Abstract
Background
Establishing the diagnosis of peri-prosthetic shoulder infection prior to revision shoulder arthroplasty can be difficult. The objectives of the present study were (i) to describe the technique of fluoroscopic capsular needle biopsy for the diagnosis of peri-prosthetic shoulder infection and (ii) to determine the feasibility and preliminary accuracy of the test in a pilot sample of patients undergoing revision shoulder arthroplasty.
Methods
Eighteen patients, comprising eight females and nine males with a mean age of 61 years (range 37 years to 81 years) underwent capsular needle biopsy during the work-up of suspected chronic arthroplasty-related glenohumeral infection. Intra-operative tissue samples were taken from a minimum of three regions of the joint capsule during revision surgery. Standard serum indices were obtained.
Results
Of 17 patients with possible infection, five had confirmed culture positive infections based on intra-operative biopsies. Of these five patients, four (80%) had positive cultures from fluoroscopic capsular needle biopsy, with matching cultures. There were no complications. No culture-positive patients had elevated serum indices for infection.
Conclusions
The technique for fluoroscopic capsular needle biopsy appears to be feasible and the preliminary results for this technique appear to be promising, with a sensitivity of 80% and a specificity of 100%.
Level of evidence: Level II: diagnostic test
Keywords: capsular needle biopsy, infection, open biopsy, Proprionibacterium acnes, periprosthetic shoulder infection, shoulder joint, shoulder arthroplasty
Introduction
Infection following implant-related shoulder surgery can be a catastrophic complication, with a reported prevalence of deep peri-prosthetic infection in the range of 0% to 15.4%.1–3 At 20-year follow-up, the infection rate in shoulder hemiarthroplasty is 1.3%, with an underlying diagnosis of trauma being associated with a higher risk of periprosthetic infection.4 The incidence of infection in total shoulder arthroplasty at 20 years is 0% to 5.1%.2,5
Proprionibacterium acnes is the most commonly occurring organism in prosthesis-related shoulder infections.2,4,6 However, it has proven difficult to diagnose with aspiration, with a reported detection rate of 10% to 50%.7,8 Proprionibacterium acnes is a slow growing indolent organism and, in contrast to periprosthetic infections in other joints, the clinical symptoms of infection in the shoulder are often nonspecific. Patients most commonly present with pain and stiffness in the absence of systemic symptoms of fever, malaise or chills.6,7,9,10 Although several factors have been shown to correlate with the presence of positive cultures in shoulder arthroplasty, including male sex and humeral loosening, a reliable means of pre-operative diagnosis of implant-related shoulder infections remains elusive.9
Serum markers including C-reactive protein (CRP), erythrocyte sedimention rate (ESR) and white blood cell count (WBC) may indicate the presence of infection. However they do not localize the site of infection and have poor sensitivity and specificity.11,12 Open tissue culture is the accepted gold standard for the diagnosis of prosthetic shoulder infections.
Historically, synovial tissue biopsy has been used in the knee using blind needle or arthroscopy-guided techniques.13 To our knowledge, shoulder capsular needle biopsy or fluoroscopy-guided techniques have not been previously described.
The primary objective of the present study was to describe the technique of fluoroscopy-guided capsular needle biopsy for the diagnosis of periprosthetic shoulder infection. The secondary objective was to determine the feasibility and preliminary accuracy of the test in a pilot sample of patients undergoing revision shoulder arthroplasty.
Materials and methods
This was a pilot feasibility study. A prospective, consecutive series of patients were enrolled in the study and underwent capsular needle biopsy prior to revision surgery during the workup of a painful shoulder arthroplasty from 2013 to 2014. Regional ethics board approval was obtained prior to the initiation of the study.
Inclusion criteria included males or females of greater than 18 years of age who had a painful shoulder arthroplasty, in whom there was a suspicion of infection including severe pain, and who required revision surgery. Patients were excluded if they had less than 6 months of follow-up following their revision procedure.
Key demographic variables included patient age at time of capsular needle biopsy, time from index surgery, presenting symptoms and symptom duration, type of index surgery and type of revision surgery, pre-operative serologic markers, and culture results.
Technique: capsular needle biopsy
One musculoskeletal radiologist performed all capsular needle biopsies after obtaining informed consent for the procedure from the patient. No patients were on antibiotics prior to or at the time of capsular needle biopsy.
A sterile set-up with chlorhexidine preperation was used and the shoulder was draped in a sterile fashion. The radiologist was gowned and sterile gloves were used. Capsular needle biopsies were performed using an anterior approach with fluoroscopic guidance following the administration of local anesthesia, 1% lidocaine hydrochloride (Xylocaine; Astra USA, Westborough, MA, USA) alone, without conscious sedation. The needles used for the definitive procedure were different from those used for local anesthesia.
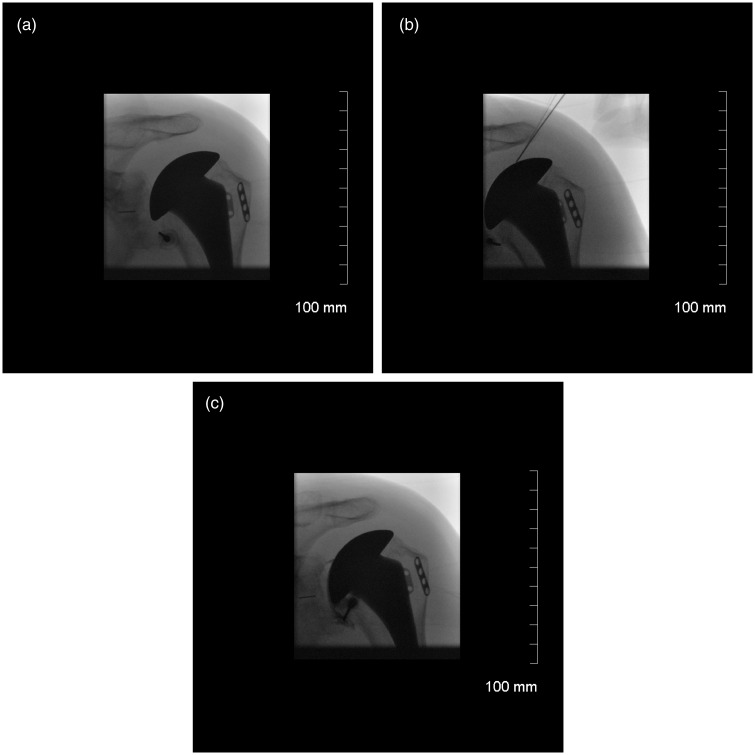
An anteroposterior fluoroscopic image was obtained for localization (Fig. 1). The capsular needle biopsy was performed using a coaxial needle biopsy technique using an 18-gauge spinal needle (Braun Medical, Bethlehem, PA, USA) A needle was placed anteriorly and was advanced to the inferior recess of the glenohumeral joint and into the rotator interval. If fluid was present, a joint aspirate was obtained. If fluid was not present, normal saline was injected and then aspirated.
Figure 1.
(a) Needle is localized in the axillary recess. (b) Needle is placed in the rotator interval. (c) Contrast dye is injected to confirm the position of the needle.
A 22-gauge Chiba needle (Cook Medical, Bloomington, IN, USA) was then advanced through the outer co-axial needle and a sample of pericapsular tissue from the axillary recess and two additional biopsies from the rotator interval were obtained. Precautions were taken to minimize the risk of cross-contamination including the use of a fresh needles for each biopsy.
Two milliltres of contrast was then injected through the outer co-axial spinal needle and an image was taken to confirm that there was an intra-articular blush (Fig. 1). A minimum of three capsular needle biopsy tissue samples were obtained in all cases and one capsular needle aspirate/wash was sent for bacteriologic analysis. All samples were incubated for a minimum of 14 days. Cultures were considered ‘positive’ if two or more samples yielded bacterial growth. In addition, 10 consecutive patients in the series had one additional biopsy specimen for histological analysis.
Technique: surgery
All surgical interventions were performed by a single surgeon. Cultures were obtained in all cases during open revision shoulder surgery. Open revision biopsies were obtained between 14 days and 40 days following capsular needle biopsy.
A minimum of three biopsy samples was obtained. Prophylactic antibiotics were held until after the samples were obtained and no patients were on antibiotics prior to surgery. Intra-operative tissue samples were taken from the inferior capsule and from the rotator interval consistent with the locations used during capsular needle biopsy and were then sent for tissue culture. Precautions were taken to minimize the risk of cross-contamination including the use of new scalpel blades for each biopsy. In addition, the 10 patients who had additional tissue sent for histological analysis during capsular needle biopsy also had tissue sent for histological analysis during open surgery.
All capsular needle and open biopsy specimens were transported in sterile containers immediately to the microbiology laboratory for aerobic and anaerobic culture. Cultures were examined every 24 h and all cultures were maintained for a minimum of 14 days to determine the presence of P. acnes, in accordance with our institutional policy for shoulder cultures. The specimen cultures were performed using standard anaerobic methods. Specimens were inoculated onto various bacteriologic media including pre-reduced CDC agar, phenylethyl alcohol agar, laked kanamycin-vancomycin agar and thioglycollate broth media. All bacterial isolates were identified using standard microbiological methods and the API Rapid ID 32 A (bioMBrieux, Marcy-I'Etoile, France) was used to identify anaerobes including P. acnes. Routine susceptibility testing of P. acnes was not performed.
Infection was considered ‘positive’ for both the capsular needle biopsy and open surgical biopsies in accordance with the the guidelines established by the Musculoskeletal Infection Society.14 Amongst other criteria, this definition requires the presence of two or more positive cultures.
Analysis
The primary outcome was a comparison of capsular needle biopsy culture results against open biopsy results as a gold standard. For the purpose of this pilot study, the sensitivity and specificity of the diagnostic test (capsular needle biopsy), as well as positive and negative predictive values, were determined.
Results
Seventeen patients, comprising eight females and nine males, were included with a mean age of 61 years (range 37 years to 81 years) (Table 1). The mean (SD) time from index surgery to the time to capsular needle biopsy was 55 (62) months (range 7 months to 204 months). The mean (SD) symptom duration was 10 (12) months (range 0.5 months to 36 months). All patients had prior shoulder arthroplasty, consisting of humeral head replacement (n = 6), resurfacing (n = 2), total shoulder arthroplasty (n = 8) or reverse shoulder arthroplasty (n = 1). The mean (SD) number of surgical procedures prior to the index arthroplasty was 1.6 (0.9) (range 1 to 4). One patient had previously undergone revision arthroplasty prior to inclusion in the study. The presenting symptoms included severe pain either at rest or with motion and in three cases, night sweats.
Table 1.
Patient demographics.
| Patient | Sex | Age at surgery | Time from index surgery (months) | Duration of symptoms (months) | Presenting symptoms | Index surgery |
|---|---|---|---|---|---|---|
| 1 | Female | 81 | 7 | 1.5 | Pain | RSA |
| 2 | Male | 52 | 12 | 0.5 | Pain/night sweats | TSA |
| 3 | Male | 54 | 9 | 2 | Pain/night sweats | HHR |
| 4 | Female | 65 | 15 | 2 | Pain | HHR |
| 5 | Female | 51 | 29 | 12 | Pain | TSA |
| 6 | Male | 51 | 12 | 4 | Pain/stiffness | HHR |
| 7 | Male | 62 | 204 | 6 | Pain/night sweats | HHR |
| 8 | Female | 75 | 42 | 3 | Pain | TSA |
| 9 | Male | 67 | 38 | 36 | Pain | Resurfacing |
| 10 | Male | 52 | 204 | 6 | Pain | TSA |
| 11 | Male | 60 | 70 | 36 | Pain | HHR |
| 12 | Male | 62 | 108 | 36 | Pain | TSA |
| 13 | Male | 61 | 7 | 0.5 | Pain/stiffness | HHR |
| 14 | Male | 70 | 36 | 6 | Pain | TSA |
| 15 | Female | 70 | 24 | 2 | Pain | TSA |
| 16 | Male | 61 | 61 | 12 | Pain | TSA |
| 17 | Male | 51 | 72 | 12 | Pain | Resurfacing |
HHR, humeral head replacement; RSA, reverse shoulder arthroplasty; TSA, total shoulder.
The mean (SD) WBC was 7.7 (2.2) cells µL × 103 (range 7 cells/µL × 103 to 12.5 cells/µL × 103; normal 3.5 cells/µL × 103 to 10.5 cells/µL × 103). The mean ESR was 14.5 (22.8) mm/h (range 0 mm/h to 90 mm/h; normal 0 mg/L to 6 mg/L). The mean (SD) CRP was 5.9 (8.1) mg/L (range 0.5 mg/L to 25 mg/L; normal < 10 mg/L). None of the patients demonstrating positive cultures had elevation of WBC, ESR or CRP values prior to the procedure.
Fluoroscopically-guided synovial fluid aspiration was performed prior to capsular needle biopsy in 13 of 17 patients. There was a single positive culture from capsular needle fluid aspiration of the 13 patients who underwent aspiration (7%). Capsular needle biopsy tissue cultures were positive in four of 17 cases (24%) (Table 2). All patients underwent open revision surgery. The open cultures yielded positive cultures in eight cases, although three cases were considered contaminants as a result of the presence of only a single positive culture. Thus, five patients (29%) had positive cultures obtained by open biopsy. Of the four positive results by capsular needle biopsy, the bacteria matched the bacteria obtained by open technique. There were three cases of P. acnes, and one case of Staphylococcus aureus.
Table 2.
Laboratory/culture results.
| Patient | Index surgery | WBC | ESR | CRP | Aspirate | Capsular needle biopsy | Open culture (positive/total) |
|---|---|---|---|---|---|---|---|
| 1 | RSA | 12.5 | 6 | 1 | Neg | Neg | CNS in 1/4 (contaminant) |
| 2 | TSA | 5.4 | 1 | 1.5 | Neg | Propionibacterium acnes | Propionibacterium acnes 4/4 |
| 3 | HHR | 5.7 | 0 | 0.5 | Neg | Neg | Propionibacterium acnes in 2/4 |
| 4 | HHR | 9.2 | 40 | 19.8 | Neg | Neg | Neg |
| 5 | TSA | 5 | 2 | 1 | Neg | Neg | Neg |
| 6 | HHR | 11.7 | 16 | 2.2 | Neg | Neg | Propionibacterium acnes in 1/5 (contaminant) |
| 7 | HHR | 9.8 | 4 | 4.3 | Neg | Neg | Neg |
| 8 | TSA | 6.1 | 8 | 2.3 | Neg | Propionibacterium acnes | Propionibacterium acnes in 4/4 |
| 9 | Resurf | 9.4 | 10 | 7 | Neg | Neg | Neg |
| 10 | TSA | 8 | 2 | 3.7 | NA | Neg | Neg (CNS in 1/4, (contaminant) |
| 11 | HHR | 6.3 | 9 | 5.7 | CNS | CNS | CNS in 2/4 |
| 12 | TSA | 7 | 90 | 22.4 | Neg | Neg | Neg |
| 13 | HHR | 7 | 35 | 25 | NA | Neg | Neg |
| 14 | TSA | 7.5 | 2 | 0.6 | NA | Neg | CNS in 1/5 (contaminant) |
| 15 | TSA | 6 | 5 | 0.5 | Neg | Neg | Neg |
| 16 | TSA | 6 | 2 | 2 | Neg | Neg | Neg |
| 17 | Resurf | 7 | 1 | 1 | Neg | Propionibacterium acnes | Propionibacterium acnes (4/6) |
CNS, coagulase negative staphylococcus; HHR, humeral head replacement; NA, not available; Neg, negative culture; Resurf, resurfacing arthroplasty; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
Fluoroscopically-assisted synovial fluid aspiration did not identify four of the five patients with positive open cultures, resulting in a sensitivity of 20%, a specificity of 100%, a positive predictive value of 100% and a negative predictive value of 69%.
Capsular needle biopsy correctly identified four patients of five with positive open cultures, resulting in a sensitivity for capsular needle biopsy of 80%, a specificity of 100%, a positive predictive value of 100% and a negative predictive value of 92% in this series. No complications occurred as a result of capsular needle biopsy.
Histological analysis of both capsular needle tissue biopsy and open tissue biopsy specimens revealed similar tissue composition: fibrous, fibroadipose and fibrovascular tissue was identified, frequently with reactive inflammatory changes. Some specimens demonstrated mixed inflammatory cells including neutrophils with few lymphocytes. Occasional synovial lining cells were observed in both capsular needle biopsy and open biopsy specimens.
All 17 patients underwent revision surgery. Four of the infections were identified pre-operatively with capsular needle biopsy and were treated with two-stage revision arthroplasty. In the fifth case, the patient underwent revision to a total shoulder arthroplasty and the open biopsy cultures identified the presence of infection postoperatively. In this case, a second revision was immediately carried out with the insertion of a spacer. Following the insertion of the spacer, patients with confirmed infection received 6 weeks of intravenous antibiotics followed by 6 weeks of oral antibiotics, with an infectious diseases specialist monitoring all patients. Following an antibiotic holiday of 6 weeks to 8 weeks, patients underwent repeat synovial biopsy or arthroscopic biopsy or both and all cultures were negative prior to embarking on the second-stage revision. Two patients underwent revision to humeral head replacement, one to a total shoulder replacement, one maintained the spacer in situ and has declined any further surgery, and the last was revised to a reverse shoulder arthroplasty. The mean (SD) time to follow-up following initial capsular biopsy to latest evaluation was 31.6 (16) months (range 12 months to 48 months). Two patients developed recurrence of infection and ultimately underwent resection arthroplasty.
Twelve patients were culture negative. One patient underwent insertion of an antibiotic spacer as a result of uncertainty regarding the presence of infection at the time of revision. This patient has not sought additional surgery because of health concerns unrelated to the shoulder. Two patients underwent revision to total shoulder arthroplasty as a result of pain from glenoid erosion, six underwent revision to reverse shoulder arthroplasty as a result of loosening of the glenoid, one underwent bone grafting to the glenoid (with revision of the humeral head only), one was revised to humeral head replacement and one underwent a subscapularis repair with no revision of the prosthesis.
The mean (SD) time to follow-up following capsular biopsy to the latest evaluation was 27 (9) months (range 8 months to 36 months). All patients who underwent a single-stage revision with a negative capsular biopsy had pain-free shoulders with no evidence of infection at the latest clinical follow-up.
Discussion
The results of the present feasibility study demonstrate that fluoroscopically-assisted capsular needle tissue biopsy has the potential to identify the presence of infection pre-operatively, with a sensitivity of 80%, a specificity of 100%, a positive predictive value of 100% and negative predictive value of 92%. This is in contrast to synovial fluid aspirate, which only correctly identified one of five patients with peri-prosthetic infection.
Although no complications were observed related to the procedure in the present study, the technique is associated with some degree of short-term pain. Although the pain was in keeping with that normally experienced with synovial fluid aspiration, synovial tissue biopsy requires multiple passes through capsule to obtain multiple separate biopsies.
The needle biopsies in the present study were performed under fluoroscopic guidance. This modality was chosen to obtain confirmation about the position of the intra-articular needle using contrast dye after puncturing the capsule. However, the biopsies could also reasonably be performed under ultrasound guidance if this modality is more readily available with the added benefit of avoiding additional radiation.
Proprionibacterium acnes is a Gram-positive, nonspore-forming anaerobic bacillus found in the cutaneous, upper respiratory and digestive mucosae.15 It preferentially colonizes areas rich in sebaceous glands, such as the axilla, scalp and face, in close proximity to shoulder surgical sites compared to the hip and knee.16 The diagnosis of post-surgical and implant-related shoulder infection is notoriously difficult in large part because P. acnes is often implicated. Given that no reliable diagnostic test currently exists for the diagnosis of P. acnes-related infection other than arthroscopic17 or open biopsy, the management of patients with shoulder implants and pain can be very difficult. Patients may be subjected to further surgery17 or revision before a firm diagnosis of infection can be established. We hypothesized that capsular needle biopsy, a minimally invasive means of obtaining tissue samples for the purpose of establishing a diagnosis of infection, has possible utility as a diagnostic test. Our goal was to compare the culture results obtained in a small series of patients with possible infection who have undergone capsular needle biopsy against the culture results of arthroscopic or open biopsy in a pilot feasibility study. Our preliminary results indicate that the results of the capsular needle biopsy have a high concordance with the results of open biopsy. Dilisio et al.17 reported on 19 patients who underwent arthroscopic biopsies prior to revision surgery with 100% sensitivity, specificity, positive predictive value and negative predictive values compared to open biopsy cultures. There are two main advantages of fluroscopically-guided synovial biopsy over arthroscopic biopsy: the first is that patients are not subjected to an additional surgical procedure and the second is the potential benefit of a percutaneous needle biopsy compared to the cost associated with operating room utilization.
In rheumatology, synovial biopsy has been used in the setting of suspected lower extremity infection when synovial fluid cannot be aspirated and/or antibiotic treatment has already been initiated, or for bacteria that are difficult to culture or grow slowly.8,13
Proprionibacterium acnes infection has proven difficult to diagnose with aspiration with a reported 10% to 50% detection rate.7,8 Often, there is a paucity of synovial fluid to aspirate or none at all. This is in contrast to hip and knee prosthetic joint infection where joint aspiration has been shown to be very helpful in diagnosis, allowing accurate pre-operative planning.18 In the present study, only one patient who was culture-positive by open biopsy had a positive culture by synovial fluid aspirate.
Tissue culture is the accepted gold standard for the diagnosis of prosthetic shoulder joint infections. Current recommendations for improvements in culture diagnosis of periprosthetic joint infections include collection of multiple samples (five or six) from the site of infection and prolongation of incubation of cultures for up to 14 days.9,19 The average time for growth of P. acnes in culture is 3.5 days to 9 days, although there are reports of positive cultures up to 4 weeks.1,8,9,20–23 Unfortunately, even tissue culture is less successful in the diagnosis of prosthetic shoulder infection than it is for other joints with a reported sensitivity as low as 54.5% and a specificity of 95.1%.24 Tissue culture by any means as a diagnostic tool has certain limitations, including sampling errors, inadequate quantities of vital bacteria, and inappropriate transport and fastidious organisms. Despite open tissue culture being the gold standard, as many as 20% of prosthetic joint infections are culture negative. In the present study, the decision to proceed with two-stage revision in four cases was based on the pre-operative synovial biopsy results. The 80% concordance rate with open cultures appears to indicate that the synovial biopsy results were reliable and that our surgical decision making was justified. However, the treatment of patients demonstrating positive pre-operative synovial biopsy cultures with a single-stage revision and extended antiobiotic coverage until open biopsy culture results are available remains as a viable option.
Given the relatively high preponderance for low-grade infection in the setting of painful shoulder arthroplasty, our current practice is to perform an infection work-up prior to revision surgery in all cases. Although there is a risk of introducing infection into the joint with synovial biopy, this risk also exists with needle aspiration alone and the higher test accuracy with synovial biopsy appears promising.
The need to treat patients with one positive culture with no other clinical signs of infection with prolonged antibiotic therapy remains controversial.25 Isolation of a single low-virulence pathogen such as coagulase-negative Staphylococcus, P acnes or Corynebacteria in the absence of other criteria is not cosnidered to represent a definite infection.14 In the present study, three patients had a positive result in a single culture, and each of these results was treated as a contaminant; none were actively treated for infection and none subsequently developed clinical evidence of infection. The absence of elevated serum indices in the present study is in accordance with previous reports.6,7
The primary limitation of the present study is its small sample size. Our goal was to determine the feasibility of this diagnostic technique in a small sample of patients and determine whether the technique had the potential to establish a diagnosis of infection. The results of the present study appear to demonstrate that the technique indeed has the potential to provide capsular tissue and, in addition, the preliminary results obtained using this technique are both sensitive and specific. The tissue was analyzed histologically to confirm that capsular/pericapsular tissue was obtained. Capsular tissue following shoulder arthroplasty consists mainly of fibrous tissue: the histology demonstrated mainly the presence of fibrous tissue and synovial lining cells were only occasionally seen in both capsular needle biopsy and open biopsy specimens. The interpretation of positive cultures in revision shoulder arthroplasty is controversial. Recent reports have indicated that up to one-third of patients undergoing first-time shoulder surgery have had positive cultures.22,26 Therefore any positive biopsy results must be interpreted carefully and considered in the context of the overall clinical picture given that there remains a possibility of false positive results.
Finally, only three synovial and open biopsies were obtained in each patient. It is possible that the accuracy could increase if more specimens were obtained.
In conclusion, capsular needle biopsy has demonstrated feasibility and potential utility as a test in the diagnosis of peri-prosthetic shoulder infection. Further studies are required to fully clarify the validity of this technique.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Review and Patient Consent
Full ethics board approval was obtained prior to initiation of the study. Patient consent was obtained prior to enrolment.
References
- 1.Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg 2010; 19: 303–307. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg 2012; 21: 1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Florschutz AV, Lane PD, Crosby LA. Infection after primary anatomic versus primary reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 1296–1301. [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Sperling JW, Schleck C, Harmsen W, Cofield RH. Periprosthetic infections after shoulder hemiarthroplasty. J Shoulder Elbow Surg 2012; 21: 1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beekman PD, Katusic D, Berghs BM, Karelse A, De Wilde L. One-stage revision for patients with a chronically infected reverse total shoulder replacement. J Bone Joint Surg Br 2010; 92: 817–822. [DOI] [PubMed] [Google Scholar]
- 6.Topolski MS, Chin PY, Sperling JW, Cofield RH. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Shoulder Elbow Surg 2006; 15: 402–406. [DOI] [PubMed] [Google Scholar]
- 7.Schneeberger AG, Gilbart MK, Sheikh R, Gerber C, Ruef C. Non-purulent low-grade infection as cause of pain following shoulder surgery: preliminary results. La Chirurgia degli organi di movimento 2009; 93(Suppl 1): S71–S77. [DOI] [PubMed] [Google Scholar]
- 8.Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res 2011; 469: 2824–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foruria AM, Fox TJ, Sperling JW, Cofield RH. Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Shoulder Elbow Surg 2013; 22: 620–627. [DOI] [PubMed] [Google Scholar]
- 10.Amaravathi RS, Kany J, Melet M, et al. Analysis of infection in shoulder arthroplasty: a multicentre study. Eur J Orthop Surg Traumatol 2011; 22: 145–150.26662767 [Google Scholar]
- 11.Gallo J, Raska M, Dendis M, Florschutz AV, Kolar M. Molecular diagnosis of prosthetic joint infection. A review of evidence. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2004; 148: 123–129. [DOI] [PubMed] [Google Scholar]
- 12.Saper D, Capiro N, Ma R, Li X. Management of Propionibacterium acnes infection after shoulder surgery. Curr Rev Musculoskelet Med 2015; 8: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bresnihan B. Are synovial biopsies of diagnostic value? Arthritis Res Ther 2003; 5: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011; 469: 2992–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brook I, Frazier EH. Infections caused by Propionibacterium species. Rev Infect Dis 1991; 13: 819–822. [DOI] [PubMed] [Google Scholar]
- 16.Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg 2001; 20: 139–143. [DOI] [PubMed] [Google Scholar]
- 17.Dilisio MF, Miller LR, Warner JJ, Higgins LD. Arthroscopic tissue culture for the evaluation of periprosthetic shoulder infection. J Bone Joint Surg Am 2014; 96: 1952–1958. [DOI] [PubMed] [Google Scholar]
- 18.Font-Vizcarra L, Garcia S, Martinez-Pastor JC, Sierra JM, Soriano A. Blood culture flasks for culturing synovial fluid in prosthetic joint infections. Clin Orthop Relat Res 2010; 468: 2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergidis P, Patel R. Novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect Dis Clin N Am 2012; 26: 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pottinger P, Butler-Wu S, Neradilek MB, et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am 2012; 94: 2075–2083. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JD, II, Hobgood ER. Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res 2009; 467: 2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy O, Iyer S, Atoun E, et al. Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg 2013; 22: 505–511. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Toye B, Desjardins M, Lapner P, Lee C. A 7-year retrospective review from 2005 to 2011 of Propionibacterium acnes shoulder infections in Ottawa, Ontario, Canada. Diagn Microbiol Infect Dis 2013; 75: 195–199. [DOI] [PubMed] [Google Scholar]
- 24.Piper KE, Fernandez-Sampedro M, Steckelberg KE, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PloS ONE 2010; 5: e9358–e9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grosso MJ, Sabesan VJ, Ho JC, Ricchetti ET, Iannotti JP. Reinfection rates after 1-stage revision shoulder arthroplasty for patients with unexpected positive intraoperative cultures. J Shoulder Elbow Surg 2012; 21: 754–758. [DOI] [PubMed] [Google Scholar]
- 26.Hudek R, Sommer F, Kerwat M, Abdelkawi AF, Loos F, Gohlke F. Propionibacterium acnes in shoulder surgery: true infection, contamination, or commensal of the deep tissue? J Shoulder Elbow Surg 2014; 23: 1763–1771. [DOI] [PubMed] [Google Scholar]