Abstract
In Pompe disease, anti-drug antibodies (ADA) to acid alpha-glucosidase (GAA) enzyme replacement therapy contribute to early mortality. Assessing individual risk for ADA development is notoriously difficult in (CRIM-positive) patients expressing endogenous GAA. The individualized T cell epitope measure (iTEM) scoring method predicts patient-specific risk of developing ADA against therapeutic recombinant human GAA (rhGAA) using individualized HLA-binding predictions and GAA genotype. CRIM-negative patients were six times more likely to develop high ADA titers than CRIM-positive patients in this retrospective study, whereas patients with high GAA-iTEM scores were 50 times more likely to develop high ADA titers than patients with low GAA-iTEM scores. This approach identifies high-risk IOPD patients requiring immune tolerance induction therapy to prevent significant ADA response to rhGAA leading to a poor clinical outcome and can assess ADA risk in patients receiving replacement therapy for other enzyme or blood factor deficiency disorders.
Keywords: Pompe Disease, ADA, Immunogenicity, Immunoinformatics, enzyme replacement therapy
1.0. Introduction
1.1. Infantile-onset Pompe Disease
Pompe Disease (glycogen storage disease type II), an autosomal recessive disorder, is caused by mutations in the acid alpha-glucosidase (GAA) gene which lead to absent or reduced GAA activity. GAA normally breaks down lysosomal glycogen [1], and GAA deficiency leads to accumulation of glycogen in lysosomes, primarily in skeletal, cardiac, and smooth muscles, leading to hypotonia and hypertrophic cardiomyopathy in infants born with the disease. Without treatment, infantile-onset Pompe disease (IOPD) leads to cardiorespiratory failure and death by 2 years of age [2].
IOPD patients are typically subdivided into two groups: those with detectible endogenous GAA on Western blot (cross-reactive immunologic material [CRIM]-positive) and those without (CRIM-negative). CRIM-positive patients are assumed to be exposed to a partial or mutated endogenous GAA sequence, which may lead to partial tolerance to exogenous GAA, while CRIM-negative patients are assumed to be completely naïve to endogenous GAA (native GAA, nGAA) and are not expected to have immune tolerance to exogenous GAA.
1.2. Enzyme Replacement Therapy and Anti-Drug Antibodies
Enzyme replacement therapy (ERT) with recombinant human alglucosidase alfa (recombinant human GAA, rhGAA, alglucosidase alfa, Myozyme®, Lumizyme®) is currently the only FDA-approved treatment for Pompe disease. ERT with rhGAA improves clinical outcomes for patients with Pompe disease and increases the average patient lifespan [3-5]. However, despite an increase in survival rate and quality of life, ERT still results in the formation of anti-drug antibodies (ADA) in the great majority (85%, where measurable) of CRIM-negative patients and in roughly 32% of CRIM-positive IOPD patients [6-9]. High ADA has been characterized as either “sustained intermediate titers” (SIT; defined as antibody titers of ≥ 12,800 and < 51,200 within first year of ERT) or high sustained antibody titers (HSAT; defined as antibody titers of ≥ 51,200 more than once at or beyond 6 months on ERT). In general, sustained antibody responses decrease ERT efficacy and are associated with poor clinical response to rhGAA treatment [6, 7]. For example, pharmacokinetic studies in patients with IOPD have shown that clearance of infused rhGAA increases by 50% (on average) from week 1 to week 12 in patients with antibody titers of ≥12,800 at week 12 [10].
1.3. Immune Tolerance Risk Assessment
A prophylactic immune tolerance induction (ITI) protocol using rituximab, methotrexate, and/or IVIG has been successful in preventing the development of HSAT in CRIM-negative IOPD patients especially when administered in the ERT-naïve setting, and has become the standard of care for CRIM-negative patients, in whom HSAT is expected [11-13]. ADA responses have proven to be difficult to predict in CRIM-positive patients, and there is currently no way to accurately determine which CRIM-positive patients are at risk of developing HSAT and a subsequent clinical decline. Treating all CRIM-positive patients with prophylactic ITI may unnecessarily expose more than two thirds of CRIM-positive patients to immune-modulating drugs and potential adverse effects associated with the treatment.
1.4. Proposed ADA Risk Assessment Approach
T cells contribute to the development of anti-drug antibodies by driving the maturation of B cell response. T cell epitopes are short linear sequences derived from proteins that are presented by HLA-DRB1 molecules on antigen-presenting cells. Treatment of patients with protein therapeutics that contain large numbers of foreign (non-self) HLA-DR-restricted T cell epitopes is known to be associated with the development of ADA [14]. The presence or absence of a matched T cell epitope in the native GAA (nGAA) may determine tolerance or lack of tolerance to rhGAA ERT. Comparing HLA-DR restricted T cell epitopes in the native GAA sequence to HLA-DR restricted T cell epitopes in rhGAA, for each individual patient, may provide a better estimate of the risk for immunogenicity than CRIM status alone. We evaluated an individualized T cell epitope measure (iTEM) approach to ADA prediction in this study [15]. The EpiMatrix T cell epitope prediction algorithm [16] was used to identify potential T cell epitopes within rhGAA that were absent in the native GAA for each individual in a cohort of 24 IOPD subjects, based on their HLA-DRB1 alleles and the pathogenic variants in their individual GAA gene. For each subject, a sum of the predicted individual T cell epitope content in rhGAA (as compared to their own nGAA) was calculated, and this GAA-iTEM score was used to determine each subject’s risk of developing ADA.
2.0. Material and Methods
2.1. Clinical Data for the Cohort of 24 IOPD Subjects
A waiver of written consent was obtained by the Duke University Medical Center Institutional Review Board. The detailed GAA genotype and ADA data for 24 HLA-DRB1 phenotyped IOPD subjects who received ERT and none of whom had received immune tolerance induction (Table 1) was made available for this analysis. The amino acid sequences of the pathogenic GAA gene variants, CRIM status, and longitudinal anti-rhGAA IgG antibody titers for each subject were obtained as described previously [4]. CRIM status was assessed by Western blot reactivity to a pool of monoclonal and/or polyclonal anti-GAA antibodies capable of recognizing both native and recombinant GAA [4, 17] from subject’s fibroblast cultures and/or PBMC and confirmed based on subject’s pathogenic GAA variants. Subject HLA haplotypes were determined by PCR using a sequence-specific oligonucleotide probe (SSP) typing test (One Lambda, Inc.). ADA titers were obtained at baseline using enzyme-linked immunosorbent assays and confirmed using radio-immunoprecipitation, and measured monthly thereafter for six months, and every three months following, as described previously [10]. Subjects whose ADA titers repeatedly exceeded 51,200 at ≥ six months on ERT were classified as “HSAT”, or “High ADA”, for this study [4]. Subjects whose ADA titers fell between 12,800 and 51,200 within the first year of ERT were classified as “SIT” and were also considered “High ADA”. Subjects who did not fall into either of these categories were classified as “Low ADA”.
Table 1.
IOPD Cohort Characteristics
| High ADA (n=15) | Low ADA (n=9) | |
|---|---|---|
| % CRIM Positive | 67% | 100% |
| Average age at first treatment | 6.9 months | 4.9 months |
| Average months of treatment | 89.5 | 91.1 |
| Average # of sequenced mutations | 2.1 | 2.2 |
| Average GAA-iTEM Score | 68.5 | 4.5 |
The goal of this study was to compare a novel method for ADA risk assessment (GAA-iTEM) to the traditional method for predicting ADA in IOPD, which is based on CRIM status. Using the traditional method for predicting ADA, the 24 subjects in this study would be divided into ADA risk groups by CRIM status, with CRIM-negative subjects predicted to have a High ADA response to rhGAA and CRIM-positive subjects predicted to have a Low ADA response (see above for definition of High and Low ADA). As expected, clinical outcomes for the CRIM-positive cohort were not concordant with the traditional classification: 10 of 19 CRIM-positive subjects had High ADA titers, while nine CRIM-positive subjects had Low ADA titers. The traditional method of predicting ADA was concordant with ADA titers for the five subjects who were CRIM-negative; all of these subjects had High ADA antibody titers. There were no CRIM-negative subjects who had Low ADA in this study cohort.
2.2. GAA-iTEM in silico approach and calculation
The initial in silico analysis was performed blinded to the ADA status of the study subjects. First, the EpiMatrix T cell epitope mapping algorithm [16] parsed each subject’s nGAA sequence and the reference sequence for rhGAA into overlapping 9-mer frames. Each frame was then evaluated for likelihood of binding to each individual subject’s HLA alleles, and assessments of binding potential rendered as Z-scores. Z-scores in the top 5% of random peptide assessments (≥1.64) are defined as EpiMatrix “hits” and considered significantly likely to bind HLA. An individualized T cell epitope measure (iTEM) has previously been described for short peptides [15, 18].
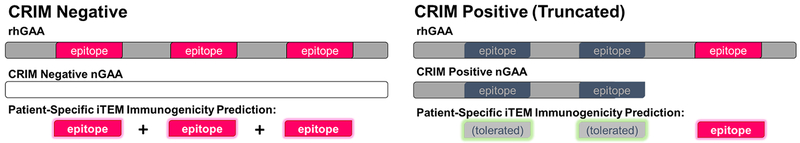
For this full-length protein, all 9-mer-to-HLA hits were compiled for the complete rhGAA sequence and compared to each subject’s nGAA to adapt a GAA-iTEM score adjusted for mutations and truncations. The GAA-iTEM score for each subject is therefore based on the number of T cell epitopes that are restricted by the individual subject’s HLA-DR, that are found in the rhGAA reference sequence and are not found in the subject’s nGAA due to mutations and/or truncations in the corresponding region(s) of their individual GAA genes. As shown in Figure 1, predicted rhGAA epitopes found to be identical in a subject’s nGAA were assumed to be tolerated. All predicted rhGAA epitopes within nGAA mutated or truncated regions that were predicted to be immunogenic for the subject’s specific alleles were summed to obtain a GAA-iTEM score. Any predicted epitopes, whether found in one allele or both alleles of the nGAA, were included in the GAA-iTEM score calculation. In regions with multiple HLA ligands predicted by EpiMatrix within the same cluster or region of higher density HLA binding (a ‘cluster’), a 10% deduction in the EpiMatrix score was applied to the lower scoring hit(s) to account for potential competition between epitopes for the same HLA-DR molecule in the same antigen presenting cell. Whereas the original iTEM publication [15] was optimized with a strong discount for overlapping binding motifs in peptides, in this case, the deduction was reduced to 10% to reflect that the impact of competition between close-proximity ligands should be limited by differential processing of an unknown number of complete antigens. For CRIM-negative subjects, assumed to have no endogenous GAA present, and therefore no immune tolerance to rhGAA, scores for all predicted epitopes for the entire sequence of GAA, for their HLA, were included in their GAA-iTEM calculations.
Figure 1. Foreign vs. self: prediction of epitopes likely to generate an inflammatory response in IOPD ERT recipients.
CRIM negative patients receiving ERT are unlikely to tolerate any T cell epitopes contained within rhGAA and restricted by their HLA due to incomplete thymic education (left), resulting in all rhGAA epitopes being perceived as “foreign”. CRIM positive patients, who may express residual protein, may tolerate those “self” rhGAA epitopes present in their nGAA, but are likely to generate inflammatory responses to “foreign” rhGAA T cell epitopes present in regions corresponding to truncated or mutated portions of their specific nGAA sequences (right).
2.3. Statistical analysis
Association between predictors of ADA response and outcome were evaluated by Chi-square test, or Fisher’s Exact test in the case of small sample sizes, using GraphPad online tools (GraphPad Software [2018], La Jolla California USA, www.graphpad.com/quickcalcs/contingency2/). Prediction metrics (sensitivity, specificity, positive predictive value, negative predictive value, odds ratio) were evaluated as per [19] using Microsoft Excel (2016).
4.0. Results
4.1. CRIM status as a predictor of ADA response (Traditional method)
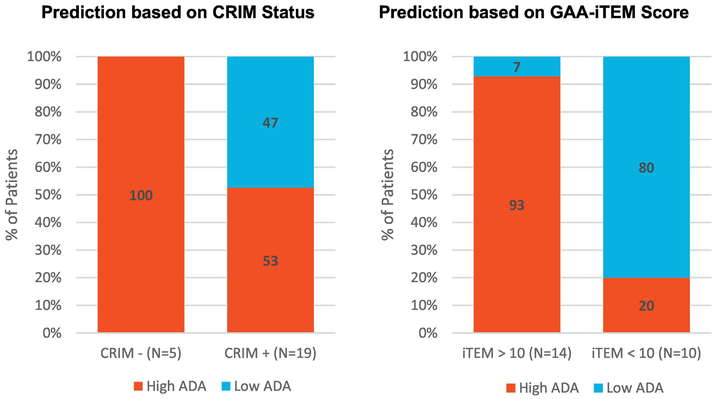
While we are aware that most clinicians use gene expression and mutation information to predict ADA in CRIM-positive patients, for the purpose of this comparison with GAA-iTEM, we used an approach described as the “traditional prediction method”. This method classifies patients based on CRIM status. Using CRIM status alone (where CRIM-negative = High ADA and CRIM-positive = Low ADA), CRIM status was a poor predictor of ADA for the study subject cohort. Although 100% of CRIM-negative subjects in this cohort developed High ADA, considering the entire cohort, CRIM Status accurately predicted ADA status in 63% of subjects, with 100% specificity and 36% sensitivity (Table 2). Overall, the association between CRIM status and ADA status was not significant (p = 0.12), with nearly 50% of CRIM-positive subjects developing a High ADA response despite having native GAA.
Table 2.
Specificity and Sensitivity Analysis using GAA-iTEM>10 and CRIM status
| Predictors for High ADA | ||
|---|---|---|
| GAA-iTEM >10 | CRIM Negative | |
| Overall Agreement | 88% | 63% |
| Sensitivity | 87% | 36% |
| Specificity | 89% | 100% |
| Positive Predictive Value | 93% | 100% |
| Negative Predictive Value | 80% | 53% |
| Odds Ratio compared to GAA-iTEM <10 or CRIM-positive | 52 | 6.6* |
Odds Ratio estimate due to zero-denominator
4.2. GAA-iTEM as a predictor of ADA response
GAA-iTEM scores were calculated for each of the study subjects. At the time of the analysis, analysts were blinded with respect to the patient ADA status. GAA-iTEM scores ranged from 0 to 182.73 for this group of 24 subjects, with a mean of 59.00 (± 72.11). All five CRIM-negative subjects had highly elevated GAA-iTEM scores (between 158.43 and 182.73), reflecting the GAA-iTEM prediction that there was a high density of ‘foreign’ T cell epitopes in the rhGAA as compared to their nGAA sequence. As predicted, all five CRIM-negative subjects had High ADA responses to rhGAA.
The GAA-iTEM scores of the remaining 19 CRIM-positive subjects ranged from 0 to 160.80. Based on natural separation in this small dataset, a GAA-iTEM score of +10 was chosen as the initial “threshold”, over which a subject would be considered at risk for High ADA. A total of nine subjects had GAA-iTEM scores above the defined threshold of +10, with GAA-iTEM scores ranging from 12.61 to 160.80. Of these nine subjects predicted to develop a High ADA response, eight demonstrated High ADA in the clinic (seven HSAT, one SIT). The remaining 10 CRIM-positive subjects had GAA-iTEM scores below +10 (ranging from 0 to 8.60). Of these 10 subjects, eight demonstrated low ADA response in the clinic, while two demonstrated High ADA response (both HSAT). Defining risk of High ADA as a GAA-iTEM score above 10, the overall agreement between GAA-iTEM score and High ADA was 88% (p=0.0005) within this data set (sensitivity: 87%; specificity: 89%). Notably for this small sample, moving the cutoff to include one more low iTEM score patient or one more high iTEM score patient reduced the predictive value of the iTEM score.
5.0. Discussion
Infantile-onset Pompe disease (IOPD) is a fatal neuromuscular disorder, with a life expectancy of less than 1-2 years in untreated patients [2]. Treating Pompe disease with rhGAA enzyme replacement therapy (ERT) allows patients to live longer and is the current standard of care and the only FDA approved treatment. However, ADA to rhGAA may limit the effectiveness of rhGAA treatment, especially in cases with SIT and HSAT, where the outcome of ERT is poor. Prophylactic immune tolerance induction (ITI) protocol with rituximab, methotrexate, and/or IVIG is the current clinical approach to prevent ADA in CRIM-negative patients. Various combinations of rituximab, cyclophosphamide, IVIG, plasmapheresis, and increased doses of ERT have been tried without success in patients with IOPD who developed HSAT prior to implementation of ITI. ITI (rituximab, methotrexate, and/or IVIG) with the addition of bortezomib, which targets antibody-secreting plasma cells, successfully reduced antibody titers and stabilized clinical decline in at least four HSAT patients, three of whom were CRIM-positive, as previously published [20-22]. However, such a “rescue” approach is not ideal, due to prolonged immunosuppression arising from the use of maintenance doses of rituximab and methotrexate along with bortezomib compared to a shorter 5-week course of ITI administered in an ERT-naive setting [22]. Hence, identifying patients likely to develop High ADA at the outset is of utmost importance. There is currently no accurate means of identifying which CRIM-positive patients are at a greater risk of developing High ADA and should be placed on prophylactic ITI therapy. Sixty-eight percent of Pompe patients are CRIM-positive, and high titer ADA develop in 32% of CRIM-positive patients [7, 9]. These are the patients that would benefit from improved ADA predictions, since high level of ADA results in a poor response to ERT therapy and a subsequent clinical decline. Patients who do not go on to develop ADA would also benefit from improved predictions, as they might not be placed on immune tolerance induction drugs, avoiding the potential risks of long-term immune suppression.
Using GAA genotype and HLA-DR haplotype, we developed a new approach to assess ADA risk for IOPD patients. The clinical implications of using the traditional method for predicting ADA in IOPD is evident for this cohort. Based on CRIM status alone, five CRIM-negative subjects at risk of High ADA would be appropriately selected for treatment with immune tolerance induction (ITI). The remaining 19 CRIM-positive subjects would not have been treated with immune-modulating drugs, and ten of these 19 CRIM-positive patients would have developed subsequent High ADA. The alternative being investigated in the last couple of years, treating all patients with ITI, would unnecessarily expose nine CRIM-positive patients to immune modulating drugs.
Had the decision to use ITI been based on GAA-iTEM score for this cohort of 24 IOPD subjects, 14 subjects would be treated with immune-modulating drugs, of whom 13 developed ADA in the absence of ITI. A total of 10 subjects would not be treated with immune-modulating drugs, of whom only two developed High ADA. This decision matrix would provide significant benefits to patients.
The false positive predictions in this dataset (CRIM+ subjects who were predicted to but did not develop ADA) highlight refinements that can be made to the GAA-iTEM risk assessment method in the future. Individual cases highlight where improvements could be made: One individual’s GAA-iTEM score was just over our threshold for significance (12.61) and was therefore predicted to have a High ADA response. However, the subject developed low levels of ADA in the clinic. Thus, our threshold may be set too low, which may be redefined in a larger cohort. Two subjects developed High ADA responses in the clinic, despite having very low GAA-iTEM scores of 2.04 and 0. More detailed investigations of individual T cell epitope response to rhGAA, or to clinical conditions (unrelated to HLA) that might have contributed to the development of ADA for subjects that have GAA-iTEM scores close to the pre-defined threshold may lead to improved classifications in future iterations of the GAA-iTEM method. It is possible that discrepancies for patients with low GAA-iTEM scores may be due to T-independent immune response to rhGAA, or to low-level expression of subject CRIM, or to presentation of conserved epitopes by an HLA allele other than the DRB1 alleles that are used in the GAA-iTEM calculation.
It was interesting to note that including novel (non-conserved) epitopes that were present in only one allele of the native GAA (heterozygous mutation) provided an improved correlation despite the fact that the epitope would still be present in the other GAA gene. This may suggest that peripheral, and not central tolerance is more relevant for driving effector T cell responses that trigger epitope-spreading, and that the presence of a single T effector epitope, in the right context, may be sufficient for initiating ADA response, despite the presence of a conserved, or neutral epitope in the other GAA allele. This observation definitely deserves further investigation.
In this preliminary study, we tested several variations of the GAA-iTEM calculation with no, or negligible, improvement in the ADA predictions for this cohort of subjects. These variations include: considering only homozygous mutations from the subject’s nGAA, considering only one of the subjects’ alleles, considering only the higher scoring of the subjects’ alleles, adjusting for TCR facing-T cell epitope homology to the subjects’ native sequence using the JanusMatrix algorithm [23], and adjusting for TCR facing homology to the human proteome. Other approaches, such as accounting for epitopes restricted by other HLA such as DP and DQ or reducing the GAA-iTEM score for epitopes that have TCR-facing residues are highly conserved in other (non-GAA) proteins in the human proteome may improve the prediction of ADA. Future studies will explore whether these and additional variations on the GAA-iTEM calculation will improve the correlation with ADA status.
In this retrospective study, the ADA response correlate was classified as simply “High” or “Low”. Future studies with larger cohorts may lead to a better correlation between the GAA-iTEM score and relative titer of ADA response. Other factors that may have an effect on predictive value that were not considered in this study include: age of subject when starting therapeutic, dose of ERT, time on therapeutic, time to ADA response, level of CRIM, and relative protein expression by allele. These factors will be explored in future studies of CRIM-positive patient cohorts.
The novel approach described here individualizes risk assessment in IOPD patients and may be useful as an additional method for identifying CRIM-positive patients that would benefit from ITI therapy. For patients at low risk of ADA, applying GAA-iTEM may reduce the need for unnecessary immune suppression and might also reduce the adverse effects associated with immune-modulating drugs. Obtaining HLA information during newborn screening, combined with GAA sequence, and performing GAA-iTEM analysis could enable earlier identification of high-risk patients. “Watchful waiting” could be considered as an option for subjects with very low GAA-iTEM scores. Further, we believe a similar GAA-iTEM approach could be applied for other gene-deficiency diseases that are treated with enzyme or factor replacement therapy, for which ADA may contribute to clinical decline.
Supplementary Material
Figure 2. Predictive results: CRIM status vs. GAA-iTEM.
Using an GAA-iTEM score threshold of +10 (right) results in correct predictions for 92% of patients with GAA-iTEM scores > 10 and 80% of patients with GAA-iTEM scores <10 whereas using CRIM status as a predictor of High ADA response to ERT (left) only results in correct predictions for 55% of CRIM positive patients.
Highlights:
CRIM Status is the current method for identifying IOPD patients at high risk of ADA.
Determinants of ADA include individual patient HLA and GAA gene sequence.
Individualization of the ADA prediction can be performed using in silico tools described here (GAA-iTEM), provided that patient HLA-DRB1 typing and patient GAA sequence is available.
GAA-iTEM score predictions have better overall agreement than CRIM status predictions, in this cohort.
The GAA-iTEM approach may be useful for predicting ADA to other ERTs.
Acknowledgements
We are grateful for the participation of IOPD subjects, their families, and for the contributions of the many clinicians at Duke University, and to Gail Skowron, M.D. for her careful review of this manuscript.
Abbreviations:
- ADA
Anti-Drug Antibody
- ITI
Immune Tolerance Induction
- GAA
acid alpha-glucosidase
- rhGAA
recombinant human GAA
- nGAA
native GAA
- CRIM
Cross Reactive Immunological Material
- iTEM
Individualized T Cell Epitope Measure
- GAA-iTEM
for acid alpha-glucosidase
- ERT
enzyme-replacement therapy
- IOPD
infantile-onset Pompe disease
- HLA
human leukocyte antigen
Footnotes
Study Approval
The Duke University Medical Center IRB approved this study (Protocol 00043093).
Disclosure of Conflicts of Interest
Zoheb B. Kazi: Grant support: Lysosomal Disease Network; Sanofi Genzyme
Ankit K. Desai: Grant support: Sanofi Genzyme
Priya S. Kishnani: Research support, honoraria, and Pompe and Gaucher Disease Registries’ advisory board membership: Sanofi Genzyme; grants: Shire Pharmaceuticals, Valerion; Amicus; personal fees: Alexion Pharmaceuticals, Inc., Amicus Therapeutics, Shire Pharmaceuticals; advisory board membership: Baebies, Inc,
Anne S. De Groot, Bill Martin, Frances Terry and Rebecca Martin work for EpiVax, Inc., a small biotech company that is focused on personalizing medical therapy using immunoinformatics tools. These authors attest that the study was performed with the intent to minimize or eliminate any potential conflict of interest, to the best of their ability, but that there remains potential inherent conflict of interest due to their intent to develop the GAA-iTEM tool for clinical use. These authors also attest that the analysis was reported as performed, avoiding over-reach and using all available clinical data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirschhorn R and Reuser AJJ, Glycogen storage disease type II: acid a-glucosidase (acid maltase) deficiency, in Scriver's OMMBID the online metabolic & molecular bases of inherited disease, Valle D and Scriver CR, Editors. 2009, McGraw-Hill: New York. [Google Scholar]
- 2.Kishnani PS, et al. , A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr, 2006. 148(5): p. 671–676. [DOI] [PubMed] [Google Scholar]
- 3.Nicolino M, et al. , Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med, 2009. 11(3): p. 210–9. [DOI] [PubMed] [Google Scholar]
- 4.Kishnani PS, et al. , Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr, 2006. 149(1): p. 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishnani PS, et al. , Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology, 2007. 68(2): p. 99–109. [DOI] [PubMed] [Google Scholar]
- 6.Banugaria SG, et al. , The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med, 2011. 13(8): p. 729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berrier KL, et al. , CRIM-negative infantile Pompe disease: characterization of immune responses in patients treated with ERT monotherapy. Genet Med, 2015. 17(11): p. 912–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel TT, et al. , The impact of antibodies in late-onset Pompe disease: a case series and literature review. Mol Genet Metab, 2012. 106(3): p. 301–9. [DOI] [PubMed] [Google Scholar]
- 9.Desai AK, Kazi ZB, and Kishnani PS, Cross-reactive immunologic material positive infantile Pompe disease: Characterization of immune responses in patient treated with enzyme replacement therapy. Molecular Genetics and Metabolism. 117(2): p. S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumizyme R [Package Insert] Genzyme Corporation, Cambridge, MA: October 2016. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/125291lbl.pdf. [Google Scholar]
- 11.Messinger YH, et al. , Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med, 2012. 14(1): p.135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banugaria SG, et al. , Algorithm for the early diagnosis and treatment of patients with cross reactive immunologic material-negative classic infantile pompe disease: a step towards improving the efficacy of ERT. PLoS One, 2013. 8(6): p. e67052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazi ZB, et al. , Sustained immune tolerance induction in enzyme replacement therapy–treated CRIM-negative patients with infantile Pompe disease. JCI Insight, 2017. 2(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Groot AS and Scott DW, Immunogenicity of protein therapeutics. Trends Immunol, 2007. 28(11): p. 482–90. [DOI] [PubMed] [Google Scholar]
- 15.Cohen T, et al. , A method for individualizing the prediction of immunogenicity of protein vaccines and biologic therapeutics: individualized T cell epitope measure (iTEM). J Biomed Biotechnol, 2010. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schafer JR, et al. , Prediction of well-conserved HIV-1 ligands using a matrix-based algorithm, EpiMatrix. Vaccine, 1998. 16(19): p. 1880–4. [DOI] [PubMed] [Google Scholar]
- 17.Klinge L, et al. , Safety and efficacy of recombinant acid alpha-glucosidase (rhGAA) in patients with classical infantile Pompe disease: results of a phase II clinical trial. Neuromuscul Disord, 2005. 15(1): p. 24–31. [DOI] [PubMed] [Google Scholar]
- 18.Schanen BC, et al. , Coupling sensitive in vitro and in silico techniques to assess cross-reactive CD4(+) T cells against the swine-origin H1N1 influenza virus. Vaccine, 2011. 29(17): p. 3299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simundic AM, Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC, 2009. 19(4): p. 203–11. [PMC free article] [PubMed] [Google Scholar]
- 20.Banugaria SG, et al. , Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from Pompe disease. Genet Med, 2013. 15(2): p. 123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazi ZB, et al. , Durable and sustained immune tolerance to ERT in Pompe disease with entrenched immune responses. JCI Insight, 2016. 1(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenger EO, et al. , Immune Tolerance Strategies in Siblings with Infantile Pompe Disease-Advantages for a Preemptive Approach to High-Sustained Antibody Titers. Mol Genet Metab Rep, 2015. 4: p. 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moise L, et al. , The two-faced T cell epitope: examining the host-microbe interface with JanusMatrix. Hum Vaccin Immunother, 2013. 9(7): p. 1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.