Abstract
Background
Treatment persistence is an important consideration when selecting a therapy for chronic conditions such as rheumatoid arthritis (RA). We assessed the long-term persistence of abatacept or a tumor necrosis factor inhibitor (TNFi) following (1) inadequate response to a conventional synthetic disease-modifying antirheumatic drug (first-line biologic agent) and (2) inadequate response to a first biologic DMARD (second-line biologic agent).
Methods
Data were extracted from the Rhumadata® registry for patients with RA prescribed either abatacept or a TNFi (adalimumab, certolizumab, etanercept, golimumab, or infliximab) who met the study selection criteria. The primary outcome was persistence to abatacept and TNFi treatment, as first- or second-line biologics. Secondary outcomes included the proportion of patients discontinuing therapy, reasons for discontinuation, and predictors of discontinuation. Persistence was defined as the time from initiation to discontinuation of biologic therapy. Baseline characteristics were compared using descriptive statistics; cumulative persistence rates were estimated using Kaplan-Meier methods, compared using the log-rank test. Multivariate Cox proportional hazard models were used to compare the persistence between treatments, controlling for baseline covariates.
Results
Overall, 705 patients met the selection criteria for first-line biologic agent initiation (abatacept, n = 92; TNFi, n = 613) and 317 patients met the criteria for second-line biologic agent initiation (abatacept, n = 105; TNFi, n = 212). There were no clinically significant differences in baseline characteristics between the treatments with either first- or second-line biologics. Persistence was similar between the first-line biologic treatments (p = 0.7406) but significantly higher for abatacept compared with TNFi as a second-line biologic (p = 0.0001). Mean (SD) times on first-line biologic abatacept and TNFi use were 4.53 (0.41) and 5.35 (0.20) years, and 4.80 (0.45) and 2.82 (0.24) years, respectively, as second-line biologic agents. The proportion of patients discontinuing abatacept and TNFi in first-line was 51.1% vs. 59.5% (p = 0.1404), respectively. In second-line, it was 57.1% vs. 74.1% (p = 0.0031). The main reasons for stopping both treatments were inefficacy and adverse events.
Conclusions
Abatacept and TNFi use demonstrated similar persistence rates at 9 years as a first-line biologic agent. As a second-line biologic agent, abatacept had better persistence rates over a TNFi.
Keywords: Rheumatoid arthritis, Registry, Disease-modifying antirheumatic drugs (biologic), Persistence, Abatacept, TNF inhibitor
Background
The goal of rheumatoid arthritis (RA) therapy, according to the Canadian Rheumatology Association Recommendations, is to achieve a target of sustained remission or low disease activity [1]. This should be achieved using a treat-to-target approach, based on shared decision-making between the patient and the rheumatologist regarding disease activity, and other patient factors, such as structural damage, comorbidities, and safety issues, as well as individual medical and societal costs [1, 2]. The current treatment strategy in Canada is to use a conventional synthetic disease-modifying antirheumatic drug (csDMARD), such as methotrexate (MTX), as a first-line treatment, in combination with either hydroxychloroquine or sulfasalazine and then add or switch to a biologic DMARD (bDMARD) or targeted-synthetic DMARD (tofacitinib) if the treatment target is not achieved. Tumor necrosis factor inhibitors (TNFis) are typically used as the first choice of biologic agent, although current guidelines do not stipulate any preference regarding which bDMARDs should be used [1, 2]. The Quebec general reimbursement policy states that reimbursement is available for the first biologic agent after the failure of two csDMARDs [3].
Approved treatments for the management of RA have multiple modes of action, and an understanding of how to use these treatments in clinical practice would benefit both clinicians and patients [2]. Several real-world studies have investigated the differences in the efficacy, after the first-line therapy had failed, between a strategy of cycling to another agent with the same mode of action and switching to an agent with a different mode of action [4–7]. Using a second-line TNFi after a first-line TNFi has failed can be an effective treatment strategy [8, 9], although second-line TNFi use often results in a lower response [4, 6, 7]. These results are supported by several studies reporting that switching to a different mode of action when first-line therapy fails is more effective than cycling between agents with the same mode of action [10–15]. Although these studies investigated the differences between cycling and switching in the real world, no long-term data are available to assess the long-term persistence rates between the two treatment strategies.
Abatacept, a selective T cell co-stimulation modulator [16], was one of the first approved alternatives to TNFi agents and, as such, has substantial effectiveness and safety data collected in both clinical trials and real-world practice, including information on the long-term follow-up of patients [17–24].
The aim of this analysis was to assess the long-term persistence of abatacept and a TNFi following an inadequate response to a csDMARD (first-line biologic agent initiation cohort) or a first bDMARD (second-line biologic agent initiation cohort), using data from patients with RA in Quebec, Canada, enrolled in the Rhumadata® registry.
Methods
Data source and patient population
The Rhumadata® registry is an observational clinical practice registry of approximately 7500 patients diagnosed with RA who fulfill the ACR criteria and were seen between January 1, 1999, and February 21, 2018, by 15 rheumatologists at either the Institute of Rheumatology of Montreal or the Center for Osteoporosis and Rheumatology of Quebec in Canada; 3471 patients are being actively followed. Patients included in Rhumadata® represent the standard referral to university or community rheumatology practice of the province of Québec.
Rhumadata® is a complete tabular electronic database built in MySQL language, an open source database provided by ORACLE® (Oracle Corporation, Redwood Shores, CA, USA). All fields are built as mandatory, limiting possible errors and variations of entry, with less than 5% of the information recorded as free text. A restricted verifiable personal usage code is allocated to all those who interact with the database. The data collected are saved twice daily on multiple secured servers. Data collected at baseline and/or at each visit are shown in Table 1.
Table 1.
Summary of information collected in the Rhumadata® registry at baseline and/or each visit
| Patient demographics | Age, gender, height, weight, date of appearance of first symptoms, date of diagnosis, and smoking status |
| Patient-reported outcomes | HAQ Disability Index, morning stiffness (minutes), pain (VAS), patient fatigue (VAS), and patient global evaluation of the impact of disease (VAS) |
| Physician-derived outcomes | PGA of disease activity (VAS), joint counts in 28 joints (tender joint count, swollen joint count), medications used for the control of the disease, and comorbidities and their pharmacologic treatment |
| Laboratory values | Complete blood count, ESR, CRP, liver function testing, creatinine level, RF, and anti-CCP (at baseline or once if not documented previously) |
| Safety information | AEs, SAEs, deaths, non-serious and serious infectious events, and antibiotic usage |
| Hospitalization | Surgeries, recent visits and length of stay in days to the emergency room or hospitalization at their local hospital |
AE adverse event, CCP cyclic citrullinated peptide, CRP C-reactive protein, ESR erythrocyte sedimentation rate, HAQ Health Assessment Questionnaire, PGA physician global assessment, RF rheumatoid factor, SAE serious adverse event, VAS visual analog scale
The Rhumadata® registry was established in accordance with the Declaration of Helsinki and is approved on an annual basis by an ethics committee (IRB services); all patients provided written informed consent.
Study population
This analysis includes all patients in the Rhumadata® database with a primary diagnosis of RA (based on the clinical judgment of the clinician) who were prescribed either abatacept or a TNFi (adalimumab, certolizumab, etanercept, golimumab, or infliximab) as a first or second biologic agent on or after January 1, 2006 (date of abatacept approval in Canada); all patients in the Rhumadata® registry meeting these selection criteria were included. Patients were followed from baseline, defined as the initiation of a first or second biologic agent, until the cessation of treatment, loss to follow-up, or the end of the analysis period (February 21, 2018), whichever comes first. Treatment assignment was based on clinical practice and determined by a rheumatologist.
Study outcomes
The primary outcome was persistence to abatacept and TNFi treatments when used as first- or second-line biologic agents. Secondary outcomes included the proportion of patients discontinuing the treatment, reasons for treatment discontinuation, and predictors of treatment discontinuation.
Persistence was defined as the time on treatment and was calculated from initiation to discontinuation of biologic therapy; patients remaining on treatment at the time of data extraction and patients who were lost to follow-up were included in the analysis and were said to have a censored discontinuation time. Data from all patients who had temporary treatment interruption and subsequently resumed biologic treatment were also included in the analysis of the primary outcome, regardless of the length of the interruption. Time to treatment discontinuation was defined as the time taken until patients permanently stopped study treatments. Reasons for treatment discontinuation were recorded as well as secondary diagnoses and comorbidities reported at first or second biologic agent initiation and infections while on treatment.
Statistical analysis
For baseline characteristics, data are presented as the number (%) for categorical variables and mean (SD) for continuous variables. Differences in categorical variables were tested using Fisher’s exact or chi-square tests and continuous variables using Student’s t test or ANOVA. DMARD persistence rates are presented using Kaplan-Meier survival curves, adjusted for censoring (i.e., for patients not experiencing biologic cessation during the study time frame for whatever reason) and compared using log-rank tests. These curves represent the attrition over time associated with drug persistence in the patient cohorts. A multivariate analysis was conducted using a subset of variables deemed to be univariately associated with DMARD persistence and/or a stepwise regression approach. Hazard ratios (HR) and 95% confidence intervals (CI) for time to treatment discontinuation were adjusted for age at diagnosis, disease duration, and age-adjusted Charlson Comorbidity Index.
Stepwise selection proportional hazard models (Cox regressions) were used to determine which, among all baseline variables measured, were associated with biologic discontinuation. Baseline characteristics potentially associated with DMARD persistence were included, one at a time, in proportional hazard models. Variables had to have a p value of 0.25 or less to enter the model and of 0.15 or less to remain in the model. The secondary diagnoses and comorbidities reported at first or second biologic agent initiation were tabulated for each treatment group, as were the infections reported while on treatment and the reasons for biologic discontinuation. Statistical analyses were performed using SAS version 9.4.
Results
Patient disposition and baseline characteristics
Overall, 705 patients were selected for first-line biologic agent initiation in this study following an inadequate response to a csDMARD; of these, 92 patients received abatacept and 613 received a TNFi. A total of 317 patients were selected for second-line biologic agent initiation, 105 of whom received abatacept and 212 of whom received a TNFi. No clinically significant differences were seen between the treatment groups for the majority of baseline characteristics, in both the first- and the second-line biologic agent initiation cohorts (Table 2); however, significant differences in a few key characteristics were noted (e.g., Clinical Disease Activity Index and Simplified Disease Activity Index scores and concomitant medications); these differences were controlled for using a multivariate analysis. In both first- and second-line biologic agent initiation cohorts, the majority of patients were female and received concomitant csDMARDs. In the first-line biologic agent initiation cohort, patients had a mean age of ~ 48 years and had been diagnosed with RA for ~ 7 years. In the second-line biologic agent initiation cohort, patients had a mean age at entry of 45 years and had been diagnosed with RA for 10–12 years.
Table 2.
Baseline characteristics
| Use following csDMARD-IR | Use following first bDMARD-IR | |||||
|---|---|---|---|---|---|---|
| Abatacept | TNFi | p value | Abatacept | TNFi | p value | |
| n | 92 | 613 | – | 105 | 212 | – |
| Age, years | 49.3 (13.6) | 47.3 (13.2) | 0.1871 | 45.9 (14.4) | 45.5 (13.0) | 0.8197 |
| Disease duration, years | 7.0 (7.8) | 6.8 (7.7) | 0.7980 | 12.1 (10.4) | 10.0 (8.8) | 0.0660 |
| Female, n (%) | 71 (77.2) | 476 (77.7) | 0.8940 | 84 (80.0) | 156 (73.6) | 0.2655 |
| Concomitant medication use, n (%) | ||||||
| csDMARDs | 87 (94.6) | 567 (92.5) | 1.0000 | 87 (82.9) | 174 (82.1) | 1.0000 |
| MTX | 69 (75.0) | 466 (76.0) | 0.7955 | 69 (65.7) | 142 (67.0) | 0.8994 |
| HCQ | 72 (78.3) | 350 (57.1) | < 0.0001 | 43 (41.0) | 72 (34.0) | 0.2640 |
| SSZ | 11 (12.0) | 45 (7.3) | 0.1455 | 9 (8.6) | 10 (4.7) | 0.2095 |
| LEF | 7 (7.6) | 54 (8.8) | 0.8434 | 7 (6.7) | 16 (7.5) | 1.0000 |
| Corticosteroids | 45 (48.9) | 282 (46.0) | 0.6542 | 73 (69.5) | 93 (43.9) | < 0.0001 |
| Number of oral corticosteroid prescriptions per 100 person-years of treatment | 81.24 | 44.02 | 99.03 | 76.23 | ||
| TNFi used, n (%) | N/A | N/A | ||||
| Adalimumab | 146 (23.8) | 58 (27.4) | ||||
| Certolizumab | 62 (10.1) | 27 (12.7) | ||||
| Etanercept | 239 (39.0) | 69 (32.5) | ||||
| Golimumab | 105 (17.1) | 29 (13.7) | ||||
| Infliximab (Remicade) | 60 (9.8) | 26 (12.3) | ||||
| Infliximab (Inflectra) | 1 (0.2) | 3 (1.4) | ||||
| Comorbidities | ||||||
| Age-adjusted CCI | 2.8 (1.4) | 2.4 (1.3) | 0.2403 | 2.9 (1.8) | 2.6 (1.5) | 0.2228 |
| Hyperlipidemia, n (%) | 28 (30.4) | 193 (31.5) | 0.9044 | 42 (40.0) | 75 (35.4) | 0.4589 |
| Hyperglycemia, n (%) | 13 (14.1) | 82 (13.4) | 0.8699 | 11 (10.5) | 32 (15.1) | 0.2987 |
| Hypertension, n (%) | 37 (40.2) | 290 (47.3) | 0.2187 | 58 (55.2) | 110 (51.9) | 0.6329 |
| COPD, n (%) | 37 (40.2) | 194 (31.6) | 0.1211 | 43 (41.0) | 76 (35.8) | 0.3905 |
| CVD, n (%) | 11 (12.0) | 75 (12.2) | 1.0000 | 19 (18.1) | 37 (17.5) | 0.8769 |
| Charlson Comorbidity Index | 1.4 (0.9) | 1.2 (0.7) | 0.0039 | 1.4 (1.2) | 1.3 (0.8) | 0.59 |
| RF+, n (%) | 69 (75.0) | 424 (69.2) | 0.2680 | 73 (69.5) | 141 (66.5) | 0.7020 |
| ACPA+, n (%) | 56 (60.9) | 358 (58.4) | 1.0000 | 55 (52.4) | 107 (50.5) | 1.0000 |
| ESR, mm/h | 18.7 (16.0) | 24.5 (19.8) | 0.0080 | 21.9 (19.2) | 24.8 (20.8) | 0.3164 |
| CRP, mg/L | 14.30 (20.20) | 12.90 (20.40) | 0.5875 | 16.30 (23.40) | 11.80 (19.40) | 0.1226 |
| Patient-reported outcomes | ||||||
| Patient global, VAS 0–10 | 5.6 (2.4) | 4.8 (2.8) | 0.0430 | 5.2 (2.7) | 4.4 (2.9) | 0.0324 |
| Patient pain, VAS 0–10 | 6.1 (2.6) | 5.3 (3.0) | 0.0401 | 5.7 (3.0) | 5.0 (3.1) | 0.1157 |
| Patient fatigue, VAS 0–10 | 5.8 (2.8) | 4.7 (3.2) | 0.0099 | 5.7 (2.8) | 4.8 (3.3) | 0.0597 |
| Morning stiffness, min | 155.0 (308.3) | 120.9 (274.1) | 0.3676 | 127.3 (294.7) | 93.0 (244.5) | 0.3597 |
| HAQ | 1.31 (0.61) | 1.24 (0.60) | 0.3661 | 1.51 (0.60) | 1.18 (0.66) | 0.0006 |
| Physician global, VAS 0–10 | 4.8 (2.7) | 4.0 (2.6) | 0.0370 | 4.0 (2.6) | 3.4 (2.6) | 0.2256 |
| Swollen joint count, 0–28 | 8.5 (5.2) | 7.4 (5.3) | 0.1409 | 7.7 (5.3) | 6.5 (6.0) | 0.1755 |
| Tender joint count, 0–28 | 6.8 (6.4) | 6.6 (5.7) | 0.8451 | 7.3 (6.1) | 5.4 (5.9) | 0.0481 |
| Disease activity measures | ||||||
| CDAI | 26.3 (12.7) | 23.5 (11.7) | 0.1416 | 24.8 (11.0) | 18.8 (12.5) | 0.0069 |
| SDAI | 28.6 (13.1) | 24.7 (12.0) | 0.0639 | 26.3 (11.7) | 20.4 (12.6) | 0.0140 |
| DAS28-4, ESR | 4.8 (1.3) | 4.7 (1.3) | 0.7557 | 4.8 (1.2) | 4.5 (1.4) | 0.3621 |
Data are mean (SD), unless stated otherwise
ACPA anti-citrullinated protein antibody, bDMARD biologic disease-modifying antirheumatic drug, CCI Charlson Comorbidity Index, CDAI Clinical Disease Activity Index, COPD chronic obstructive pulmonary disease, CRP C-reactive protein, csDMARD conventional synthetic disease-modifying antirheumatic drug, CVD cardiovascular disease, DAS28-4 disease activity score in 28 joints (four variables), ESR erythrocyte sedimentation rate, HAQ Health Assessment Questionnaire, HCQ hydroxychloroquine, IR inadequate response, LEF leflunomide, MTX methotrexate, RF rheumatoid factor, SDAI Simplified Disease Activity Index, SSZ sulfasalazine, TNFi tumor necrosis factor inhibitor, VAS visual analog scale
Primary outcome
In patients with an inadequate response to a prior csDMARD (first-line biologic agent initiation), there were no significant differences in the primary outcome of persistence during the 9-year study period between abatacept- and TNFi-treated patients (p = 0.7406; Fig. 1a); however, persistence was significantly higher with abatacept than with TNFi in patients who had an inadequate response to a prior bDMARD (second-line biologic agent initiation, p = 0.0001) (Fig. 1b). The mean (SD) biologic persistence times for the first-line biologic agent initiation were 4.53 (0.41) (median [95% CI], 3.25 [1.95, 8.59]) and 5.35 (0.20) (median [95% CI], 3.72 [2.71, 4.51]) years for the abatacept and TNFi groups, respectively. For the second-line biologic agent initiation, these values were 4.80 (0.45) (median [95% CI], 3.03 [1.92, 4.78]) and 2.82 (0.24) (median [95% CI], 1.08 [0.77, 1.60]) years, respectively. The mean (SD) treatment interruption time was 44.4 (133.1) days and 20.6 (64.6) days for first- and second-line TNFi use, respectively. For abatacept, the mean treatment interruption time was 34.7 (84.3) days and 37.4 (84.2) days for first-and second-line treatment, respectively.
Fig. 1.
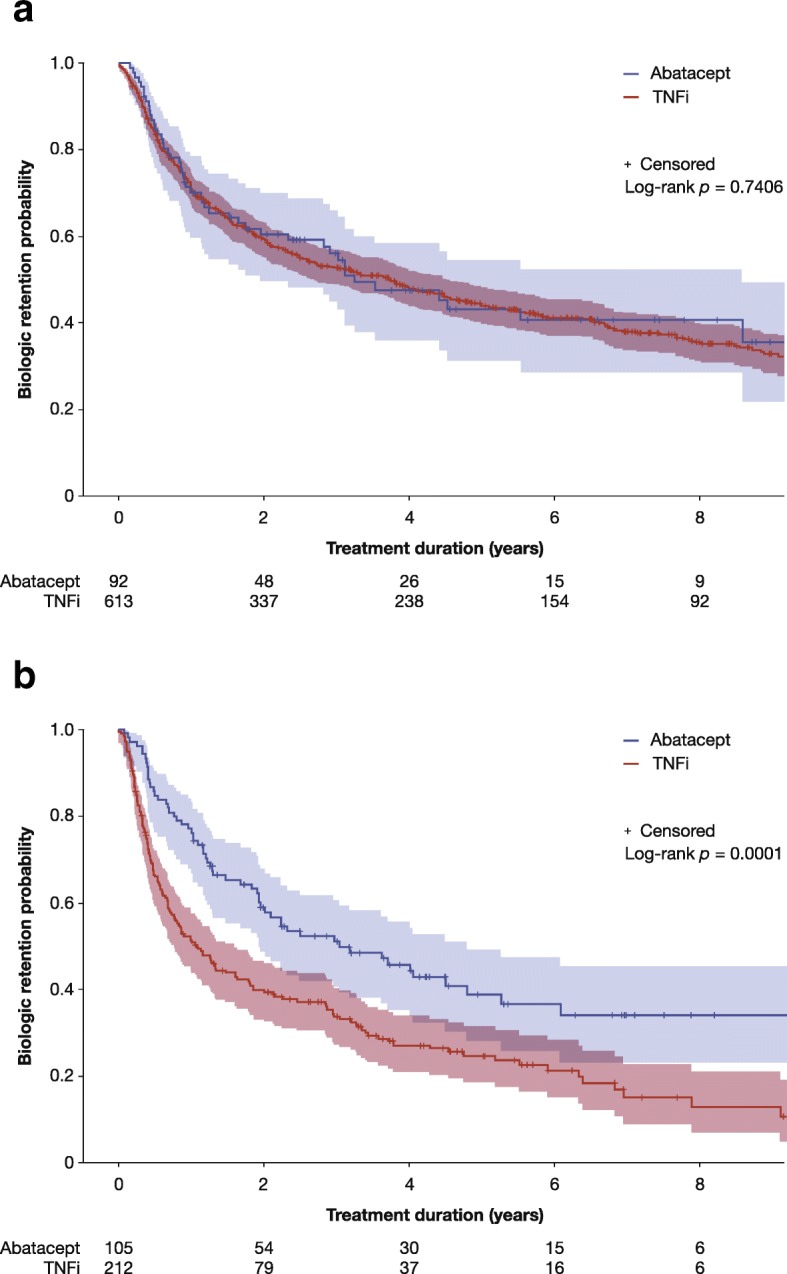
Kaplan-Meier retention curves in patients failing a csDMARDs and b a first bDMARD. bDMARD, biologic disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; TNFi, tumor necrosis factor inhibitor
Over the 9-year study period, a similar percentage of patients with an inadequate response to a prior csDMARD permanently discontinued both abatacept and a TNFi (51.1% vs. 59.5%, p = 0.1404); however, treatment discontinuation was significantly lower for abatacept than for TNFis in patients who had failed a first bDMARD (57.1% vs. 74.1%, p = 0.0031) (Table 3).
Table 3.
Treatment status
| Treatment status | Use following csDMARD-IR (first-line biologic agent) | Use following first bDMARD-IR (second-line biologic agent) | ||||
|---|---|---|---|---|---|---|
| Abatacept | TNFi | p value | Abatacept | TNFi | p value | |
| n | 92 | 613 | – | 105 | 212 | – |
| Stopped treatment, n (%) | 47 (51.1) | 365 (59.5) | 0.1404 | 60 (57.1) | 157 (74.1) | 0.0031 |
| Treatment duration, years, mean (SD) | 1.57 (1.68) | 2.01 (2.23) | 0.1068 | 1.76 (1.78) | 1.33 (1.70) | 0.1045 |
| Reasons for stopping, n (%) | 0.1351 | 0.4409 | ||||
| Inefficacy | 28 (59.6) | 171 (46.8) | 38 (63.3) | 86 (54.8) | ||
| Adverse event | 8 (17.0) | 76 (20.8) | 12 (20.0) | 27 (17.2) | ||
| Lost to follow-up | 2 (4.3) | 21 (5.8) | 0 | 6 (3.8) | ||
| Treatment stopped by the patient | 3 (6.4) | 14 (3.8) | 0 | 4 (2.5) | ||
| Infections | 3 (6.4) | 22 (6.0) | 2 (3.3) | 9 (5.7) | ||
| Death | 0 | 7 (1.9) | 3 (5.0) | 4 (2.5) | ||
| Ongoing treatment | 45 (48.9) | 248 (40.5) | 0.1404 | 45 (42.9) | 55 (25.9) | 0.0031 |
| Treatment duration, years, mean (SD) | 4.46 (2.99) | 6.25 (3.25) | 0.0007 | 4.57 (2.69) | 4.15 (2.68) | 0.4364 |
bDMARD biologic disease-modifying antirheumatic drug, csDMARD conventional synthetic disease-modifying antirheumatic drug, IR inadequate response, TNFi tumor necrosis factor inhibitor
Secondary outcomes
In both first- and second-line biologic agent initiation cohorts, the main reasons for stopping both abatacept and TNFi treatments were inefficacy (first-line, 59.6% vs. 46.8% [p = 0.1210]; second-line, 63.3% vs. 54.8% [p = 0.2851]) and adverse events (AEs) (first-line, 17.0% vs. 20.8% [p = 0.7006]; second-line, 20.0% vs. 17.2% [p = 0.7544]) (Table 3). In the first-line biologic agent initiation cohort, the mean (SD) time to treatment discontinuation was 1.57 (1.68) years for abatacept-treated and 2.01 (2.23) years for TNFi-treated (adjusted HR [95% CI], 0.932 [0.678, 1.252]) patients. In the second-line biologic agent initiation cohort, the mean (SD) time to treatment discontinuation was 1.76 (1.78) years for abatacept-treated and 1.33 (1.70) years for TNFi-treated (adjusted HR, 0.553; 95% CI, 0.403, 0.746) patients.
Multivariate analysis showed that in patients with an inadequate response to a first bDMARD (second-line biologic initiation cohort), treatment with abatacept (vs. TNFi) (HR [95% CI], 0.506 [0.319, 0.804], p = 0.0039) and concomitant treatment with a cyclooxygenase 2 inhibitor (HR [95% CI], 0.524 [0.305, 0.901], p = 0.0194) were significant predictors of improved retention, whereas female (vs. male) sex (HR [95% CI], 1.933 [1.117, 3.345], p = 0.0185) and baseline disease activity score in 28 joints (four variables) (erythrocyte sedimentation rate) (DAS28-4 [ESR]) (HR [95% CI], 1.244 [1.064, 1.454], p = 0.0061) were significant predictors of biologic treatment failure (Fig. 2).
Fig. 2.
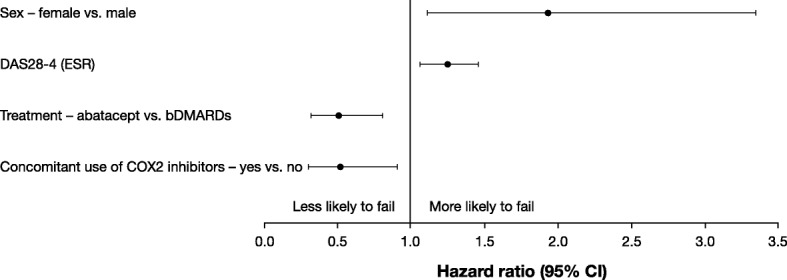
Multivariate analysis showing the predictors of biologic failure when used in patients with an inadequate response to a first bDMARD (second-line biologic initiation cohort). CI, confidence interval; COX2, cyclooxygenase 2; DAS28-4, disease activity score in 28 joints (four variables); ESR, erythrocyte sedimentation rate
Discussion
The results from this analysis of the Rhumadata® registry, using real-world data from all patients with a primary diagnosis of RA who were prescribed either abatacept or a TNFi as a first or second biologic agent in Canadian clinical practice, demonstrate that there was no difference in persistence during the 9-year study period between abatacept and a TNFi when used as a first-line biologic agent. As a second-line biologic agent, patients treated with abatacept had a greater persistence than those treated with a TNFi, based on both univariate (Kaplan-Meier) and multivariate (controlling for all baseline covariates using Cox proportional hazard model) analyses.
These findings are consistent with clinical trial data from the phase III AMPLE (NCT00929864) and ATTEST (NCT00095147) trials [22–24]. In the AMPLE trial, subcutaneous (SC) abatacept + MTX was non-inferior to SC adalimumab + MTX in terms of American College of Rheumatology 20% improvement (ACR20) response at 1 year, and the treatments remained comparable over the 2-year follow-up [23, 24]. Similarly, the ATTEST trial showed that ACR20 response rates were significantly greater with both abatacept + MTX and infliximab + MTX than with placebo + MTX at 6 months [22]. In both trials, similar safety profiles were shown between agents, although abatacept-treated patients had fewer serious adverse events and serious infections [22–24].
Registries complement data from randomized controlled trials, especially as more long-term experience, including persistence data, can be collected in real-world settings than is possible in clinical trials [25–27]. Several observational studies and biologic registries have contributed to a wealth of available data regarding the long-term efficacy and safety of these agents [18, 19, 21, 23, 25]. Furthermore, observational studies have highlighted the importance of real-world persistence, defined as the time from the index dose to the time of switching to a different biologic or the time of the last dose/censoring, as a surrogate for treatment effectiveness and a factor to consider when choosing between agents [20, 28]. These studies showed that first-line abatacept treatment had higher persistence rates than TNFis [20, 28].
Our results in patients treated with abatacept or a TNFi as a second-line biologic agent are supported by several real-world studies [12–14]. These studies showed that switching treatment to a biologic with a different mode of action after the first-line treatment (with TNFi) has failed is associated with better outcomes than cycling to a different TNFi [12–14]. A systematic review and Bayesian analysis comparing the effectiveness of switching to a non-TNFi vs. cycling between TNFi agents showed that switching to a therapy with a new mode of action was more effective than TNFi cycling in patients with RA and an inadequate response to an initial TNFi [29]. The cause of TNFi discontinuation may influence the performance of the cycling strategy as the differences were particularly evident in patients who failed the previous treatment due to inefficacy [12–14]. Our results further support this growing body of evidence, showing that as a second-line biologic agent, abatacept was associated with greater persistence than a TNFi over the 9-year follow-up period. In addition to better effectiveness, switching to a new mode of action rather than cycling to a different TNFi has been associated with a reduction in overall economic burden and healthcare costs [11, 30].
In our multivariate analysis of patients with an inadequate response to a first bDMARD (second-line biologic initiation cohort), treatment with abatacept (vs. TNFi) and concomitant treatment with a cyclooxygenase 2 inhibitor were significant predictors of improved retention, whereas female (vs. male) sex and DAS28-4 (ESR), measured continuously, were significant predictors of treatment failure. The published literature looking at predictors of retention in patients with RA treated with a first bDMARD (second-line biologic initiation) is limited. Two studies showed that changing to a non-TNFi rather than a TNFi after the failure of a first biologic was a predictor of improved retention [31, 32]. In addition, a multivariate analysis [6] of the ACTION study showed that abatacept-treated patients who had received at least one prior biologic had a significantly lower risk of discontinuation if they were anti-cyclic citrullinated peptide (CCP) positive, had failed < 2 anti-TNF agents, or had a cardiovascular comorbidity at abatacept initiation [6]. Factors that were not predictors of abatacept discontinuation included baseline C-reactive protein value, baseline rheumatoid factor status, type of previous TNFi failure, and abatacept treatment patterns (monotherapy, combination with MTX or other csDMARDs) [6].
Our study has several strengths: we utilized a large Canadian-based registry of patients with RA, over a 9-year follow-up period, to examine the persistence with abatacept vs. a TNFi as either the first- or second-line biologic agents. To the best of our knowledge, this is the longest follow-up period examining the persistence in these patients. The results from the unselected population in this study support the clinical trial data and are more generalizable to patients found in clinical practice. Further, the Rhumadata® registry is an electronic database that contains fields that are built as an obligatory menu, limiting the possible errors and variations of data entry. This study has some limitations: as with all observational studies, there may be some bias with regard to the assignment of treatment and patient selection. Confounding by indication may be present owing to the lack of randomization. Data were collected at two academic centers and may not be generalizable to Canadian clinical practice.
Conclusions
The results from this analysis of the Rhumadata® registry showed that abatacept and TNFis demonstrated similar persistence over a 9-year follow-up period as a first-line biologic agent in patients who have failed one prior csDMARD. As a second-line biologic agent, abatacept had a better persistence over a TNFi in patients who had failed one prior bDMARD.
Acknowledgements
Professional medical writing support and editorial assistance was provided by Claire Line, PhD, at Caudex and was funded by Bristol-Myers Squibb. The authors would like to thank Edith Villenueve, Angèle Turcotte, Frédéric Massicotte, Jean-Pierre Pelletier, and Marie-Anaïs Remillard for making substantial contributions to the acquisition of patient data and to Diane Sauvageau for her substantial contributions to the analysis and interpretation of the data.
Abbreviations
- ACPA
Anti-citrullinated protein antibody
- ACR
American College of Rheumatology
- ACR20
American College of Rheumatology 20% improvement
- AE
Adverse event
- bDMARD
Biologic disease-modifying antirheumatic drug
- CCI
Charlson Comorbidity Index
- CCP
Cyclic citrullinated peptide
- CDAI
Clinical Disease Activity Index
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- COX2
Cyclooxygenase 2
- CRP
C-reactive protein
- csDMARD
Conventional synthetic disease-modifying antirheumatic drug
- CVD
Cardiovascular disease
- DAS28-4
Disease activity score in 28 joints (four variables)
- ESR
Erythrocyte sedimentation rate
- HAQ
Health assessment questionnaire
- HCQ
Hydroxychloroquine
- HR
Hazard ratio
- IR
Inadequate response
- LEF
Leflunomide
- MTX
Methotrexate
- PGA
Physician global assessment
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- SAE
Serious adverse event
- SC
Subcutaneous
- SDAI
Simplified Disease Activity Index
- SSZ
Sulfasalazine
- TNFi
Tumor necrosis factor inhibitor
- VAS
Visual analog scale
Authors’ contributions
DC, LB, BH, J-PR, and LC provided substantial contributions to the study conception and design, acquisition of the data, and analysis and interpretation of the data. EA and RP provided substantial contributions to the analysis and interpretation of the data. All authors were involved in the drafting, critical review, and approval of all versions of this manuscript, its content, and its submission, and take public responsibility for appropriate portions of the content.
Funding
Professional medical writing support and editorial assistance for this manuscript was funded by Bristol-Myers Squibb. Rhumadata® is supported by unrestricted grants from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, Sandoz, and UCB.
Availability of data and materials
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Rhumadata® is approved on an annual basis by an ethics committee (Institutional Review Board Services, Aurora, Ontario, Canada). All registry subjects were required to provide written informed consent prior to participating.
Consent for publication
Not applicable.
Competing interests
DC is a consultant and speaker for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Merck, Novartis, Pfizer, Roche, and Sandoz. LB is a consultant and speaker for, and has received research support from, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer, Roche, and UCB; in addition, LB has received speaker fees from Merck and research support from Merck and Sanofi. EA and RP are employees of and/or shareholders of and/or hold stock options in Bristol-Myers Squibb. BH is a consultant for AbbVie, Amgen, Lilly, Merck, Pfizer, and UCB; in addition, BH is a speaker for Pfizer and has received research support from AbbVie. J-PR is a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, and UCB; in addition, J-PR has received consulting fees and research support from Arthrovision Inc. LC declares no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Denis Choquette, Phone: +1 514 523 8603, Email: denis.choquette.irm@videotron.ca.
Louis Bessette, Email: louis.bessette@crchul.ulaval.ca.
Evo Alemao, Email: Evo.Alemao@bms.com.
Boulos Haraoui, Email: paulharaoui@videotron.ca.
Roelien Postema, Email: Roelien.Postema@bms.com.
Jean-Pierre Raynauld, Email: jp.raynauld@videotron.ca.
Louis Coupal, Email: louis.coupal@mail.mcgill.ca.
References
- 1.Bykerk VP, Akhavan P, Hazlewood GS, Schieir O, Dooley A, Haraoui B, et al. Canadian Rheumatology Association Recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol. 2012;39(8):1559–1582. doi: 10.3899/jrheum.110207. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 3.Régie de l’assurance maladie Québec. Liste des médicaments – Établissements: Dépôt légal — Bibliothèque et Archives nationales du Québec; 2018 [updated 24 mai 2018. Available from: http://www.ramq.gouv.qc.ca/SiteCollectionDocuments/liste_med/2019/liste_med_2019_05_23_fr.pdf. Accessed 14 Aug 2018.
- 4.Codullo V, Iannone F, Sinigaglia L, Favalli EG, Sarzi-Puttini P, Atzeni F, et al. Comparison of efficacy of first- versus second-line adalimumab in patients with rheumatoid arthritis: experience of the Italian biologics registries. Clin Exp Rheumatol. 2017;35(4):660–665. [PubMed] [Google Scholar]
- 5.Gottenberg JE, Brocq O, Perdriger A, Lassoued S, Berthelot JM, Wendling D, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA. 2016;316(11):1172–1180. doi: 10.1001/jama.2016.13512. [DOI] [PubMed] [Google Scholar]
- 6.Nüßlein HG, Alten R, Galeazzi M, Lorenz HM, Nurmohamed MT, Bensen WG, et al. Prognostic factors for abatacept retention in patients who received at least one prior biologic agent: an interim analysis from the observational, prospective ACTION study. BMC Musculoskelet Disord. 2015;16:176. doi: 10.1186/s12891-015-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiff M, Le Bars M, Gaillez C, Wu G, Poncet C, Genovese M. Efficacy and safety of abatacept in patients with rheumatoid arthritis and an inadequate response to anti-TNF therapy by number of prior anti-TNF therapies used. Ann Rheum Dis. 2009;68(Suppl 3):574. [Google Scholar]
- 8.Schiff MH, von Kempis J, Goldblum R, Tesser JR, Mueller RB. Rheumatoid arthritis secondary non-responders to TNF can attain an efficacious and safe response by switching to certolizumab pegol: a phase IV, randomised, multicentre, double-blind, 12-week study, followed by a 12-week open-label phase. Ann Rheum Dis. 2014;73(12):2174–2177. doi: 10.1136/annrheumdis-2014-205325. [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Kay J, Doyle MK, Landewe R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374(9685):210–221. doi: 10.1016/S0140-6736(09)60506-7. [DOI] [PubMed] [Google Scholar]
- 10.Cantini F, Niccoli L, Nannini C, Cassara E, Kaloudi O, Giulio Favalli E, et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin Arthritis Rheum. 2017;47(2):183–192. doi: 10.1016/j.semarthrit.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Chastek B, Chen CI, Proudfoot C, Shinde S, Kuznik A, Wei W. Treatment persistence and healthcare costs among patients with rheumatoid arthritis changing biologics in the USA. Adv Ther. 2017;34(11):2422–2435. doi: 10.1007/s12325-017-0617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emery P, Gottenberg JE, Rubbert-Roth A, Sarzi-Puttini P, Choquette D, Taboada VM, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74(6):979–984. doi: 10.1136/annrheumdis-2013-203993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology (Oxford) 2014;53(9):1664–1668. doi: 10.1093/rheumatology/keu158. [DOI] [PubMed] [Google Scholar]
- 14.Wei W, Knapp K, Wang L, Chen CI, Craig GL, Ferguson K, et al. Treatment persistence and clinical outcomes of tumor necrosis factor inhibitor cycling or switching to a new mechanism of action therapy: real-world observational study of rheumatoid arthritis patients in the United States with prior tumor necrosis factor inhibitor therapy. Adv Ther. 2017;34(8):1936–1952. doi: 10.1007/s12325-017-0578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonafede MM, Curtis JR, McMorrow D, Mahajan P, Chen CI. Treatment effectiveness and treatment patterns among rheumatoid arthritis patients after switching from a tumor necrosis factor inhibitor to another medication. Clinicoecon Outcomes Res. 2016;8:707–715. doi: 10.2147/CEOR.S115706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada A, Salama AD, Sayegh MH. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J Am Soc Nephrol. 2002;13(2):559–575. doi: 10.1681/ASN.V132559. [DOI] [PubMed] [Google Scholar]
- 17.Alten R, Mariette X, Lorenz HM, Galeazzi M, Cantagrel A, Nusslein HG, et al. Real-world predictors of 12-month intravenous abatacept retention in patients with rheumatoid arthritis in the ACTION observational study. RMD Open. 2017;3(2):e000538. doi: 10.1136/rmdopen-2017-000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alten R, Nusslein HG, Mariette X, Galeazzi M, Lorenz HM, Cantagrel A, et al. Baseline autoantibodies preferentially impact abatacept efficacy in patients with rheumatoid arthritis who are biologic naive: 6-month results from a real-world, international, prospective study. RMD Open. 2017;3(1):e000345. doi: 10.1136/rmdopen-2016-000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrold LR, Reed GW, Solomon DH, Curtis JR, Liu M, Greenberg JD, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18(1):280. doi: 10.1186/s13075-016-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones Graeme, Hall Stephen, Bird Paul, Littlejohn Geoff, Tymms Kathleen, Youssef Peter, Chung Eric, Barrett Rina, Button Peter. A retrospective review of the persistence on bDMARDs prescribed for the treatment of rheumatoid arthritis in the Australian population. International Journal of Rheumatic Diseases. 2017;21(8):1581–1590. doi: 10.1111/1756-185X.13243. [DOI] [PubMed] [Google Scholar]
- 21.Lahaye C, Soubrier M, Mulliez A, Bardin T, Cantagrel A, Combe B, et al. Effectiveness and safety of abatacept in elderly patients with rheumatoid arthritis enrolled in the French Society of Rheumatology’s ORA registry. Rheumatology (Oxford) 2016;55(5):874–882. doi: 10.1093/rheumatology/kev437. [DOI] [PubMed] [Google Scholar]
- 22.Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67(8):1096–1103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiff M, Weinblatt ME, Valente R, van der Heijde D, Citera G, Elegbe A, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2014;73(1):86–94. doi: 10.1136/annrheumdis-2013-203843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinblatt ME, Schiff M, Valente R, van der Heijde D, Citera G, Zhao C, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65(1):28–38. doi: 10.1002/art.37711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikiphorou E, Buch MH, Hyrich KL. Biologics registers in RA: methodological aspects, current role and future applications. Nat Rev Rheumatol. 2017;13(8):503–510. doi: 10.1038/nrrheum.2017.81. [DOI] [PubMed] [Google Scholar]
- 26.van Vollenhoven RF, Askling J. Rheumatoid arthritis registries in Sweden. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S195–S200. [PubMed] [Google Scholar]
- 27.Takahashi N, Kojima T, Kaneko A, Kida D, Hirano Y, Fujibayashi T, et al. Longterm efficacy and safety of abatacept in patients with rheumatoid arthritis treated in routine clinical practice: effect of concomitant methotrexate after 24 weeks. J Rheumatol. 2015;42(5):786–793. doi: 10.3899/jrheum.141288. [DOI] [PubMed] [Google Scholar]
- 28.Nadkarni A, McMorrow D, Fowler R, Smith D. Comparing biologic persistence and healthcare costs in rheumatoid arthritis patients initiating subcutaneous biologics. J Comp Eff Res. 2017;6(8):659–669. doi: 10.2217/cer-2017-0010. [DOI] [PubMed] [Google Scholar]
- 29.Kim HL, Lee MY, Park SY, Park SK, Byun JH, Kwon S, et al. Comparative effectiveness of cycling of tumor necrosis factor-alpha (TNF-alpha) inhibitors versus switching to non-TNF biologics in rheumatoid arthritis patients with inadequate response to TNF-alpha inhibitor using a Bayesian approach. Arch Pharm Res. 2014;37(5):662–670. doi: 10.1007/s12272-014-0337-1. [DOI] [PubMed] [Google Scholar]
- 30.Betts KA, Griffith J, Ganguli A, Li N, Douglas K, Wu EQ. Economic burden and treatment patterns of cycling between conventional synthetic disease-modifying antirheumatic drugs among biologic-treated patients with rheumatoid arthritis. Clin Ther. 2016;38(5):1205–1216. doi: 10.1016/j.clinthera.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Lauper Kim, Nordström Dan C, Pavelka Karel, Hernández Maria Victoria, Kvien Tore K, Kristianslund Eirik Klami, Santos Maria Jose, Rotar Žiga, Iannone Florenzo, Codreanu Catalin, Lukina Galina, Gale Sara L, Sarsour Khaled, Luder Yves, Courvoisier Delphine Sophie, Gabay Cem. Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: analyses from the pan-European TOCERRA register collaboration. Annals of the Rheumatic Diseases. 2018;77(9):1276–1282. doi: 10.1136/annrheumdis-2017-212845. [DOI] [PubMed] [Google Scholar]
- 32.Rotar Z, Hocevar A, Rebolj KA, Praprotnik S, Tomsic M. Retention of the second-line biologic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis failing one tumor necrosis factor alpha inhibitor: data from the BioRx.si registry. Clin Rheumatol. 2015;34(10):1787–1793. doi: 10.1007/s10067-015-3066-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.


