Abstract
BACKGROUND:
The mechanisms underlying spontaneous atrial fibrillation (AF) associated with atrial ischemia/infarction are incompletely elucidated. Here, we investigate the mechanisms underlying spontaneous AF in an ovine model of left atrial myocardial infarction (LAMI).
METHODS AND RESULTS:
LAMI was created by ligating the atrial branch of the left anterior descending coronary artery. ECG loop recorders were implanted to monitor AF episodes. In 7 sheep, dantrolene—a ryanodine receptor blocker—was administered in vivo during the 8-day observation period (LAMI-D, 2.5 mg/kg, IV, BID). LAMI animals experienced numerous spontaneous AF episodes during the 8-day monitoring period that were suppressed by dantrolene (LAMI, 26.1±5.1; sham, 4.3±1.1;
LAMI-D, 2.8±0.8; mean±SEM episodes per sheep, P<0.01). Optical mapping showed spontaneous focal discharges (SFDs) originating from the ischemic/normal-zone border. SFDs were calcium driven, rate dependent, and enhanced by isoproterenol (0.03 μmol/L, from 210±87 to 3816±1450, SFDs per sheep) but suppressed by dantrolene (to 55.8±32.8, SFDs per sheep, mean±SEM). SFDs initiated AF-maintaining reentrant rotors anchored by marked conduction delays at the ischemic/ normal-zone border. NOS1 (NO synthase-1) protein expression decreased in ischemic zone myocytes, whereas NADPH (nicotinamide adenine dinucleotide phosphate, reduced form) oxidase and xanthine oxidase enzyme activities and reactive oxygen species (DCF [6-carboxy-2’,7’- dichlorodihydrofluorescein diacetate]-fiuorescence) increased. CaM (calmodulin) aberrantly increased [3H]ryanodine binding to cardiac RyR2 (ryanodine receptors) in the ischemic zone. Dantrolene restored the physiological binding of CaM to RyR2.
CONCLUSIONS:
Atrial ischemia causes spontaneous AF episodes in sheep, caused by SFDs that initiate reentry. Nitroso-redox imbalance in the ischemic zone is associated with intense reactive oxygen species production and altered RyR2 responses to CaM. Dantrolene administration normalizes the CaM response, prevents LAMI-related SFDs, and AF initiation. These findings provide novel insights into the mechanisms underlying ischemia-related atrial arrhythmias.
Keywords: arrhythmias, cardiac, calcium, dantrolene, heart atria, infarction
Graphical Abstract

Atrial fibrillation (AF) is the most common arrhythmia in adults, affecting >4 million Americans.1,2 AF is associated with a range of potential adverse consequences like decreased quality of life, decompensated cardiovascular status, and stroke.3,4 AF may be caused by cardiac conditions, such as heart failure and coronary artery disease (CAD),1,5–7 or may occur de novo (lone AF). Clinical and experimental studies show that both atrial and ventricular CAD-related insults lead to AF onset and perpetuation.7–17 It has been suggested that the pathophysiological role of atrial ischemia/infarction in AF onset is greatly underestimated.18,19 For instance, AF is a well-known complication of acute myocardial infarction,11 and CAD is a significant risk factor for AF.20 Although AF after ventricular myocardial infarction might also be triggered by an increase in intra-atrial pressure in the context of acute ventricular dysfunction,21,22 various studies have shown that isolated atrial infarction is common, representing ≈20% of autopsy-proven atrial infarctions.23,24 In patients with both paroxysmal and persistent AF but no proven atrial infarction, isolated atrial myocardial perfusion abnormalities, coronary flow reserve impairment, and microvascular dysfunction can also occur.14–16 A recent report indicated that selective atrial coronary artery occlusion during elective percutaneous transluminal coronary angioplasty is associated with myocardial ischemic damage, atrial arrhythmias, and intra-atrial conduction delay.25
At the mechanistic level, Nishida et al26 demonstrated a greater occurrence of spontaneous sarcoplasmic reticulum (SR) Ca2+-release events (SCEs) in ischemic zone (IZ) cardiomyocytes and AF initiation in an 8-day canine regional right atrial myocardial infarction model. In that study, however, the molecular basis of SCEs was not established. In particular, the manner in which cardiac RyR2 (ryanodine receptor) function may be altered by atrial ischemia was not examined, and its relationship to spontaneous AF in vivo and to ectopic firing was not directly determined. Novel insights into such mechanisms are needed to develop new, mechanism-based management approaches. Here, we studied chronic left atrial (LA) myocardial infarction (LAMI)-related AF in an ovine model in which regional impairment in atrial coronary perfusion causes spontaneous AF in vivo. As suggested previously in other settings,27–29 we hypothesized that atrial infarction predisposes cardiomyocytes to SCEs and local reentry, leading to spontaneous focal discharges that trigger AF. In addition, we tested whether dantrolene, which has been shown to stabilize RyR2,30–33 may prevent this cascade of events.
METHODS
Data, Materials, and Code Disclosure
The authors declare that all supporting data are available within the article (and its Data Supplement). Any further data, if available, that support the findings of this study are available from the corresponding author on reasonable request.
Ovine LAMI Model
All sheep were castrated male sheep in the range of 35 to 40 kg body weight and 3 to 4 months of age. The protocol that was used conforms to the Guide for Care and Use of Laboratory Animals published by the United States National Institutes of Health, publication no. 85–23, revised 1996. We implemented an innovative chronic ovine LAMI model generated by surgical ligation of small coronary artery branches that irrigate specific regions of the posterior left atrium. After left thoracotomy at the fifth intercostal space, the left anterior atrial coronary branch was identified and ligated with a 4–0 silk suture (N=30). Depending on the anatomy of the specimen, smaller branches arising from the circumflex artery and irrigating the LA roof were also ligated (Figure 1, average arteries ligated per animal, 1.69 in LAMI versus 1.65 in dantrolene group). Thereafter, the pericardial sac and the chest were closed. Sham-operated animals (N=7) were similarly prepared, but the left coronary branch/branches were not sutured. ECG loop recorders (Reveal; Medtronic, Inc) were implanted subcutaneously to monitor AF episodes. Spontaneous AF episodes were defined as AF episodes occurring spontaneously and lasting >2 minutes. Sheep were followed postoperatively for 7 to 8 days. In 7 sheep, dantrolene—a RyR2 blocker—was administered during the 8-day observation period (LAMI-D, 2.5 mg/kg, IV, BID). The experimental protocol is shown in Figure 2. After heart removal, the IZ was defined visually by its red exudative appearance and lack of contractility.
Figure 1. Left atrial myocardial infarction (LAMI) model.

A, Schematic of an LAMI sheep left atrial appendage (LAA) showing the left atrial (LA) coronary branches that were ligated (white knots). B, LAMI-induced PQ-segment changes. Twenty-four hours after LAMI, the P waves, which were positive before surgery (stars, left), became negative (stars, right). C, Eight-day LAMI specimen: a regional increase in inflammation at the LAA anterior portion in the ischemic zone (IZ) region extends to the LA roof-PV region. The IZ-non-IZ (NIZ) boundary is shown by red dashed line. D, Representative example of Picrosirius red collagen staining of NIZ, IZ, and dantrolene-treated IZ samples and quantification of fibrosis. Data are mean±SEM. LAMI IZ and NIZ, N=5 hearts; and LAMI- D, N=4 hearts. BZ indicates border zone; LAD, left anterior descending artery; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; and RA, right atrium. *P<0.05.
Figure 2. Experimental protocol.

Seven sheep were treated with dantrolene (left atrial myocardial infarction [LAMI]+D) of total 30 sheep that had coronary ligation (LAMI). Seven sheep underwent sham surgeries without any coronary ligations. Twenty-four sheep were monitored with loop recorders (12 LAMI, 7 LAMI+D, 5 Sham). A total of 11 sheep were used for optical mapping studies, including 4 with dual mapping; 5 sheep were subjected to histological analysis; 3 for reactive oxygen species (ROS) measurement; 4 for NADPH (nicotinamide adenine dinucleotide phosphate, reduced form); and 3 for XO assay. n=5 in the [3H]ryanodine-binding assay is the number of assay repetitions done on each of the 4 sheep (N=4). Some sheep samples were used for analysis in >1 subcategory. AF indicates atrial fibrillation; and DCF, 6-carboxy-2’,7’-dichlorodihydrofluorescein diacetate.
Optical Mapping and Pacing Protocol
Total 11 sheep were heparinized (200 U/kg, IV) and then anesthetized with propofol (5–10 mg/kg). Hearts were excised and Langendorff perfused with warm oxygenated Tyrode solution (pH 7.4; 95% O2, 5% CO2; 36–38°C). In 7 sheep, optical mapping was conducted as previously with 1 charge-coupled device (CCD) camera directed toward the LA and 1 CCD camera toward right atrium (Di-4-ANEPS, 500 frames per second and 5-second movies).34,35 In 4 LAMI hearts, both the CCD cameras were used to dual map LA appendage (LAA) with voltage-sensitive (Vm) dye, RH237, and intracellular calcium (Cai)-sensitive dye, Rhod-2-AM. LAA was paced with cycle lengths 150 to 300 ms for 10 seconds, and optical recording of Vm and Cai was performed to record the last paced beats and subsequent postpacing interval.
Caffeine-Induced Ca2+ Release
Atrial myocytes were enzymatically dissociated from non-IZ (NIZ) and IZ of the heart using the Langendorff retrograde perfusion method.36 Myocytes were cultured in M199 medium with 3% penicillin/streptomycin, loaded with fluo-4 AM, and field-stimulated to record Ca2+-release amplitudes (basal and 2-mmol/L caffeine-induced peak) by confocal line scan. Fluorescence peak (F) is calculated as a ratio of delta F over F0 (resting diastolic calcium fluorescence). Delta F is calculated as Fmax-Fmin (resting fluorescence during pacing). Calcium spark analysis was obtained using SparkMaster ImageJ plugin.
Collagen Analysis
Myocardial-tissue samples were collected from NIZ and IZ. The tissue was then fixed in 10% formalin, paraffin embedded in, sectioned at a thickness of 4 pm. Fibrosis was quantified on Picrosirius red-stained images. Images were photographed (10× objective), with either a digital 3-chip color CCD camera (DC-330, DAGE-MTI) on a Zeiss Axioplan2e microscope or a 12-bit color CCD camera (Qicam; Qimaging) on a Zeiss ACIO imager A1 microscope. Digital images were processed using ImageJ (National Institutes of Health), with additional threshold color plugins to process JPEG images. Pixels stained in red were normalized to the total pixel area of the image, and the results were expressed as a percentage. Perivascular, endocardial, and epicardial fibroses were excluded from the analysis.
[3H]Ryanodine-Binding Assay
The effect of CaM (calmodulin) on [3H]ryanodine binding was assessed as described.37,38 SR-enriched vesicles were obtained from IZ and NIZ, using Tris-buffered solution (0.9% NaCl, 10 mmol/L Tris-HCl, pH 6.8) plus protease inhibitors (PIs) and collected by 3 steps of centrifugation. The 40 000-g pellets were resuspended in Tris-buffered solution with PI, plus 10% sucrose. After protein quantification, the [3H]ryanodine-bind-ing assay was performed. Briefly, 100 μl of [3H]ryanodine-binding buffer containing 200 mmol/L KCl, 25 mmol/L Tris, 50 mmol/L Hepes (pH 7.4), 1 mmol/L EGTA, 5 nmol/L [3H]ryano-dine (68.4 Ci-mmol-1; Dupont NEN), and CaCl2 was added to set free [Ca2+] to pCa 5. Ca2+/EGTA ratio was calculated with Max-Chelator (www.stanford.edu/~cpatton/maxc.html), and 50 μg of SR-enriched solution was incubated for 2 hours at 37°C, filtered on GF/B glass filters (Whatman) presoaked with water, and washed twice with 5 mL of distilled water with an M24-R cell harvester (Brandel). Nonspecific binding was determined in the presence of 20 μM unlabeled ryanodine and subtracted from each sample.
Western Blot
Sheep IZ and NIZ tissue samples were washed with PBS containing PIs (Roche, PI tablet) and flash-frozen in liquid nitrogen. Frozen tissue (50–100 mg) was homogenized in 1 mL of lysis buffer containing PI. The homogenate was centrifuged at 1000 rpm for 3 minutes and the supernatant used for Western blotting. The tissue homogenates (50 μg) were then subjected to 1-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis. The blots were incubated overnight in a cold room with nNOS antibody (1:2000; Millipore, CA) and rabbit anti-GAPDH antibody (1:5000; Sigma-Aldrich, St. Louis, MO). The protein bands were visualized using enhanced chemilumi-nescence (Thermo Scientific, Rockford, IL). AntiCaM primary antibody was purchased from Abcam (Cat No.105498).
Nitroso-Redox Imbalance
Xanthine Oxidase and NADPH Oxidase Assay
Xanthine oxidase (XO) assay kit was purchased from Abcam, Cambridge, MA, and the assay was performed per the protocol provided by the vendor. NADPH (nicotinamide adenine dinucleotide phosphate, reduced form) oxidase assay was performed by the University of Michigan Metabolomics Core.39
DCF Assay
Fresh single 20-mm3 tissue samples from the IZ and NIZ were frozen in OCT (optimum cutting temperature formulation)-filled cassettes. Thereafter, they were incorporated in 50-μM slices, washed with PBS, and incubated with 20-μM/L DCF (6-carboxy-2’,7’-dichlorodihydrofiuorescein diacetate) for 30 minutes at 37°C. Fluorescent light from the preparation was filtered with a 520-nm optical bandpass filter after DCF loading (confocal microscope; Zeiss, Inc).
Hearts that were used for optical mapping were not used for any other subsequent studies. For the metabolomics analysis, fresh, frozen samples of heart tissue from distinct animals were collected immediately after heart isolation.
Statistical Analysis
Data are represented as mean±SEM values. Statistical comparisons were performed by nonparametric t test (Wilcoxon signed-rank test) and nonparametric ANOVA (Kruskal-Wallis test followed by post hoc Dunn multiple comparison test) when appropriate. Differences were considered significant when P<0.05.
RESULTS
LAMI-Induced PQ-Segment Changes and Extent of Lesions
We previously reported the distribution of coronary branches in sheep.40 As previously shown in human atrial infarction,41 P-wave polarity and shape changed postoperatively (Figure 1B). After 8 days, visual examination of explanted LAMI hearts revealed a large red exudative region over a large portion of the LA free wall (Figure 1C, left), the LA roof, and the posterior LA-pulmonary vein region (Figure 1C, right). The IZ exhibited a nearly complete absence of contractility (Movie I in the Data Supplement). Histological evaluation of the IZ and NIZ regions showed that the normal myocardium is replaced by extensive, loose, immature connective tissue spanning the entire wall of most of the roof of the atrium (Figure I in the Data Supplement). Picrosirius red staining showed a significant increase in interstitial fibrosis in the IZ compared with the NIZ. Fibrous tissue was interspersed with viable cardiomyocytes in the IZ, suggesting that the arterial occlusions caused patchy necrosis interspersed with viable but underperfused and hypocontractile cardiomyocytes. There was no significant effect of dantrolene on the extent of fibrosis in the IZ (Figure 1D). No statistically significant heart rate changes are seen in the dantrolene-treated and nontreated LAMI groups. Mean arterial pressure did not change significantly after the coronary ligation (Figure II in the Data Supplement). Extent and size of the ischemic region in the dantrolene-treated and nontreated LAMI groups are not significantly different (Figure III in the Data Supplement).
Spontaneous AF Occurrence and Effect of Dantrolene
The implanted loop recorder showed premature atrial contractions and spontaneous AF episodes, particularly after LAMI. A representative AF episode is shown in Figure 3A. LAMI animals experienced significantly more and longer lasting spontaneous AF episodes than sham-operated animals (Figure 3B and 3C; Figure IV in the Data Supplement). In the LAMI-D group, the number of spontaneous AF episodes was significantly reduced versus LAMI animals and comparable with sham-operated animals (Figure 3B, top). Dantrolene led to large decreases in the number of AF episodes from days 2 to 8 (Figure 3B, bottom).
Figure 3. Left atrial myocardial infarction (LAMI)-induced spontaneous atrial fibrillation (AF), effect of in vivo administration of dantrolene.

A, Representative example of a spontaneous AF episode 24 h after LAMI. B, Top, Total number of in vivo spontaneous AF episodes per sheep during the 8-d follow-up period in sham-operated sheep, LAMI animals, and LAMI animals treated with dantrolene, 2.5 mg/kg IV, BID. Mean±SEM. **P<0.01. Bottom, Details of daily number of in vivo spontaneous AF episodes during the follow-up period in the dantrolene group. Note that on PO-1 and PO-8, the sheep did not receive dantrolene. Mean±SEM. *P<0.05, compared with PO-1. Both short and long episodes that occurred in LAMI animals were prevented by dantrolene administration. PO indicates postoperative day
Dual Optical Mapping and Postpacing SFDs
Langendorff-perfused LAMI hearts were subjected to dual optical mapping with RH237 (membrane potential [Vm] sensitive) and Rhod-2-AM (Ca2+ sensitive), respectively (N=4). A photographic image and the corresponding RH237-fluorescence background image delineating the NIZ and IZ are shown in Figure 4A (top). Ten-second movies (including the last second of the pacing train and 9 seconds post-pacing) were recorded (Figure 4A, bottom) at progressively shorter cycle lengths (300–150 ms in 25 ms decrements). A representative recording is shown in Figure 4B: the pacing train elicited 4 postpacing SFDs: 1 from the LAA IZ-NIZ border and 3 from the posterior LA IZ region. Importantly, SFDs were calcium driven (Figure 4C; Figure V in the Data Supplement) and rate dependent (Figure 4D, bottom), occurring principally at pacing intervals of 175 or 150 ms. A simultaneous Vm/Ca2+ single-pixel time sequence at the site of SFD onset (LAA IZ-border) is shown in Figure 4C. During pacing, the Ca2+ transient follows Vm. However, the SFD Ca2+ transient precedes Vm. Figure 4D shows that the number of postpacing SFDs increased with isoproterenol perfusion (0.03 μM), and SFD numbers were markedly decreased after dantrolene (10–20 μM). Figure 4E shows sequential Vm snapshots of the SFD that occurred at the LAA. The origin at the IZ-NIZ border is best seen at 0, 8, 16, and 22 ms (see also a distinct example in Movie II in the Data Supplement).
Figure 4. Optical mapping and postpacing spontaneous focal discharges (SFDs) in a Langendorff-perfused 8-d left atrial myocardial infarction ovine heart.

A, Left atrial photographic snapshot (left) and corresponding RH237-fluorescence background snapshot (right) delineating the nonischemic zone (NIZ) and ischemic zone (IZ). B, Left atrial appendage (LAA) electrogram during sinus rhythm (SR), 10-s pacing at 175 ms cycle length and post-pacing (red line). The movie recording was started ≈1 s before the end of the 10-s pacing train. In this representative example, the pacing train elicited 4 postpacing SFDs: 1 from the LAA IZ-border and 3 from the posterior left atrial (PLA) IZ region. C, Simultaneous membrane voltage and calcium transients (Vm/Cai) single-pixel time sequence at the site of onset of a representative LAA IZ SFD. During pacing, the Ca2+ transient follows Vm. However, the SFD Ca2+ transient precedes Vm. D, Top, Average number of postpacing SFDs before and after isoproterenol (ISO) and before and after dantrolene in 7 hearts. Bottom, Rate dependency of SFDs. Most of the SFDs occurred at the pacing interval of 175 ms. At the 150-ms interval, the pacing often induced atrial fibrillation. E, Sequential Vm snapshots of the LAA IZ SFDs onset. The IZ origin of the SFD is best seen at 0, 8, 16, and 22 ms. Vm/Cai: Membrane voltage and calcium transients. *P<0.05.
The electrophysiological properties of the preparation are illustrated in Figure 5. Figure 5A shows the conduction properties, with clear slowing in the IZ region, Figure 5B shows representative maps of activation isochrones, action potential duration (APD), and Ca2+ transient duration as a function of cycle length from 1 heart. IZ conduction abnormalities were clearly exaggerated as rate increased. Figure 5C shows voltage-calcium activation delay maps during pacing and during SFDs. Mean data (Figure 5D) indicate that the voltage-Ca2+ activation delay (defined as the time from the initial Cai-signal rise to the voltage upstroke) was negative for paced complexes (reflecting the fact that the voltage rise occurred before the Ca2+-fluorescence increase) but was positive for SFDs, indicating that the Ca2+ signal preceded the voltage increase.
Figure 5. Dual mapping, conduction slowing, and voltage-calcium activation delay maps.
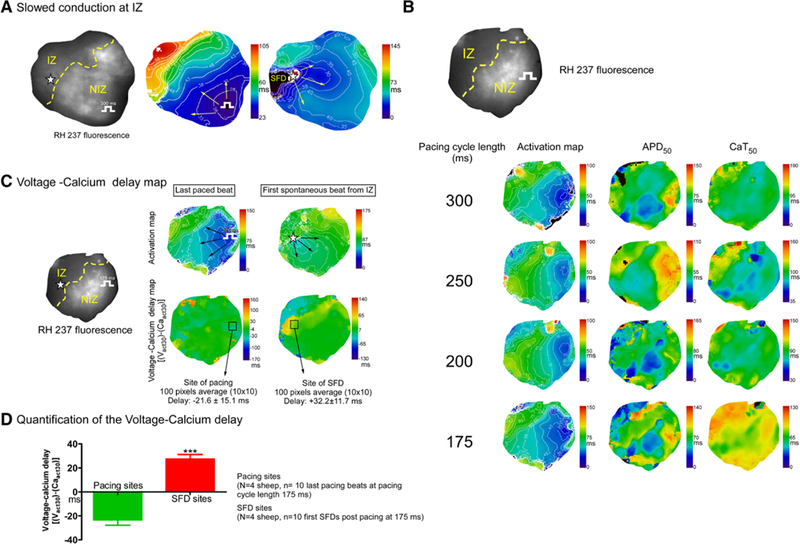
A, Activation maps showing that the impulse conduction is slowed at the ischemic zone (IZ) during both paced and spontaneous beats. B, Activation maps, action potential duration to 50% repolarization (APD50), and calcium transient durations to 50% relaxation (CaT50) are shown at different pacing cycle lengths. C, Voltage-calcium activation delay maps are shown during a paced and a spontaneous beat from the IZ. D, Quantification of voltage-calcium activation delays for paced and spontaneous beats (spontaneous focal discharges [SFDs]). ***P<0.001 vs paced beats.
Figure VI in the Data Supplement shows that the perfusion of dantrolene did not significantly modulate APD or conduction velocity. At baseline, burst pacing induced AF episodes: 1 during pacing and 1 post-pacing. After isoproterenol, 4 AF episodes occurred during pacing and 2 post-pacing. In contrast, all 3 AF episodes induced after dantrolene perfusion occurred during pacing. This observation suggests that dantrolene prevented AF initiation by SFDs but did not affect the reentry substrate. Similar phenomena were observed in all 7 hearts subjected to this protocol (including arrhythmia induction with both isoproterenol and programmed stimulation).
Spatiotemporal Stability of Rotors and SFDs in the Ovine Heart
Rotors and SFDs recorded with optical mapping techniques in LAMI Langendorff-perfused hearts demonstrate that in a given heart, rotors or SFDs appeared at spatiotemporally consistent locations. Figure 6A shows a rotor that formed shortly after AF initiation and anchored at the edge of the IZ. An isochronal map presented in Figure VII in the Data Supplement further illustrates the reentrant nature of the activation occurring at the IZ/NIZ junction in this heart. Analysis of SFDs in 7 LAMI hearts shows that postpacing-elicited SFDs originated at a maximum of 3 spatially consistent IZ-border locations/heart (Figure 6B).
Figure 6. Optical mapping of rotors and spontaneous focal discharges (SFDs) in left atrial myocardial infarction (LAMI) hearts.

A, Left, Example of a rotor that anchored at the border of the ischemic zone (IZ). Right, Bipolar electrograms at the IZ/ non-IZ (NIZ)-junction, right atrial appendage (RAA), and left superior pulmonary vein (LSPV) show that the rotor location corresponded to the DFmax region. An isochronal map of the reentrant activity is presented in Figure VI in the Data Supplement. B, Composite maps of the sites of origin of postpacing SFDs. Red dots and numbers show the number of SFDs/atrial site in 7 distinct LAMI hearts.
Intracellular Ca2+ Dynamics
Atrial myocytes were enzymatically dissociated from NIZ and IZ of Langendorff-perfused LAMI hearts. Con-focal line scan of the myocytes after fluo-4 AM loading shows an increased frequency and amplitude of Ca2+ sparks (Figure 7A). Caffeine-induced Ca2+-transient amplitudes in IZ myocytes were not significantly different from NIZ myocytes (Figure 7B), indicating an unchanged overall SR Ca2+ load.
Figure 7. Intracellular calcium dynamics.

A, Confocal line scan fluo-4 fluorescence in nonischemic zone (NIZ)- and ischemic zone (IZ)-isolated myocytes. Calcium spark frequency and amplitude are increased in the IZ (N=3, n=6 IZ, and n=6 NIZ). B, Caffeine-induced Ca2+-transient amplitude (ΔF) in IZ and NIZ myocytes (N=3, n=9 IZ, and n=11 NIZ). ΔF=Fmax-Fmin. F0 indicates resting diastolic calcium fluorescence in the absence of pacing; Fmax, maximal fluorescence during the Ca2+ transient; Fmin, resting fluorescence during pacing; and ns, nonsignificant. *P<0.05.
Nitroso-Redox Imbalance in the IZ
Figure 8A shows fluorescence levels of the reactive oxygen species (ROS)-sensitive dye, DCF,42 in fresh LAMI tissue samples. DCF fluorescence in the IZ was significantly greater than in the NIZ. After adding H2O2, all regions exhibited equal DCF fluorescence. The xanthine content and NADP/NADPH ratios were significantly increased in the IZ versus NIZ (Figure 8B), and Western blot analysis in 7 LAMI and 3 sham heart tissue samples showed significantly reduced level of nNOS (NO synthase-1) protein expression in the IZ (Figure 8C). The decreased NOS1 expression, abnormally high levels of NADPH and XO activity, and excessive ROS production in the IZ show nitroso-redox imbalance, which could potentially cause RyR2 dysfunction, SCEs, and AF
Figure 8. Reactive oxygen species and nitroso-redox imbalance in left atrial myocardial infarction (LAMI) hearts.

A, DCF (6-carboxy-2’,7’-dichlorodihydrofluorescein diacetate) fluorescence in the ischemic zone (IZ). Top, Representative series of con-focal microscope DCF fluorescence snapshots across a single LAMI sample. Bottom, DCF fluorescence quantification in 3 LAMI hearts. B, Tissue xanthine content (top) and NADP/NADPH (nicotinamide adenine dinucleotide phosphate/reduced form) ratios (bottom) are significantly larger in the IZ, indicating increased xanthine oxidase and NADPH oxidase activities, respectively. C, Top, Quantification of NOS1 (NO synthase-1) protein expression relative to GAPDH. Non-IZ (NIZ) value normalized to 1. IZsham and NIZsham are corresponding regions in sham-operated animals. Bottom, Representative blots. D, CaM modulation of [3H]ryanodine binding in NIZ and IZ tissue homogenates tissue from 4 animals and effect of dantrolene. Five distinct counts were obtained in IZ, NIZ, IZ+dantrolene, and NIZ+dantrolene samples from each animal. DIC indicates differential interference contrast microscopy. *P<0.05, **P<0.01.
Differential CaM Modulation of [3H] Ryanodine Binding
We adapted a [3H]ryanodine-binding assay43,44 to add progressively larger CaM concentrations (0–0.3 μM) to tubes containing homogenized tissue (NIZ, IZ, or IZ+dantrolene 20 μM). CaM aberrantly increased [3H] ryanodine binding to RyR2 in the IZ (red). This effect was abolished by dantrolene (green), suggesting that dantrolene restored the physiological binding of CaM to RyR2 in the IZ. In contrast, little change in CaM modulation was seen in the NIZ (black), as would be expected if native CaM was physiologically bound to RyR2 at baseline (Figure 8D).
We have measured CaM protein expression levels in NIZ and IZ samples (N=3, n=4). The results shown in Figure VIII in the Data Supplement indicate that CaM protein expression levels are significantly increased in the IZ versus NIZ group. The increase in CaM expression may be a compensatory mechanism of the cell to quench the hyperactivity of the RyR2 that was caused by ischemia. In addition, the increase in CaM expression indirectly supports our contention that CaM aberrantly stimulates the RyR2 channel.
DISCUSSION
Our study shows that regional atrial ischemia sets the stage for SCE-induced SFDs that initiate spontaneous AF and that this cascade of events is prevented by the administration of dantrolene. Our principal specific findings are that (1) regional LAMI in the sheep is a reproducible model of ischemia-related spontaneous AF, in agreement with previous findings in dogs41; (2) intracellular calcium load is increased in myocytes from the IZ; (3) dantrolene—a drug that suppresses RyR2 unzipping and SCEs33,45–47—prevents spontaneous AF in LAMI sheep; (4) dantrolene prevents the onset of spatio-temporally stable postpacing SFDs emanating from the IZ; (5) dantrolene also reverses aberrant CaM-induced increases in [3H]ryanodine binding to RyR2 in IZ myocytes; and (6) ROS are increased in IZ myocytes. In addition, we found that in IZ cardiomyocytes, NOS1 (nNOS) protein expression is significantly reduced, and XO and NADPH oxidase activity is significantly increased, providing a potential candidate molecular basis for LAMI-induced RyR2 dysfunction.
Role of Atrial Infarction in AF Pathophysiology
Recent reports have suggested a key role for spontaneous SR Ca2+-release events (SCEs) in both paroxysmal and persistent AF pathophysiology. In persistent AF cardiomyocytes, the NCX1 (sodium-calcium exchanger type-1) expressions were unaltered.48 There was RyR2 dysregulation and increased SR Ca2+ uptake, resulting in enhanced SR Ca2+ load.48 In contrast, in persistent AF myocyte NCX1 expression was increased,49 and SR Ca2+ leak was caused by Ca2+/CaMKII (CaM-dependent protein kinase II)-mediated increases in the open probability of RyR2.49 Similarly, enhanced CaMKII activity was detected in the fibrillating atria of animal models50 and patients,51 causing RyR2 hyperphosphorylation at Ser2814 leading to increased diastolic SR Ca2+ leak.50–53
However, it remains unclear whether these mechanisms are relevant to patients with AF associated with CAD and atrial infarction. In right atrial infarction myocytes, Nishida et al26 showed a larger occurrence of SCEs in IZ cardiomyocytes, although the relationship to ectopic activity and spontaneous AF was not probed and the mechanisms underlying SCEs were not addressed in any detail. Studies in infarcted ventricular tissue after 48 hours54 and 3 weeks postinfarction55 demonstrated increased Ca2+ spark and Ca2+-wave activities.56,57 However, the mechanisms by which CAD and atrial infarction lead to SCEs and AF remain largely unknown. For the first time, we provide here clear data implicating SCEs caused by RyR2 dysfunction in SFDs that initiate reentry and spontaneous AF in a CAD model. In addition, our results point to nitroso-redox imbalance as a potential underlying mechanism.
Role of Nitroso-Redox Imbalance
O2•‒ generation during ischemia and reperfusion is caused by multiple potential cellular sources that include cellular cytochrome p450 activity, mitochondrial electron transport damage and uncoupling, the conversion of cellular xanthine dehydrogenase to XO, NADPH oxidase activity, and uncoupled NO synthase activity.58 Recent studies have indicated that NADPH oxidase is responsible for a large proportion of the O2•‒ produced by the atria in AF.59 Rather than redox imbalance alone, it was suggested that nitroso-redox imbalance may contribute to RyR2 dysfunction.29,60 Similar to redox signaling, NO can also modify cysteine residues. This occurs by the addition of the NO moiety to a cysteine thiol to form an SNO (S-nitrosothiol) and is termed S-nitrosylation. NOS1 colocalizes with RyR2, and it was found that NOS1 signaling may increase RyR2 activity via S-nitrosylation61 but that a decreased NOS1 activity may result in an increased O2•‒ production via XO or NADPH oxidases,62–65 also leading to increased RyR2 activity.27 NO usually inhibits XO and NADPH oxidases and limits O2•‒ production.63,66,67 But with the loss of NOS1 and NO, oxidases are no longer inhibited and produce an excess of O2^. In this context, the 89 cysteine residues60 of RyR2 are prime targets for regulation by ROS.68,69 In addition to direct regulation, ROS may also regulate RyR2 Ca2+ release through indirect pathways by altering CaM binding.70,71 As presented here, NOS1 protein expression levels in LAMI IZ myocytes are reduced, whereas, at the same time, there is an increased XO and NADPH oxidase activity and abnormally high levels of ROS. Thus, our results suggest that nitroso-redox imbalance is present in our model and underlies ROS-induced RyR2 dysfunction. Besides, our results suggest that RyR2 N-terminal/central domain interaction is under the control of redox-sensing mechanisms built into these domains. Previously, it was demonstrated that the mode of interdomain interaction of 2 specific domains within the RyR2 (N-terminal and central domains) plays a key role in Ca2+ channel regulation. Under normal conditions, the interaction of these domains is tight (zipped), stabilizing the closed state of the channel; on stimulation of the RyR2, the interdomain interaction becomes loose (unzipped), and the channel opens.72 In diseased conditions, a mutation in either of these domains weakens the interdomain interaction even in resting or nonactivating conditions, and diseased channels remain partially open. Based on sequence similarities with RyR1,73 an oxidoreductase-like domain is present in the N-terminal region 41 to 420 and may function as a redox sensor. This region corresponds to the N-terminal domain—one of the key domains involved in the interdomain interaction. Interestingly, Cys residues in the RyR2 (2403, 2532, 2573, and 2578) are clustered in the region that is partially overlapping with the central domain—another key domain involved in the interdomain interaction.74
CaM Binding to RyR2
CaM—a classical RyR2 regulator75–77—binds to RyR2 stoichiometrically (4 CaMs per tetrameric RyR2).78–80 CaM inhibits RyR2 opening at all Ca2+ concentrations and, as such, regulates SR Ca2+ release.78,80 CaM binding to RyR2 is reduced after myocardial infarction,81 heart failure,82,83 or by CPVT1 (catecholaminergic polymorphic ventricular tachycardia-1) mutations,84 rendering RyR2 channels hyperactive. Recently, evidence was presented that in 1-month infarction heart failure versus normal myocytes, the CaM-RyR2 affinity measured by fluorescence resonance energy transfer is ≈3-fold lower.81 Another recent study, however, showed that arrhythmia-causing mutant CaM proteins aberrantly bind to RyR2 with a relatively higher affinity; this interaction, however, changes the CaM effect on RyR2 from inhibitory to stimulatory.28 This is reminiscent of the dual action of CaM on skeletal ryanodine receptor (RyR1): activating effect at nanomolar [Ca2+] but inhibitory effect at high micromolar [Ca2+].85 Our data suggest that similar to the findings of this study, there is an aberrant CaM-RyR2 binding in IZ myocytes. In our view, the increase of CaM expression may be a compensatory mechanism of the cell to quench the hyperactivity of the RyR2 that was caused by ischemia. Also, the increase of CaM expression indirectly supports our contention that CaM aberrantly stimulates the RyR2 channel. Our results also extend previous work showing that defective CaM binding may be restored by dantrolene.32,83 Dantrolene is a hydantoin derivative that acts as muscle relaxant and is the most effective treatment for malignant hyperthermia—a rare life-threatening familial disorder caused by mutations in the RyR1.45 The therapeutic action of dantrolene is at least partly because of its binding to an amino-terminal sequence of RyR1, which restores interdomain interactions critical for the closed state of the channel.86 Recently, dantrolene has been shown to target a corresponding sequence in RyR2.45,86 Dantrolene corrects defective RyR2 interdomain interaction (unzipping) in failing myocytes.45 This action in turn inhibits spontaneous Ca2+ leak/Ca2+ sparks and improves cardiomyocyte function.45 It was also suggested that dantrolene treatment ameliorates RyR2 unzipping and aberrant Ca2+ events after prolonged endo-thelin-1 exposure and after having reduced nuclear translocation of CaM.46,87 Dantrolene improves intracellular Ca2+ handling and arrhythmias in a mouse model of CPVT147 and prolongs survival after ventricular fibrillation by mitigating impaired calcium handling in pigs and rabbits.33 The previous evidence, in combination with our results, points toward RyR2 unzipping and aberrant CaM binding in atrial infarction as the cause of SCEs and SFDs in IZ cardiomyocytes.
Limitations
Early studies suggested that dantrolene significantly prolongs APD.88,89 This effect, however, was not statistically significant in atrial fibers.88 Further, it was also suggested that dantrolene effects in vivo may be mediated by modulation of sympathetic tone, which may result in an excessive opening of L-type Ca2+ channels.90 Therefore, it remains unclear whether dantrolene modulates individual sarcolemmal ion channel conductance in atrial cardiomyocytes. In addition, because Ca2+-transient measurements in the intact heart cannot be accurately calibrated in terms of absolute intracellular Ca2+ concentrations, we are unable to quantify any differences in Ca2+-transient amplitudes between dantrolene-treated and control LAMI hearts. Thus, whether dantrolene directly affects Ca2+-transient amplitudes in our LAMI sheep remains unknown. The results presented in Figures I and II in the Data Supplement, however, suggest no effects on APD, conduction velocity, or heart rate, as would have been expected if dantrolene acted by modulating sympathetic tone. On the contrary, dantrolene has been suggested to display antioxidant effects91 or to act as a modulator of antioxidant enzyme activity.92,93 Therefore, we cannot exclude the possibility that part of the antifibrillatory action of dantrolene was mediated by decreased nitroso-redox imbalance. We performed nNOS and DCF measurements in sham and LAMI cells to assess the possible role of nitroso-redox imbalance in ischemic atrial tissues; however, we did not measure these indices in dantrolene-treated LAMI sheep. This measurement would be useful to perform in future studies. As previously shown by Rivard et al,94 autonomic neural output affects the AF substrate after atrial ischemia. It would be interesting in future studies to assess whether the results would be changed by chemical autonomic blockade or autonomic denervation and to assess any potential contribution of autonomic tone to AF triggering.
We showed in this study that dantrolene suppresses Ca2+-mishandling and prevents spontaneous AF in LAMI sheep. However, dantrolene did not appear to suppress AF induction by programmed stimulation. This observation is compatible with the notion that Ca2+-handling abnormalities are central to the triggered activity that causes spontaneous ectopic arrhythmias, which initiate AF-maintaining reentry in a vulnerable substrate, but that factors other than Ca2+ mishandling, like fibrosis and scarring in the atrial IZ, underlie the reentry substrate as suggested previously.26
Finally, we did not perform imaging studies to assess possible cardiac function changes in our sheep. Although such studies might provide useful information, they would have required general anesthesia to immobilize the animals, which would have interfered with the other measurements we obtained and were, therefore, not performed. Absence of any clinical changes or alterations in heart rate or blood pressure (Figure II in the Data Supplement) in our sheep, nor of any specific reason to suspect altered cardiac function, we decided that the use of additional animals only to perform such studies was not sufficiently justified.
Supplementary Material
WHAT IS KNOWN?
Atrial and ventricular coronary artery disease-related myocardial insults lead to atrial fibrillation (AF) onset and perpetuation.
AF initiation may be caused by right atrial myocardial infarction. Spontaneous sarcoplasmic reticulum Ca2+-release events preferentially occur in cardiomyocytes located in the ischemic atrium.
WHAT THE STUDY ADDS?
Left atrial ischemia/infarction causes spontaneous AF episodes in sheep. Ischemia/infarction-related AF episodes are initiated by spontaneous focal discharges and maintained by reentry.
Nitroso-redox imbalance in the ischemic zone is associated with intense reactive oxygen species production and an altered RyR2 (ryanodine receptor) response to calmodulin.
Dantrolene administration normalizes the calmodulin response, prevents ischemia-related spontaneous focal discharges, and averts AF initiation.
ACKNOWLEDGMENTS
We thank L. Mundada, G. Guerrero-Serna, T. Herron, and F Alvarado for their helpful suggestions.
SOURCES OF FUNDING
This study was supported by American Heart Association Grant-in-Aid 13GRNT16820063 (to Dr Kalifa); National Institutes of Health grants HL05548 and HL120108 (to Dr Valdivia); Canadian Institutes of Health Research and Quebec Heart and Stroke Foundation (to Dr Nattel); and JSPS (Japan Society for the Promotion of Science) Grant-in-Aid for Scientific Research (C) 15K09077 and Joint International Research Grant 15KK0341 (to Dr Yamazaki).
Footnotes
DISCLOSURES
None.
The Data Supplement is available athttp://circep.ahajournals.org/lookup/suppl/doi:10.1161/CIRCEP.117.005659/-/DC1.
Circ Arrhythm Electrophysiol is available athttp://circep.ahajournals.org.
REFERENCES
- 1.Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17, ix-40, ix. doi: 10.1016/j.mcna.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, Acqui-stapace I, Stella A, Bonforte G, DeVecchi A, DeCristofaro V, Buccianti G, Vincenti A. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46:897–902. doi: 10.1053/j.ajkd.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 3.Sanoski CA. Clinical, economic, and quality of life impact of atrial fibrillation. J Manag Care Pharm. 2009;15(6 suppl B):S4–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prystowsky EN, Benson DW Jr, Fuster V, Hart RG, Kay GN, Myerburg RJ, Naccarelli GV, Wyse DG. Management of patients with atrial fibrillation. A statement for healthcare professionals. From the subcommittee on electrocardiography and electrophysiology, American Heart Association. Circulation. 1996;93:1262–1277. [DOI] [PubMed] [Google Scholar]
- 5.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the manitoba follow-up study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 6.Roy D, Talajic M, Dubuc M, Thibault B, Guerra P, Macle L, Khairy P Atrial fibrillation and congestive heart failure. Curr Opin Cardiol. 2009;24:29–34. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Abbott RD, Savage DD, McNamara PM. Coronary heart disease and atrial fibrillation: the Framingham Study. Am Heart J. 1983;106:389–396. [DOI] [PubMed] [Google Scholar]
- 8.James TN. Myocardial infarction and atrial arrhythmias. Circulation. 1961;24:761–776. [DOI] [PubMed] [Google Scholar]
- 9.Crenshaw M, Brian S, Ward M, Samuel R, Granger M, Christopher B, Stebbins M, Amanda L, Topol M, Eric J. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. 1997;30:406–413. [DOI] [PubMed] [Google Scholar]
- 10.Wong CK, White HD, Wilcox RG, Criger DA, Califf RM, Topol EJ, Ohman EM. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am Heart J. 2000;140:878–885. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg RJ, Yarzebski J, Lessard D, Wu J, Gore JM. Recent trends in the incidence rates of and death rates from atrial fibrillation complicating initial acute myocardial infarction: a community-wide perspective. Am Heart J. 2002;143:519–527. [DOI] [PubMed] [Google Scholar]
- 12.Lazar EJ, Goldberger J, Peled H, Sherman M, Frishman WH. Atrial infarction: diagnosis and management. Am Heart J. 1988;116:1058–1063. [DOI] [PubMed] [Google Scholar]
- 13.Wong AK, Marais HJ, Jutzy K, Capestany GA, Marais GE. Isolated atrial infarction in a patients with single vessel disease of the sinus node artery. Chest. 1991;100:255–256. [DOI] [PubMed] [Google Scholar]
- 14.Kochiadakis GE, Skalidis EI, Kalebubas MD, Igoumenidis NE, Chrysosto-makis SI, Kanoupakis EM, Simantirakis EN, Vardas PE. Effect of acute atrial fibrillation on phasic coronary blood flow pattern and flow reserve in humans. Eur Heart J. 2002;23:734–741. doi: 10.1053/euhj.2001.2894. [DOI] [PubMed] [Google Scholar]
- 15.Skalidis EI, Hamilos MI, Karalis IK, Chlouverakis G, Kochiadakis GE, Vardas PE. Isolated atrial microvascular dysfunction in patients with lone recurrent atrial fibrillation. J Am Coll Cardiol. 2008;51:2053–2057. doi: 10.1016/j.jacc.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 16.Skalidis EI, Kochiadakis GE, Igoumenidis NE, Vardakis KE, Vardas PE. Phasic coronary blood flow velocity pattern and flow reserve in the atrium: regulation of left atrial myocardial perfusion. J Am Coll Cardiol. 2003;41:674–680. [DOI] [PubMed] [Google Scholar]
- 17.Alasady M, Abhayaratna WP, Leong DP, Lim HS, Abed HS, Brooks AG, Mattchoss S, Roberts-Thomson KC, Worthley MI, Chew DP, Sanders P. Coronary artery disease affecting the atrial branches is an independent determinant of atrial fibrillation after myocardial infarction. Heart Rhythm. 2011;8:955–960. doi: 10.1016/j.hrthm.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Bunc M, Starc R, Podbregar M, Bruĉan A. Conversion of atrial fibrillation into a sinus rhythm by coronary angioplasty in a patient with acute myocardial infarction. Eur J Emerg Med. 2001;8:141–145. [DOI] [PubMed] [Google Scholar]
- 19.Pehkonen E, Honkonen E, Mäkynen P, Kataja M, Tarkka M. Stenosis of the right coronary artery and retrograde cardioplegia predispose patients to atrial fibrillation after coronary artery bypass grafting. Thorac Cardiovasc Surg. 1998;46:115–120. doi: 10.1055/s-2007-1010206. [DOI] [PubMed] [Google Scholar]
- 20.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 21.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW, Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 22.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 23.Cushing EH, Feil HS, Stanton EJ, Wartman WB. Infarction of the cardiac auricles (atria): clinical, pathological, and experimental studies. Br Heart J. 1942;4:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wartman WB, Souders JC. Localization of myocardial infarcts with respect to the muscle bundles of the heart. Arch Pathol (Chic). 1950;50:329–346. [PubMed] [Google Scholar]
- 25.Álvarez-García J, Vives-Borrás M, Gomis P, Ordoñez-Llanos J, Ferrero-Gre-gori A, Serra-Peñaranda A, Cinca J. Electrophysiological effects of selective atrial coronary artery occlusion in humans. Circulation. 2016;133:2235–2242. doi: 10.1161/CIRCULATIONAHA.116.021700. [DOI] [PubMed] [Google Scholar]
- 26.Nishida K, Qi XY, Wakili R, Comtois P, Chartier D, Harada M, Iwasaki YK, Romeo P, Maguy A, Dobrev D, Michael G, Talajic M, Nattel S. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. 2011;123:137–146. doi: 10.1161/CIR-CULATIONAHA.110.972778. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhyth-mogenesis in cardiomyocytes. Proc Natl Acad Sci USA. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang HS, Nitu FR, Yang Y, Walweel K, Pereira L, Johnson CN, Faggioni M, Chazin WJ, Laver D, George AL Jr, Cornea RL, Bers DM, Knollmann BC. Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circ Res. 2014;114:1114–1124. doi: 10.1161/CIRCRESAHA.114.303391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler MJ, Plummer BN, Wan X, Sun QA, Hess D, Liu H, Deschenes I, Rosenbaum DS, Stamler JS, Laurita KR. Aberrant S-nitrosylation mediates calcium-triggered ventricular arrhythmia in the intact heart. Proc Natl Acad Sci USA. 2012;109:18186–18191. doi: 10.1073/pnas.1210565109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N. Dantrolene stabilizes domain interactions within the ryanodine receptor. J Biol Chem. 2005;280:6580–6587. doi: 10.1074/jbc.M408375200. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell JT, Domeier TL, Blatter LA. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol. 2012;302:H953–H963. doi: 10.1152/ajpheart.00936.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roden DM, Knollmann BC. Dantrolene: from better bacon to a treatment for ventricular fibrillation. Circulation. 2014;129:834–836. doi: 10.1161/CIRCULATIONAHA.113.007657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamiri N, Massé S, Ramadeen A, Kusha M, Hu X, Azam MA, Liu J, Lai PF, Vigmond EJ, Boyle PM, Behradfar E, Al-Hesayen A, Waxman MB, Backx P, Dorian P, Nanthakumar K. Dantrolene improves survival after ventricular fibrillation by mitigating impaired calcium handling in animal models. Circulation. 2014;129:875–885. doi: 10.1161/CIRCULA-TIONAHA.113.005443. [DOI] [PubMed] [Google Scholar]
- 34.Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, Berenfeld O, Nattel S. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–671. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 35.Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006;113:626–633. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 36.Callewaert G, Cleemann L, Morad M. Caffeine-induced Ca2+ release activates Ca2+ extrusion via Na+-Ca2+ exchanger in cardiac myocytes. Am J Physiol. 1989;257(1 pt 1):C147–C152. doi: 10.1152/ajpcell.1989.257.1.C147. [DOI] [PubMed] [Google Scholar]
- 37.Loaiza R, Benkusky NA, Powers PP, Hacker T, Noujaim S, Ackerman MJ, Jalife J, Valdivia HH. Heterogeneity of ryanodine receptor dysfunction in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2013;112:298–308. doi: 10.1161/CIRCRESAHA.112.274803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao YT, Valdivia CR, Gurrola GB, Powers PP, Willis BC, Moss RL, Jalife J, Valdivia HH. Arrhythmogenesis in a catecholaminergic polymorphic ventricular tachycardia mutation that depresses ryanodine receptor function. Proc Natl Acad Sci USA. 2015;112:E1669–E1677. doi: 10.1073/pnas.1419795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trammell SA, Brenner C. Targeted, lcms-based metabolomics for quantitative measurement of nad+ metabolites. Comput Struct Biotechnol J. 2013;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamazaki M, Morgenstern S, Klos M, Campbell K, Buerkel D, Kalifa J. Left atrial coronary perfusion territories in isolated sheep hearts: implications for atrial fibrillation maintenance. Heart Rhythm. 2010;7:1501–1508. doi: 10.1016/j.hrthm.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shakir DK, Arafa SOE. Right atrial infarction, atrial arrhythmia and inferior myocardial infarction form a missed triad: a case report and review of the literature. Can J Cardiol. 2007;23:995–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leutner S, Eckert A, Müller WE. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J Neural Transm (Vienna). 2001;108:955–967. doi: 10.1007/s007020170015. [DOI] [PubMed] [Google Scholar]
- 43.Valdivia HH, Fuentes O, el-Hayek R, Morrissette J, Coronado R. Activation of the ryanodine receptor Ca2+ release channel of sarcoplasmic reticulum by a novel scorpion venom. J Biol Chem. 1991;266:19135–19138. [PubMed] [Google Scholar]
- 44.Valdivia HH, Coronado R. Inhibition of dihydropyridine-sensitive calcium channels by the plant alkaloid ryanodine. FEBS Lett. 1989;244:333–337. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, Xu X, Uchinoumi H, Okuda S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53:1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bossuyt J, Bers DM. Visualizing CaMKII and CaM activity: a paradigm of compartmentalized signaling. J Mol Med (Berl). 2013;91:907–916. doi: 10.1007/s00109-013-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, Xu X, Tatei-shi H, Oda T, Okuda S, Doi M, Yamamoto T, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. CircJ. 2010;74:2579–2584. [DOI] [PubMed] [Google Scholar]
- 48.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhyth-mogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATI0NAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULA-TI0NAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Müller FU, Schmitz W, Schotten U, Anderson ME, Valder-rábano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin invest. 2009;119:1940–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schöndube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 52.Greiser M, Neuberger HR, Harks E, El-Armouche A, Boknik P, de Haan S, Verheyen F, Verheule S, Schmitz W, Ravens U, Nattel S, Allessie MA, Dobrev D, Schotten U. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2009;46:385–394. doi: 10.1016/j.yjmcc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Rokita AG, Anderson ME. New therapeutic targets in cardiology: arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII). Circulation. 2012;126:2125–2139. doi: 10.1161/CIRCULATIONAHA.112.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyden PA, Dun W, Barbhaiya C, Ter Keurs HE. 2APB- and JTV519(K201)-sensitive micro Ca2+ waves in arrhythmogenic Purkinje cells that survive in infarcted canine heart. Heart Rhythm. 2004;1:218–226. doi: 10.1016/j.hrthm.2004.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca(2+) sparks in myocytes from infarcted hearts. Circ Res. 2000;87:1040–1047. [DOI] [PubMed] [Google Scholar]
- 56.Litwin SE, Bridge JH. Enhanced Na(+)-Ca2+ exchange in the infarct-ed heart. Implications for excitation-contraction coupling. Circ Res. 1997;81:1083–1093. [DOI] [PubMed] [Google Scholar]
- 57.Gómez AM, Schwaller B, Porzig H, Vassort G, Niggli E, Egger M. Increased exchange current but normal Ca2+ transport via Na+-Ca2+ exchange during cardiac hypertrophy after myocardial infarction. Circ Res. 2002;91:323–330. [DOI] [PubMed] [Google Scholar]
- 58.Raedschelders K, Ansley DM, Chen DD. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Ther. 2012;133:230–255. doi: 10.1016/j.phar-mthera.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Dudley SC Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 60.Ziolo MT, Houser SR. Abnormal Ca(2+) cycling in failing ventricular myocytes: role of NOS1-mediated nitroso-redox balance. Antioxid Redox Signal. 2014;21:2044–2059. doi: 10.1089/ars.2014.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Viatchenko-Karpinski S, Sun J, Györke I, Benkusky NA, Kohr MJ, Valdivia HH, Murphy E, Györke S, Ziolo MT. Regulation of myocyte contraction via neuronal nitric oxide synthase: role of ryanodine receptor S-nitrosylation. J Physiol. 2010;588(pt 15):2905–2917. doi: 10.1113/jphysiol.2010.192617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan SA, Lee K, Minhas KM, Gonzalez DR, Raju SV, Tejani AD, Li D, Berkowitz DE, Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci USA. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hare JM. Nitrosoredox balance in the cardiovascular system. N Engl J Med. 2004;351:2112–2114. doi: 10.1056/NEJMe048269. [DOI] [PubMed] [Google Scholar]
- 64.Kinugawa S, Huang H, Wang Z, Kaminski PM, Wolin MS, Hintze TH. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ Res. 2005;96:355–362. doi: 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- 65.Traynham CJ, Roof SR, Wang H, Prosak RA, Tang L, Viatchenko-Karpinski S, Ho HT, Racoma IO, Catalano DJ, Huang X, Han Y, Kim SU, Gyorke S, Billman GE, Villamena FA, Ziolo MT. Diesterified nitrone rescues nitroso-re-dox levels and increases myocyte contraction via increased SR Ca(2+) handling. PLoS One. 2012;7:e52005. doi: 10.1371/journal.pone.0052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hassoun PM, Yu FS, Zulueta JJ, White AC, Lanzillo JJ. Effect of nitric oxide and cell redox status on the regulation of endothelial cell xanthine dehydrogenase. Am J Physiol. 1995;268(5 pt 1):L809–L817. doi: 10.1152/ajplung.1995.268.5.L809. [DOI] [PubMed] [Google Scholar]
- 67.Lee CI, Liu X, Zweier JL. Regulation of xanthine oxidase by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:9369–9376. [DOI] [PubMed] [Google Scholar]
- 68.Xie H, Zhu PH. Biphasic modulation of ryanodine receptors by sulfhydryl oxidation in rat ventricular myocytes. BiophysJ 2006;91:2882–2891. doi: 10.1529/biophysj.106.087338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 70.Okabe E, Tsujimoto Y, Kobayashi Y. Calmodulin and cyclic ADP-ribose interaction in Ca2+ signaling related to cardiac sarcoplasmic reticulum: superoxide anion radical-triggered Ca2+ release. Antioxid Redox Signal. 2000;2:47–54. doi: 10.1089/ars.2000.2.1-47. [DOI] [PubMed] [Google Scholar]
- 71.Zhang JZ, Wu Y, Williams BY, Rodney G, Mandel F, Strasburg GM, Hamilton SL. Oxidation of the skeletal muscle Ca2+ release channel alters calmodulin binding. Am J Physiol. 1999;276(1 pt 1):C46–C53. [DOI] [PubMed] [Google Scholar]
- 72.Ikemoto N, Yamamoto T. Postulated role of inter-domain interaction within the ryanodine receptor in Ca(2+) channel regulation. Trends Cardiovasc Med. 2000;10:310–316. [DOI] [PubMed] [Google Scholar]
- 73.Baker ML, Serysheva II, Sencer S, Wu Y, Ludtke SJ, Jiang W, Hamilton SL, Chiu W. The skeletal muscle Ca2+ release channel has an oxidoreduc-tase-like domain. Proc Natl Acad Sci USA. 2002;99:12155–12160. doi: 10.1073/pnas.182058899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voss AA, Lango J, Ernst-Russell M, Morin D, Pessah IN. Identification of hyperreactive cysteines within ryanodine receptor type 1 by mass spectrometry. J Biol Chem. 2004;279:34514–34520. doi: 10.1074/jbc.M404290200. [DOI] [PubMed] [Google Scholar]
- 75.Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. J Mol Cell Cardiol. 2002;34:919– 939. [DOI] [PubMed] [Google Scholar]
- 76.Means AR, VanBerkum MF, Bagchi I, Lu KP, Rasmussen CD. Regulatory functions of calmodulin. Pharmacol Ther. 1991;50:255–270. [DOI] [PubMed] [Google Scholar]
- 77.Weinstein H, Mehler EL. Ca(2+)-binding and structural dynamics in the functions of calmodulin. Annu Rev Physiol. 1994;56:213–236. doi: 10.1146/annurev.ph.56.030194.001241. [DOI] [PubMed] [Google Scholar]
- 78.Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca release channel. J Clin invest. 2007;1 17:1344–1353. doi: 10.1172/JCI29515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca(2+) release channel (ryanodine receptor). J Biol Chem. 2003;278:23480–23486. doi: 10.1074/jbc.M301125200. [DOI] [PubMed] [Google Scholar]
- 80.Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J Biol Chem. 2001;276:20144–20153. doi: 10.1074/jbc.M010771200. [DOI] [PubMed] [Google Scholar]
- 81.Yang Y, Guo T, Oda T, Chakraborty A, Chen L, Uchinoumi H, Knowlton AA, Fruen BR, Cornea RL, Meissner G, Bers DM. Cardiac myocyte Z-line calmodulin is mainly RyR2-bound, and reduction is arrhythmogenic and occurs in heart failure. Circ Res. 2014;114:295–306. doi: 10.1161/CIR-CRESAHA.114.302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin- dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 83.Ono M, Yano M, Hino A, Suetomi T, Xu X, Susa T, Uchinoumi H, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Koseki N, Kyushiki H, Ikemoto N, Matsuzaki M. Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca(2+) release in heart failure. Cardiovasc Res. 2010:87:609–617. doi: 10.1093/cvr/cvq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu X, Yano M, Uchinoumi H, Hino A, Suetomi T, Ono M, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ikemoto N, Mat-suzaki M. Defective calmodulin binding to the cardiac ryanodine receptor plays a key role in CPVT-associated channel dysfunction. Biochem Biophys Res Commun. 2010;394:660–666. doi: 10.1016/j.bbrc.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balshaw DM, Yamaguchi N, Meissner G. Modulation of intracellular calcium-release channels by calmodulin. J Membr Biol. 2002;185:1–8. doi: 10.1007/s00232-001-011 1–4. [DOI] [PubMed] [Google Scholar]
- 86.Paul-Pletzer K, Yamamoto T, Bhat MB, Ma J, Ikemoto N, Jimenez LS, Morimoto H, Williams PG, Parness J. Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J Biol Chem. 2002;277:34918–34923. doi: 10.1074/jbc.M205487200. [DOI] [PubMed] [Google Scholar]
- 87.Gangopadhyay JP, Ikemoto N. Intracellular translocation of calmodulin and Ca2+/calmodulin-dependent protein kinase II during the development of hypertrophy in neonatal cardiomyocytes. Biochem Biophys Res Commun. 2010;396:515–521. doi: 10.1016/j.bbrc.2010.04.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salata JJ, Wasserstrom JA, Jalife J. Dantrolene sodium: effects on isolated cardiac tissues. J Mol Cell Cardiol. 1983;15:233–243. [DOI] [PubMed] [Google Scholar]
- 89.Salata JJ, Jalife J. Effects of dantrolene sodium on the electrophysiological properties of canine cardiac Purkinje fibers. J Pharmacol Exp Ther. 1982;220:157–166. [PubMed] [Google Scholar]
- 90.Acsai K, Nagy N, Marton Z, Oravecz K, Varro A. Antiarrhythmic potential of drugs targeting the cardiac ryanodine receptor Ca2+ release channel: case study of dantrolene. Curr Pharm Des. 2015;21:1062–1072. [DOI] [PubMed] [Google Scholar]
- 91.Büyükokuroglu ME, GülçIn I, Oktay M, Küfrevioğlu OI. In vitro antioxidant properties of dantrolene sodium. Pharmacol Res. 2001;44:491–494. doi: 10.1006/phrs.2001.0890. [DOI] [PubMed] [Google Scholar]
- 92.Büyükokuroğlu ME, Taysi S, Polat F, GöÇer F. Mechanism of the beneficial effects of dantrolene sodium on ethanol-induced acute gastric mucosal injury in rats. Pharmacol Res. 2002;45:421–425. [DOI] [PubMed] [Google Scholar]
- 93.Ucuncu H, Taysi S, Aktan B, Buyukokuroglu ME, Elmastas M. Effect of dantrolene on lipid peroxidation, lutathione and glutathione-dependent enzyme activities in experimental otitis media with effusion in guinea pigs. Hum Exp Toxicol. 2005;24:567–571. doi: 10.1191/0960327105ht569oa. [DOI] [PubMed] [Google Scholar]
- 94.Rivard L, Sinno H, Shiroshita-Takeshita A, Schram G, Leung TK, Nattel S. The pharmacological response of ischemia-related atrial fibrillation in dogs: evidence for substrate-specific efficacy. Cardiovasc Res. 2007;74:104–113. doi: 10.1016/j.cardiores.2007.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


