Abstract
Recent advancements in bioprinting techniques have enabled convenient fabrication of micro-tissues in organ-on-a-chip platforms. In a sense, the success of bioprinted micro-tissues depends on how close their architectures are to the anatomical features of their native counterparts. The bioprinting resolution largely relates to the technical specifications of the bioprinter platforms and the physicochemical properties of the bioinks. In this article, we compare inkjet, extrusion, and light-assisted bioprinting technologies for fabrication of micro-tissues towards construction of biomimetic organ-on-a-chip platforms. Our theoretical analysis shows that for a given printhead diameter, surface contact angle dominates inkjet bioprinting resolution, while nozzle moving speed and the nonlinearity of viscosity for bioinks regulate extrusion bioprinting resolution. The resolution of light-assisted bioprinting is strongly affected by the photocrosslinking behavior and light characteristics. Our tutorial guideline for optimizing bioprinting resolution would help model the complex microenvironment of biological tissues in organ-on-a-chip platforms.
Keywords: Inkjet bioprinting, extrusion bioprinting, stereolithography, resolution, bioink, organoid
Table of contents entry
We compare current bioprinting technologies for their effective resolutions in the fabrication of micro-tissues towards construction of biomimetic microphysiological systems.
1. Introduction
Organ-on-a-chip (OoC) platforms aim to replicate the function of tissues and organs, bridging the gap between the conventional models based on planar cell cultures/animals and the complex human system. In most cases, an OoC platform consists of a patterned extracellular matrix (ECM)-like tissue model with embedded cellular components, which regulate the phenotypes and biological behaviors of the cells.1 Due to their biomimicry to human tissues, OoC platforms allow for more accurate testing of the effects of pharmaceutical agents for predicting human responses. To this end, the performance of the OoC platforms largely depend on how the engineered micro-tissues resemble their native architecture. In addition to soft lithography and other techniques such as photo-patterning and self-assembly, three-dimensional (3D) bioprinting has been recently adapted to generating miniaturized tissue and organ models towards the construction of OoC models in a controllable and automated manner (e.g., models of liver,2 kidney,3 thrombosis,4 heart,5 and neuron6 summarized in Table 1 and depicted in Fig. 1). These OoC models may be connected in a single microfluidic circuitry, which can enable investigation of multi-tissue interactions for improved drug screening.7
Table 1.
Selected OoC platforms utilizing bioprinting techniques.
| Organ/Disease Model |
Bioprinted Component |
Bioprinting Technique |
Feature Size |
Comments | Ref |
|---|---|---|---|---|---|
| Liver | Patterns of cell suspensions | Inkjet (DeskViewer™) |
~ 400 μm | Bioprinted co-culture of endothelial and hepatocytes | 2 |
| Thrombosis | Bifurcation blood vessels (chip) | Extrusion (Allevi) |
~ 200 μm | Bioprinted sacrificial Pluronic-F127 in gelatin-based hydrogel | 4 |
| Heart | Core/shell pattern loaded by cells | Extrusion (Organovo) |
~ 150 μm | Tubular fibers of alginate/gelatin hydrogel | 5 |
| Nervous system | Multiple cells & microchannels | Extrusion (customized) |
~ 150 μm | Bioprinting of parallel microchannels, a sealant layer, and a chamber | 6 |
| Kidney | Renal proximal tubules | Extrusion (customized) |
~ 100 μm | Bioprinted sacrificial Pluronic-F127 in gelatin/fibrin hydrogel | 3 |
| Liver | Cell-encapsulated hydrogels | Light-assisted (customized) |
~ 50 μm | Dual-material bioprinting of gelatin and hyaluronic acid | 8 |
Figure 1.
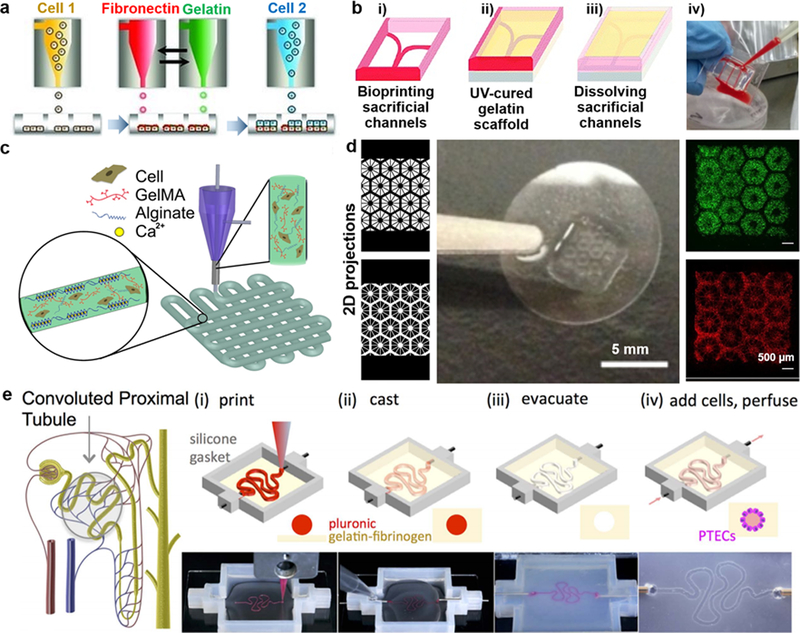
Representative OoC models: a) Schematic of the development of liver micro‐tissue (as spheroids) by inkjet bioprinting of endothelial cells (cell 1), a thin layer of fibronectin and gelatin, and hepatocytes (HPCs; cell 2); reprinted with permission from Matsusaki et al. 2013.2 b) Schematic of the blood-vessel-on-a-chip using bioprinting process of sacrificial channel (i), adding GelMA (gelatin methacryloyl) (ii) and removing channels (iii), along with the infusion of human whole blood into the endothelialized microchannels (iv); adapted with permission from Zhang et al. 2016.4 c) Schematic showing the extrusion of a mixture of alginate and GelMA for the heart-on-a-chip; reprinted with permission from Zhang et al. 2016.5 d) Liver model in which human induced pluripotent stem cells (hiPSCs)-HPCs were patterned by the first digital mask (or two-dimensional projection) followed by the patterning of supporting cells using a second digital mask, shown with fluorescent images showing patterns of fluorescently labeled hiPSC-HPCs (green) and supporting cells (red), along with a photograph of the final chip (center); adapted with permission from Ma et al. 2016.8 e) Schematic of a nephron highlighting the convoluted proximal tubule, along with corresponding schematic and images of different steps in the fabrication of proximal tubules: a sacrificial ink is first printed on a gelatin-fibrinogen extracellular matrix (i), additional matrix is cast around the printed feature (ii), the sacrificial ink is evacuated to create an open tubule (iii), and Proximal tubule epithelial cells (PTECs) are seeded within the tubule and perfused for long time periods (iv); reprinted with permission from Homan et al. 2016.3
More specifically, recent 3D bioprinting techniques9 allow for the generation of micro-tissues and organoids by using cell-laden bioinks while preserving the structural configuration of the desired organ.10, 11 In addition, 3D printing techniques have been applied to creating the supporting framework and microfluidic encasement in OoC platforms (e.g., blood vessel-on-a-chip in Fig. 1b and proximal tubule-on-a-chip in Fig. 1e).12 One relevant success criterion in the fabrication of OoC platforms is the structural fidelity of the bioprinted micro-tissues, partly determined by the minimum feature size known as the bioprinting resolution. A bioprinting resolution of nearly 200 μm has been shown to be suitable for blood vessel models and organoids (e.g., Fig. 1b and Fig. 1c), although it seems inadequate for replicating capillaries and heterogeneous models such as tumor angiogenesis.13 This is discussed in details in the next section. In general, bioprinting resolutions below 100 μm provide precise patterning of cell-laden constructs in OoC platforms for high-throughput experiments and improved drug screening (e.g., Fig. 1d), while a resolution of nearly 10 μm allows creating microfluidic encasements and connecting channels for directing circulation flow across the chip. Nanoscale resolutions may also be relevant for generating miniaturized building blocks or microparticles used in OoC platforms.14 In this article, we discuss features, advantages, and disadvantages of inkjet, extrusion and light-assisted bioprinting along with two-photon lithography (TPL). We then report the practical bioprinting resolutions for micro-tissues and OoC platforms, supplemented with numerical simulations of bioprinting resolution in inkjet and extrusion bioprinting techniques.
While it is apparent that tissue functions do not solely depend on the resolution, the lower limit of bioprinting resolution does play a major role in the fabrication strategy for micro-tissues and organoids. The lower limit in inkjet and extrusion bioprinting is controlled by the printhead size that dispenses the bioink and geometry of the bioprinted micro-tissue, in addition to the physical properties of the cell-laden bioink, such as the example of cell suspensions in Fig. 1a). The physical integrity of the bioprinted micro-tissue is further linked to the continuity in the flow of the bioink droplets. Hence the pneumatic pumps or pistons used in extrusion-based technologies can be more beneficial in fabricating micro-tissues with superior integrity along the vertical direction when compared to inkjet-based methods, such as the thick blood vessel construct bioprinted in Fig. 1b, core-shell bioprinted vascular bed shown in Fig. 1c, and the proximal tubule bioprinted in Fig. 1e. On the other hand, light-assisted bioprinting uses a light source to either selectively crosslink a vat of photocurable bioinks onto a moveable stage or eject bioink droplets from a light-absorbing layer onto a substrate. The lower limit in light-assisted technologies is controlled by the illumination light parameters and optical properties of the bioink such as the refractive index (Fig. 1d). In addition to these conventional techniques, a sophisticated photocrosslinking process using nonlinear laser absorption (i.e., TPL) offering nanometer-scale bioprinting resolutions has been employed for potential applications in fabricating small-scale constructs,15 with great potentials for OoC platforms.
This article aims to identify key parameters in controlling effective bioprinting resolution for improving the fabrication fidelity in OoC platforms. This effective resolution represents the overall impacts of the bioprinting process, bioink properties, and post-printing factors. We employ engineering fundamentals and numerical simulations to predict the weight of each physical parameter in the resolution of selected bioprinting process in a bioprinting modality-specific manner. We finally rank the governing parameters for practical resolution of each bioprinting modality. This knowledge is anticipated to contribute to the success of bioprinting towards fabrication of improved OoC platforms.
2. Role of Bioprinting Resolution in Tissue Functionality
Recent applications of bioprinting techniques for OoC platforms have experienced challenges in creating physiologically relevant constructs at desired resolutions dictated by the detailed architectural organizations in the biological tissues. An ideal ECM-like microenvironment should include several properties such as: pore size in the range of several times of the cell size to provide contractile force for cell motility,18, 19 pore interconnection to allow the diffusion of nutrients and metabolites,20 and proper surface chemistry.21 More importantly, it is necessary to provide the accuracy of cell patterning when the OoC platforms are used for drug screening and related applications. We discuss some of the common examples here on this mutual relationship and then summarize for major tissue types (Table 3).
Table 3.
Desired tissue (patho)physiological activities, proposed building blocks, feature sizes, and cited resolutions (reported for cell-laden or acellular bioinks) for major tissue models. (NA: not available)
| Organ Model |
Primary (Patho)- physiological Activity |
Proposed Building Block | Desired Feature Size42 | Cited Resolution | Ref |
|---|---|---|---|---|---|
| Bone (compact) | Provides mechanical strength of the skeletal system | Cylindrical osteon of osteocytes and mineralized ECM | ~200 μm | 150–400 μm; acellular |
43,44 |
| Cartilage | Provides structural support and lubricates joints between bones | Zonal layers of ECM hydrogel and chondrocytes | 50–500 μm | 100–200 μm; cell-laden |
45 |
| Skeletal muscle | Provides forces for movements | Aligned multi-nucleated skeletal muscle bundles/fibers | ~20–50 μm | 50–200 μm; acellular |
43,46 |
| Brain | Generates electrical signaling for cognition and for controlling rest of the body | Directionally oriented neuronal cells supported by astrocytes | <5 μm (neurites) | 350 μm; cell-laden |
6 |
| Eye cornea | Allows light penetration and focusing for visualization | Transparent membrane seeded by epithelial/endothelial cells | ~500 μm (thickness) | 150–200 μm; cell-laden |
47 |
| Cardiac muscle | Generates contractions for driving the circulatory system | Aligned cardiomyocytes bundles | 50–100 μm | 100–200 μm; cell-laden |
5 |
| Lung | Provides gas exchange through respiratory movements | Hierarchical airways and spherical alveoli at terminal ends with epithelial cells on the surfaces and endothelial cells in the junctions | ~200 μm (diameter of alveolus); single cell (alveolus thickness) | NA | |
| Gut | Enables food digestion and absorption | Membrane-like ECM containing villi surfaces with epithelial cells | 50–150 μm (villus size) ~500 μm (villus length) |
500 μm; acellular |
48 |
| Liver | Metabolizes and detoxifies | Hexagonal lobule of primarily hepatocytes | ~500 μm | 50–200 μm; cell-laden |
8 |
| Kidney | Provides excretion of waste molecules and reabsorption of functional molecules | Various nephron structures (e.g., renal corpuscle, renal tubule) | ~15–250 μm | 100–200 μm; acellular |
3 |
| Blood vessel | Carries nutrients/oxygen to the organs and wastes away | Tubular layers of cells and ECMs | 30 μm (arteriole/venule); 8 μm (capillary) | 50–500 μm; acellular |
4 |
| Tumors | Feature hypoxic, uncontrolled proliferative and dense tumor microenvironments | Tumor type-dependent cells in ECMs | 10–500 μm (tumor type-dependent) | 50–500 μm; cell-laden |
40 |
Feature size is defined as the smallest size over which the tissue can be built upon, while maintaining its overall function. This parameter highlights the structural complexity of the tissue and dictates the level of accuracy required for its fabrication. The smallest feature sizes in the structures of some tissue types are limited to the submillimeter scales. An example is the multi-layer skin in which three almost uniform, submillimeter-thick layers (i.e., epidermis, dermis, and hypodermis) are stacked together with different cell/ECM types across the layers.22 Epidermis has a thickness of approximately 100 μm and is filled with keratinocytes, tightly interconnected to serve as a barrier. Dermis is the thickest layer (from 1 to 4 mm in thickness) and it contains fibroblasts, macrophages, and adipocytes. The hypodermis layer is mainly for fat storage and it contains loose connective tissues. In this sense, multiple cell-laden bioinks made of collagen, fibroblasts, and keratinocytes have been used, which led to the formation of the basement membrane after in vitro culture.23 With regard to the thickness of the different skin layers, resolutions in the range of ~100 μm are sufficient to make a one-to-one projection of the host tissue.
The feature size lowers in other target tissues, such as the skeletal muscle. The muscle cells, known as myocytes, are elongated cells that contain myofibrils. A myofibril that contains long chains of sarcomeres is embedded within the cytoplasm of a multi-nucleated syncytium and forms the building block of a skeletal muscle.24 The aligned assembly of myofibrils provides spatially distributed active forces for obtaining muscle functionality.25 Some attempts have been made to generate larger muscle tissues with aligned muscle cells on thin sheets of collagen matrix, such as those reported by Yan et al,26 which indicated that the micro-architecture of muscle bundles and cell alignments demanded a bioprinting resolution of <50 μm. Acellular bioinks that mimic muscle composition are suggested for creating ECM-like microenvironments that direct the behaviors of subsequently seeded muscle cells.27
The bioprinting resolution is also critical in the case of cardiac muscle because of the high sensitivity of cardiac functionality with respect to cell alignment. Myocardium contains cardiomyocytes and other cell types that are embedded in a well-ordered ECM, which provides electrical coupling between the cardiac and non-cardiac cells.28 The in vivo structure of the cardiac muscle has a reticular-like basket arrangement and the cardiomyocytes are rectangular shape bundled into fibers with diameters of ~100 μm.28 To obtain functional cardiac models, cardiomyocytes should be aligned into anisotropic arrangements for recapitulating electrophysiological and mechanical properties.29, 30 A fabrication control at the level of cardiac bundles or smaller (50–100 μm in effective resolution) in cell patterning and over the cell-material interface at the microscale (i.e., a cell-laden bioink5) facilitates the creation of biomimetic tissue constructs that recapitulate the structural aspects of the in vivo myocardial phenotype.
The challenge of patterning and organization is further raised by multicomponent design required in the fabrication. Generally (including the examples illustrated above), vascular networks are required for the delivery of nutrients and oxygen to cellular components and removal of metabolic waste from the same for maintaining the viability and functions of almost all tissue types. Such networks can range from micrometer-sized capillaries to millimeter-sized vessels accompanied by their compositional complexity (e.g., endothelial cells, smooth muscle cells, fibroblasts, ECM, etc.) and hierarchically assembled architectures, which should ideally, also be built into the corresponding tissue models to be fabricated.31 The degree of control over the composition, size, and structure of the vascular networks thus not only affects the survival but also regulates the drug screening performance of the tissue models. For example, cellular patterning plays a crucial role in liver tissue biofabrication as a functional liver consists of multiple cell types organized in a unique microstructure (i.e., multiple cell-laden bioinks).8 Hepatic sinusoids, the special microvascular systems (with diameters less than 10 μm) lined by sinusoid endothelial cells that form a radiating pattern, are critical for normal liver function and hepatocyte survival.32 To this end, a bioprinting resolution on the similar order perhaps becomes vital for the spatial positioning of both hepatic and endothelial cells to reconstruct the liver lobules. According to specific applications, the bioinks can be acellular (followed by endothelial cell injection/seeding) for fabricating relatively simple vascular or vascularized tissue models while more complex constructs may require cell-laden bioinks during the bioprinting processes.
Moreover, nephrotoxicity is a key player in drug testing and the kidney proximal tubules provide the main site of action in renal toxicity.33,34 Kidney micro-tissues can be used to model native biology and its architecture for a reproducible generation of cellular function and transporter activity. Bioprinted architectures may provide an enabling tool to study tubular transport of xenobiotics, different proteins, and ions across (tight) junctions between epithelial cells. Nephron structures would dictate the relevant bioprinting resolution; for example, renal tubules (500 to 750 μm in average) would demand a bioprinting resolution of around 200 μm while renal corpuscles (150 to 250 μm in average) and proximal tubules (20 to 50 μm in average) have smaller feature sizes.3,35
Another example is that of tumors. A tumor microenvironment is multiscale, and is highly dynamic with distinctive key features present at the different stages of the disease.36, 37 In particular, the long-term growth of a tumor requires sufficient blood supply, in which tumor cells activate angiogenic factors within the microenvironment of the tumor and induce a fine capillary network (i.e., tumor angiogenesis).38, 39 To grasp the structural details of the tumor microenvironment and angiogenesis, it has been realized that high-resolution bioprinting is strongly desired as the conventional models cannot recapitulate the in vivo physiology.40 Innovations in bioprinting resolution around the dimension of capillaries would facilitate the modeling of tumor angiogenesis in human patients and recapitulate other key parameters that match both the type and stage of the disease.5, 41
A summary of tissue models is presented in Table 3. A systematic analysis of governing parameters in bioprinting resolution can help the design and development of such tissue models. An ideal bioprinting resolution may be dictated by the application of an OoC platform, but for most cases it is close to the finest features of tissue microarchitecture (i.e., ~10 μm to a few hundred micrometers), which oftentimes are correlated with their desired functions. Since cellular components reorganize within the ECM over time, higher resolutions may be relevant to reproduce the micro-/nanoscale topography of the ECMs in tissue models. It is thus desirable to understand the practical limitations of common bioprinting techniques before designing the experiment.16
There are other challenges in bioprinting tissue models, such as increasing bioprinting speed, adding multi-material capacity, and reducing mechanical stresses on encapsulated cells (in the case of cell-laden bioinks), which hamper the bioprinting resolutions. For cell-laden bioinks, mechanical stresses on cellular components are reduced by lowering bioink viscosity or enlarging the nozzle size, and such considerations impede the final desired resolution. When bioprinting is used to generate the supporting framework and microfluidic channels to direct fluid circulation, in addition to the hosted micro-tissue, the resolution further plays a key role in the performance of the constructed OoC platform.1 We discuss how various bioprinting process parameters affect and regulate the effective resolution.
3. Inkjet Bioprinting
3.1. Mechanism of performance in inkjet printers
This subsection is devoted to the principles of inkjet printing systems and the governing factors in their performance. There are two forms of inkjet printing: drop-on-demand (DOD) and continuous inkjet (CI).49 In the case of DOD printing, the desired ink is pushed under pressure through a small orifice causing the resulting jet to break up into droplets through Rayleigh instability. These droplets become electrically charged and an electrical field is used to control the position where each droplet is deposited. In the case of CI printing, droplets are generated by transmitting a pressure pulse in an ink-filled container. Droplets not used for bioprinting are steered out of the printer stage and allowed to continue to a collection gutter for recirculating into the ink-filled container. In practice, a small fraction of the droplets is deposited on the substrate while a majority of droplets is recycled. In some CI printers, a micro-heater is used to heat up a small volume of the ink, while a mechanical pulse is used to form vapor bubbles.49, 50 In other CI printers, a piezoelectric element is used to mechanically actuate the chamber and create a pulse. Small inkjet printers, such as those commonly used as desktop printers, use the heating approach while larger industrial-scale printers usually rely on a piezoelectric actuator.49 Due to contamination issues by recirculating a portion of bioink droplets post-ejection, CI systems are generally limited to applications in product-making and low-resolution fabrication in industrial settings. Since the DOD method does not suffer from similar cross-contamination problems between the sample and the substrate (as bioink droplets are ejected only when required), it is often the preferred technology amongst material scientists.
The mechanism of inkjet bioprinters is similar to their classical counterparts, where the ink in the cartridge is replaced by a bioink consisting of cells and other biological materials necessary for maintaining cell growth and viability during biofabrication. Two subtypes of DOD bioprinters, including those based on thermal and piezoelectric printheads, have been used for bioprinting of molecules onto desired surfaces (Fig. 2a).51–53 For thermal printheads, a micro-resistor near the nozzle is heated to temperatures of 200–3000 °C by electric pulses that are delivered within microseconds, to rapidly form bioink vapor bubbles and thus create a pressure pulse to eject droplets.49 Although no evidence of denaturing of biological materials during the heating process has been reported, this method suffers from the lack of precise directionality and control in droplet size, as well as thermal stress imposed on cells, nozzle clogging, and unpredictable cell encapsulation. For piezoelectric printheads, an array of piezoelectric plates is used to drive the movement of bioinks. One major concern with this approach is the high frequencies (15–25 kHz) employed in such bioprinters, which can potentially induce cellular damage.54 The resolution of inkjet bioprinters is described by the droplet footprint,49 hence measuring droplet spreading on flat surfaces is used to determine the lateral and vertical resolution.
Figure 2.
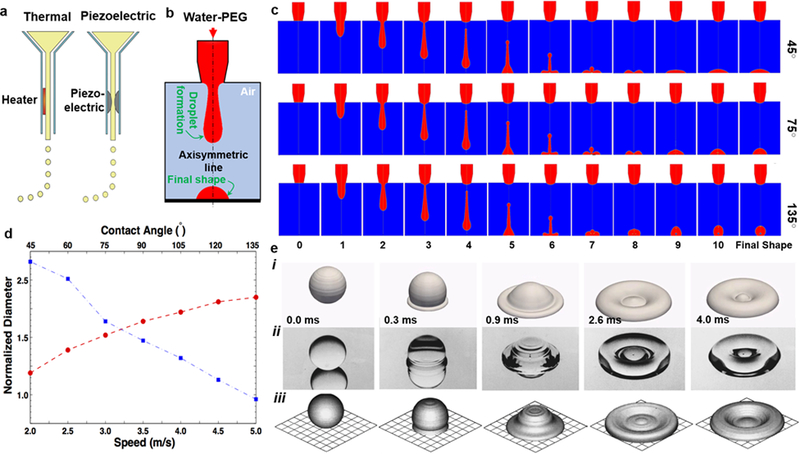
a) Overview of the most widespread inkjet bioprinting approaches; reprinted with permission from Hölzl et al. 2016.57 b) Schematic of numerical simulation and an example of the final shape. c) Computed drop shape after leaving the nozzle over time for three different surface contact angles. d) Normalized projection diameter (divided by nozzle size) versus input-bioink speed (circle, red) and surface contact angle (square; blue). e) Deposition of 2-mm water droplet on a stainless steel surface at different time points: i) simulation in this study, ii) photographs of the experimental study by Fard et al. 1996, iii) simulation by Fard et al. 1996; reprinted with permission from Fard et al. 1996.58
3.2. Theoretical resolution
Continuous inkjet (CI) or drop-on-demand (DOD) bioprinting technologies can create droplets ranging from 20 to 300 μm (the minimum size can be reduced to even smaller when working with acellular bioinks) regulated by bioprinter parameters, such as droplet deposition rate and nozzle size, as well as bioink physical properties.51–53 While CI has been used for producing constructs with a droplet diameter of nearly 100 μm, DOD bioprinting can generate smaller droplets, typically around 20–50 μm in diameter.54 In the former type, the Rayleigh instability yields a stream of droplets expelled out of a small nozzle. Since individual droplets are spread over the surface, the final diameter is typically larger than the nozzle diameter. The nozzle diameter regulates the printing resolution and the bioink conditions dictate the printout size. In general, traditional inkjet printing involves high shear rates in the nozzle (higher than 1×104 1/s), short residence times (10–200 μs), and high frequencies of droplet deposition (10–100 kHz).
A typical DOD printhead (Fig. 2a) consists of a long capillary attached to a piezoelectric actuator (or a heating component) in the interior and an orifice-like tip. Input voltage stimulates the piezoelectric element to induce a sudden volume change, and this generates pressure waves within the capillary to push the fluid out.49 The kinetic energy transmitted to the fluid should exceed the surface energy of droplet formation for the ejection of droplets onto the surface.54 The surface tension between the bioink and substrate can regulate the droplet size after ejection. The efficiency of bioprinting also relies on the rate of polymerization or stabilization upon deposition. According to Fromm et al., bioprinting is only possible if the inverse of Ohnesorge number is larger than 1:55
| (1) |
In Eq. (1), We denotes the Weber number, Re represents the Reynolds number, τ is the surface tension, ρ denotes the bioink density, η represents the dynamic viscosity, and ι is a characteristic length (i.e., nozzle diameter in our case). Very low Z denotes high viscous dissipation for droplet formation, while Z larger than 14 means a trend towards satellite droplets or a large droplet with tails of small droplets. The principal parameter in this case is the dynamic viscosity as other factors are similar for most biological fluids. According to previous studies, a fluid viscosity of around ~100 mPa∙s denotes a realistic upper bound (to ensure the formation of bioink droplets) for printability with current bioprinters.54 For ink viscosity higher than 1000 mPa·s, physical constrains over the deposition process are necessary. More recently, acoustophoretic printing by use of nonlinear effects of highly localized acoustic pressure at the nozzle tip was employed to offer monodisperse DOD printing of aqueous poly(ethylene glycol) (PEG, a good representative material system of bioinks) within a broad range of Z from ~ 0.1 to ~200, while reaching ~100-μm resolution.56
3.3. Numerical simulation
We used a numerical model based on the method of volume of fluid (VOF) to obtain some insights on the droplet generation in inkjet bioprinting. We present the governing equations to emphasize the physical parameters in our model. Assuming Newtonian and incompressible flow, the governing equations include the continuity of fluid and Navier-Stokes relations:59
| (2) |
| (3) |
, whereis the velocity vector, is the density, is the dynamic viscosity, p is the pressure, and is the vector of body forces per unit volume acting on the fluid. We adapted VOF to capture the interface deformation of bubbles, where the liquid volume fraction of a two-phase fluid should follow the preservation of total volume.60 The surface tension can be assumed to be constant and the shear stress becomes zero along the liquid-gas interface; thus the boundary condition at the liquid-gas interface is imposed as:61
| (4) |
, where , , , and are the pressure terms on both the liquid and gas sides of the interface, surface tension, and local interface curvature, respectively. A typical continuum surface force model62 can be used to employ Eq. (4) in our numerical simulations. We performed the numerical simulations in the two-dimensional (2D) axisymmetric domain presented in Fig. 2b, where the source is at the top of the computational domain. The physical properties of PEG aqueous solution are assumed by the general mixture law, while density, viscosity, and surface tension of PEG are assumed as 1056.1 kg/m3, 3.7×10−3 Pa∙s, and 49.92×10−3 N/m, respectively (provided by Dynalene Inc., Whitehall, PA). The computational domain is 20×20 mm2 in the radial and axial directions.
Our simulations have revealed the spatial and temporal features of the droplets before they impact the substrate, which is highly difficult to observe through experiments. The schematic of axisymmetric computational (2D) domain is shown in Fig. 2b. It is assumed that the selected bioink, PEG aqueous solution, has a periodic, cos-sign, temporal velocity at the top of the computational domain.59 The cos-sign velocity inlet results in releasing a portion of the bioink at a time. The released portion, while departing the injection nozzle, goes through shape deformations and forms a microscale droplet followed by a droplet-tail that matches well with experimental and computational results reported in the literature.54 We also performed a validation study by simulating the impact of water droplets on a stainless steel surface and compared with existing data in literature. Fard et al.58 generated droplets by pushing water through a hypodermic needle. Water droplets of 2 mm in diameter fell onto a polished stainless steel substrate with an impact velocity of approximately 1 m/s. This process was photographed using a high-speed camera. The static contact angle of water droplets on the surface was 165° with advancing and receding contact angles of 168° and 163°, respectively. The experimental results and our numerical simulation (along with the numerical simulation performed by Fard et al.58) are shown in Fig. 2e to demonstrate the accuracy of our numerical model in predicting the impact of water droplets. The slight differences might have come from the mesh size selected in our study.
For the selected bioink, our preliminary simulation showed that a velocity range of 1 mm/s to 100 mm/s would lead to continuous droplet formation. Fig. 2c depicts the role of contact angle between the bioink and collecting substrate highlighting the role of surface tension. High hydrophobicity of the substrate yields a better bioprinting resolution and lower stability for bioprinting. The extent of the droplet-tail is a function of inlet velocity and physical properties of the PEG aqueous solution, such as surface tension, viscosity, and density (see also (Eq. 1)). Departed droplet from the nozzle then impacts the bottom surface and forms a final steady shape. In general, the final shape of the droplet is a function of all the physical properties and the droplet-surface contact angle. The final shape of small-scale droplets, however, is dominated by the contact angle, which is due to very high surface tension forces compared to gravitational forces. Dependence of the final shape on the contact angle is well depicted in Fig. 2c. In this panel, three different cases are modeled for a PEG weight ratio of 72% in the aqueous solution with contact angles of 45°, 75°, and 135°. The differences in the final shapes can be observed in these three cases.
The bioink properties and contact angle of the collecting substrate are then depicted in Fig. 2d, which shows the droplet diameter divided by the nozzle diameter versus the injection speed of PEG aqueous solution and contact angle values of the bottom surface. The speed has a sharper effect, as expected, while contact angle interestingly regulates the diameter of the final shape as a linear function. The contact angle represents the surface tension between the droplet and the surrounding gas as well as the surface tension between the droplet and the solid substrate (see (Eq. 4)). The linear correlation between the contact angle (when >75°) shown here can be related to the underlying physics (Supplementary Materials). In summary, the bioprinting resolution is dependent on the viscosity and the affinity of the substrate with respect to bioink (i.e., higher viscosity and lower hydrophilicity will lead to a better resolution) as well as the nozzle diameter, which controls the droplet volume. Our discussions are typically valid for 2D/pseudo-3D resolution as it is not usually straightforward in deposition of 3D constructs using inkjet bioprinting. This may also, to a certain extent, limit the usage of inkjet bioprinting to planar OoC platforms with minimal thickness variations.
3.4. Resolution in practice
Inkjet bioprinting has been mainly used to generate two and half-dimensional (2.5D) constructs by introducing pre-defined mass concentrations of different components, such as cells, tissue growth factors, and therapeutic agents.46 Phillippi et al. used this technique to deliver volumes of nearly 15 pL to generate 75-μm-diameter droplets.46 Line patterns of single droplets of greater than 50 μm in diameter have been made using this technology.63 Some disadvantages of this method include lengthy processing times associated with very low droplet volumes and ineffective (or non-uniform) deposition of selected volumes due to high viscosities of the bioinks. In a pioneering work,64 a customized bioprinter was used for rapid biofabrication of cell-laden collagen patches using smooth muscle cells. This work showed a spatial resolution of 18.0 ± 7.0 μm along the proximal axis and 0.5 ± 4.9 μm along the distal axis. The resolution of cell seeding in this case is of interest considering the speed of droplet formation (160 droplets/s). In another work,65 researchers used inkjet bioprinting and post-printing gelation of alginate and fibrin hydrogels to construct 3D tissue models (rather than 2.5D) with multiple cell types towards bone and muscle tissue models. Inkjet bioprinting was used along with simultaneous photopolymerization for 3D cartilage tissue constructs, composed of PEG-diacrylate (PEGDA) and human chondrocytes.45 This study showed direct cartilage repair using high-resolution bioprinting on the order of 85 μm (i.e., droplet size) with a 20-μm layer thickness, proposing new solutions for the articular cartilage (approximately 200 μm in thickness). These studies showed the capacity of inkjet bioprinting for generation of cellularized tissue models, commonly with bioink viscosities of lower than 10 mPa∙s and cell concentrations of lower than 10 million cells/mL. There are some recent efforts to improve the capacity of inkjet bioprinting to produce 3D constructs (see recent reviews on this topic66), such as liquid support‐based bioprinting proposed by Christensen et al.67 and stabilization by horseradish peroxidase-catalyzed crosslinking proposed by Sakai et al.68
In addition, inkjet-based bioprinting was used to fabricate a liver-on-a-chip by patterning multiple arrays of human liver tissue (Fig. 1a).2 These arrays were stacked with human umbilical vein endothelial cells (HUVECs), the fibronectin-gelatin layers as a glue and the layers of hepatocyte-like cells (HepG2 cells). The work showed a resolution of approximately 400 μm in the bioprinted cell suspensions. The bioprinting resolution could be improve if they used a more hydrophobic substrate (i.e., larger contact angle). They confirmed the efficiency of the chip through the use of a hepatotoxic drug troglitazone, and measured cytochrome-mediated metabolism in the model. Similarly, a human liver micro‐tissue was also fabricated using inkjet bioprinting for sandwich-like deposition of human induced pluripotent stem cells (hiPSCs) and endothelial cells.2 They improved the cell-ECM adhesion using thin layers of fibronectin and gelatin and evaluated the model by measuring drug metabolism and toxicity. Despite these models, inkjet-based bioprinting suffers from the limitation in generating complex structures in 3D and it has been less used for OoC micro-tissue models so far.
4. Extrusion Bioprinting
4.1. Mechanism of extrusion bioprinters
Extrusion bioprinting is a solid free-form biofabrication technique that involves pressure or plunger-actuated dispenser to push out a viscoelastic fluid (or gel) containing biomaterials and/or cells.69 These platforms deposit viscous bioinks to fabricate complex 3D tissue models containing living components.16 This method is quite effective in bioprinting high concentrations of cells to accelerate growth and tissue formation.70 However, possible detrimental effects of polymerization and shear forces on cell viability and functionality must be considered before optimizing the relevant parameters.57 An ideal bioink for extrusion bioprinting should possess characteristics such as shear-thinning that minimizes resistance under shear flow but must also reestablish its mechanical property relatively fast after ejection from the printhead. This technology is particularly well-suited for biological applications as it is able to deposit heterogeneous materials with wide-ranging properties.69 Scaffolds printed using this technology are usually soft and the constructs must be subjected to some forms of gelation post-print to support their integrity.
Among the physical properties of bioinks, dynamic viscosity plays a major role in bioprinting resolution as well as the printing threshold (i.e., initiation of bioink ejection from the nozzle and stable deposition on the surface). Extrusion bioprinting works with a wide range of viscosities ranging from 1×102 to 1×109 mPa∙s.71 Bioinks such as those based on PEG polymers, hyaluronic acid, and alginate have been used to demonstrate the capacity of extrusion bioprinting in creating tissues and tissue models.70 The other major factor in bioprinting resolution is the velocity of bioink inside the nozzle, affected by the pneumatic pressure or plunger speed. It has been discussed that cell viability also relates to the flow rate of bioinks; for example, a flow rate of 0.5 mm3/s was found to be optimum for cell viability while the extruding period and shear stress on cells were considered as two governing factors.72 However, they did not study the correlation between resolution of the constructs and bioink viscosity. Bruneaux et al. investigated the relation between viscosity, shear rate inside the nozzle, and bioink flow rate, where they quantified how viscosity and nozzle size controlled cell mortality.73
4.2. Theoretical resolution of extrusion bioprinting
To understand the role of bioink physical properties on the bioprinting resolution, simple closed-form solutions can provide novel insights.74 We have thus developed an approximate equation for calculating bioprinting resolution while neglecting the surface tension effects, bioink elasticity, and inhomogeneity at microscales, such as fibrous composition in some bioinks (e.g., liquid crystal behavior of collagen precursor solutions75). It is assumed that the printhead (nozzle) is a uniform cylindrical duct of a constant diameter, D, under steady pneumatic pressure or plunger speed. One can define the average shear rate on the bioink by:
| (5) |
, where Q denotes the volume rate of the bioink extrusion. This quantity relates to bioink speed in the nozzle, v (i.e., mass preservation):
| (6) |
, with d as the strand diameter. The combination of Eqs. (5) and (6) gives:
| (7) |
The constitutive relation of the bioink dictates how viscosity is related to the shear rate. These parameters are commonly obtained using rheological measurements. Bioink viscosity, η, relates to the shear rate by K, a material-based constant. General power-law constitutive law reads:
| (8) |
The mathematical manipulations of Eqs. (7) and (8) yield:
| (9) |
Hence, the fiber diameter printed from the nozzle is written as:
| (10) |
Based on this relation, the fiber diameter has direct correlations to D3/2, v−1/2, and η1/2(n−1). For shear-thinning bioinks, n should be lower than 1 and for shear-thickening bioinks, it is larger than 1. We have used the material properties of Pluronic-F127 (30% w/v) to investigate the behavior of the above formulation. Pluronic-F127 properties are available from the literature.76 As shown in Fig. 3c, the nozzle moving speed has a direct correlation with the fiber diameter although there is a practical limitation where the fiber loses the continuity. The variation of strand diameter versus bioink viscosity depicts how the viscosity regulates the fiber diameter for shear-thinning and -thickening bioinks. For the case of shear-thinning, higher viscosity leads to a better resolution while for the case of shear-thickening it yields an opposite effect. The case of Newtonian bioink cannot be well described here as n=1 eliminates the power term in the equation.
Figure 3.
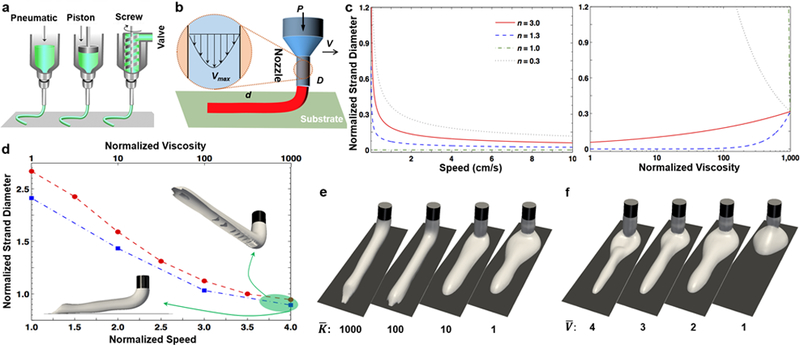
a) An overview of the most common extrusion bioprinting approaches; reprinted with permission from Malda et al. 201377. b) Schematic of the theoretical modeling, with an inset showing a realistic velocity profile inside the nozzle. c) Theoretical simulation of normalized strand diameter (d/D) versus normalized viscosity (: K divided by water viscosity) and theoretical simulation of normalized strand diameter (d/D) versus nozzle moving speed (V) for four power-law constant values. d) Numerical simulation of normalized strand diameter (d/D) versus normalized speed (= V divided by inlet velocity of bioink ~ Vmax/2; for n=0.3 and =100; circle; red) and normalized viscosity (for n=0.3 and =3; square; blue), where 3D perspectives of extruded bioinks are shown for the extreme case. e) 3D perspective view of bioink extruded for four viscosity ratios () and n=0.3. f) 3D perspective view of bioink extruded for four velocity ratios () and n=0.3.
We have used numerical simulation to provide further insights into extrusion bioprinting resolution. The simulations are performed in a 3D domain, as shown in Fig. 3d–f, in which bioink is deposited on the target substrate. The computational domain and nozzle dimensions can be found in Fig. 3d. Here, it is assumed that the nozzle moves with a fixed velocity on the target surface. The computation showed good convergence when we used implicit solutions of Navier-Stokes’ solutions and VOF equations (similar to the previous section) for large viscosities. In contrast to our mathematical formulation, the numerical simulation considers surface tension effects.
In contrast to inkjet bioprinting, the viscosity has been found to be a dominant factor for flow properties inside the nozzle and after deposition in extrusion bioprinting (Fig. 3d–e). Regarding the physical integrity of the bioink inside the nozzle, a lengthy (at least ten times the nozzle diameter) nozzle results in a parabolic fully developed velocity profile, such as the velocity profile shown in Fig. 3b, and this magnifies the role of viscosity in the bioprinting process. In contrary, a uniform velocity profile (i.e., when there is a slight difference between maximum and minimum flow velocities) eliminates the effects of non-Newtonian viscosity behavior. The viscosity dependency of shear rates also affects the surface deformation of the bioink when it leaves the nozzle and the combination of flow-induced stresses with surface tension forces dictates the form of the spherical residue formation shown in Fig. 3e. The values reported in Fig. 3d show an almost negative-exponential relation between bioink viscosity and bioprinting resolution, with a slower rate compared to that of Fig. 3c.
The printing speed (parameter V in Fig. 3b) is the major kinematics factor in controlling the bioprinting resolution, through variation of the local shear flow and volume of the bioink (Fig. 3f). The nozzle movement velocity defines how far we can have a continuous flow with minimal distortion. The variation in nozzle velocity also dictates the time required to deposit the entire construct. The range of this speed also depends on technical specifications of the hardware used in 3D printers. Finally, the numerical simulation has shown that the hydrophobicity of the substrate has a smaller effect than the nozzle velocity and bioink viscosity on the bioprinting resolution of the selected material system. This could be due to the weight of the bioink after deposition and inability of the numerical model in considering bioink self-healing. However, such an effect only affects the first layer of the bioprinted filament and is mitigated as the structure builds up.
4.3. Resolution in practice
There are several reviews on extrusion-based bioprinting of tissue models for potential use towards OoC platforms.7, 78–80 The translation of extrusion-based bioprinting to OoC fabrication was recently shown by a liver-on-a-chip platform.81 The chip was fabricated by dispensing a biocompatible polymer (polylactic acid [PLA]; PCL) as the housing with associated microfluidic channels, and two bioinks as the biological components: HepG2-laden collagen and HUVEC-laden gelatin. They showed the various designs of the bioprinted liver-on-a-chip and favorable liver functionality at a resolution of approximately 300 μm. Johnson et al. used extrusion-based bioprinting in modeling the nervous system with a resolution of close to 200 μm.6 They deposited PCL onto the dish to first make microchannels and added grease and silicone across the channels to build chambers. Then, they bioprinted three bioinks into each chamber: i) rat embryonic hippocampal neurons, ii) rat embryonic sensory neurons and Schwann cells, and iii) porcine kidney epithelial cells. This chip was used to investigate the transmission behavior of the pseudorabies virus to the cell body.
In different set of works, sacrificial bioinks and extrusion-based bioprinting have been used to generate perfusable models in chip devices. Fugitive or sacrificial bioinks should possess not only shear-thinning properties but also suitable biocompatibility to allow for subsequent encapsulation with cell-laden matrices and/or seeding with cells on the surface of the microchannels. In addition, the sizes of the templates formed by the bioprinted sacrificial bioinks also determine the feature size or resolution of the generated microchannels in the tissue constructs. One example is a kidney proximal tubule-on-a-chip by printing the silicon gasket onto a substrate, and dispensing the fugitive bioink of the thermosensitive Pluronic F127 (at a resolution of 200 μm) as shown in Fig. 1e.3 The chip was filled by a fibrinogen-gelatin-CaCl2-transglutaminase bioink containing fibroblasts to mimic the kidney ECM. After gelation of the matrix, they perfused the culture medium to wash out Pluronic F127 and to generate the hollow channel. After seeding the epithelial cells, the chip showed in vivo-like morphology formation of the proximal tubule and improved albumin uptake capacity. This chip was then used to test nephrotoxicity against cyclosporine A, an immunosuppressive drug. In a separate work, a thrombosis-on-a-chip model was fabricated using a similar technique, at a resolution of 250 μm.4 Pluronic F127 was used as the sacrificial material within a gelatin methacryloyl (GelMA) matrix encapsulating fibroblasts, while the generated channels after removal of Pluronic F127 were subsequently seeded with HUVECs (Fig. 1b). This chip was used to probe non-fibrotic and fibrotic human blood clots by perfusing tissue plasminogen activator (tPA), a known thrombolytic agent. In both models, the lower limit of the fabrication process made the chips impractical for smaller blood vessel systems, such as capillaries. Another approach to replicate the structural configuration of the vasculature systems is to employ core-shell bioprinting of bioinks that enable dual-stage crosslinking, as shown in Fig. 1c.5 The small distance between the two layers of the nozzles in core-shell extrusion has led to a better bioprinting resolution of smaller than 150 μm, further modulated by the volumes of the bioink and the crosslinker delivered through the inner/outer phases. In conclusion, extrusion-based bioprinting has shown great capacity in rapid fabrication of OoC platforms.
5. Light-Assisted Bioprinting
5.1. Principles of operation
While previously described methods use thermal, piezoelectric, shear, or acoustic forces to deposit and pattern bioinks in a controlled 3D pattern, light-assisted bioprinting uses a light source that is tuned to control the basic properties of the bioinks to either polymerize a photocurable bioink or facilitate the transfer of the bioink from a donor slide onto a substrate. For the purpose of this article we have selected three main technologies that use a light source: laser-induced forward transfer (LIFT), conventional stereolithography (SLA), and digital micromirror device (DMD). The process in each method along with the advantages, disadvantages, and resolution are explained here and summarized in Table 4.
Table 4.
Overview of current light-assisted bioprinters.
| Technology | Resolution | Viability | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| LIFT | >100 μm | 90–100% | Nozzle-free transfer of material allows for a wide range of bioinks; absorbing layer minimizes laser effects | Slow printing; lower mechanical strength of materials; difficulty printing multi-material constructs | 23 |
| SLA | >50 μm | 85–90% | Fast printing speed; high mechanical strength | Photoinitiator cytotoxicity; limited printable materials | 82 |
| DMD | <50 μm | 75–90% | Very fast printing speed | Photoinitiator cytotoxicity; limited printable materials; limited control on layer thickness | 83 |
| TPL | <5 μm | 70–90% | Very high resolution | Very slow printing speed, limited volume, limited printable materials | 84 |
LIFT has been a promising technique for fabricating 2D and 3D constructs such as the bone, skin, and nervous tissues.81 The basic setup for LIFT includes a laser that can produce pulsed beams, an absorbent slide where a layer of bioink is coated, and a collecting substrate on which the biological material is deposited (Fig. 4ai). The pulsed laser energy is deposited in a thin layer of an absorbing material on a transparent substrate. The absorption of the pulsed laser energy at the interface between the transparent donor and the absorbing layer yields vapor bubbles or mechanical waves that break the donor layer around the irradiated zone, thus transferring a bioink droplet into the collecting substrate. The morphology and size of the transferred droplet can be controlled by tuning the properties of the incident laser spot (e.g., laser wavelength and pulse energy) and the chemical properties of the bioink. The dynamics of the bubble expansion and jet formation depends first on the laser fluence (i.e., the optical energy delivered per unit area).
Figure 4.
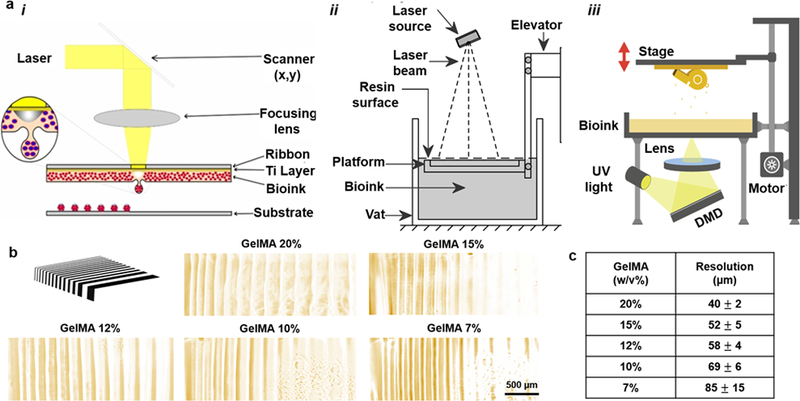
a) Common methods of light-assisted bioprinting: i) LIFT; schematic reprinted with permission from Catros et al. 201144. ii) SLA. iii) DMD. Both schematics in (ii) and (iii) reprinted from 3dPrintingIndustry website with permission. b) Pattern of photolithography and printed lines with GelMA hydrogels at different concentrations (by the authors); c) Summary of bioprinting resolution (from b) using the GelMA bioink.
There are other considerations for the LIFT process. The droplet dimension increases with the pulsed laser energy at a defined focusing conditions from a minimum value corresponding to a minimum energy below which no transfer takes place. Higher resolutions can be achieved by finding the energy threshold for the selected bioink. Furthermore, increase in the temperature and pressure within the confined volume, which pushes the droplet away from the absorbing layer, can lead to a shock wave during the LIFT process.85 For bioprinting applications, we should limit the heat diffusion into the bioink to avoid cell damage.
Although this method has been widely used to deliver and pattern biological materials23 with high cell viability and density86 as well as a bioprinting resolution of within 20–30 μm,82 it has a few disadvantages. Due to the complexity of the laser source and the control of the laser, this method continues to be costly. Also, it suffers from unwanted deposition of residue from the absorbent layer because of low flow rates that are required for the controlled deposition of the bioink. Laser fluencies need also to be carefully selected to limit the heat diffusion generated by the laser pulse into the absorbing layer, avoiding the transfer of melted debris on the receiving substrates.
Conventional SLA was in fact the first technology introduced for creating 3D constructs. This technology, invented by Charles Hull in 1980s,87 uses a light source to selectively polymerize a photocrosslinkable resin (Fig. 4aii). Although this approach is popular in manufacturing industries, it has only been recently adopted for tissue engineering applications for creating biodegradable scaffolds and tissue models.88 Most SLA printers are configured so that the light source polymerizes a bath filled with liquid resin onto a moving stage. Once one layer is polymerized, the stage descends a predefined distance (based on the vertical resolution of the SLA printer) for another layer to be polymerized, as shown in Fig. 4aii. Alternatively, another more recent approach uses a light source that is located below the bath of liquid resin, where it is capable of selectively polymerizing the photocurable resin onto a moving stage. The stage is then elevated by a distance equivalent to the thickness of the layer and another layer is selectively polymerized. Even though printing speeds are higher in SLA, this method suffers from a lower resolution and poor biocompatibility.16 Using commercially available laser beams of nearly 25 μm diameter, a pioneer SLA-based bioprinting work reported the smallest printed feature size in the order of 150 μm per layer, with an axial resolution down to 250 μm (perpendicular to the light pathway).44, 89, 90 The resolution has thereafter been improved to the order of 50 μm in hydrogels.91
While SLA uses a point source to polymerize a large area of photocurable resin, newer technologies such as continuous digital light processing (DLP) or DMD generate dynamic masks using computer-controlled array of micromirrors to polymerize a unit volume of the resin all at once thus significantly expediting the curing process (Fig. 4aiii).88 The DMD is composed of millions of micromirrors aligned in columns and rows. During the exposure time, the DMD mirrors can be selected independently to deflect the light or to project it, which results in a light pattern. Each layer is solidified based on the pattern generated by the DMD chip and the light is modulated and transferred through a reduction lens onto the liquid resin. After patterning each layer, the substrate is lowered by a translation stage into the bath of liquid resin to cure the next layer. Resolution for this method is comparable to cell sizes and falls in the micrometer region to as low as 5 μm by varying the laser power.91 This method is also able to maintain very high cell density (upwards of millions of cells per mL) compared to techniques that use nozzles where physical contacts and shear stresses can often cause cell lysis or functional loss.31 The DMD chip has the potential to generate multiple digital masks in the range of microsecond being a high-resolution tool for spatial light modulation. For example, DMD-based highly demagnified image projection has also been used in LIFT and for multiphoton polymerization.92
Compared to other 3D bioprinting technologies, light-assisted 3D bioprinters have the advantage of higher printing speed and better bioprinting resolution.13 There are, however, disadvantages involved with this technology especially when it is used in tissue engineering or bioprinting applications. Although reports of over 90% cell viability have been shown using UV-based SLA systems,93 the light-source and the photoinitiator used in most of these technologies can be damaging to cells.83, 94 Aside from the effect of the laser energy on cells, the hydrogel viscosity and stiffness have also been known to affect cell viability, attachment, differentiation, and proliferation.83
Due to the high degree of directionality in laser light and less light scattering perpendicular to laser light, the bioprinting resolution in SLA-and DMD-based bioprinting is predominantly determined by the thickness of the photocured resin. The thickness can be tuned by modifying laser characteristics such as pulse duration, wavelength and energy, repetition rate, and beam focus diameter, as well as the resin properties such as viscosity and surface tension.95 Although the polymerization kinetics of the curing process can be very complex, the following relationship is often used to describe the thickness of the photocured resin:96
| (11) |
, where Cd, DP, Ei, and Ec represent the curing depth (μm), penetration depth (μm), light irradiation dose (mJ/cm2), and critical energy for the liquid resin to reach gelation point (mJ/cm2), respectively. As Ei approaches Ec, the layer is cured and the resin is solidified.
When applying (Eq. 11) to hydrogel systems, the material parameters depend on the capacity of the hydrogel to absorb and scatter the emitted light. We have tested the concept of curing thickness to define bioprinting resolution for SLA-based bioprinting of GelMA mixed by 0.5% w/v lithium phenyl-2,4,6-trimethyl-benzoylphosphinate (LAP), as shown in Fig. 4b–c. A photomask made of parallel lines of different thicknesses, ranging from 20 μm to 200 μm, was made and then used to mask GelMA layers with a 0.5-mm thickness under focused UV light (with wavelength of 380 nm and intensity of 500 W/cm2 for 5 s).97 Selected examples of GelMA hydrogel patterns are shown in Fig. 4b and summarized in Fig. 4c. From high-to-low concentrations of GelMA, the resolution changed from nearly 40 μm to 85 μm. The presence of GelMA polymeric network helps absorption of emitted light and faster crosslinking, thus improving the resolution. The concentration of the polymer network in the hydrogel system indeed regulates DP and Ec.
5.2. Resolution in practice
When using light-assisted bioprinting, a photocurable bioink should be designed to possess proper mechanical, chemical, and biological properties before and after polymerization to assist with cell adhesion, survival, functionality, and differentiation.44, 90 There are many naturally derived bioinks such as agarose, alginate, collagen, GelMA, and hyaluronic acid that can be used to produce photocurable bioinks. Although these hydrogels tend to be inherently more biocompatible and promote cellular function, they are often mixed with synthetic bioinks, such as PEG, to modulate the mechanical, chemical, and biological properties of the substrate before and/or after the gelation process.98
The LIFT technique has been utilized to achieve high-resolution patterning of cellular compositions with almost any desired cell density and suspension viscosity.96 In a study by Castros et al., the authors demonstrated that by using an infrared laser focused on a quartz ribbon coated with titanium and a layer of hydrogel bioink, nano-hydroxyapatite and osteoprogenitor cells could be patterned into single-and multilayer constructs for bone tissue models.44 This study showed high cell viability over a time course of 2 weeks and proper bioactivity. In the study, they minimized the viscosity of the bioink by reducing hydroxyapatite concentration to prepare 20–30 μm layers of bioink over the ribbon and achieved a resolution of 100 μm. In another study, LIFT was used to print 60-μm layers of fibroblasts-and keratinocytes-laden collagen for skin tissue formation.99 Towards creating 3D tissue models, they printed 10 and 20 layers of the bioink with a resolution in the order of 150 μm. Wu and Ringeisen have fabricated tissue models loaded by human umbilical vein smooth muscle cells (HUVSMCs) and HUVECs using small droplets with diameters of approximately 50 μm.100 These works showed that the LIFT technique can be used for tissue biofabrication and it can be scalable to manufacturing macroscale constructs. Nevertheless, the difficulty of controlled droplet deposition in LIFT is considered as one of the major disadvantages of the technique and has limited its resolution to the order of 100 μm.
SLA bioprinting has been initially used for creating bone tissue models. In a study, the authors used a 100-μm-resolution SLA printer to solidify a biodegradable bioink made of diethyl fumarate, poly(propylene fumarate), and bisacylphosphine oxide (325-nm wavelength UV) towards healing critical-sized bone defects (around 4 mm in thickness and 50 mm in diameter).44 In another work, PEG-based nerve guidance conduits of millimeter-size were fabricated using SLA. This work achieved a resolution of >50 μm and nearly-rapid fabrication of large constructs.89 SLA has been recently employed to the rapid fabrication of microfluidic systems using transparent biocompatible polymers, with potential outlooks in OoC platforms.101 Since SLA eliminates the need of expensive molds and it has the advantage of rapid prototyping, SLA-based microfluidic systems may lead to an efficient commercialization of OoC platforms.
The printing speed of light-based printing technology can be greatly enhanced by maskless DMD-based bioprinting. Pre-defined woodpile and hexagonal structures of GelMA scaffolds (15% w/v) were bioprinted and then seeded uniformly by HUVECs.8 This work reached a resolution of approximately 30 μm and a layer thickness of 200–300 μm. This technique was then used to fabricate liver-on-a-chip as a hexagonal lobule structure, with a resolution of approximately nearly 50 μm, as shown in Fig. 1d.8 A manual exchange of two bioinks was used, including GelMA 5% w/v) as the parenchymal tissue and GelMA-glycidal hyaluronic acid (25% w/v GelMA and 1%w/v hyaluronic acid) as the vasculature. The bioinks were mixed with human-induced pluripotent stem cell-derived hepatic progenitor cells and HUVECs. A recent study by Zhu et al. demonstrated DMD bioprinting of prevascularized tissue models with complex geometries of varying widths (50 μm and down) and heights of 50 μm using endothelial cells that were encapsulated in a mixture of glycidal methacrylate-hyaluronic acid and GelMA.83 This study not only highlighted the versatility and the accuracy of the maskless method, but since all tissue models were bioprinted under 1 min, it also emphasized the high speed of bioprinting associated with DMD.13
5.3. Two-Photon Lithography
5.3.1. Principle of operation
TPL is one of the many new additions to the light-assisted bioprinting technologies that offers perhaps the finest resolution for the biofabrication of 3D scaffolds.102 As opposed to traditional light-assisted systems, this technique does not rely on the use of complex optical systems or photomasks to print in a photosensitive bioink. This method involves the use of laser light, mainly a near-infrared ultrafast femtosecond laser as compared to more conventional UV light sources used in SLA bioprinters. A multi-photon absorption process occurs in a medium that allows penetration of the emitted light and does not absorb light at the defined wavelength of the laser. The freedom that laser systems offer in terms of scanning and tightly modulating the energies at the point of interest allows for submicron features. The beam can be controlled to create an arbitrary 3D periodic or non-periodic pattern, leading to fine structures to be bioprinted rapidly with high precision. The resolution of bioprinting using TPL relates with the square of intensity of the incident laser light. The highest amount of absorption happens at the focal point of laser beam.
Following light incidence, a molecule is excited from its ground state Gs to an excited state Es separated with an energy difference of Ef (above the Gs) by absorbing two single photons (upward solid arrows), as shown in Fig. 5. The absorption of the two photons creates a virtual state Vs, in which the two photons can both have the same level of energy, E1 (degenerate, Ef = 2E1), or different energies, E1 and E2 (nondegenerate, Ef = E1 + E2). After excitation of the molecule, the system loosens into another state R, the lowest vibrational level of the lowest-energy Es by vibrational relaxation (dashed arrow in Fig. 5). The system then returns to the ground state through radiative or non-radiative pathways (bold dashed arrow in Fig. 5). The absorption rate of a photon is determined by Beer’s law:103
| (12) |
, where I(x) is the intensity of the light after passing through a medium of cross section x, is the concentration of the solution, I0 is the intensity of the light before entering the medium, and α is the absorption coefficient that becomes β in a two-photon absorption process where the absorption rate is given by:
| (13) |
Figure 5.
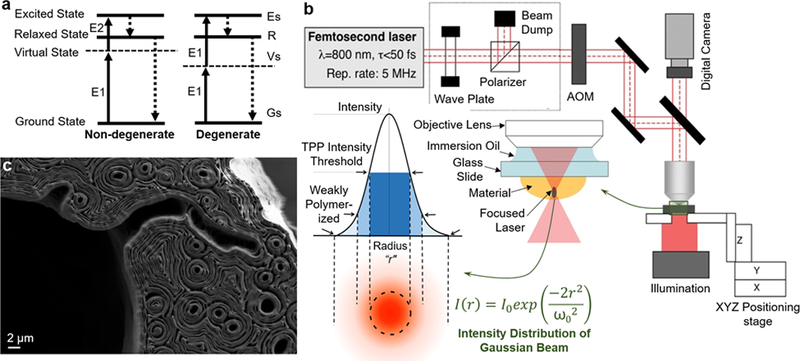
a) The principle of two-photon absorption. b) Schematic illustration of the experimental setup for two-photon bioprinting along with a zoomed description of focal plane and distribution of light intensity in the laser focus of a Gaussian beam is shown. Reprinted with permission from American Institute of Physics. c) The cross section of TPL-bioprinted mouse paw bone imaged using scanning electron microscopy and intricate contours within the structure arose from the bioprinting process. Image provided by Elise Morgan and Alice White, Department of Mechanical Engineering, Boston University, Boston, USA.
This relation indicates how optical properties of the medium (through and β) can affect the intensity of laser light required to induce photocrosslinking in the medium.
The resolution of TPL can be largely defined based on the imaging resolution of the two-photon system. In TPL the width of light absorption, which denotes the resolution, differs between the direction of penetration (axial resolution, rz) and the perpendicular plane (lateral resolution, rxy):
| (14) |
, in which λ denotes light wavelength, represents the refractive index of the bioink, and NA is the numerical aperture of the objective. Equation (14) shows that the lateral resolution of TPL only depends on the system characteristics while the optical properties of the bioink regulate the axial resolution.
Another major factor in TPL is the length of laser penetration that limits the overall size of the constructs, a main drawback of such systems. In two-photon setup, the laser can penetrate a longer distance (normally two or three times greater) than that in confocal setup, due to the reduced scattering of photons at longer wavelengths.104 This distance represents the size limit of bioprinted constructs and also depends on the optical properties of bioinks. Higher refractive index hampers the axial resolution of TPL (see Eq. (14)) but allows bioprinting of a larger construct.
5.3.2. Resolution in practice
Resolution is a key parameter representing the feature size of the nanostructures fabricated by TPL. The challenges for two-photon polymerization was reported by Kawata et al. 2001.105 They fabricated micro-bulls from resin with dimensions approximately the size of a red blood cell (10-μm long and 7-μm high). Since then, a variety of methods have been published to improve the processing accuracy, which includes the modification of the spatial resolution by changing the process parameters and by optimizing the photoinitiator properties. It is well known that the spatial resolution of TPL is related with the pulse energy, which can be enhanced to improve the resolution by using highly sensitive photoinitiators.106, 107 As shown in Fig. 5b, based on the energy distribution at focus of a laser Gaussian beam, the polymerization regions can be divided into three different parts: i) an unpolymerized section, ii) a weakly polymerized section with low degree of crosslinking (low molecular weight), and iii) a completely polymerized section with high molecular weight and degree of crosslinking. In general, unpolymerized parts can be eliminated by proper rinsing materials in the developing process.
Xing et al. investigated ways to improve the lateral spatial resolution of TPL to 80 nm, by using an anthracene derivative (9,10-bis-pentyloxy-2,7-bis [2-(4-dimethylamino-phenyl)-vinyl] anthracene (BPDPA) as a highly sensitive and efficient photoinitiator, which contributes to a sharper crosslinking and short exposure time.103 Low-intensity threshold results in more precise features according to the mechanism of material the photoinitiation. The density of radicals, , produced by femtosecond laser pulses can be calculated by solving a simple rate Eq. (15)104 and if we assume the initial density of radicals equals to zero, the solution can be stated as Eq. (16):
| (15) |
| (16) |
, where
| (17) |
, where σ2 is the effective two-photon cross-section for the generation of radicals, σ2a the ordinary two-photon absorption cross-section, η the efficiency of the initiation process, the primary initiator particle density, and I the photon flux intensity. Presume that the laser beam is a Gaussian beam and the distribution of photon flux intensity I(r, z) at distances of r along the cross-section and of z in the propagation direction from the center can be expressed as follows:105
| (18) |
, where I0 is the photon flux intensity at the beam center (r = 0, z = 0), ω0 is the beam waist, and ω(z) is the beam radius in the plane with distance of z. The average photon flux intensity at the focus plane Ifocus is supposed as follows:108
| (19) |
, where W, ς, f, h, and υ represent the average power, pulse width, repetition frequency, Planck constant, and frequency of light, respectively. The photon-polymerization is originated where the density of radicals (r, z) exceeds the threshold th, that is (r, z) ≥ th. The photon flux intensity at the focal plane (z = 0) reaches the maximum, where
| (20) |
Even though TPL has been developed for almost two decades, it has only been adopted for bioprinting applications relatively recently.109 In 2002, Watanabe et al. introduced TPL and generated 3D hydrogels using acrylamide, acryloylacetone, and N,N-methylene bisacrylamide.110 Since then TPL has been used for generating 3D hydrogels.104 Applegate et al. prepared 3D multiscale patterns in protein hydrogels made of soft silk with low-energy femtosecond lasers without the use of exogenous or chemical crosslinkers.103 This work showed a lateral resolution of 5 μm and an axial resolution of nearly 50 μm in millimeter-sized hydrogel constructs, as well as a penetration distance of 1 cm. Since then several improvements, such as multi-material bioprinting and patterned geometries, have been made in the direction of manufacturing of tissue models via TPL. For example, Wylie et al. spatially immobilized multiple growth factors within agarose hydrogels with a 40-μm resolution and a penetration of approximately 500 μm.109, 111 High-resolution (at a lateral resolution of approximately 1 μm and an axial resolution of approximately 10 μm) patterns of homing ligands were fabricated by selectively photobleaching fluorescein inside a bulk collagen gel (having a size of 100 × 100 × 2 μm3) to study cell behavior under biomimetic conditions.110 Another group applied TPL along with novel photoinitiator formulations to fabricate cell-loaded GelMA constructs containing osteosarcoma cells.84 While reaching a feature size of ~1 μm, they showed that the cell damage was attributed to a chemical origin rather than laser radiations, and minimized the cell damage by changing photoinitiator. Indirect bioprinting based on TPL has been applied to generate hollow models. In a recent work, multiscale channels were created within PEG tetrabi-cyclononyne hydrogels by photodegrading the bioink at the target pattern.112 They showed a lateral resolution of approximately 1 μm and a penetration depth of approximately 500 μm. TPL has shown great promises as a 3D bioprinting technology since it allows for the creation of micro-tissue models with submicron resolution (e.g., 3D-bioprinted osteon model in Fig. 5c), which could otherwise not be conveniently achieved with other bioprinting strategies. However, its use in the generation of OoC platforms remain to be explored.
6. Concluding remarks
In the past few years, the 3D bioprinting techniques have shown their potential in fabricating biomimetic micro-tissues. The ability to pattern cells and ECM materials, along with the capacity of scaling down, helps the fabrication of micro-tissue models with improved physiological and pathophysiological relevance for drug screening and personalized medicine. While bioprinting for transplantation and regenerative medicine have faced clinical obstacles, micro-tissue models seem a favorable technological area where regulatory requirements are not as severe. However, the main challenges for bioprinting micro-tissue models for OoC applications may include the integration of micro-tissues with surrounding encasements and microfluidic connections, maintaining the biological viability of cellular components, and sustaining long-term phenotypic stability.
The architectural resemblance of the OoC platforms to their native counterparts may be improved by development of tissue-specific bioinks and enhancement of the state-of-the-art bioprinting resolution. The ideal resolution depends on the desired OoC pattern but it is commonly limited to the geometry of encapsulated cells when they are aligned within the construct (>10 μm). High-fidelity bioprinting will lead to generation of fine features in the micro-tissue models mimicking those of their native counterparts. As a summary of the discussion on bioprinting resolution, the governing factors for each bioprinting category are presented in Table 5. These items may provide a guide map for the selection of varying parameters in different bioprinting categories. For design purposes, the effects of bioprinting governing factors on time cost are also shown in Table 5. Time consideration can be critical when the higher-throughput processes are required or the bioink includes time-dependent biological components.
Table 5.
Major factors (when elevated in values) in controlling bioprinting resolution for a given printhead (their effects on print time are shown through symbols ↓ denotes reduction, ↔ no change, and ↑ increase in time).
| Item | Inkjet | Extrusion | Light-assisted |
|---|---|---|---|
| 1 | Surface contact angle (↔) | Nozzle moving speed (↓) | Light alignment/wavelength (↓) |
| 2 | Bioink viscosity (↑) | Bioink viscosity behavior (↑) | Photocrosslinking density (↓) |
| 3 | Pressure or heat field (↔) | Pressure or piston speed (↓) | Optical opaqueness (↑) |
| 4 | Nozzle moving speed (↓) | Surface contact angle (↔) | Bioink viscosity (↑) |
Our numerical simulations in Section 2.3 showed that surface contact angle is the key parameter in regulating inkjet bioprinting resolution. The contact angle is a result of molecular interactions among the bioink, surrounding air, and solid surface. The numerical simulations in Section 3.2 showed that the nozzle moving speed and bioink viscosity play counterpart roles in controlling the extrusion bioprinting resolution, while the nozzle diameter regulates the lower limit. For a given printhead diameter, by selecting proper bioink physical properties, the resolution can be improved in extrusion and light-assisted bioprinting techniques, reaching ideal bioprinting resolutions down to 10–50 μm. The physical properties of bioinks represent their molecular weight, water percentage, material composition, and cell size and density. For example, high cell densities result in higher levels of bioink viscosity. In addition to physical properties of the bioinks, light-assisted 3D bioprinting strategies require superior optical characteristics of the bioinks as well as suitable incident light characteristics. Among the different techniques, SLA has shown micrometer-sized bioprinting resolutions, making them ideal for the fabrication of microfluidic parts in OoC platforms. TPL techniques could provide a fabrication tool for micro-particle systems in such platforms.
To conclude, the rapid growth and disruptive potential of 3D bioprinting demand further research that addresses the principles of 3D bioprinting and likewise enables engineers to realize its full capabilities. We can use these capabilities to develop novel platforms that recapitulate the unique microenvironment of biological tissues as organoids and OoC platforms to further investigate the cause and development of abnormal conditions as well as the structural and functional changes resulting from pathological manifestations. The future challenges for biofabrication may involve micrometer sized resolution relevant for organoids and disease models, and the design of bioinks to be easily prepared for rapid prototyping. The systematic evaluation of governing parameters may allow optimization of bioinks and bioprinting process. An optimized selection of biophysical properties of bioinks and bioprinter setup could improve the resolution of the construct while preserving cell viability and minimizing operative costs. For example, optimized nozzle shape and bioink viscosity, along with proper numerical simulations of bioink behavior within printing process, can possibly provide improvements in resolution without exceeding mechanical stresses that might hamper cell viability. Regarding bioprinter setups, we may advance the interface software that control bioprinter platforms and the technical features of bioprinter platforms, which can improve the bioprinting processes or may minimize platform inaccuracies (e.g., in terms of positioning and vibrations). In conclusion, a combination of materials science, cell biology, and physics is necessary to take on this challenge to move bioprinting from the realm of research laboratories into real-world biomedical applications.
Supplementary Material
Table 2.
An overview of common bioprinting categories. Adapted from Murphy & Atala 201416 and Ji & Guvendiren 2017.17
| Parameter | Inkjet | Extrusion | Light-assisted |
|---|---|---|---|
| Speed | Medium | Low | High |
| Cost | Low | Low | Medium |
| Resolution | 100–500 μm | 100–500 μm | 20–100 μm* |
| Flexibility | Low | High | High |
| Commercialization | Medium | High | Low |
For TPL this resolution will be from 0.1 to 10 μm.
Acknowledgements
The authors also acknowledge funding from the National Institutes of Health (EB021857, AR066193, AR057837, CA214411, HL137193, EB024403, EB023052, HL140618, AR069564). A.K.M. acknowledges the fellowship support from Canadian Institutes of Health Research (CIHR). S.H. acknowledges support from the Swiss National Science Foundation (SNSF). Y.S.Z. acknowledges funding from the National Cancer Institute of the National Institutes of Health (K99CA201603, R00CA201603, R21EB025270, R21EB026175) and the New England Anti-Vivisection Society (NEAVS). Finally, the authors thank the anonymous reviewers for their constructive comments and suggestions to improve the manuscript.
References Uncategorized References
- 1.Huh D, Hamilton GA and Ingber DE, Trends Cell Biol., 2011, 21, 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsusaki M, Sakaue K, Kadowaki K and Akashi M, Advanced healthcare materials, 2013, 2, 534–539. [DOI] [PubMed] [Google Scholar]
- 3.Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A and Lewis JA, Scientific reports, 2016, 6, 34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang YS, Davoudi F, Walch P, Manbachi A, Luo X, Dell’Erba V, Miri AK, Albadawi H, Arneri A, Li X, Wang X, Dokmeci MR, Khademhosseini A and Oklu R, Lab on a Chip, 2016, 16, 4097–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YS, Arneri A, Bersini S, Shin S-R, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A and Khademhosseini A, Biomaterials, 2016, 110, 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson BN, Lancaster KZ, Hogue IB, Meng F, Kong YL, Enquist LW and McAlpine MC, Lab on a Chip, 2016, 16, 1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prendergast ME, Montoya G, Pereira T, Lewicki J, Solorzano R and Atala A, Microphysiological Systems, 2018, 2. [Google Scholar]
- 8.Ma X, Qu X, Zhu W, Li Y-S, Yuan S, Zhang H, Liu J, Wang P, Lai CSE and Zanella F, Proceedings of the National Academy of Sciences, 2016, 113, 2206–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garreta E, Oria R, Tarantino C, Pla-Roca M, Prado P, Fernández-Avilés F, Campistol JM, Samitier J and Montserrat N, Materials Today, 2017, 20, 166–178. [Google Scholar]
- 10.Yeong W-Y, Chua C-K, Leong K-F and Chandrasekaran M, Trends in biotechnology, 2004, 22, 643–652. [DOI] [PubMed] [Google Scholar]
- 11.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ and Atala A, Nat Biotech, 2016, 34, 312–319. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharjee N, Urrios A, Kang S and Folch A, Lab on a Chip, 2016, 16, 1720–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miri AK, Nieto D, Iglesias L, Goodarzi Hosseinabadi H, Maharjan S, Ruiz‐Esparza GU, Khoshakhlagh P, Manbachi A, Dokmeci MR, Chen S, Zhang Yu S and Khademhosseini A, Advanced Materials, 2018, 1800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Shi Y, Lao Z, Ni J, Li G, Hu Y, Li J, Chu J, Wu D and Sugioka K, Lab on a Chip, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Skylar-Scott MA, Liu M-C, Wu Y, Dixit A and Yanik MF, Advanced Healthcare Materials, 2016, 5, 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy SV and Atala A, Nature biotechnology, 2014, 32, 773–785. [DOI] [PubMed] [Google Scholar]
- 17.Ji S and Guvendiren M, Frontiers in bioengineering and biotechnology, 2017, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle AD, Carvajal N, Jin A, Matsumoto K and Yamada KM, Nature Communications, 2015, 6, 8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee M, Wu BM and Dunn JC, Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 2008, 87, 1010–1016. [DOI] [PubMed] [Google Scholar]
- 20.Aydin H, El Haj A, Pişkin E and Yang Y, Journal of tissue engineering and regenerative medicine, 2009, 3, 470–476. [DOI] [PubMed] [Google Scholar]
- 21.Hutmacher DW, in The Biomaterials: Silver Jubilee Compendium, ed. Williams DF, Elsevier Science, Oxford, 2000, DOI: 10.1016/B978-008045154-1.50021-6, pp. 175–189. [DOI] [Google Scholar]
- 22.Yousuf Y, Amini-Nik S and Jeschke MG, in Skin Tissue Models, Elsevier, 2018, pp. 39–54. [Google Scholar]
- 23.Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V, Zychlinski D, Schambach A, Reimers K, Vogt Peter M and Chichkov B, Biotechnology and Bioengineering, 2012, 109, 1855–1863. [DOI] [PubMed] [Google Scholar]
- 24.Cittadella Vigodarzere G and Mantero S, Frontiers in Physiology, 2014, 5, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y-C, Dennis RG, Larkin L and Baar K, Journal of Applied Physiology, 2005, 98, 706–713. [DOI] [PubMed] [Google Scholar]
- 26.Yan W, George S, Fotadar U, Tyhovych N, Kamer A, Yost MJ, Price RL, Haggart CR, Holmes JW and Terracio L, Tissue engineering, 2007, 13, 2781–2790. [DOI] [PubMed] [Google Scholar]
- 27.Laternser S, Keller H, Leupin O, Rausch M, Graf-Hausner U and Rimann M, SLAS TECHNOLOGY: Translating Life Sciences Innovation, 2018, 23, 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghista DN and Ng EY-K, Cardiac perfusion and pumping engineering, World Scientific Hackensack, 2007. [Google Scholar]
- 29.Fast VG, Darrow BJ and Saffitz JE, Circulation research, 1996, 79, 115–127. [DOI] [PubMed] [Google Scholar]
- 30.Bien H, Yin L and Entcheva E, IEEE engineering in medicine and biology magazine, 2003, 22, 108–112. [DOI] [PubMed] [Google Scholar]
- 31.Miri AK, Khalilpour A, Cecen B, Maharjan S, Shin S-R and Khademhosseini A, Biomaterials, 2018, DOI: 10.1016/j.biomaterials.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hautekeete ML and Geerts A, Virchows Archiv, 1997, 430, 195–207. [DOI] [PubMed] [Google Scholar]
- 33.Wessely O, Cerqueira DM, Tran U, Kumar V, Hassey JM and Romaker D, Pediatric nephrology (Berlin, Germany), 2014, 29, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashammakhi N, Wesseling-Perry K, Hasan A, Elkhammas E and Zhang YS, Kidney International, 2018, 94, 1073–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nitta K, Recent Advances in the Pathogenesis and Treatment of Kidney Diseases, Karger Medical and Scientific Publishers, 2018. [Google Scholar]
- 36.Cao Y, Biomedecine & Pharmacotherapy, 2005, 59, S340–S343. [DOI] [PubMed] [Google Scholar]
- 37.Fukumura D and Jain RK, J. Cell. Biochem, 2007, 101, 937–949. [DOI] [PubMed] [Google Scholar]
- 38.Carmeliet P and Jain RK, Nature, 2011, 473, 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weis SM and Cheresh DA, Nature medicine, 2011, 17, 1359–1370. [DOI] [PubMed] [Google Scholar]
- 40.Asghar W, El Assal R, Shafiee H, Pitteri S, Paulmurugan R and Demirci U, Materials Today, 2015, 18, 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatia SN and Ingber DE, Nat. Biotechnol, 2014, 32, 760–772. [DOI] [PubMed] [Google Scholar]
- 42.http://virtualslides.med.umich.edu/).
- 43.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ and Atala A, Nature biotechnology, 2016, 34, 312. [DOI] [PubMed] [Google Scholar]
- 44.Catros S, Fricain J-C, Guillotin B, Pippenger B, Bareille R, Remy M, Lebraud E, Desbat B, Amédée J and Guillemot F, Biofabrication, 2011, 3, 025001. [DOI] [PubMed] [Google Scholar]
- 45.Cui X, Breitenkamp K, Finn M, Lotz M and D’Lima DD, Tissue Engineering Part A, 2012, 18, 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A and Campbell P, Stem cells, 2008, 26, 127–134. [DOI] [PubMed] [Google Scholar]
- 47.Isaacson A, Swioklo S and Connon CJ, Experimental Eye Research, 2018, 173, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madden LR, Nguyen TV, Garcia-Mojica S, Shah V, Le AV, Peier A, Visconti R, Parker EM, Presnell SC, Nguyen DG and Retting KN, iScience, 2018, 2, 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derby B, Journal of Materials Chemistry, 2008, 18, 5717–5721. [Google Scholar]
- 50.Zhang Y, Tse C, Rouholamin D and Smith P, Open Engineering, 2012, 2, 325–335. [Google Scholar]
- 51.Le HP, Journal of Imaging Science and Technology, 1998, 42, 49–62. [Google Scholar]
- 52.Guo Y, Guo Y, Patanwala HS, Patanwala HS, Bognet B, Bognet B, Ma AW and Ma AW, Rapid Prototyping Journal, 2017, 23, 562–576. [Google Scholar]
- 53.Hasenbank MS, Edwards T, Fu E, Garzon R, Kosar TF, Look M, Mashadi-Hossein A and Yager P, Analytica chimica acta, 2008, 611, 80–88. [DOI] [PubMed] [Google Scholar]
- 54.Saunders RE and Derby B, International Materials Reviews, 2014, 59, 430–448. [Google Scholar]
- 55.Fromm J, IBM Journal of Research and Development, 1984, 28, 322–333. [Google Scholar]
- 56.Foresti D, Kroll KT, Amissah R, Sillani F, Homan KA, Poulikakos D and Lewis JA, Science Advances, 2018, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L and Ovsianikov A, Biofabrication, 2016, 8, 032002. [DOI] [PubMed] [Google Scholar]
- 58.Pasandideh‐Fard M, Qiao Y, Chandra S and Mostaghimi J, Physics of fluids, 1996, 8, 650–659. [Google Scholar]
- 59.Chorin AJ, Mathematics of computation, 1968, 22, 745–762. [Google Scholar]
- 60.Mirzaee I, Charmchi M and Sun H, Numerical Heat Transfer, Part A: Applications, 2016, 70, 460–491. [Google Scholar]
- 61.Landau L and Lifshitz E, Journal, 1987. [Google Scholar]
- 62.Mirzaii I and Passandideh-Fard M, International Journal of Multiphase Flow, 2012, 39, 216–226. [Google Scholar]
- 63.Zhang X and Zhang Y, Cell biochemistry and biophysics, 2015, 72, 777–782. [DOI] [PubMed] [Google Scholar]
- 64.Moon S, Hasan SK, Song YS, Xu F, Keles HO, Manzur F, Mikkilineni S, Hong JW, Nagatomi J and Haeggstrom E, Tissue Engineering Part C: Methods, 2009, 16, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura M, Iwanaga S, Henmi C, Arai K and Nishiyama Y, Biofabrication, 2010, 2, 014110. [DOI] [PubMed] [Google Scholar]
- 66.Chameettachal S and Pati F, 3D Bioprinting in Regenerative Engineering:: Principles and Applications, 2018. [Google Scholar]
- 67.Christensen K, Xu C, Chai W, Zhang Z, Fu J and Huang Y, Biotechnology and Bioengineering, 2015, 112, 1047–1055. [DOI] [PubMed] [Google Scholar]
- 68.Sakai S, Ueda K, Gantumur E, Taya M and Nakamura M, Macromolecular Rapid Communications, 2017, 39, 1700534. [DOI] [PubMed] [Google Scholar]
- 69.Ozbolat IT and Hospodiuk M, Biomaterials, 2016, 76, 321–343. [DOI] [PubMed] [Google Scholar]
- 70.Chua CK and Yeong WY, Bioprinting: principles and applications, World Scientific Publishing Co Inc, 2014. [Google Scholar]
- 71.Jungst T, Smolan W, Schacht K, Scheibel T and Groll J. r., Chemical reviews, 2015, 116, 1496–1539. [DOI] [PubMed] [Google Scholar]
- 72.Cheng J, Lin F, Liu H, Yan Y, Wang X, Zhang R and Xiong Z, Journal of Manufacturing Science and Engineering, 2008, 130, 021014. [Google Scholar]
- 73.Bruneaux J, Therriault D and Heuzey M-C, Journal of Micromechanics and Microengineering, 2008, 18, 115020. [Google Scholar]
- 74.Suntornnond R, Tan EYS, An J and Chua CK, Materials, 2016, 9, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamranpour NO, Miri AK, James-Bhasin M and Nazhat SN, Biofabrication, 2016, 8, 015018. [DOI] [PubMed] [Google Scholar]
- 76.Liu S, Guo D and Xie G, Journal of Applied Physics, 2012, 112, 104309. [Google Scholar]
- 77.Malda J, Visser J, Melchels Ferry P, Jüngst T, Hennink Wim E, Dhert Wouter JA, Groll J and Hutmacher Dietmar W, Advanced Materials, 2013, 25, 5011–5028. [DOI] [PubMed] [Google Scholar]
- 78.Skardal A, Shupe T and Atala A, Drug Discovery Today, 2016, 21, 1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng W, Unutmaz D and Ozbolat IT, Trends in biotechnology, 2016, 34, 722–732. [DOI] [PubMed] [Google Scholar]
- 80.Murphy SV and Atala A, Regenerative Medicine Technology: On-a-Chip Applications for Disease Modeling, Drug Discovery and Personalized Medicine, CRC Press, 2016. [Google Scholar]
- 81.Lee H and Cho D-W, Lab on a Chip, 2016, 16, 2618–2625. [DOI] [PubMed] [Google Scholar]
- 82.Arcaute K, Mann BK and Wicker RB, Tissue Engineering Part C: Methods, 2010, 17, 27–38. [DOI] [PubMed] [Google Scholar]
- 83.Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, Wang P, Lai CSE, Gou M and Xu Y, Biomaterials, 2017, 124, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ovsianikov A, Mühleder S, Torgersen J, Li Z, Qin X-H, Van Vlierberghe S, Dubruel P, Holnthoner W, Redl H, Liska R and Stampfl J, Langmuir, 2014, 30, 3787–3794. [DOI] [PubMed] [Google Scholar]
- 85.Karaiskou A, Zergioti I, Fotakis C, Kapsetaki M and Kafetzopoulos D, Applied Surface Science, 2003, 208–209, 245–249. [Google Scholar]
- 86.Hopp B, Smausz T, Kresz N, Barna N, Bor Z, Kolozsvári L, Chrisey DB, Szabó A and Nógrádi A, Tissue engineering, 2005, 11, 1817–1823. [DOI] [PubMed] [Google Scholar]
- 87.Hull CW, Journal, 1986. [Google Scholar]
- 88.Skoog SA, Goering PL and Narayan RJ, Journal of Materials Science: Materials in Medicine, 2014, 25, 845–856. [DOI] [PubMed] [Google Scholar]
- 89.Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V, Zychlinski D, Schambach A, Reimers K and Vogt PM, Biotechnology and bioengineering, 2012, 109, 1855–1863. [DOI] [PubMed] [Google Scholar]
- 90.Gruene M, Pflaum M, Hess C, Diamantouros S, Schlie S, Deiwick A, Koch L, Wilhelmi M, Jockenhoevel S and Haverich A, Tissue Engineering Part C: Methods, 2011, 17, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Odde DJ and Renn MJ, Trends in biotechnology, 1999, 17, 385–389. [DOI] [PubMed] [Google Scholar]
- 92.Zhang R and Larsen NB, Lab on a chip, 2017, 17, 4273–4282. [DOI] [PubMed] [Google Scholar]
- 93.Grogan SP, Chung PH, Soman P, Chen P, Lotz MK, Chen S and D’Lima DD, Acta biomaterialia, 2013, 9, 7218–7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hribar KC, Soman P, Warner J, Chung P and Chen S, Lab on a chip, 2014, 14, 268–275. [DOI] [PubMed] [Google Scholar]
- 95.Yih J-N, Hu YY, Da Sie Y, Cheng L-C, Lien C-H and Chen S-J, Optics letters, 2014, 39, 3134–3137. [DOI] [PubMed] [Google Scholar]
- 96.Jacobs PF, Rapid prototyping & manufacturing: fundamentals of stereolithography, Society of Manufacturing Engineers, 1992. [Google Scholar]
- 97.Miri AK, Hosseinabadi HG, Cecen B, Hassan S and Zhang YS, Acta Biomaterialia, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melchels FP, Feijen J and Grijpma DW, Biomaterials, 2009, 30, 3801–3809. [DOI] [PubMed] [Google Scholar]
- 99.Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V, Zychlinski D, Schambach A, Reimers K, Vogt PM and Chichkov B, Biotechnology and Bioengineering, 2012, 109, 1855–1863. [DOI] [PubMed] [Google Scholar]
- 100.Wu P and Ringeisen B, Biofabrication, 2010, 2, 014111. [DOI] [PubMed] [Google Scholar]
- 101.Au AK, Lee W and Folch A, Lab on a Chip, 2014, 14, 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farsari M and Chichkov BN, Nature photonics, 2009, 3, 450. [Google Scholar]
- 103.Swinehart D, J. Chem. Educ, 1962, 39, 333. [Google Scholar]
- 104.Zipfel WR, Williams RM and Webb WW, Nature biotechnology, 2003, 21, 1369–1377. [DOI] [PubMed] [Google Scholar]
- 105.Kawata S, Sun H-B, Tanaka T and Takada K, Nature, 2001, 412, 697. [DOI] [PubMed] [Google Scholar]
- 106.Deubel M, von Freymann G, Wegener M, Pereira S, Busch K and Soukoulis CM, Nat Mater, 2004, 3, 444–447. [DOI] [PubMed] [Google Scholar]
- 107.Farsari M and Chichkov BN, Nat Photon, 2009, 3, 450–452. [Google Scholar]
- 108.Cao YY, Takeyasu N, Tanaka T, Duan XM and Kawata S, Small, 2009, 5, 1144–1148. [DOI] [PubMed] [Google Scholar]
- 109.Xing J, Liu J, Zhang T, Zhang L, Zheng M and Duan X, Journal of Materials Chemistry B, 2014, 2, 4318–4323. [DOI] [PubMed] [Google Scholar]
- 110.Watanabe T, Akiyama M, Totani K, Kuebler SM, Stellacci F, Wenseleers W, Braun K, Marder SR and Perry JW, Advanced Functional Materials, 2002, 12, 611–614. [Google Scholar]
- 111.Applegate MB, Coburn J, Partlow BP, Moreau JE, Mondia JP, Marelli B, Kaplan DL and Omenetto FG, Proceedings of the National Academy of Sciences, 2015, 112, 12052–12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Torgersen J, Ovsianikov A, Mironov V, Pucher N, Qin X, Li Z, Cicha K, Machacek T, Liska R, Jantsch V and Stampfl J, BIOMEDO, 2012, 17, 105008–105008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


