Abstract
Regulation of human keratinocyte (HK) migration is critical for skin wound healing. Profiling HK migration-specific genes could help us gain a comprehensive understanding of the process. The main challenge is to separate genes that are unrelated to migration, but simultaneously induced by the same growth factor. In this study, we took advantage of a unique response of HKs to transforming growth factor-β (TGF-β), which inhibits proliferation but not migration of HKs, to suppress selectively the proliferation-related genes. Furthermore we stimulated HKs independently with TGF-α or insulin and identified the common genes and eliminated TGF-α- or insulin-specific genes. Under these conditions, we obtained profiles of the immediate-early genes (IEGs, at 30 minutes), early genes (EGs, at 60minutes), and delayed-early genes (DEGs, at 120 minutes) by microarray analyses, followed by quantitative real-time reverse transcription-PCR (QRT-PCR) validation and functional characterization by RNA interference (RNAi). Our results revealed the following: (1) 25 upregulated and 1 downregulated IEGs;(2) 58 upregulated and 15 downregulated EGs, and (3) 13 upregulated and 3 downregulated DEGs in both TGF-α- and insulin-stimulated HKs. Three genes, all encoding secreted molecules, were investigated in HK migration. These cell motility-specific gene profiles may prove useful to skin wound healing.
INTRODUCTION
Cell migration is the result of repeated cycles of cytoskeleton-mediated protrusion and polarization, formation of adhesive contacts, cell contraction, and retraction at the trailing edge (Lauffenburger and Horwitz, 1996). These sequential events are initiated and continuously driven by two extracellular environmental cues, extracellular matrices (ECMs) and soluble growth factors (GFs) (Eliceiri, 2001;Schwartz and Ginsberg, 2002). In response to a motility signal, a cell on an ECM substratum begins to show an initial protrusion of the plasma membrane at the cell’s leading edge by polymerizing actin filaments into a membrane structure called lamellipodia. Adhesive complexes, which are composed of clusters of integrin receptors, actin filaments, and associated proteins at the plasma membrane, are then established. As the cell migrates, the adhesive complexes at the leading edge of the cell develop into larger, more organized complexes, called focal adhesions (Gumbiner, 1996). The focal adhesions then serve as points of traction over which the body of the cell translocates toward the leading edge. Subsequent release from the ECM substratum at the rear of the cell allows the cell body to be displaced and consequently achieve a net step in the forward direction (Mitchison and Cramer, 1996). Between the two distinct migration cues, that is, ECMs versus GFs, Li et al. showed that ECMs, but not GFs, initiate cell migration. In contrast, GFs optimize the ECM-initiated cell migration and provide moving directionality (Li et al., 2004b, c). These studies suggest that haptotaxis (ECM-driven) is a prerequisite for chemotaxis (GF-induced). Haptotaxis can occur without chemotaxis, as previously reported (Carter, 1967). However, the reverse is not true. Chemotaxis cannot take place in the absence of ECM.
This regulation of cell migration is extremely important in the process of healing a human skin wound, since epidermal and dermal, as well as non-resident bone marrow-derived, cells must all migrate over or into the wound bed. In unwounded skin, the resident skin cells are nourished by a filtrate of plasma from dermal blood vessels. When skin is wounded, the resident cells in the wound encounter an acute environmental transition from plasma to serum for the first time. Then, as the wound heals, wound remodeling initiates new blood vessel formation and a subsequent transition from serum back to plasma. Interestingly, the “plasma-to-serum-to-plasma” transition coincides with the three classical phases of skin wound healing as previously described (Singer and Clark, 1999). We reported previously that human serum, but not human plasma, promotes keratinocyte migration (Henry et al., 2003).
Re-epithelialization, the process by which human keratinocytes (HKs) migrate over the wound bed and resurface the wound, is a central event that resolves problems associated with skin wounds, that is, infection, water loss, metabolic disturbance, nutritional loss, and pain. Re-epithelialization largely depends upon the migration of HKs across a wound bed, in which the microenvironment (GFs and connective tissue components) is very different from that experienced by the same HKs in unwounded skin (Odland and Ross, 1968; Woodley et al., 1993). The physiological factors that induce HKs to migrate from the wound margins are not fully defined. However, we compared all reported HK pro-motility factors and demonstrated that transforming growth factor-α (TGF-α) and insulin are the most potent “mitogens” in human serum that enhance ECM-initiated HK migration (Li et al., 2006). HK migration occurs long before cell division, which occurs only after a 36–48 hour lag phase (Li et al., 2004c). Within the wound bed, fibronectin and collagen are two ECM components capable of initiating HK migration. Odland and Ross (1968) demonstrated by electron microscopy that dramatic morphological changes occur when HKs transform from a stationary mode to a migratory mode. Stationary cells are polar and cuboidal and exhibit desmosomal and hemidesmosomal connections. When the same cells are in a migratory mode, they flatten out, extend lamellipodia with a ruffled plasma membrane, dramatically decrease surface desmosomes and hemidesmosomes, retract their tonofilaments to a perinuclear location, increase surface gap junctions, and dramatically assemble actin cables that form belt-like structures related to the inner aspects of the plasma membrane (Woodley et al., 1986).
GFs stimulate cell migration by signal transduction from their cell-surface receptors to cytoplasmic signaling networks and then to gene expression in the nucleus. Products of the newly expressed genes in turn act either intracellularly or extracellularly to execute the motility signal initiated at the cell surface, resulting in cell migration. While a great many previous studies focused on individual signaling pathways or genes involved in the control of cell migration, there have been few studies that have systematically analyzed the gene profiles of cell motility-specific signals. A major reason was the technical problems of sorting out the inseparable pleiotropic effects of a given GF, such as mitogenic, motogenic, and factor-specific effects. Therefore, genes previously reported in GF-treated cells were actually a mixture of genes induced by multiple distinct signals from the same GF. In this study, we have designed and used a unique approach to separate migration signal-regulated genes from genes unrelated to migration and yet induced by the same GF.
RESULTS
Strategy No. 1 for identification of HK motility-specific gene expression: dual pro-motility GF stimulation
A major challenge in identifying a cell migration-specific gene profile is to separate migration signal-regulated genes from genes that are migration-unrelated, but often coexpressed in response to the same GF stimulation, such as proliferation-related genes or genes involved in factor-specific responses (such as glucose-uptake genes specifically induced by insulin and not by other GFs). We have previously shown that migration of primary human HKs could be equally stimulated by more than one GF, specifically TGF-α and insulin (Li et al., 2006). This property would allow us to select for the genes commonly regulated by both GFs and, at the same time, to eliminate factor-specific genes. Therefore, we selected TGF-α and insulin as two parallel migration-promoting cues to independently induce gene expression in HKs.
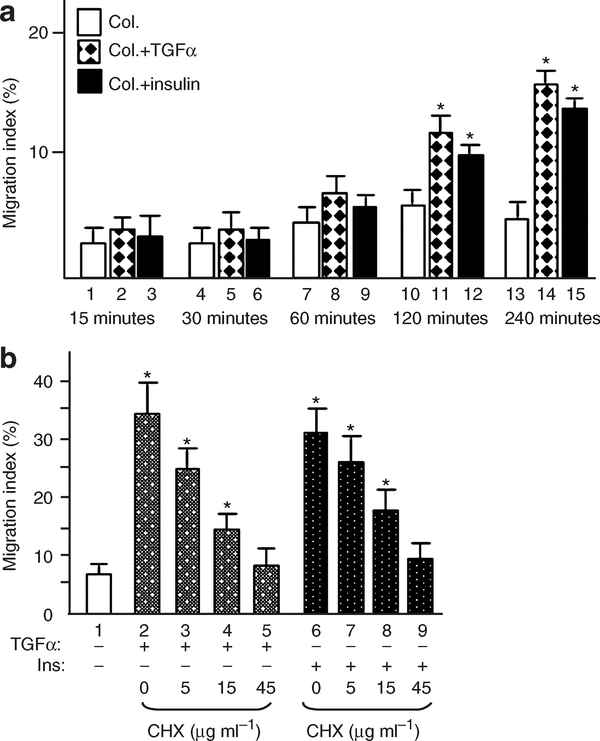
We first wanted to determine what time points of the GF stimulation should be used and why. The time course of TGF-α and insulin-stimulated HK migration, as shown in Figure 1a, revealed that, for the initial 60 minutes following stimulation, there was little detectable enhancement of HK migration in response to either TGF-α (diamond-filled bars) or insulin (solid black bars) over collagen control (open bars). A significant stimulation of HK migration became evident 120 minutes after TGF-α or insulin stimulation (bars 11 and 12 vs 10). These observations suggested that, following addition of a GF, quiescent HKs need at least 60 minutes of “cellular preparation” prior to exhibiting induced migration. To test if de novo protein synthesis is required, we treated HKs with increasing concentrations of the protein synthesis inhibitor cycloheximide and subjected the cells to migration assays in response to TGF-α or insulin. As shown in Figure 1b, cycloheximide inhibited both TGF-α-stimulated (bars 3–5) and insulin-stimulated (bars 7–9) HK migration in a dose-dependent manner. The inhibitory effects were not due to cellular cytotoxicity, because more than 94% of the cells survived in fresh medium after removal of cycloheximide. We decided to focus on the gene expression profiles following 30 minutes (to detect immediately-early genes, or IEGs), 60 minutes (early genes, or EGs), and 120 minutes (delayed-early genes, or DEGs) stimulation of HKs by TGF-α and insulin. GF-induced IEGs, EGs, and DEGs have previously been well defined by Stiles and Herschman (Rollins and Stiles, 1989; Herschman, 1991).
Figure 1. TGF-α and insulin-stimulated keratinocyte migration on type I collagen.
HKs were serum-starved overnight and subjected to colloidal gold migration assays in the presence or absence of TGF-α and insulin. Only migration index (MI) is presented, as previously described (Li et al., 2004a). MI is defined as the percentage of the track area compared with the total area under the microscope and is quantified by computer-assisted analyses. (a) The migration of keratinocytes was time-dependent in response to TGF-α or insulin. HKs showed significant migration after 2 hours of GF stimulation. This experiment was repeated more than three times. (b) Cycloheximide was added at the indicated concentrations to the cells 30 minutes prior to GF stimulation. Presence of cycloheximide inhibited HK migration stimulated by TGF-α or insulin in a dose-dependent manner, in comparison with control (lanes 1, 2, 6) (*P<0.001). The experiment was repeated twice.
Strategy No. 2 for identification of migration-specific gene expression: using TGF-β to suppress proliferation signal-induced genes
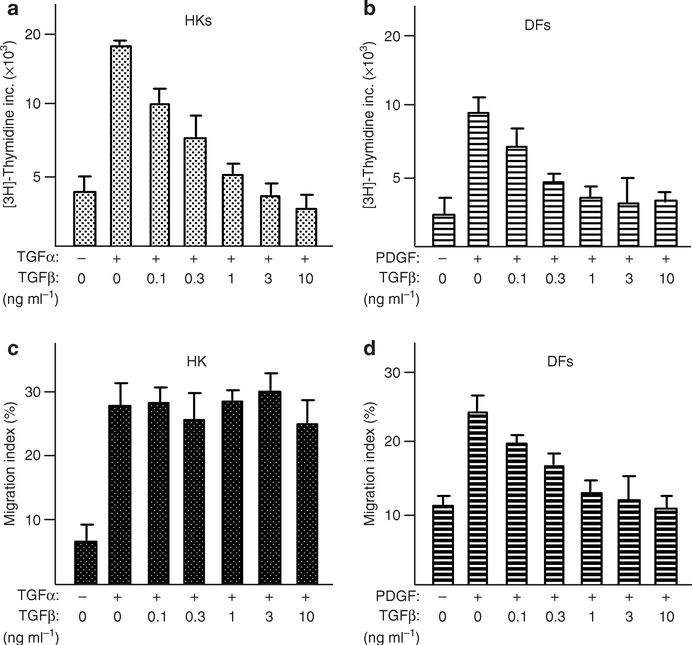
As mentioned previously, many GFs stimulate both proliferation and migration in HKs, like TGF-α and insulin. To separate these two major signaling events and focus on the migration-specific genes, we took advantage of our previous observation that TGF-β inhibits serum-stimulated HK proliferation but not migration (Sarret et al., 1992; Bandyopadhyay et al., 2006). To verify if this is also true for TGF-α- and insulin-stimulated proliferation versus migration in HKs, we carried out HK migration and proliferation assays in the presence or absence of TGF-β. In the same experiments, human dermal fibroblasts were included as control. As shown in Figure 2, TGF-β inhibited the proliferation of both TGF-α-stimulated HKs (Figure 2a) and platelet-derived growth factor-BB-stimulated human dermal fibroblasts (Figure 2b) in a dose-dependent manner. As shown previously (Bandyopadhyay et al., 2006), TGF-β also inhibited platelet-derived growth factor-BB-stimulated human dermal fibroblast migration (Figure 2c). However, TGF-β was unable to block TGF-α-stimulated HK migration (Figure 2d). Similar results were obtained for insulin-stimulated HK migration (data not shown). Thus, in HKs, TGF-α and insulin signaling to promote proliferation and migration can be separated by the presence of TGF-β. This unique property of HKs became extremely useful for our purpose of identifying the migration-specific gene profiles in the cells.
Figure 2. TGF-β blocked GF-stimulated HK proliferation, but not migration.
Colloidal gold migration assay and DNA synthesis assay were carried out as described previously by us (Bandyopadhyay et al., 2006). (a–d) TGF-β blocked GF-induced proliferation of HKs and dermal fibroblasts (DFs) in a dose-dependent manner (a and b). However, TGF-β selectively blocked GF-induced migration of DFs and not HKs (c and d). The experiments were repeated more than three times.
Final design of approach
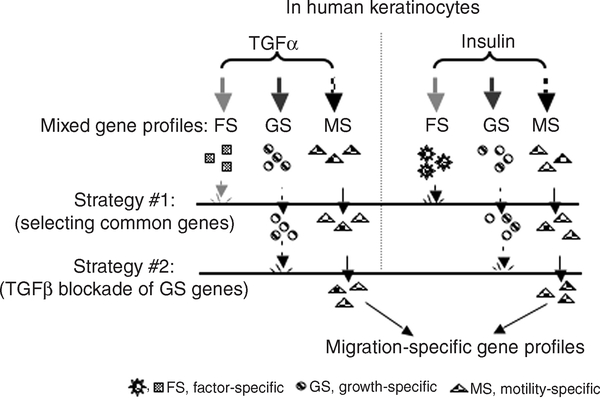
On the basis of (i) dual GF stimulation and (ii) selective inhibition of HK proliferation by TGF-β, the entire design of our approach is schematically shown in Figure 3. Therefore, we carried out the following sequential experimental procedures, aiming to identify migration-specific gene profiles: (1) HKs were to be pretreated with TGF-β to block cell proliferation and, therefore, the expression of key growth-related genes; (2) the cells, then, were to be stimulated with TGF-α or insulin for 30, 60, and 120 minutes; (3) total RNA was to be isolated from the cells and subjected to DNA microarray analyses; (4) commonly regulated genes and factor-specific genes in response to both TGF-α and insulin were to be identified. Steps 1–4 were to be repeated using fresh cell cultures, prior to step 5: (5) 12 genes were to be randomly selected and subjected to quantitative real-time reverse transcription–PCR (QRT–PCR) verification.
Figure 3. A schematic representation of the overall design of approach.
TGF-α and insulin are two potent stimulators of human keratinocyte migration. Therefore, dual stimulation with both GFs was used to eliminate the factor-specific (FS) genes, such as insulin-specific genes involved in glucose uptake. TGF-β selectively inhibits TGF-α- or insulin-stimulated proliferation, and not migration, of human keratinocytes, preventing growth-specific (GS) gene expression. After two layers of “gene filtration”, motility-specific (MS) gene profiles are anticipated.
To investigate whether the early signaling pathways (that is, prior to transcription activation) of TGF-α and insulin are affected by the co-presence of TGF-β, the lysates of cells that were serum-starved and GF-stimulated in the absence or presence of TGF-β were subjected to immunoblotting analyses with anti-phosphotyrosine, anti-phospho-Smad, or a loading control antibody. As shown in Figure 4, TGF-α stimulation for 30, 60, and 120 minutes caused increased tyrosine phosphorylation of several previously known protein species, including the 170-kDa epidermal growth factor receptor, the 140-kDa phospholipase-C-γ, the 62-kDa Dok, and the 42/44-kDa ERK½ (Figure 4a, lanes 2–4 vs lane 1). Weak detection of the epidermal growth factor receptor tyrosine phosphorylation was expected because it peaks around 5 minutes after TGF-α stimulation and then declines (data not shown). In addition, we used a physiological concentration of 20ngml−1 of TGF-α (in contrast to the 200–500 ng ml−1 frequently used for short periods of stimulation; see Li et al., 2006 and references therein). Co-presence of TGF-β did not affect the pattern or intensity of the phosphotyrosine proteins (Figure 4a, lanes 6–8 vs lane 5). Similarly, 5 μgml−1 insulin stimulation caused increased phosphorylation of the well-characterized 70-kDa β subunit of the insulin receptor (Figure 4b, lanes 2–4 vs lane 1). As expected, co-presence of TGF-β did not interfere with the insulin-stimulated receptor activation (Figure 4b, lanes 6–8 vs lane 5). These data suggest that TGF-β’s blocking point is likely at downstream transcription control levels. For example, it has previously been shown that TGF-β blocks the expression of the IEG c-myc (Pietenpol et al., 1990). To make sure that TGF-β signaling remains active during the entire 120-minute period of GF stimulation, we tested the activation of Smad2/3, a downstream signaling molecule for TGF-β. As shown, TGF-β-induced phosphorylation of Smad2/3 remained detectable for the entire 2 hours in both TGF-α-stimulated (Figure 4c, lanes 5–8) and insulin-stimulated (Figure 4e, lanes 5–8) cells. In contrast, neither TGF-α nor insulin stimulation alone caused any detectable phosphorylation of Smad2/3 (Figure 4c and d, lanes 1–4). Anti-p38 blotting of the same membrane was used as an equal sample loading control (Figure 4d and f).
Figure 4. TGF-β did not affect the early signaling by TGF-α or insulin.
(a and b) Serum-starved HKs were unstimulated or stimulated with TGF-α (20ngml−1) or insulin (5 μgml−1) in the absence or presence of TGF-β. Total lysates of the cells were subjected to immunoblotting with anti-phosphotyrosine antibodies. TGF-α or insulin stimulated tyrosine phosphorylation of the previously reported proteins (lanes 1–4). None of the phosphotyrosine proteins were affected by the presence of TGF-β (lanes 5–8). The effectiveness of TGF-β presence was indicated by increased Smad phosphorylation in both TGF-α- (c and d) and insulin- (e and f) treated cells. The experiments were repeated three times.
To estimate how many TGF-α-regulated genes were blocked in TGF-β-pretreated HKs, HKs were pretreated with or without TGF-β prior to TGF-α stimulation, which promotes both migration and proliferation. Total RNA isolated from the cells was analyzed by DNA microarray analysis. We found that TGF-β pretreatment blocked 124 of the total 500 TGF-α-regulated (up- and downregulated) genes at the 60-minute time point (data not shown). These data indicate that the approach of using TGF-β to block cell-proliferation-related gene expression is feasible.
Commonly up- and downregulated IEG, EG, and DEG profiles in migrating, but not proliferating, HKs
We used SScore to compare the expression levels of each gene before and after GF stimulation at each of the three time points. SScore determines the significance of the relative expression level by probe-to-probe comparison (Kennedy et al., 2006). The results are shown in Table 1. At 30-minute stimulation (to detect IEGs), TGF-α upregulated a total of 71 genes and downregulated a total of 46 genes. At the same time point, insulin upregulated 75 genes and downregulated 28 genes. Among them, 25 upregulated genes and one downregulated gene were detected in both TGF-α- and insulin-stimulated cells. Therefore, among the IEGs, approximately 35% of the genes upregulated and 2% of the genes downregulated by TGF-α were shared by insulin stimulation, whereas 33% of the genes upregulated and 4% of the genes downregulated by insulin were shared by TGF-α stimulation.
Table 1.
TGF-α- and insulin-regulated total and common genes
| Upregulated genes |
Downregulated genes |
|||||
|---|---|---|---|---|---|---|
| Growth factor | 30 min | 60 min | 120 min | 30 min | 60 min | 120 min |
| TGF-α | 71 | 174 | 209 | 46 | 41 | 74 |
| Insulin | 75 | 109 | 42 | 28 | 47 | 39 |
| Shared genes | 25 | 58 | 13 | 1 | 15 | 3 |
| % | 35 (TGF-α) 33 (ins.) | 33 (TGF-α) 53 (ins.) | 6 (TGF-α) 31 (ins.) | 2 (TGF-α) 4 (ins.) | 37 (TGF-α) 32 (ins.) | 4 (TGF-α) 8 (ins.) |
ins., insulin; TGF, transforming growth factor.
The result of DNA microarray analysis is shown, on the basis of the significance of gene expression (P<0.05) in the probe-based analysis calculated by using SScore software. The numbers shown from TGF-α- or insulin-stimulated cells for the indicated times are the total numbers of up- or downregulated genes. Among them, the commonly up- or downregulated genes are referred to as “shared genes”. The percentage of shared genes over the total number of genes regulated under TGF-α or insulin treatment is shown as “%”.
At 60-minute stimulation (to detect EGs), TGF-α caused upregulation of 174 genes and downregulation of 41 genes. Insulin caused upregulation of 109 genes and downregulation of 47 genes. Among them, 58 upregulated and 15 downregulated genes were detected in both TGF-α- and insulin-stimulated cells. Among the EGs, 33% of the genes upregulated and 37% of the genes downregulated by TGF-α were shared by insulin stimulation, whereas 53% of the genes upregulated and 32% of the genes downregulated by insulin were shared by TGF-α stimulation.
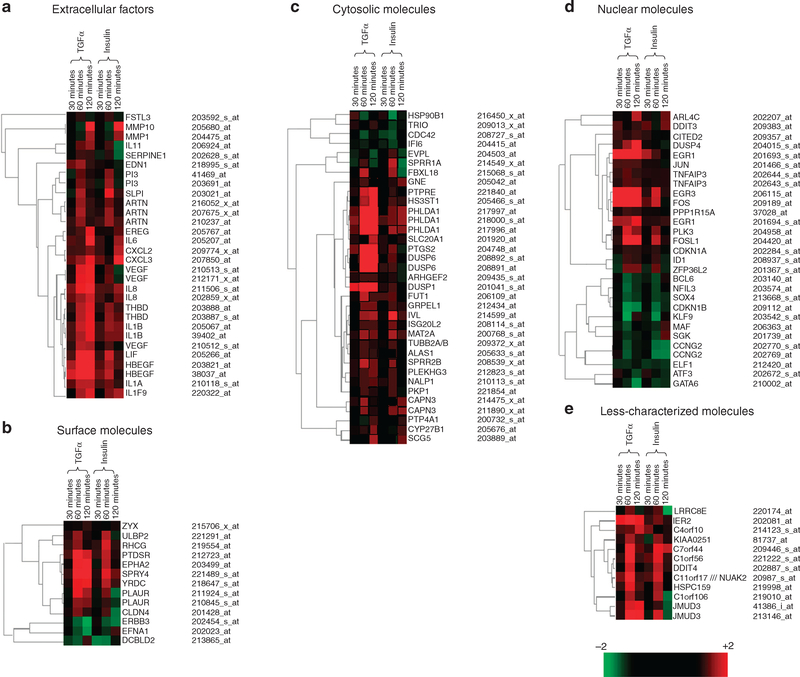
At 120-minute stimulation, TGF-α upregulated 209 genes and downregulated 74 genes. Insulin upregulated 42 genes and downregulated 39 genes. Among them, 13 upregulated and 3 downregulated genes were detected in both TGF-α- and insulin-stimulated cells. Among these DEGs, 6% of the genes upregulated and 4% of the genes downregulated by TGF-α were shared by insulin, whereas 31% of the genes upregulated and 8% of the genes downregulated by insulin were shared by TGF-α stimulation. The genes that were significantly regulated (P<0.05) by both TGF-α and insulin are presented in Figure 5. As shown, we classified the identified genes into five different categories on the basis of the cellular location of the genes’ products. The five categories are the following: extracellular factors, cell-surface molecules, cytosolic molecules, nuclear molecules, and less-well-characterized proteins. We generated a dendrogram for the genes in each category and, therefore, created a total of five dendrograms. These analyses revealed the kinetics of the gene expression and a comparison of their expression patterns. The upregulated genes are shown in red, and the downregulated genes are shown in green. The unchanged genes are shown in black. The degree of the change is shown by an increasing intensity of the color, as indicated by the scale bar.
Figure 5. The DNA microarray gene profile.
The relative expression levels of the genes in HKs in response to TGF-α or insulin stimulation for 30, 60, and 120 minutes are shown by dendrograms. Only those genes that were significantly regulated by both TGF-α and insulin for at least one time point are shown. These genes were classified as follows into different categories on the basis of the locations of their protein products: extracellular factors (a), surface molecules (b), cytosolic molecules (c), nuclear molecules (d), and less characterized molecules (e). The genes that were upregulated at certain time points are shown in red, and those downregulated at certain time points are shown in green. Genes with similar kinetic expression patterns in response to both TGF-α and insulin were clustered together. Similarities of the expression patterns are indicated by the hierarchy lines on the left-hand side. For certain genes, there were more than one probe set that detected the expression of the genes. The probe sets, which detected a significant difference in response to the GF stimulation for at least one time point, are presented here. The final presented data resulted from the consensus of DNA array analysis of mRNA samples from two independent cultures for each time point.
To understand the expression profiles, we analyzed IEGs, EGs, and DEGs according to the cellular locations and functions of their encoded gene products (Table S1). Among the IEGs upregulated, 10 genes were extracellular factors (40%), one gene was a cell-surface protein (4%), five genes were cytosolic molecules (20%), eight genes were nuclear molecules (32%), and one was less characterized (4%). Only one IEG, a nuclear protein, was downregulated.
Among the EGs upregulated, 13 were secreted extracellular factors (23%), eight were cell-surface molecules (14%), 21 were cytosolic molecules (36%), 6 were nuclear molecules (10%), and 10 were less characterized (17%). Among the EGs downregulated, 2 were cell-surface molecules (13%), 4 were cytosolic molecules (27%), and 9 were nuclear molecules (60%). For the DEGs up-regulated, 7 were extracellular factors (54%), 5 were cytosolic molecules (38%), and 1 was a nuclear molecule (8%). For the DEGs downregulated, 1 was a cell-surface molecule (33%), 1 was a cytosolic molecule (33%), and 1 was a nuclear molecule (33%).
Verification of DNA microarray data by QRT–PCR
To verify the gene profiles of the microarray analyses, we randomly selected 12 genes that were either up- or downregulated at at least one of the three stimulation time points by both TGF-α and insulin. Expression of these genes in the original total RNA samples was analyzed by QRT–PCR analyses. A total of 34 reactions for both TGF-α (17 reactions) and insulin (17 reactions) were carried out. The DNA sequences of the 12 pairs of real time PCR primers plus a pair of glyceraldehyde-3-phosphate dehydrogenase primers are shown in Table S2. As shown in Table S3, data from the QRT-PCR analyses on all the genes selected were qualitatively consistent with those of DNA microarray analyses. Quantitatively, however, among the 34 reactions, 28 (82%) exhibited greater-fold changes by QRT–PCR than by microarray analyses. The remaining six reactions showed less-fold changes between QRT–PCR and microarray analyses.
Functional characterization of three secreted gene products
Finally, to investigate whether the “migration signal-regulated” genes indeed play a role in HK migration, we selected three genes that all encode secreted polypeptides: heparin-binding EGF-like GF (HB-EGF), stromelysin-2 (matrix metalloproteinase-10 (MMP-10)), and chemokine (CXC family) ligand 3 (CXCL3). Both HB-EGF and MMP-10 have previously been reported to promote HK migration and wound healing (Hashimoto et al., 1994; Krampert et al., 2004; Shirakata et al., 2005). While little is known about the role of CXCL3 in HK migration, mice that are deficient in CXCR2, a receptor for CXC family ligands, show delayed wound healing (Devalaraja et al., 2000). We designed small hairpin RNAs against each of the three genes and cloned them into the lentiviral small interfering RNA delivery system, FG12 (see Materials and Methods). As previously demonstrated by us, the FG12 system offers more than 95% RNA interference (RNAi) transduction efficiency and permanent gene knockdown in HKs (Bandyopadhyay et al., 2006; Fan et al., 2006).
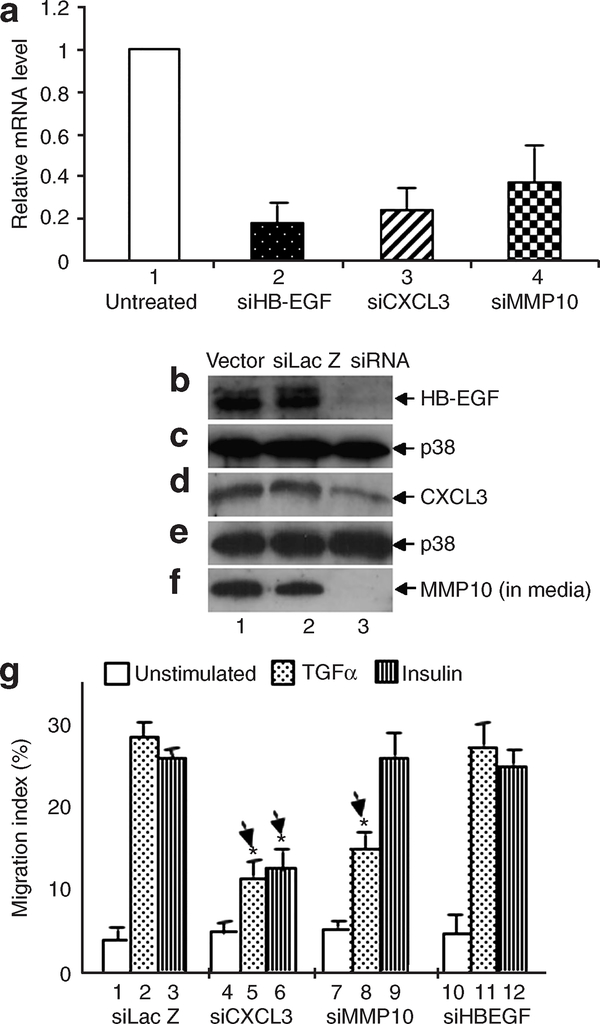
To verify the effectiveness of the RNAi constructs, total RNA was extracted from the cells and subjected to QRT–PCR analyses. As shown in Figure 6a, in siRNA against heparin binding EGF-like growth factor (siHB-EGF)-infected HKs, the HB-EGF mRNA level was reduced by 83% (bar 2) in comparison with that in HKs infected with control siLac Z (bar 1). Similarly, the mRNA levels of CXCL3 and siMMP-10 were reduced by more than 60–70% (bars 3 and 4). At the protein level, cell lysate or conditioned medium was collected from the gene knockdown or control (vector alone or siLacZ) cells and subjected to immunoblotting assays with antibodies against each of the three gene products. Consistently, the level of HB-EGF protein was reduced more than 95% (Figure 6b, lane 3 vs lanes 1 and 2). Anti-p38 antibody immunoblots were used as sample loading controls. The protein level of CXCL3 in the cells was reduced by more than 70% in comparison with that in HKs infected with empty vector or siLacZ (Figure 6d, lane 3 vs lanes 1 and 2).
Figure 6. Functional characterization of three secreted gene products in HK migration.
HKs, infected with FG12 system carrying vector alone, control siLac-Z, or small interfering RNA against each of the three candidate genes, were subjected to RT-PCR, immunoblotting, and colloidal gold migration assays. (a) Total RNA from these cells was isolated 5 days following infection and was subjected to RT-PCR. (b–f) To detect the protein expression levels, lysates of the HKs, starved in serum-free medium overnight, were extracted and immunoblotted with antibodies against HB-EGF or CXCL3. To detect secreted MMP-10 proteins, infected HKs were starved in serum-free medium overnight. Conditioned medium (CM) was collected and concentrated 100 times. The CM was analyzed by western blot with an anti-MMP-10 antibody (f). (g) After the effectiveness of the RNAi was confirmed, colloidal gold migration assay was carried out to functionally characterize the importance of these genes in HK migration. The experiments were repeated three times (*P<0.05).
Since we were unable to detect the intracellular protein level of MMP-10 in HKs, we collected TGF-α-stimulated conditioned media of HKs infected with either control constructs or siMMP-10 and subjected them to immunoblotting analysis with anti-MMP-10 antibodies. We detected a significant amount of secreted MMP-10 from conditioned media of the control cells (Figure 6f, lanes 1 and 2). However, MMP-10 knockdown almost completely blocked MMP-10 secretion (Figure 5f, lane 3 vs lanes 1 and 2). Therefore, our RNAi approach effectively downregulated the genes of interest. As mentioned previously, this downregulation was permanent throughout the life span of the cells in culture.
The above gene-knockdown HKs were then subjected to cell migration assays in response to TGF-α and insulin. As expected, both TGF-α and insulin strongly stimulated the migration of HKs infected with empty vector lentivirus (Figure 6g, bars 2 and 3 vs bars 1). However, the GF-induced migration of siCXCL3-lentiviral-infected HKs was decreased by 40–50% in comparison to the control HKs (bars 5, 6 vs bar 2, 3). These data were quantitatively consistent with the immunoblotting results in which the cellular protein level of CXCL3 was downregulated approximately 70% by siCXCL3 (see Figure 6c). Therefore, CXCL3 is required for HK migration in response to the GFs. Surprisingly, the GF-stimulated HK migration was not affected by HB-EGF knockdown (bars 11 and 12). On the other hand, MMP-10 knockdown only slightly, but significantly, lowered TGF-α-stimulated HK migration (bar 8 vs bar 2), but did not affect insulin-induced HK migration (bar 9 vs bar 3). These results suggest that MMP-10 and HB-EGF, although secreted by HKs, are not essential for HK migration. We postulate that when HK-derived MMP-10 and HB-EGF are secreted into the wound environment, they in turn influence migration of other skin cell types such as dermal fibroblasts and dermal microvascular endothelial cells, whose migration is equally critical for wound healing and remodeling.
DISCUSSION
Cell migration and cell proliferation are two mutually exclusive events. Migrating cells are not proliferating and, similarly, proliferating cells are not simultaneously migrating. In cell migration assays, mitomycin C is often used to eliminate the potential contribution of cell proliferation to the results of these assays and, therefore, it might be a useful alternative to block proliferation-related gene expression. However, since the blocking point of mitomycin C in proliferating cells lies in the very downstream G2/M transition phase of the cell cycle, it will not block the growth signal-induced EG expression. Therefore, mitomycin C does not help to identify migration signal-specific genes. The central idea of this study is to make use of the fact that TGF-β blocks HK proliferation but not migration (Sarret et al., 1992; Bandyopadhyay et al., 2006). In addition, however, TGF-β is known to block the expression of both IEGs, such as c-myc (Pietenpol et al., 1990), and Rb-regulated DEGs (Koike et al., 1994). Furthermore, to further ensure the “purity” of the migration-related gene profiles, we stimulated HKs with two distinct pro-motility factors. We have shown that TGF-α and insulin are the main keratinocyte pro-motility factors in human serum (Li et al., 2006).
It is well established that TGF-β′s effects can be divided into primary and secondary effects. Its primary effects on most cell types include antiproliferation and/or antimotility. Its secondary and main effect is to induce expression of many genes, including ECMs and mitogenic/motogenic GFs, cytokines, and MMPs. These newly produced factors could in turn act in either an autocrine or a paracrine manner to affect various responses of the cells. Our migration assays, colloidal gold and “scratch” assays, last for 12–16 hours. Within this period, the physiological concentrations of TGF-β did not cause any significant change in human keratinocyte migration stimulated by TGF-α, as we and others have previously reported, (Sarret et al., 1992;Tsuboi et al., 1992; Garlick and Taichman 1994;Ashcroft et al., 1999). However, as mentioned earlier, the ECM and GFs produced by the cells in response to TGF-β may later act to stimulate cell migration (for example, >48 hours) (Hebda 1988; Nickoloff et al., 1988).
Microarray analysis allows profiling large-scale and comparative gene expression under two different conditions. Therefore, this approach can provide a useful global picture of the gene expression involved in a given activity that takes place inside the cells. In conjunction with the available knowledge on the kinetics and functions of a given gene, the technique is helpful to reveal intracellular signaling networks that execute a specific cellular response such as motility. Here, we have identified 82 genes commonly upregulated and 19 genes commonly downregulated by both TGF-α and insulin following stimulation for up to 2 hours. Among them, 31 of the upregulated genes (38%) have previously been reported to play a role in cell migration, and 9 of the downregulated genes (47%) were reported for cell migration. Among the 14,500 well-characterized genes in the Affymetrix HU-133A 2.0 array, there are only 187 probes representing 72 genes (0.5%) that are known to relate to cell migration. Therefore, our strategy “enriched” the pool of migration signal-specific genes more than 80-fold. In analyzing the DNA microarray data, we used the SScore program to select genes significantly regulated by both TGF-α and insulin and generated dendrograms based on this selection. In the dendrograms, genes with more than one probe on the microarray chip were shown more than once, such as HB-EGF and PHLDA1. These different probe sets for the same genes tend to cluster together, indicating that their expression patterns are similar. Results of this analysis further confirmed the induction or repression of the genes by TGF-α and insulin, since multiple probe sets for the same genes showed similar expression patterns.
Our study focused on the migration-specific gene profiles in primary human keratinocytes in vitro. Are these profiles relevant to those in HKs in vivo? Unfortunately, this kind of study using human skin wounds is technically impossible. It is not possible to wound human skin, take biopsies at different time points, isolate the RNA from them, and obtain gene expression profiles. Even in mice, it is not feasible to isolate the wound cells/tissues without contaminating the specimens with unwounded tissue. Since the latter is not in the environment of the wound, the gene expression profiles from unwounded tissue do not reflect wound healing. Furthermore, gene profiles from mouse tissue may not be at all relevant to the gene profiles of human skin wounds. Second, in a real skin wound, the environment is a mixture of multiple factors (cytokines, GFs, ECMs, MMPs, and so on). It is not possible to distinguish what genes are inflammation-related, proliferation-related, migration-related, differentiation-related, metabolism-related, and so on. It is impossible to know which gene is induced by what factor.
While microarray has been widely used to determine gene expression patterns under various environmental and cellular conditions, to our knowledge this is the first report of gene expression profiles designed in response to a specific type of cell-surface signal. Several previous studies also used a DNA microarray approach to identify gene profiles associated with wound healing. Fitsialos et al. surveyed the gene expression profiles during the course of an in vitro scratch assay using cultured HKs. They used inhibitors of extracellular signalregulated kinase, p38-mitogen-activated protein kinase, and phosphatidylinositol 3-kinase to narrow down the pathway-specific gene expression profiles (Fitsialos et al., 2007). They identified several extracellular factors that were induced at early time points (1–3 hours), such as HB-EGF, vascular endothelial growth factor, epiregulin (EREG), LIF, SERPINE-1, and IL-8, and transcription factors, such as Fos, Jun, and EGR1. These results are consistent with our observations. Roy et al. (2007) compared the gene expression profiles in wound-derived blood vessels and blood vessels from intact human skin. They demonstrated that the extracellular proteinase MMP-1 was upregulated, which was also observed in our study. In conclusion, our study has provided comprehensive profiles of the EG expression induced by HK pro-motility signals. These profiles may serve as a foundation to gain new insights into the mechanism of the re-epithelialization process during wound healing.
MATERIALS AND METHODS
Primary HKs were purchased from Cascade Biologics (Portland, OR) and cultured in Epilife supplemented with human keratinocyte growth supplements, according to the manufacturer’s instruction. HKs at passages 4–5 were used in all experiments. Native rat-tail type I collagen was from BD Biosciences (Bedford, MA). Recombinant human TGF-α was from R&D Systems, Inc. (Minneapolis, MN). Recombinant human insulin was from Gibco (Grand Island, NY). The Human Genome U133A 2.0 GeneChip Array was obtained from Affymetrix (Santa Clara, CA). Colloidal gold (gold chloride, G4022) was from Sigma (St Louis, MO). The anti-phospho-Smad2/3 antibody was from Cell Signaling (Danvers, MA). The anti-human MMP10 antibody was from Chemicon (Temecula, CA). The anti-human HB-EGF antibody was from R&D Systems. The anti-human CXCL3 antibody was from Aviva Systems Biology (San Diego, CA). Restriction enzymes and T4 DNA ligase were from New England BioLabs (Beverly, MA). Plasmid Midi kit was from Promega (Madison, WI). XL-10 Gold Ultra competent cells (XL-10 Gold) were from Stratagene (Kingsport, TN). All other reagents and supplies, unless indicated, were from VWR (Bristol, CT) or Sigma.
Treatment of HKs with stimulators and inhibitors
HKs were seeded on collagen I-precoated tissue culture plates (150 mm) at around 45% confluence (~2.5 × 106cells) in complete medium and incubated overnight. The next day, the cells (~4.5 × 106 cells) were deprived of GFs and incubated in serumfree medium for 16 hours to arrest the cells at G0/G1 phase. The cells were then washed with pre-warmed serum-free medium and incubated in fresh serum-free medium containing 20ngml−1 TGF-β for 20minutes at 37°C to block the expression of growth signal-related genes. TGF-α or insulin was added (in the continued presence of TGF-β) for 30, 60, and 120 minutes. At the end of each stimulation time point, the cells were washed with ice-cold phosphate-buffered saline to halt the stimulation and subjected to RNA isolation, as described below. Duplicate plates (100 mm) under the same conditions were subjected to immunoblotting analyses for phosphotyrosine and phospho-Smad proteins.
RNA extraction and microarray
Total RNA was extracted from HKs using the RNeasy mini kit (Qiagen) following the manufacturer’s user manual. After the RNA’s quality was confirmed by the 260/280 nm ratio of spectrometer readings and by RNA electrophoresis, the RNA was used for microarray processing on a Human Genome U133A 2.0 Array containing 22,000 oligonucleotide probe sets. This array represents 18,400 transcripts and variants, including 14,500 well-characterized human genes. Briefly, RNA was hybridized with a T7-(dT) promoter and reverse-transcribed to cDNA using Superscript II reverse transcriptase (200 U μl−1). Second-strand cDNA was synthesized by DNA polymerase I. For in vitro reverse transcription, T7 RNA polymerase and biotin-labeled ribonucleotides were also added. The synthesized biotin-labeled cRNA was fragmented and then hybridized to probe the array at 45 °C for 16 hours. The data were analyzed using DCHIP software (Li and Hung Wong, 2001). The signal for each probe was verified by the PM/MM ratio and reported as present, marginal, or absent. One-to-one comparisons were performed with samples from control versus growth-factor-treated cells. The data were discarded if signals were absent from both the groups. The comparison of the expression level of each gene between GF treatment and control was analyzed by SScore, a program from Bioconductor (Kennedy et al., 2006), and genes with P<0.05 were selected. The expression patterns of selected genes were summarized by hierarchical clustering analysis (Eisen et al., 1998). The entire DNA array experiment was repeated from cell culture to data analyses and the data of consensus among the experiments are presented.
QRT-PCR
Extracted total RNA was first subjected to reverse-transcription assays using TaqMan Reverse Transcription Reagents with oligo d(T)16 as the primer. The detailed procedure was as described in the user manual (Applied Biosystems, Branchburg, NJ). Real-time PCR was performed using SYBR Green PCR Master Mix. The reactions were performed in 384-well clear optical reaction plates in the 7900HT system and exported by the SDS 2.1 program. All the instruments and reagents related to reverse-transcription reaction and real-time PCR were from Applied Biosystems. Primers for realtime PCR were designed using the on-line program PrimerQuest from Integrated DNA Technology Inc. (Coralville, IA).
RNAi design and delivery by lentiviral system FG12
The RNAi Selection Program, as described previously (Yuan et al., 2004), was used to identify possible RNAi target sequences, which were then scored as reported (Reynolds et al., 2004). The selected RNAi sequences against MMP10 and HB-EGF, two well-known pro-motility factors, and CXCL3, a less characterized gene in HK migration, were synthesized and cloned into the lentiviral RNAi delivery vector, FG12 (Qin et al., 2003). Transfection and virus stock production were as described previously by us (Guan et al., 2007). The gene transduction efficiency was analyzed with a fluorescence microscope for a coexpressed green fluorescent protein marker gene on the same vector. The selected RNAi sequences (sense) against the three selected genes were as follows: for human MMP10, as previously reported (Meyer et al., 2005); for human HB-EGF, GAAGUUGGGCAUGACUAAU; and for human CXCL3, GUCCGU GGUCACUGAACUG. Downregulation of the gene products was verified by immunoblotting analyses with antibodies against MMP-10, HB-EGF, or CXCL3.
Cell migration and DNA synthesis assays
The colloidal gold migration assay and [3H]thymidine incorporation assay were as previously described (Li et al., 2004a; Bandyopadhyay et al., 2006).
Patient consent for experiments was not required because French laws consider human tissue left over from surgery as discarded material. All the above studies received USC institutional approval and adhered to the Declaration of Helsinki Principles. The GEO accession number is GSE853.
Supplementary Material
Table S1. Human keratinocyte motility gene profiles.
Table S2. Primer sequences for quantitative PCR.
Table S3. Gene profile verification by QRT–PCR.
ACKNOWLEDGMENTS
We thank the USC-CHLA genome core lab for processing microarray and data analyses. This study was supported by NIH Grants GM/AR67100-01 (to WL) and AR46538 (to DTW).
Abbreviations
- CXC
chemokine
- DEG
delayed-early gene (<120 minutes GF stimulation)
- ECM
extracellular matrix
- EG
early gene (<60 minutes GF stimulation)
- GF
growth factor
- HB-EGF
hairpin-binding EGF-like GF
- HK
human keratinocyte
- IEG
immediate-early gene (<30 minutes GF stimulation)
- MMP
matrix metalloproteinase
- QRT-PCR
quantitative real-time reverse transcriptase–PCR
- RNAi
RNA interference
- TGF-β
transforming growth factor-β
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE et al. (1999) Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1: 260–266 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay B, Fan J, Guan S, Li Y, Chen M, Woodley DT et al. (2006) A “traffic control” role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol 172: 1093–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SB (1967) Haptotaxis and the mechanism of cell motility. Nature 213:256–60 [DOI] [PubMed] [Google Scholar]
- Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN et al. (2000) Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol 115:234–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP (2001) Integrin and growth factor receptor crosstalk. Circ Res 89:1104–10 [DOI] [PubMed] [Google Scholar]
- Fan J, Guan S, Cheng CF, Cho M, Fields JW, Chen M et al. (2006) PKCdelta clustering at the leading edge and mediating growth factor-enhanced, but not ECM-initiated, dermal fibroblast migration. J Invest Dermatol 126:1233–43 [DOI] [PubMed] [Google Scholar]
- Fitsialos G, Chassot A, Turchi L, Dayem MA, LeBrigand K, Moreilhon C et al. (2007) Transcriptional signature of epidermal keratinocytes subjected to in vitro scratch wounding reveals selective roles for ERK½, P38, and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 282: 15090–102 [DOI] [PubMed] [Google Scholar]
- Garlick JA, Taichman LB (1994) Effect of TGF-β1 on re-epithelialization of human keratinocytes in vitro: an organotypic model. J Invest Dermatol 103:554–9 [DOI] [PubMed] [Google Scholar]
- Guan S, Chen M, Woodley DT, Li W (2007) Nck{beta} adapter controls neuritogenesis by maintaining cellular paxillin level. Mol Cell Biol 27: 6001–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345–57 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Higashiyama S, Asada H, Hashimura E, Kobayashi T, Sudo K et al. (1994) Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem 269:20060–6 [PubMed] [Google Scholar]
- Hebda PA (1988) Stimulatory effects of transforming growth factor-beta and epidermal growth factor on epidermal cell outgrowth from porcine skin explant cultures. J Invest Dermatol 91:440–5 [DOI] [PubMed] [Google Scholar]
- Henry G, Li W, Garner W, Woodley DT (2003) Migration of human keratinocytes in plasma and serum and wound re-epithelialization. Lancet 361:574–6 [DOI] [PubMed] [Google Scholar]
- Herschman HR (1991) Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem 60:281–319 [DOI] [PubMed] [Google Scholar]
- Kennedy RE, Kerns RT, Kong X, Archer KJ, Miles MF (2006) SScore: an R package for detecting differential gene expression without gene expression summaries. Bioinformatics 22:1272–4 [DOI] [PubMed] [Google Scholar]
- Koike M, Ishino K, Huh N, Kuroki T. (1994) Growth inhibition of SV40-transformed human keratinocytes by TGF-βeta s is not linked to dephosphorylation of the Rb gene product. Biochem Biophys Res Commun 201:673–81 [DOI] [PubMed] [Google Scholar]
- Krampert M, Bloch W, Sasaki T, Bugnon P, Rulicke T, Wolf E et al. (2004) Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol Biol Cell 15:5242–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF (1996) Cell migration: a physically integrated molecular process. Cell 84:359–69 [DOI] [PubMed] [Google Scholar]
- Li C, Hung Wong W (2001) Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol 2:RESEARCH0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Fan J, Chen M, Guan S, Sawcer D, Bokoch GM et al. (2004a) Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol Biol Cell 15: 294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Fan J, Chen M, Woodley DT (2004b) Mechanisms of human skin cell motility. Histol Histopathol 19:1311–24 [DOI] [PubMed] [Google Scholar]
- Li W, Henry G, Fan J, Bandyopadhyay B, Pang K, Garner W et al. (2004c) Signals that initiate, augment, and provide directionality for human keratinocyte motility. J Invest Dermatol 123:622–33 [DOI] [PubMed] [Google Scholar]
- Li Y, Fan J, Chen M, Li W, Woodley DT (2006) Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration. J Invest Dermatol 126:2096–105 [DOI] [PubMed] [Google Scholar]
- Meyer E, Vollmer JY, Bovey R, Stamenkovic I (2005) Matrix metalloproteinases 9 and 10 inhibit protein kinase C-potentiated, p53-mediated apoptosis. Cancer Res 65:4261–72 [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP (1996) Actin-based cell motility and cell locomotion. Cell 84:371–9 [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Mitra RS, Riser BL, Dixit VM, Varani J (1988) Modulation of keratinocyte motility: correlation with production of extracellular matrix molecules in response to growth promoting and antiproliferative factors. Am J Pathol 132:543–51 [PMC free article] [PubMed] [Google Scholar]
- Odland G, Ross R (1968) Human wound repair. I. Epidermal regeneration. J Cell Biol 39:135–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol JA, Holt JT, Stein RW, Moses HL (1990) Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation. Proc Natl Acad Sci USA 87:3758–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XF, An DS, Chen IS, Baltimore D (2003) Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA 100:183–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A (2004) Rational siRNA design for RNA interference. Nat Biotechnol 22:326–30 [DOI] [PubMed] [Google Scholar]
- Rollins BJ, Stiles CD (1989) Serum-inducible genes. Adv Cancer Res 53:1–32 [DOI] [PubMed] [Google Scholar]
- Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A et al. (2007) Transcriptome-wild analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci USA 104:14472–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret Y, Woodley DT, Grigsby K, Wynn K, O’Keefe EJ (1992) Human keratinocyte locomotion: the effect of selected cytokines. J Invest Dermatol 98:12–6 [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH (2002) Networks and crosstalk: integrin signaling spreads. Nat Cell Biol 4:E65–8 [DOI] [PubMed] [Google Scholar]
- Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C et al. (2005) Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci 118:2363–70 [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341:738–46 [DOI] [PubMed] [Google Scholar]
- Tsuboi R, Sato C, Shi CM, Ogawa H (1992) Stimulation of keratinocyte migration by growth factors. J Dermatol 19:652–3 [DOI] [PubMed] [Google Scholar]
- Woodley DT, Chen JD, Kim JP, Sarret Y, Iwasaki T, Kim YH et al. (1993) “Re-epithelialization. Human keratinocyte locomotion”. Dermatol Clin 11:641–6 [PubMed] [Google Scholar]
- Woodley DT, Kalebec T, Banes AJ, Link W, Prunieras M, Liotta L (1986) “Adult human keratinocytes migrating over nonviable dermal collagen produce collagenolytic enzymes that degrade type I and type IV collagen”. J Invest Dermatol 86:418–23 [DOI] [PubMed] [Google Scholar]
- Yuan B, Latek R, Hossbach M, Tuschl T, Lewitter F (2004) siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res 32:W130–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Human keratinocyte motility gene profiles.
Table S2. Primer sequences for quantitative PCR.
Table S3. Gene profile verification by QRT–PCR.