanoic acid. It should be emphasized that the application of new chiral ligand (S)‐4 and the corresponding glycine equivalent (S)‐2, providing for the enhanced reactivity and diastereo‐control on the alkylation step, were vital for the successful realization of this approach on the large‐scale. Excellent overall yield and enantiomeric purity of the final product coupled with operational convenience and attractive cost structure, bode well for widespread application of this methodology for large‐scale synthesis of tailor‐made amino acids.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21761132021 and 21606133), Natural Scientific Foundation of Jiangsu Province, P. R. China (No. Bk20160922) and IKERBASQUE, Basque Foundation for Science.
Z. Yin, H. Moriwaki, H. Abe, T. Miwa, J. Han, V. A. Soloshonok, ChemistryOpen 2019, 8, 701.
Contributor Information
Dr. Hiroki Moriwaki, Email: hiroki-moriwaki@hamari.co.jp
Dr. Jianlin Han, Email: hanjl@njfu.edu.cn
Prof. Dr. Vadim A. Soloshonok, Email: vadym.soloshonok@ehu.es
References
- 1.For the definition of tailor-made amino acids, see: Soloshonok V. A., Cai C., Hruby V. J., Meervelt L. V., Tetrahedron 1999, 55, 12045–12058. [Google Scholar]
- 2. Zhou Y., Wang J., Gu Z., Wang S., Zhu W., Aceña J. L., Soloshonok V. A., Izawa K., Liu H., Chem. Rev. 2016, 116, 422–518. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Wang J., Sánchez-Roselló M., Aceña J. L., del Pozo C., Sorochinsky A. E., Fustero S., Soloshonok V. A., Liu H., Chem. Rev. 2014, 114, 2432–2506; [DOI] [PubMed] [Google Scholar]
- 3b. Zhu Y., Han J. L., Wang J., Shibata N., Sodeoka M., Soloshonok V. A., Coelho J. A. S., Toste F. D., Chem. Rev. 2018, 118, 3887–3964; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3c. Zhu W., Wang J., Wang S., Gu Z., Aceña J. L., Izawa K., Liu H., Soloshonok V. A., J. Fluorine Chem. 2014, 167, 37–54. [Google Scholar]
- 4.
- 4a. Henninot A., Collins J. C., Nuss J. M., J. Med. Chem. 2018, 61, 1382–1414; [DOI] [PubMed] [Google Scholar]
- 4b. Blaskovich, chemistry M. A. T. Unusual amino acids in medicinal, J. Med. Chem. 2016, 59, 10807–10836; [DOI] [PubMed] [Google Scholar]
- 4c. Ma J. S., Chim. Oggi 2003, 21, 65–68; [Google Scholar]
- 4d. Hodgson D. R. W., Sanderson J. M., Chem. Soc. Rev. 2004, 33, 422–430; [DOI] [PubMed] [Google Scholar]
- 4e. Sato T., Izawa K., Aceña J. L., Liu H., Soloshonok V. A., Eur. J. Org. Chem. 2016, 2757–2774; [Google Scholar]
- 4f. Sorochinsky A. E., Aceña J. L., Moriwaki H., Sato T., Soloshonok V. A., Amino Acids 2013, 45, 691–718; [DOI] [PubMed] [Google Scholar]
- 4g.Asymmetric Synthesis and Application of alpha-Amino Acids, ACS Symposium Series 1009, V. A. Soloshonok, K. Izawa, Eds., Oxford University Press, 2009;
- 4h. Soloshonok V. A., Sorochinsky A. E., Synthesis 2010, 2319–2344. [Google Scholar]
- 5.For recent reviews on fluorinated α-AAs, see:
- 5a. Smits R., Cadicamo C. D., Burger K., Koksch B., Chem. Soc. Rev. 2008, 37, 1727–1739; [DOI] [PubMed] [Google Scholar]
- 5b. Kukhar V. P., Sorochinsky A. E., Soloshonok V. A., Future Med. Chem. 2009, 1, 793–819; [DOI] [PubMed] [Google Scholar]
- 5c. Sorochinsky A. E., Soloshonok V. A., J. Fluorine Chem. 2010, 131, 127–139; [Google Scholar]
- 5d. Tarui A., Sato K., Omote M., Kumadaki I., Ando A., Adv. Synth. Catal. 2010, 352, 2733–2744; [Google Scholar]
- 5e. Czekelius C., Tzschucke C. C., Synthesis 2010, 543–566; [Google Scholar]
- 5f. Qiu X.-L., Qing F.-L., Eur. J. Org. Chem. 2011, 3261–3278; [Google Scholar]
- 5g. Turcheniuk K. V., Kukhar V. P., Roeschenthaler G.-V., Acena J. L., Soloshonok V. A., Sorochinsky A. E., RSC Adv. 2013, 3, 6693–6716; [Google Scholar]
- 5h. Aceña J. L., Sorochinsky A. E., Soloshonok V. A., Synthesis 2012, 44, 1591–1602; [Google Scholar]
- 5i. Aceña J. L., Sorochinsky A. E., Moriwaki H., Sato T., Soloshonok V. A., J. Fluorine Chem. 2013, 155, 21–38. [Google Scholar]
- 6.For recent reviews on fluorinated β-AAs, see:
- 6a. Mikami K., Fustero S., Sánchez-Roselló J. L., Aceña M., Soloshonok V. A., Sorochinsky A. E., Synthesis 2011, 3045–3079; [Google Scholar]
- 6b. Han J., Sorochinsky A. E., Ono T., Soloshonok V. A., Curr. Org. Synth. 2011, 8, 281–294; [Google Scholar]
- 6c. Aceña J. L., Simon-Fuentes A., Santos F., Curr. Org. Chem. 2010, 14, 928–949. [Google Scholar]
- 7.
- 7a. Dhillon S., Drugs 2018, 78, 1509–1516; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7b. Urquhart L., Nat. Rev. Drug Discovery 2018, 17, 799; [DOI] [PubMed] [Google Scholar]
- 7c. Scott L. J., Drugs 2019, 79, 315–324; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7d.M. Shirley, Drugs 2018, 78, 1947–1953. [DOI] [PubMed]
- 8.
- 8a. Shu M., Yu R., Zhang Y., Wang J., Yang L., Wang L., Lin Z., Med. Chem. 2013, 9, 32–44; [DOI] [PubMed] [Google Scholar]
- 8b. Tsushima T., Kawada K., Ishihara S., Uchida N., Shiratori O., Higaki J., Hirata M., Tetrahedron 1988, 44, 5375–87; [Google Scholar]
- 8c. Ojima I., Jameison F. A., Pete B., Radunz H., Schittenhelm C., Lindner H. J., Emith A. E., Drug Des. Discovery 1994, 11, 91–113; [PubMed] [Google Scholar]
- 8d. Borozan S. Z., Zlatović M. V., Stojanović S. Đ., J. Biol. Inorg. Chem. 2016, 21, 357–368. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Sandberg M., Eriksson L., Jonsson J., Sjöström M., Wold S., J. Med. Chem. 1998, 41, 2481–2491; [DOI] [PubMed] [Google Scholar]
- 9b. van Hest J. C. M., Kiick K. L., Tirrell D. A., J. Am. Chem. Soc. 2000, 122, 1282–1288; [Google Scholar]
- 9c. Kiick K. L., Tirrell D. A., Tetrahedron 2000, 56, 9487–9493; [Google Scholar]
- 9d. Borozan S. Z., Stojanović S. Đ., Comput. Biol. Chem. 2013, 47, 231–239; [DOI] [PubMed] [Google Scholar]
- 9e. Wadhwani P., Strandberg E., Heidenreich N., Bürck J., Fanghänel S., Ulrich A. S., J. Am. Chem. Soc. 2012, 134, 6512–6515; [DOI] [PubMed] [Google Scholar]
- 9f. Tkachenko A. N., Mykhailiuk P. K., Afonin S., Radchenko D. S., Kubyshkin V. S., Ulrich A. S., Komarov I. V., Angew. Chem. Int. Ed. 2013, 52, 1486–1489; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 1526–1529; [Google Scholar]
- 9g. Gfeller D., Michielin O., Zoete V., J. Comput. Chem. 2012, 33, 1525–1535; [DOI] [PubMed] [Google Scholar]
- 9h. Ng V., Kuehne A. A., Chan W. C., Chem. Eur. J. 2018, 24, 9136–9147. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Li S. G., Portela-Cubillo F., Zard S. Z., Org. Lett. 2016, 18, 1888–1891; [DOI] [PubMed] [Google Scholar]
- 10b. Ojima I., Kato K., Nakahashi K., Fuchikami T., Fujita M., J. Org. Chem. 1989, 54, 4511–4522; [Google Scholar]
- 10c. Drouet F., Noisier A. F. M., Harris C. S., Furkert D. P., Brimble M. A., Eur. J. Org. Chem. 2014, 2014, 1195–1201; [Google Scholar]
- 10d. Ghosh S., Nandakumar M. V., Krautscheid H., Schneider C., Tetrahedron Lett. 2010, 51, 1860–1862. [Google Scholar]
- 11.
- 11a. Soloshonok V. A., Kukhar V. P., Tetrahedron 1997, 53, 8307–8314; [Google Scholar]
- 11b. Soloshonok V. A., Kirilenko A. G., Kukhar V. P., Resnati G., Tetrahedron Lett. 1993, 34, 3621–3624. [Google Scholar]
- 12.
- 12a. Peng W., Wan J., Xie B., Ma X., Org. Biomol. Chem. 2014, 12, 8336–8345; [DOI] [PubMed] [Google Scholar]
- 12b. Scott W. L., Alsina J., Audu C. O., Babaev E., Cook L., Dage J. L., Goodwin L. A., Martynow J. G., Matosiuk D., Royo M., Smith J. G., Strong A. T., Wickizer K., Woerly E. M., Zhou Z., O'Donnell M. J., J. Comb. Chem. 2009, 11, 14–33; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12c. Yajima T., Nagano H., Org. Lett. 2007, 9, 2513–2515; [DOI] [PubMed] [Google Scholar]
- 12d. Wang J., Lin D., Zhou S., Soloshonok V. A., Liu H., J. Org. Chem. 2011, 76, 684–687; [DOI] [PubMed] [Google Scholar]
- 12e. Larsson U., Carlson R., Leroy J., Acta Chem. Scand. 1993, 47, 380–90. [Google Scholar]
- 13.
- 13a. Soloshonok V. A., Cai C., Hruby V. J., Angew. Chem. Int. Ed. 2000, 39, 2172–2175; [PubMed] [Google Scholar]; Angew. Chem. 2000, 112, 2256–2259; [Google Scholar]
- 13b. Yamada T., Okada T., Sakaguchi K., Ohfune Y., Ueki H., Soloshonok V. A., Org. Lett. 2006, 8, 5625–5628; [DOI] [PubMed] [Google Scholar]
- 13c. Soloshonok V. A., Gerus I. I., Yagupolskii Y. L., Kukhar V. P., Zh. Org. Khim. 1987, 23, 2308–2313. [Google Scholar]
- 14.
- 14a. Shibata N., Nishimine T., Shibata N., Tokunaga E., Kawada K., Kagawa T., Sorochinsky A. E., Soloshonok V. A., Chem. Commun. 2012, 48, 4124–4126; [DOI] [PubMed] [Google Scholar]
- 14b. Röschenthaler G.-V., Kukhar V. P., Kulik I. B., Belik M. Y., Sorochinsky A. E., Rusanov E. B., Soloshonok V. A., Tetrahedron Lett. 2012, 53, 539–542; [Google Scholar]
- 14c. Turcheniuk K. V., Poliashko K. O., Kukhar V. P., Rozhenko A. B., Soloshonok V. A., Sorochinsky A. E., Chem. Commun. 2012, 48, 11519–11521. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Han J., Kitagawa O., Wzorek A., Klika K. D., Soloshonok V. A., Chem. Sci. 2018, 9, 1718–1739; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15b. Han J., Nelson D. J., Sorochinsky A. E., Soloshonok V. A., Curr. Org. Synth. 2011, 8, 310–317; [Google Scholar]
- 15c. Han J., Wzorek A., Kwiatkowska M., Soloshonok V. A., Klika K. D., Amino Acids 2019, DOI: 101007/s00726-019-02729-y. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Aceña J. L., Sorochinsky A. E., Soloshonok V., Amino Acids 2014, 46, 2047–2073; [DOI] [PubMed] [Google Scholar]
- 16b. Sorochinsky A. E., Aceña J. L., Moriwaki H., Sato T., Soloshonok V. A., Amino Acids 2013, 45, 1017–1033; [DOI] [PubMed] [Google Scholar]
- 16c. Wang Y., Song X., Wang J., Moriwaki H., Soloshonok V. A., Liu H., Amino Acids 2017, 49, 1487–1520. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Ellis T. K., Ueki H., Yamada T., Ohfune Y., Soloshonok V. A., J. Org. Chem. 2006, 71, 8572–8578; [DOI] [PubMed] [Google Scholar]
- 17b. Soloshonok V. A., Ueki H., Ellis T. K., Yamada T., Ohfune Y., Tetrahedron Lett. 2005, 46, 1107–1110. [Google Scholar]
- 18.
- 18a. Soloshonok V. A., Ellis T. K., Ueki H., Ono T., J. Am. Chem. Soc. 2009, 131, 7208–7209; [DOI] [PubMed] [Google Scholar]
- 18b. Sorochinsky A. E., Ueki H., Aceña J. L., Ellis T. K., Moriwaki H., Soloshonok V. A., Org. Biomol. Chem. 2013, 11, 4503–4507. [DOI] [PubMed] [Google Scholar]
- 19.
- 19a. Takeda R., Kawamura A., Kawashima A., Sato T., Moriwaki H., Izawa K., Akaji K., Wang S., Liu H., Aceña J. L., Soloshonok V. A., Angew. Chem. Int. Ed. 2014, 53, 12214–12217; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 12410–12413; [Google Scholar]
- 19b. Jörres M., Chen X., Aceña J. L., Merkens C., Bolm C., Liu H., Soloshonok V. A., Adv. Synth. Catal. 2014, 356, 2203–2208. [Google Scholar]
- 20. Nian Y., Wang J., Moriwaki H., Soloshonok V. A., Liu H., Dalton Trans. 2017, 46, 4191–4198. [DOI] [PubMed] [Google Scholar]
- 21.
- 21a. Nian Y., Wang J., Zhou S., Wang S., Moriwaki H., Kawashima A., Soloshonok V. A., Liu H., Angew. Chem. Int. Ed. 2015, 54, 12918–12922; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 13110–13114; [Google Scholar]
- 21b. Nian Y., Wang J., Zhou S., Dai W., Wang S., Moriwaki H., Kawashima A., Soloshonok V. A., Liu H., J. Org. Chem. 2016, 81, 3501–3508. [DOI] [PubMed] [Google Scholar]
- 22. Zhou S., Wang J., Chen X., Aceña J. L., Soloshonok V. A., Liu H., Angew. Chem. Int. Ed. 2014, 53, 7883–7886; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 8017–8020. [Google Scholar]
- 23. Mei H., Hiramatsu T., Takeda R., Moriwaki H., Abe H., Han J., Soloshonok V. A., Org. Process Res. Dev. DOI: 10.1021/acs.oprd.8b00404. [Google Scholar]
- 24. Romoff T. T., Palmer A. B., Mansour N., Creighton C. J., Miwa T., Ejima Y., Moriwaki H., Soloshonok V. A., Org. Process Res. Dev. 2017, 21, 732–739. [Google Scholar]
- 25. Ueki H., Ellis T. K., Martin C. H., Soloshonok V. A., Eur. J. Org. Chem. 2003, 1954–1957. [DOI] [PubMed] [Google Scholar]
- 26.
- 26a. Ellis T. K., Hochla V. M., Soloshonok V. A., J. Org. Chem. 2003, 68, 4973–4976; [DOI] [PubMed] [Google Scholar]
- 26b. Tang X., Soloshonok V. A., Hruby V. J., Tetrahedron: Asymmetry 2000, 11, 2917–2925. [Google Scholar]
- 27.
- 27a. Houck D., Aceña J. L., Soloshonok V. A., Helv. Chim. Acta 2012, 95, 2672–2679; [Google Scholar]
- 27b. Taylor S. M., Yamada T., Ueki H., Soloshonok V. A., Tetrahedron Lett. 2004, 45, 9159–9162. [Google Scholar]
- 28.
- 28a. Soloshonok V. A., Ueki H., J. Am. Chem. Soc. 2007, 129, 2426–2427; [DOI] [PubMed] [Google Scholar]
- 28b. Soloshonok V. A., Ono T., Ueki H., Vanthuyne N., Balaban T. S., Bürck J., Fliegl H., Klopper W., Naubron J. V., Tam T. T., Drake A. F., Roussel C., J. Am. Chem. Soc. 2010, 132, 10477–10483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


