Abstract
Background
Anti-tumour immune competence has an impact in hepatocarcinogenesis and success of anti-cancer therapies. Tumour-infiltrating lymphocytes (TILs) and monocytes/macrophages (TAMs) are proposed to have significance in cancer. However, there is only limited data concerning their impact on patient outcome and survival in hepatocellular carcinoma (HCC).
Methods
Frequencies of CD68+, CD163+ M2-polarized TAMs and TILs were measured in de novo HCC tumours in non-cirrhosis (n = 58) using immunohistology and correlated to patients’ clinicopathological characteristics and survival rates.
Results
Patients with tumours marked by appearance of TILs and CD68+ TAMs showed an improved 1-, 3- and 5-year recurrence-free survival (all p ≤ 0.05). CD68+ TAMs were associated with reduced incidence of recurrent and multifocal disease. Conversely, CD163+ TAMs were associated with multifocal HCC and lymphangiosis carcinomatosa (all p ≤ 0.05).
Conclusions
TILs and CD68+ TAMs are associated with multiple tumour characteristics and patient survival in HCC. However, there is only scarce data about the biology underlying their mechanistic involvement in human tumour progression. Thus, experimental data on functional links might help develop novel immunologic checkpoint inhibitor targets for liver cancer.
Keywords: Hepatocellular carcinoma, Monocytes/macrophages, Tumour-infiltrating lymphocytes, M2 macrophages, Biomarkers, Prognosis
Background
Tumour-related mechanisms define the host immune functions in the tumour microenvironment and decrease the efficiency of the anti-tumour immune competence. This phenomenon plays a key role in the process of hepatocarcinogenesis, where tumour infiltration with immune competent cells exerts a strong influence on prognosis [1]. In liver cancer, tumour-associated macrophages (TAMs) and monocyte subsets with distinguished functional polarization have a significant impact on cancer-related inflammation and foster tumour escape mechanisms and progression [2]. These subpopulations of immunologic phenotypes are related to as classically (M1) or alternatively activated (M2) monocytes/macrophages. These monocytes/macrophages deploy their immunoregulatory activities in close interplay with T cell-dependent responses [2].
Tumour-infiltrating lymphocytes (TILs) play a major role in the progression of solid malignancies and can have a strong influence on the success of the related anti-cancer therapies. TILs in hepatocellular carcinoma (HCC) are mainly T cells. TILs are the hallmark of the anti-cancer immunity in human cancer, on grounds of their ability to specifically interact with and neutralize tumour-related neoantigens [3]. TILs’ immune activity may influence hepatocarcinogenesis not only by direct effects on the adaptive immune system and cytokine interactions, but also further by modulating neoangiogenesis and innate immune responses, i.e. related monocyte/macrophages functions [4–8]. Therefore, the quantification of TILs in solid tumours might help to deliver novel insights concerning their role in the process of hepatocarcinogenesis and to monitor the therapy outcome with immunologic checkpoint inhibition.
Major progress in the efficacy of adjuvant therapies for hepatic malignancies has emerged. However, the restraints of these neoadjuvant regimens constitute an important clinical problem [9]. Therefore, an additional immune checkpoint blockade represents an attractive therapeutic concept that complements these current therapies in cancer. Furthermore, the pre- and on-treatment quantification of the immunologic infiltrates could deliver novel biomarkers for state of the art disease management, based on the fact that immunological checkpoint targeting is effective only in a scarce amount of the tumour patients [10]. HCC can arise de novo in non-cirrhotic hepatic environment in approximately 20% of all cases [11]. However, this subgroup of patients usually presents at an advanced stage of hepatocarcinogenesis, due to the lack of symptoms and surveillance. Therefore, our study aimed to assess the presence and abundance of tumour-infiltrating subsets of monocytes/macrophages and TILs in advanced de novo HCC in non-cirrhosis. This study investigated also their association with tumour recurrence, patient survival and outcome.
Methods
Patients and tumour specimens
Our retrospective study was conducted in 58 patients with de novo HCC in non-cirrhosis. In all patients, a major liver resection with curative intent was performed. None of the included patients had history of viral hepatitis or were treated with neoadjuvant radio- and/or chemotherapy before the tumour resection. Formalin-fixed and paraffin-embedded surgical specimens, embedding a representative tumour sample for immunohistochemical staining, were obtained from the archives of the Department of Pathology. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of the Leipzig University.
Immunohistology
The protocols for immunohistology and quantification of positive staining have been published previously [12–16]. The abundance of infiltrating TILs, CD68+ and CD163+ TAMs were measured in regard to the tumour central area (TCA) and the tumour-infiltrating front (TIF). Zeiss Axio Imager A1 Phase Contrast microscope (Carl Zeiss, Jena, Germany) was used to asses positive staining. Table 1 summarizes the antibodies and reagents used to conduct immunohistology.
Table 1.
Antibodies and reagents used for immunostaining
| Antigen | m/p | Clone | Species | Company | Secondary antibody | Company | Substrate | Antigen retrieval |
|---|---|---|---|---|---|---|---|---|
| CD68 | m | PG-M1 | Mouse | Agilent | anti-mouse-Ig/ peroxidase | Vector | DAB | Tris/EDTA pH 9.0 |
| CD163 | m | 10D6 | Mouse | Leica Biosystems, Newcastle Upon Tyne, UK | anti-mouse-Ig/ peroxidase | Vector | DAB | 10 mM citrate, pH 5.5 |
DAB 3,3′-diaminobenzidine, m monoclonal, p polyclonal, TAM tumor-associated macrophage
Density quantification of cellular infiltrates
Quantification was performed as described [13]. In brief, tumour-infiltrating immune cells were categorized as negative/absent in up to 5% positive cells (0–5% positive cells, score 0) and positive/present (> 5% positive cells, score 1). HCC patients were then assigned to two groups either to be negative or positive for CD68+ or CD163+ TAMs. Evaluation of TILs was performed using routine H&E slides as described [17]. Briefly, the extent of lymphocyte abundance in the tumour area was categorized as none (score 0), low (score 1), moderate (score 2) or high (score 3). Accordingly, HCC patients were then assigned to the TIL− (none to low infiltration) or TIL+ group (moderate to high infiltration).
Statistical analysis
The IBM SPSS statistics software was used to perform survival and univariate analysis and to produce the Kaplan-Meier curves (Version 25/Year 2017/USA). The log-rank test was applied to compare differences in survival distributions. The Cox proportional hazards model was utilized to conduct multivariate analysis for the significant parameters in the univariate analysis. The chi-square test, Fisher’s exact test or Student’s t test (independent sample) were applied to compare categorical and continuous variables. Statistical differences were considered significant for p ≤ 0.05.
Results
Table 2 summarizes the clinicopathological characteristics of all patients included in the current work. The studied population of HCC patients had 1-, 3- and 5-year survival of 76.4%, 64.1% and 62.2%, respectively. The recurrence-free survival rates 1-, 3- and 5- year after tumour resection were 63.5%, 57.7% and 53.6%, respectively. In 23/58 (39.7%) patients, a recurrent tumour disease was detected and 17/58 (29.3%) patients developed a local tumour recurrence. A metastatic disease was seen in 11/58 (19.0%) patients.
Table 2.
Clinicopathological characteristics of the patients included in the study
| Variable | Value (%) |
|---|---|
| No. of patients | 58 |
| Gender | |
| Female | 13 (22.4%) |
| Male | 45 (77.6%) |
| Patient age (years) | |
| ≤ 60 | 21 (36.2%) |
| > 60 | 37 (63.8%) |
| Pathologic T stage | |
| T1/T2 | 29 (50.0%) |
| T3/T4 | 29 (50.0%) |
| Pathologic N stage | |
| Positive | 11 (19.0%) |
| Negative | 47 (81.0%) |
| Lymphangiosis carcinomatosa | |
| Positive | 17 (29.3%) |
| Negative | 41 (70.7%) |
| Angioinvasion | |
| Positive | 30 (51.7%) |
| Negative | 28 (48.3%) |
| Multiple tumour nodules | |
| With | 13 (22.4%) |
| Without | 45 (77.6%) |
| Tumor size (mm) | |
| ≤ 50 | 11 (19.0%) |
| > 50 | 47 (81.0%) |
| Pathologic R category | |
| R0 | 49 (84.5%) |
| R1/R2 | 9 (15.5%) |
| Histologic differentiation | |
| Well | 11 (19.0%) |
| Moderate/poor | 47 (81.0%) |
| Distant metastases | |
| With | 11 (19.0%) |
| Without | 47 (81.0%) |
| Tumor recurrence | |
| With | 23 (39.7%) |
| Without | 35 (60.3%) |
| Local recurrence | |
| With | 17 (29.3%) |
| Without | 41 (70.7%) |
Distribution of monocytes/macrophages and tumour-infiltrating lymphocytes in HCC
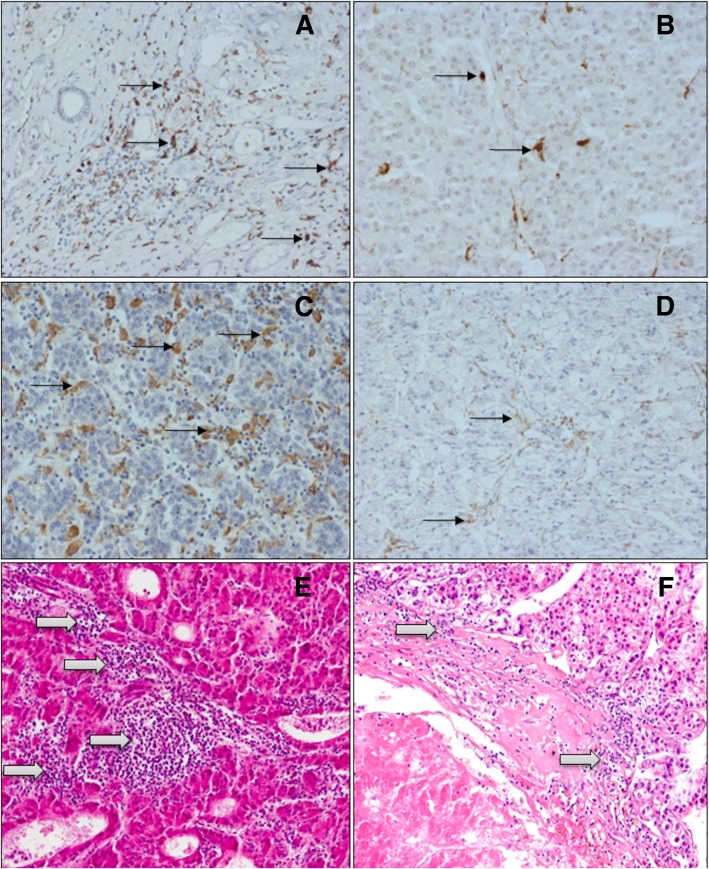
Figure 1 provides characteristic images for the abundance of TILs and CD68+ or CD163+ TAMs in HCC. Tables 2, 3, 4 and 5 summarize the respective statistical data of the patients. TILs and CD68+ or CD163+ TAMs revealed a homogeneous expression pattern in TCA and TIF and were also detected in areas of HCC necrosis (Fig. 1a–f).
Fig. 1.
Immunohistology of monocytes/macrophages and H&E staining of TILs in tumour central area (TCA) of HCC specimens. a High density of CD68+ TAMs. b Low density of CD68+ TAMs. c High density of CD163+ TAMs. d Low density of CD163+ TAMs. e High density of TILs. f Low density of TILs. Legend: left column, high density; right column, low density. Black arrows indicate monocytes/macrophages, white arrows TILs
Table 3.
Correlation of CD68+ TAMs at the tumour-infiltrating front (TIF) with clinicopathological characteristics in HCC
| Variable | CD68+/TIF | CD68−/TIF | p value |
|---|---|---|---|
| No. of patients | 53 | 5 | |
| Patient age, years | 0.324 | ||
| ≤ 60 | 33 (62.3%) | 4 (80.0%) | |
| > 60 | 20 (37.7%) | 1 (20.0%) | |
| Gender | 0.430 | ||
| Female | 42 (79.2%) | 3 (60.0%) | |
| Male | 11 (20.8%) | 2 (40.0%) | |
| Local tumour recurrence | 0.583 | ||
| Positive | 15 (28.3%) | 2 (40.0%) | |
| Negative | 38 (71.7%) | 3 (60.0%) | |
| Overall tumour recurrence | 0.050 | ||
| Positive | 19 (35.8%) | 4 (80.0%) | |
| Negative | 34 (64.2%) | 1 (20.0%) | |
| Distant Metastases | 0.209 | ||
| Positive | 9 (17.0%) | 2 (40.0%) | |
| Negative | 44 (83.0%) | 3 (60.0%) | |
| Multiple tumour nodules | 0.035 | ||
| Positive | 10 (18.9%) | 2 (40.0%) | |
| Negative | 43 (81.1%) | 3 (60.0%) | |
| Tumour size (mm) | 0.951 | ||
| ≤ 50 | 10 (10.0%) | 1 (20.0%) | |
| > 50 | 43 (90.0%) | 4 (80.0%) | |
| R status | 0.772 | ||
| Positive | 8 (15.1%) | 1 (20.0%) | |
| Negative | 45 (84.9%) | 4 (80.0%) | |
| Angioinvasion | 0.583 | ||
| Positive | 25 (47.2%) | 2 (40.0%) | |
| Negative | 28 (52.8%) | 3 (60.0%) | |
| Lymphangiosis carcinomatosa | 0.583 | ||
| Positive | 15 (28.3%) | 2 (40.0%) | |
| Negative | 38 (71.8%) | 3 (60.0%) | |
| Histologic differentiation | 0.951 | ||
| Well | 10 (10.0%) | 1 (20.0%) | |
| Moderate/poor | 43 (90.0%) | 4 (80.0%) | |
| Pathologic T stage | 0.148 | ||
| T1/T2 | 28 (53.8%) | 1 (20.0%) | |
| T3/T4 | 24 (46.2%) | 4 (80.0%) | |
| Pathologic N stage | 0.658 | ||
| Positive | 2 (03.8%) | 0 (00.0%) | |
| Negative | 51 (96.2%) | 5 (100.0%) |
Table 4.
Correlation CD163+ TAMs in the tumour central area (TCA) or tumour-infiltrating front (TIF) with clinicopathological characteristics in HCC
| Variable | CD163+/TCA | CD163−/TCA | p value |
|---|---|---|---|
| No. of patients | 42 | 16 | |
| Patient age, years | 0.177 | ||
| ≤ 60 | 13 (31.0%) | 8 (50.0%) | |
| > 60 | 29 (69.9%) | 8 (50.0%) | |
| Gender | 0.680 | ||
| Female | 10 (76.2%) | 3 (18.8%) | |
| Male | 32 (23.8%) | 13 (81.2%) | |
| Local tumour recurrence | 0.398 | ||
| Positive | 11 (26.2%) | 6 (37.5%) | |
| Negative | 31 (73.8%) | 10 (62.5%) | |
| Overall tumour recurrence | 0.320 | ||
| Positive | 15 (35.7%) | 8 (50.0%) | |
| Negative | 27 (64.3%) | 8 (50.0%) | |
| Distant metastases | 0.469 | ||
| Positive | 7 (16.7%) | 4 (25.0%) | |
| Negative | 35 (83.3%) | 12 (75.0%) | |
| Multiple tumour nodules | 0.016 | ||
| Positive | 36 (85.7%) | 9 (56.3%) | |
| Negative | 6 (14.3%) | 7 (43.8%) | |
| Tumour size (mm) | 0.438 | ||
| ≤ 50 | 9 (21.4%) | 2 (12.5%) | |
| > 50 | 33 (78.6%) | 14 (87.5%) | |
| R status | 0.695 | ||
| Positive | 35 (15.1%) | 2 (12.5%) | |
| Negative | 7 (84.9%) | 14 (87.5%) | |
| Angioinvasion | 0.311 | ||
| Positive | 20 (47.6%) | 10 (62.5%) | |
| Negative | 22 (52.4%) | 6 (37.5%) | |
| Lymphangiosis carcinomatosa | 0.656 | ||
| Positive | 13 (31.0%) | 4 (25.0%) | |
| Negative | 29 (69.0%) | 12 (75.0%) | |
| Histologic differentiation | 0.979 | ||
| Well | 8 (19.0%) | 3 (18.8%) | |
| Moderate/poor | 34 (81.0%) | 13 (81.3%) | |
| Pathologic T stage | 0.501 | ||
| T1/T2 | 22 (53.7%) | 7 (43.8%) | |
| T3/T4 | 19 (46.3%) | 9 (56.3%) | |
| Pathologic N stage | 0.470 | ||
| Positive | 1 (02.4%) | 1 (06.3%) | |
| Negative | 41 (97.6%) | 15 (93.7%) | |
| No. of patients | 19 | 39 | |
| Lymphangiosis carcinomatosa | 0.035 | ||
| Positive | 9 (47.4%) | 8 (20.5%) | |
| Negative | 10 (52.6%) | 31 (79.5%) |
Table 5.
Correlation of TILs in the tumour central area (TCA) with clinicopathological characteristics in HCC
| Variable | TIL+/TCA | TIL−/TCA | p value |
|---|---|---|---|
| No. of patients | 20 | 38 | |
| Patient age, years | 0.476 | ||
| ≤ 60 | 6 (30.0%) | 15 (39.1%) | |
| > 60 | 14 (70.0%) | 23 (60.9%) | |
| Gender | 0.732 | ||
| Female | 15 (75.0%) | 30 (78.9%) | |
| Male | 5 (25.0%) | 8 (21.1%) | |
| Local tumour recurrence | 0.933 | ||
| Positive | 6 (30.0%) | 11 (28.9%) | |
| Negative | 14 (70.0%) | 27 (71.1%) | |
| Overall tumour recurrence | 0.546 | ||
| Positive | 9 (45.0%) | 14 (36.8%) | |
| Negative | 11 (55.0%) | 24 (63.2%) | |
| Metastases | 0.884 | ||
| Positive | 4 (20.0%) | 7 (18.4%) | |
| Negative | 16 (80.0%) | 31 (81.6%) | |
| Multiple tumour nodules | 0.100 | ||
| Positive | 18 (90.0%) | 27 (71.1%) | |
| Negative | 2 (10.0%) | 11 (28.9%) | |
| Tumour size (mm) | 0.206 | ||
| ≤ 50 | 2 (10.0%) | 9 (23.7%) | |
| > 50 | 18 (90.0%) | 29 (76.3%) | |
| R status | 0.148 | ||
| Positive | 15 (75.0%) | 34 (89.5%) | |
| Negative | 5 (25.0%) | 4 (10.5%) | |
| Angioinvasion | 0.717 | ||
| Positive | 11 (55.0%) | 19 (50.0%) | |
| Negative | 9 (45.0%) | 19 (50.0%) | |
| Lymphangiosis carcinomatosa | 0.057 | ||
| Positive | 9 (45.0%) | 30 (78.9%) | |
| Negative | 11 (45.0%) | 8 (21.1%) | |
| Histologic differentiation | 0.576 | ||
| Well | 3 (15.0%) | 8 (21.1%) | |
| Moderate/poor | 17 (85.0%) | 30 (78.9%) | |
| Pathologic T stage | 0.708 | ||
| T1/T2 | 9 (47.4%) | 20 (52.6%) | |
| T3/T4 | 10 (52.6%) | 18 (47.4%) | |
| Pathologic N stage | 0.296 | ||
| Positive | 0 (00.0%) | 2 (5.3%) | |
| Negative | 20 (100.0%) | 36 (94.7%) | |
| CD68+ TAMs/TCA | 0.008 | ||
| Positive | 20 (100.0%) | 11 (28.9%) | |
| Negative | 0 (00.0%) | 27 (71.1%) |
Monocytes/macrophages are associated with reduced incidence of tumour recurrence and formation of multiple tumour nodules in HCC patients
CD68+ TAMs in TIF were associated with reduced occurrence of recurrent HCC. In the CD68+ group, only 19/53 (35.8%) patients suffered overall tumour recurrence, whereas in the CD68− group, 4/5 (80.0%) patients had recurrent disease (p = 0.05; Table 3). CD68+ TAMs in TIF were also correlated with reduced formation of multiple tumour nodules (p = 0.035). In the CD68+ group, only 10/53 (18.9%) patients showed this feature, whereas in the CD68− group, these were 2/5 (40.0%) patients (p = 0.035).
M2-polarized macrophages are associated with lymphangiosis carcinomatosa and formation of multiple tumour nodules in HCC patients
CD163+ TAMs in TCA were associated with the formation of multiple tumour nodules (p = 0.016; Table 4). In the CD163+ group, 36/42 (85.7%) patients had multiple tumour nodules; in the CD163− group in 9/16 (56.3%) patients, this was diagnosed (p = 0.016). Moreover, when considering the TIF, in the CD163− group, 31/39 (79.5%) patients had absence of lymphangiosis carcinomatosa. In the CD163+ group, these were 10/19 (52.6%) patients (p = 0.035). No significant association between CD163+ TAMs in TCA or TIF with CD68+ TAMs could be detected.
Tumour-infiltrating lymphocytes are associated with intratumoural monocytes/macrophages in HCC patients
TILs in TCA or TIF were not correlated with clinicopathological features of HCC patients (Table 5). However, in regard to the TCA, TILs and CD68+ TAMs revealed a strong correlation. In the TIL+ group in 20/20 (100%) and in the TIL− group in only 11/38 (28.9%) patients, high frequencies of CD68+ TAMs were detected (p = 0.008). No significant correlations between TILs in TCA or TIF with CD163+ TAMs could be observed.
Influence of monocytes/macrophages and tumour-infiltrating lymphocytes on survival in HCC patients
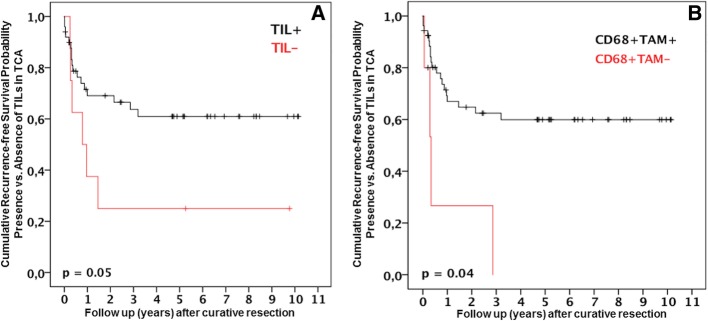
In our study, CD68+ TAMs and TILs were associated with patients’ recurrence-free survival. Figure 2 shows the Kaplan-Meier survival curves. Tables 2, 3, 4 and 5 show the statistical evaluation of all patients. Recurrence-free survival rates were significantly improved in patients with TILs in TCA (Fig. 2a). One, 3 and 5 years after surgery, these were 68.9%, 63.9% and 61.6%, respectively. Conversely, the survival was 37.8%, 23.4% and 23.4% at 1, 3 and 5 years post-surgery, respectively, in patients without TILs in TCA (p = 0.05). Similar data was obtained in regard to CD68+ TAMs in TIF (Fig. 2b). The recurrence-free survival rates were 66.9%, 63.3% and 60.0% at 1, 3 and 5 years for patients with CD68+ TAMs in TIF. Contrarily, the recurrence-free survival was 28.7% at 1 year post-surgery in HCC patients without these cells in the TIF. Of note, survival beyond 3 years after surgery could not be reached in patients without CD68+ TAMs in TIF (p = 0.04). CD163+ TAMs in TCA or TIF did not reveal any significant correlation with overall or recurrence-free survival of the HCC patients (CD163+ TAMs in TCA: overall survival p = 0.858, recurrence-free survival p = 0.283; CD163+ TAMs in TIF: overall survival p = 0.410, recurrence-free survival p = 0.405).
Fig. 2.
a Recurrence-free survival for TILs in the TCA. TIL− refers to the TIL- group. TIL+ refers to the TIL+ group. b Recurrence-free survival for CD68+ TAMs in the TIF. CD68+ TAM− refers to the CD68− group. CD68+ TAM+ refers to the CD68+ group.
Discussion
In the present work, we determined the level of abundance of monocytes/macrophages and TILs in tumour specimens of patients with de novo HCC in non-cirrhosis after oncologic resection. In addition, their association with patient’ clinicopathologic characteristics and survival was analysed. The major discoveries were (1) CD68+ TAMs were associated with decreased rates of recurrent and multifocal disease; (2) conversely, M2-polarized TAMs correlated with lymphangiosis carcinomatosa and multifocal HCC; (3) TILs and infiltrating CD68+ TAMs were strongly associated in HCC; and (4) had a potent influence on recurrence-free survival.
In this study, we demonstrated that CD68+ TAMs and M2-polarized TAMs correlate with established clinicopathologic features of advanced de novo HCC in non-cirrhosis. Of note, 29/58 (50%) of the HCC patients in our study had T3/T4 tumours and 47/58 (81%) tumours exhibited moderate/poor histologic differentiation. These results are in accordance with previously published results in HCC and other hepatobiliary tumours, which report on a negative impact of M2 polarization state of infiltrating TAMs on patient survival and outcome [18, 19]. However, when considering CD68+ TAMs, most published results demonstrate a negative value in regard to patient outcome [20]. Here, CD68+ TAMs were associated with significantly prolonged survival. A possible scenario for our results could be that TAMs comprise a diverse and heterogeneous cell population which can express markers typical not only for M1 or M2 polarization states. Their functionality strongly depends on the signals deployed by the tumour microenvironment, i.e. TAMs re-education and reprogramming as classical tumour escape mechanisms. In line with this, Li et al. demonstrated CD68+ TAMs to be also CD204+ or CD169+ cells. The tissue frequency of CD204+ TAMs associated with poor outcome. Conversely, CD169+ TAMs were associated with better survival [19]. Insofar, additional research is needed to investigate the mechanistic interplay between diverse TAMs subpopulations and the tumour microenvironment.
The predominant immune cell population in the tumour vicinity consists mainly of TILs. Accumulating scientific data demonstrates that the type, density and localization of host immunologic tumour infiltration influence its malignant behaviour and could provide clinically informative prognostic biomarkers [21–23]. Furthermore, this immunologic reaction could characterize patient outcome to a greater extent than the diagnostics for the staging of cancer, which are currently applied by conventional histopathology [24]. Consequently, TILs were identified as a reliable immunologic tool in the tumour microenvironment that could be used in clinical trials and translational research. Insofar, here we focused on the prognostic capacity of TILs in tumour specimens of HCC. Our findings indicate that the presence of TILs significantly influence survival of HCC patients after curative surgery. Of note, in our work, intratumoural prevalence of TILs in liver cancer was correlated with a high frequency of invading TAMs. Thus, it appears possible that infiltrating hepatic monocytes/macrophages and TILs comprise a coherent immunological construct that exerts a significant impact in the process of hepatocarcinogenesis.
In our work, in the setting of oncologic HCC resection, TILs and CD68+ TAMs were associated with better survival rates, which is in line with most published data about TILs’ importance in solid cancer that delineates their presence or high tumour density to be associated with improved patient survival [17, 25–27]. Insofar, in this study, we suggest TILs and CD68+ TAMs to be reliable cancer biomarkers prognosticating survival and outcome of HCC patients after surgery. However, a possible limitation of the current work is the descriptive nature of our results and the small number of HCC patients with de novo HCC in non-cirrhosis.
Conclusions
Our study demonstrates that TILs, infiltrating monocytes/macrophages and their functional polarization state associate with multiple tumour characteristics and patient survival in HCC. However, there is only scarce data about the biology underlying mechanistic involvement of TILs, monocytes/macrophages and their polarization in M1 or M2 subtypes in human tumour progression. Thus, a further examination of underlying functional mechanisms that might help develop novel immunologic checkpoint inhibitor targets for liver cancer is warranted.
Acknowledgements
We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Funds of Charité – Universitätsmedizin Berlin.
Abbreviations
- CCA
Cholangiocarcinoma
- HCC
Hepatocellular carcinoma
- PDAC
Periductal adenocarcinoma of the pancreas
- TAMs
Tumour-associated macrophages
- TCA
Tumour central area
- TIF
Tumour-infiltrating front
Authors’ contributions
GA had significant contributions to the conception of the study; to the generation, analysis, and interpretation of the data; and to preparing the manuscript. KD had significant contributions to the generation and analysis of the data and to preparing the manuscript. KS had significant contributions to the analysis and interpretation of the data and to preparing the manuscript. GA conducted a critical review of the manuscript and resources. DS and JP had significant contributions to preparing the manuscript. MS had significant contributions to the conception of the study, to the analysis and interpretation of the data and to preparing the manuscript. HMH had significant contributions to the conception of the study; to the generation, analysis and interpretation of the data; and to preparing the manuscript. All authors read and approved the final manuscript.
Funding
This work was made possible by the funding from the Berlin Institute of Health (BIH) to GA. Georgi Atanasov is a participant of the BIH Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the BIH.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Our work was conducted in accordance with the recommendations of the Ethics Committee of the Medical Faculty of the Leipzig University. The committee’s reference number is 234-14-14072014. Written informed consent for using the tissue samples was obtained from the patients.
Consent for publication
N/A
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66(6):1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Whiteside TL. Targeting adenosine in cancer immunotherapy: a review of recent progress. Expert Rev Anticancer Ther. 2017;17(6):527–535. doi: 10.1080/14737140.2017.1316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Najar HM, Ruhl S, Bru-Capdeville AC, Peters JH. Adenosine and its derivatives control human monocyte differentiation into highly accessory cells versus macrophages. J Leukoc Biol. 1990;47:429–439. doi: 10.1002/jlb.47.5.429. [DOI] [PubMed] [Google Scholar]
- 6.Xaus J, Valledor AF, Cardo M, Marques L, Beleta J, Palacios JM, et al. Adenosine inhibits macrophage colony-stimulating factor-dependent proliferation of macrophages through the induction of p27kip-1 expression. J Immunol. 1999;163:4140–4149. [PubMed] [Google Scholar]
- 7.Xaus J, Mirabet M, Lloberas J, Soler C, Lluis C, Franco R, et al. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol. 1999;162:3607–3614. [PubMed] [Google Scholar]
- 8.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 9.Chari RS, Helton WS, Marsh RD. Chemotherapy and regional therapy of hepatic colorectal metastases: expert consensus statement by Bartlett et al. Ann Surg Oncol. 2006;13:1293–1295. doi: 10.1245/s10434-006-9025-9. [DOI] [PubMed] [Google Scholar]
- 10.Finotello F, Trajanoski Z. Quantifying tumor-infiltrating immune cells from transcriptomics data. Cancer Immunol Immunother. 2018;67(7):1031–1040. doi: 10.1007/s00262-018-2150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: a comprehensive review. World J Hepatol. 2019;11(1):1–18. doi: 10.4254/wjh.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atanasov G, Hau HM, Dietel C, Benzing C, Krenzien F, Brandl A, et al. Prognostic significance of TIE2-expressing monocytes in hilar cholangiocarcinoma. J Surg Oncol. 2016;114:91–98. doi: 10.1002/jso.24249. [DOI] [PubMed] [Google Scholar]
- 13.Atanasov G, Pötner C, Aust G, Schierle K, Dietel C, Benzing C, et al. TIE2-expressing monocytes and M2-polarized macrophages impact survival and correlate with angiogenesis in adenocarcinoma of the pancreas. Oncotarget. 2018;9(51):29715–29726. doi: 10.18632/oncotarget.25690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atanasov G, Dietel C, Feldbrügge L, Benzing C, Krenzien F, Brandl A, et al. Angiogenic miRNAs, the angiopoietin axis and related TIE2-expressing monocytes affect outcomes in cholangiocarcinoma. Oncotarget. 2018;9(52):29921–29933. doi: 10.18632/oncotarget.25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atanasov G, Schierle K, Hau HM, Dietel C, Krenzien F, Brandl A, et al. Prognostic significance of tumor necrosis in hilar cholangiocarcinoma. Ann Surg Oncol. 2017;24(2):518–525. doi: 10.1245/s10434-016-5472-0. [DOI] [PubMed] [Google Scholar]
- 16.Atanasov G, Dietel C, Feldbrügge L, Benzing C, Krenzien F, Brandl A, et al. Tumor necrosis and infiltrating macrophages predict survival after curative resection for cholangiocarcinoma. OncoImmunology. 2017;6(8):e1331806. doi: 10.1080/2162402X.2017.1331806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng W, Li Y, Shen L, Cai XW, Zhu ZF, Chang JH, et al. Prognostic value of tumor-infiltrating lymphocytes for patients with completely resected stage IIIA(N2) non-small cell lung cancer. Oncotarget. 2016;7(6):7227–7240. doi: 10.18632/oncotarget.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minami K, Hiwatashi K, Ueno S, Sakoda M, Iino S, Okumura H, Hashiguchi M, Kawasaki Y, Kurahara H, Mataki Y, Maemura K, Shinchi H, Natsugoe S. Prognostic significance of CD68, CD163 and folate receptor-β positive macrophages in hepatocellular carcinoma. Exp Ther Med. 2018;15(5):4465–4476. doi: 10.3892/etm.2018.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao RR, Li JH, Zhang R, Chen RX, Wang YH. M2-polarized tumor-associated macrophages facilitated migration and epithelial-mesenchymal transition of HCC cells via the TLR4/STAT3 signaling pathway. World J Surg Oncol. 2018;16(1):9. doi: 10.1186/s12957-018-1312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JQ, Yu XJ, Wang YC, Huang LY, Liu CQ, Zheng L, Fang YJ, Xu J. Distinct patterns and prognostic values of tumor-infiltrating macrophages in hepatocellular carcinoma and gastric cancer. J Transl Med. 2017;15(1):37. doi: 10.1186/s12967-017-1139-2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès- Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 22.Loi S, MacCallum P. Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple-negative breast cancer. J Clin Oncol. 2014;32:2936–2938. doi: 10.1200/JCO.2014.56.7677. [DOI] [PubMed] [Google Scholar]
- 23.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, et al. Towards the introduction of the ‘immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 25.Rakaee M, Kilvaer TK, Dalen SM, Richardsen E, Paulsen EE, Hald SM, Al-Saad S, Andersen S, Donnem T, Bremnes RM, Busund LT. Evaluation of tumor-infiltrating lymphocytes using routine H&E slides predicts patient survival in resected non-small cell lung cancer. Hum Pathol. 2018. pii: S0046-8177(18)30185-0. [DOI] [PubMed]
- 26.Yao W, He JC, Yang Y, Wang JM, Qian YW, Yang T, Ji L. The prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep. 2017;7(1):7525. doi: 10.1038/s41598-017-08128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sideras K, Biermann K, Verheij J, Takkenberg BR, Mancham S, Hansen BE, Schutz HM, de Man RA, Sprengers D, Buschow SI, Verseput MC, Boor PP, Pan Q, van Gulik TM, Terkivatan T, Ijzermans JN, Beuers UH, Sleijfer S, Bruno MJ, Kwekkeboom J. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology. 2017;6(2):e1273309. doi: 10.1080/2162402X.2016.1273309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.