Abstract
Regular exercise is shown to exert anti-inflammatory effects, yet the effects of acute exercise on cellular inflammatory responses and its mechanisms remain unclear. We tested the hypothesis that sympathoadrenergic activation during a single bout of exercise has a suppressive effect on monocytic cytokine production mediated by β2 adrenergic receptors (AR). We investigated the effects of 20-minute moderate (65–70 % VO2 peak) exercise-induced catecholamine production on LPS-stimulated TNF production by monocytes in 47 healthy volunteers and determined AR subtypes involved. We also examined the effects of β-agonist isoproterenol and endogenous β-and α-agonists epinephrine and norepinephrine, and receptor-subtype-specific β- and α-antagonists on TNF production in a series of in vitro investigations. LPS-stimulated TNF production was determined intracellularly by flow cytometry in peripheral blood monocytes. Percent TNF-producing monocytes and per-cell TNF production with and without LPS was suppressed by exercise with moderate to large effects, which was reversed by a β2-AR antagonist in spite that plasma TNF levels did not change. This inhibitory response in TNF production by exercise was mirrored by β-AR agonists in an agonist-specific and dose-dependent manner in vitro: similar isoproterenol (EC50= 2.1–4.7×10−10M) and epinephrine (EC50= 4.4–10×10−10M) potency and higher norepinephrine concentrations (EC50= 2.6–4.3×10−8M) needed for the effects. Importantly, epinephrine levels observed during acute exercise in vivo significantly inhibited TNF production in vitro. The inhibitory effect of the AR agonists was abolished by β2-, but not by β1- or α-AR blockers. We conclude that the downregulation of monocytic TNF production during acute exercise is mediated by elevated epinephrine levels through β2-ARs. Decreased inflammatory responses during acute exercise may protect against chronic conditions with low-grade inflammation.
Keywords: adrenergic agonist, beta blocker, cytokine regulation, exercise, sympathetic activation
1. Introduction
Stimulation of monocytes and macrophages by microbial products, such as bacterial cell wall compounds (e.g. lipopolysaccharides, LPS, and peptidoglycans) triggers a complex cellular immune response that activates the production of multiple molecules, including proinflammatory cytokines such as TNF, IL-1β, and IFN-γ (Petrovsky and Aguilar, 2004). TNF plays a major role in local and systemic inflammation and helps to boost innate and adaptive immune responses to bacterial and viral infections (O’Brien et al., 1999; Strangfeld et al., 2009). However, a sustained production of TNF during persistent inflammation caused by chronic infections or obesity has been implicated in the progression of variety of human diseases, including autoimmune diseases, cardiovascular diseases and neurodegeneration (Berg and Scherer, 2005; Kollias et al., 1999). Understanding the triggers and regulatory mechanisms of monocytic production of TNF during normal to exaggerated immune responses such as during chronic infections (Kiechl et al., 2001) may contribute to developing strategies to maintain a healthy and balanced inflammatory response.
Regular physical activity is promoted for its protective effects on a wide variety of chronic inflammatory diseases, especially cardiovascular disease (Mathur and Pedersen, 2008; Smart et al., 2011). Reports of how acute exercise affect inflammatory cytokine production are inconsistent: pre- to post-acute-exercise, plasma levels of inflammatory cytokines, including TNF, increase (Moldoveanu et al., 2000; Starkie et al., 2001) or remain unchanged (Starkie et al., 2005); LPS-stimulated cytokine production in cell supernatant increases (Goebel et al., 2000), decreases (Drenth et al., 1998), or doesn’t change (Haahr et al., 1991); plasma TNF levels after LPS-injection decrease (Starkie et al., 2003); and intracellular cytokine production decreases (Starkie et al., 2001; Starkie et al., 2005) or doesn’t change (Zaldivar et al., 2006). This largely mixed state of the literature is attributed to the varying methodology (i.e., exercise intensity, duration, or type; cytokine types; or cytokine assessments: plasma, stimulated cell supernatant vs. cellular production levels). Thus, whether and in which manner acute exercise exerts immunomodulatory effects on inflammatory cytokine production remains to be clarified. Given the marked leukocytosis during an exercise challenge (Hong et al., 2005; Hong and Mills, 2008), intracellular levels of cytokines produced by a specific cell population provides more definitive evidence of cellular immune responses during exercise than total soluble levels of cytokines in mixed cell supernatant. In addition, a clear distinction should be made between plasma levels (“spontaneous production”) and immunological challenge-induced (e.g., endotoxin stimulation) cellular production of cytokines in response to exercise.
The literature suggests an increased level of muscle-derived IL-6 with its anti-inflammatory action as a mechanism for the anti-inflammatory effects of acute exercise (Petersen and Pedersen, 2006; Starkie et al., 2003). Another main mediator in inflammatory cytokine responses during exercise appears to be catecholamines. Immediate activation of the sympathetic nervous system (SNS) during exercise results in markedly increased levels of catecholamines in circulating blood (epinephrine, Epi and norepinephrine, NE) (Dimsdale and Moss, 1980) and at the vicinity of sympathetic nerve synaptic terminals (NE) (Bellinger et al., 2008). It is well documented that sympathetic nerves densely innervate cellular compartments of lymphoid organs and the adrenal glands. Sympathetic activation during acute stress (Sanders and Straub, 2002) and exercise (Pedersen and Hoffman-Goetz, 2000; Simpson et al., 2015), or catecholamine infusion (Dimitrov et al., 2010) influences leukocyte functions. Human leukocytes including monocytes possess substantial numbers of beta adrenergic receptors (β-ARs) (Elenkov et al., 2000; Maisel et al., 1989; Maisel et al., 1990). A number of in vitro studies reported blunted LPS-stimulated production of inflammatory cytokines (i.e., TNF and IL-1β) by monocytes in the presence of β-AR agonists (Severn et al., 1992; van der Poll et al., 1996). However, previously observed catecholamine effects on monocyte cytokine production were achieved by concentrations of Epi (effective concentration generating 50 % of the effect [EC50] of 1.5 × 10−8M or higher) or NE (EC50 of 3.8 × 10−8M or higher) well beyond physiological levels found in blood (Farmer and Pugin, 2000; Rontgen et al., 2004). In fact, there exists a lack of systematic investigation of β-adrenergic-mediated inhibition of cytokine production by monocytes during exercise or using physiological levels of β-adrenergic agonists in vitro. Thus, it remains to be answered whether a bout of moderate exercise inhibits proinflammatory cytokine production via sympathoadrenal activation.
Another question remains as to which AR subtype is the main mediator of the catecholamine effects on monocytic cytokine production during acute stress such as exercise. β3-ARs are not expressed on immune cells (Grisanti et al., 2010), and α-ARs appear to play little role in the effects of NE on monocytic TNF production (van der Poll et al., 1996). The literature includes mixed evidence on whether cytokine responses by monocytes are mediated by β1-AR (van der Poll et al., 1994; von Haehling et al., 2005), β2-AR, or both subtypes (Farmer and Pugin, 2000; Kavelaars et al., 1997; Sekut et al., 1995). This inconsistency may be attributed to the use of pharmacological doses of NE or Epi, as aforementioned and large doses of β-antagonists with low β1 vs. β2 selectivity in some studies (van der Poll et al., 1994; von Haehling et al., 2005). Limited systematic data of a side-by-side comparison of the agonists’ effects contributes to the confusion regarding the β1- versus β2-AR mediation of monocytic cytokine production during acute stress such as exercise.
Hence, the aims of this study were to investigate the effects of catecholamines on the LPS-stimulated TNF production by monocytes during exercise and the AR subtypes involved. Firstly, a standardized exercise challenge was employed to investigate the changes in intracellular TNF production by monocytes in relation with Epi and NE levels during an acute bout of moderate exercise among healthy volunteers. Beta-adrenergic antagonists were also used ex vivo to block the effect of catecholamines. This experiment was followed by a series of in vitro investigations using physiological doses of β-adrenergic agonists (Epi, NE, and Iso) and highly specific β1-, β2-, and α-blockers to determine the relative potency of adrenergic agonists and antagonists in human monocytic TNF production. We hypothesized that the adrenergic activation during 20-min moderate exercise would have a suppressive effect on monocytic TNF production, which would be blocked by a β2-adrenergic antagonist. These findings would be reproduced by adrenergic agonists and antagonist treatments in vitro.
2. Materials and Methods
2.1. Intracellular TNF production and detection
The dose of 200 pg/mL LPS (Escherichia coli 0111:B4, catalog # L4391, Sigma-Aldrich, St. Louis, MO) that is found previously in bacterial, chronic or recurrent infections (Kiechl et al., 2001; Wiedermann et al., 1999), was determined to be appropriate for significant activation of monocytes in preliminary experiments, with 30 to 90 % of cells producing TNF. Peripheral blood cells were incubated in sterile polypropylene plates with or without LPS for 3.5 hours at 37°C with 5 % CO2. To stop cytokine excretion, allowing intracellular detection, brefeldin A (10 µg/mL) was added during the last 3 hours of incubation.
Intracellular TNF production of monocytes was evaluated by multiparametric flow cytometry using fluorochrome-conjugated antibodies. Briefly, erythrocytes were lysed using ammonium chloride solution followed by centrifugation (5 min at 500 × g). The cell pellet was washed one time with PBS, containing 0.1 % azide and 0.5 % bovine serum albumin, prior to incubation with monoclonal antibodies (15 min) for monocyte identification: HLA-DR/PE (BD Biosciences, San Jose, CA) and CD14/APC (Biolegend, San Diego, CA). After fixation and permeabilization according to the manufacturer’s instructions (Cytofix/Cytoperm Kit; BD Biosciences), cells were stained intracellularly with TNF/FITC antibody (Biolegend). At least 10,000 gated monocytes were collected for each sample on a dual-laser FACSCalibur (BD Biosciences). Monocytes were identified by scatter parameters and then, CD14 and HLA-DR labeling (see Fig. 1A for the gating strategy). The percent of the CD14+ HLA-DR+ cell subpopulation that was positive for TNF (“% TNF+ monocytes”) and the median fluorescent intensity (MFI) of TNF were assessed. Alterations in the % TNF+ monocytes and MFI in different in vitro drug treatments are also presented as a percent of the LPS only control, thereby eliminating inter-individual differences in numbers of cytokine-producing cells.
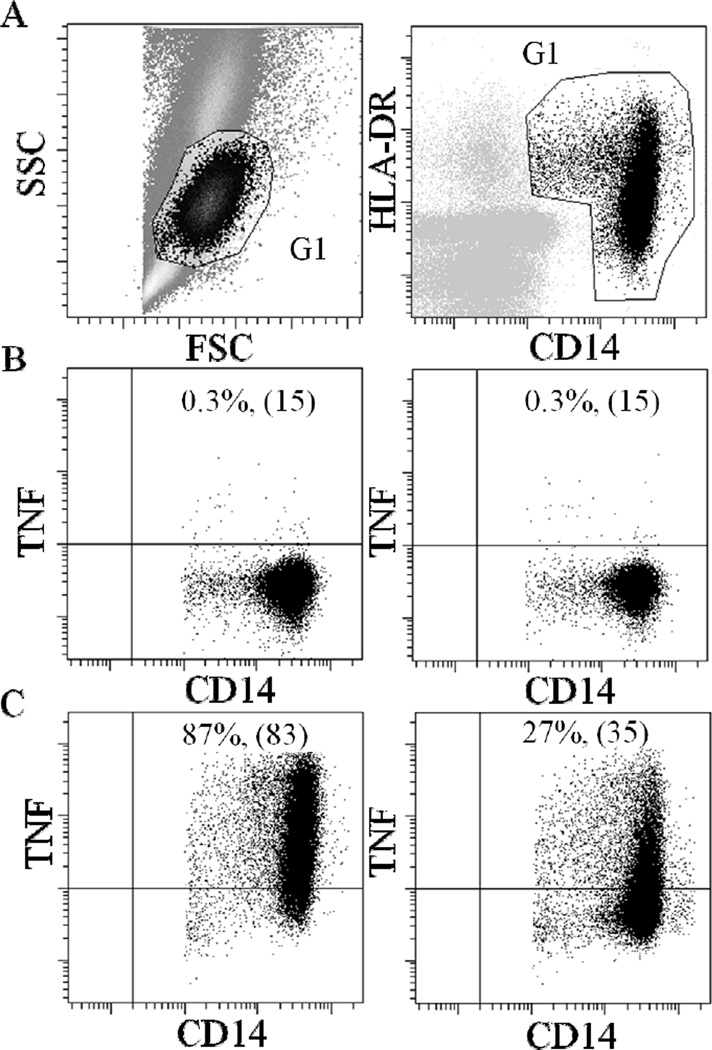
Figure 1.
Flow cytometric dot plots from a representative subject. Plots show the identification of TNF positive monocytes in whole blood cells that were either unstimulated or stimulated with LPS and in either the absence or presence of 10−8M Isoproterenol (Iso). (A) A gate (G1) is drawn around the monocyte population based on the forward and side scatter (FSC and SSC) characteristics (left panel) and the expression of CD14 and HLA-DR antigens (right panel). (B) Incubation of whole blood cells without LPS resulted only in negligible amount of TNF production by monocytes regardless of the absence (left panel) or presence (right panel) of isoproterenol. (C) LPS-stimulation led to a substantial activation of monocytes and intracellular TNF production (left panel) which was inhibited by isoproterenol (right panel). The percentage of the CD14+ HLA-DR+ cell subpopulation that was positive for TNF (% TNF+ monocytes) and the median fluorescence intensity (MFI; shown in brackets) are indicated.
Monocytes’ β-AR-mediated responsivity in TNF inhibition was calculated as the difference in % monocytes for TNF production between LPS only and LPS plus Iso. This assessment of β-AR-mediated suppression of LPS-stimulated intracellular expression of TNF by blood monocytes was termed β-AR-mediated inflammation control, “BARIC” (Hong et al., 2015). The greater values of BARIC indicate greater βAR responsivity and thus, better βAR-mediated inflammation regulation.
2.2. Ex vivo exercise experiments
2.2.1. Participants
Forty seven physically and mentally healthy subjects were studied. None had medical history of any relevant chronic disease or mental disorder or showed signs or reports of acute illness. To confirm eligibility, all subjects underwent blood tests for liver, metabolic, lipid, and thyroid panels, and a normal resting electrocardiogram (ECG) was confirmed. Individuals who had a history of heart disease, liver or renal disease, diabetes, psychosis, severe asthma, pregnancy, or ongoing inflammatory diseases (e.g., rheumatoid arthritis, multiple sclerosis, lupus), acute illness, and vaccination in last 7 days were excluded. Volunteers were recruited from the local community and compensated financially for their participation. All provided a written informed consent. The protocol was approved by the Institutional Review Board of University of California, San Diego.
2.2.2. Exercise tests
Participant’s maximal exercise capacity was determined by measuring peak oxygen consumption (VO2 peak) by having participants exercise on a treadmill (TMX 425C; Trackmaster Fitness, Eastlake, OH) until maximal exertion (voluntary cessation). The standard Bruce protocol was used where the speed and grade of the treadmill increased gradually by 1.7 mph and 10 % every 3 minutes (Hong and Mills, 2008). Subject’s expired gas was analyzed using Sensormedics metabolic cart (Sensormedics, Yorba Linda, CA) equipped with Vmax software (Version 6–2A), and the ECG was monitored using Marquette CardioSoft V.3 (GE Medical Systems, Milwaukee, WI). Participants returned between 8:30 am and 9:00 am 7–14 days later to exercise on the treadmill for 20 min at 65 – 70 % of their VO2 peak, normally rated as “somewhat hard” on Borg’s perceived exertion scale (Borg, 1970). Blood was collected before and immediately after the 20-min exercise challenge through an i.v. catheter inserted into an antecubital vein using minimal tourniquet. After insertion of the catheter the subjects rested for about 20 min prior to taking the baseline blood sample to avoid elevation of stress hormones and other factors simply due to i.v. line insertion. Participants refrained from consuming nicotine, caffeine, or alcohol, and from vigorous exercise 24-h prior to the exercise test.
2.2.3. Effect of exercise and a β2-blocker on ex vivo LPS-stimulated TNF production
Heparinized whole blood pre- and post-exercise was analyzed for LPS-stimulated monocytic intracellular TNF production. To investigate the effects of the catecholamine increase during exercise on LPS-stimulated TNF production, the whole blood was also pre-incubated with or gb without β2-blocker ICI 118551 (2.5 × 10−7M; approximately 500-fold β2 to β1 selectivity (Baker, 2005)) for 20 min prior to stimulation with LPS. In addition, to examine the responsivity of β-ARs to β-agonist stimulation, suppression of monocytic TNF production by Iso (1 × 10−8M) BARIC was analyzed in resting blood samples.
2.2.4. Catecholamine, TNF and IL-6 levels in plasma
Blood for plasma cytokines was drawn in EDTA vacutainers before and after the exercise and placed on ice. After centrifugation in a refrigerated centrifuge, plasma was stored at –80°C until the assays were done. Plasma levels of TNF and IL-6 were measured using commercially available enzyme-linked immunosorbent assay kits (Meso Scale Discovery, Gaithersburg, MD). The intra-assay variation was 4.5%. Epi and NE levels were determined by radioenzymatic assay, as described previously (Kennedy and Ziegler, 1990). The intra- and inter-assay coefficients of variation for the assay are 6.5 and 11%, respectively.
2.3. In vitro experiments
2.3.1. Participants
Peripheral venous blood (10–20 mL) was obtained from the antecubital vein of the forearm of six healthy volunteers (4 males, 2 females; mean age 40 years; range 28 to 57 years) at rest in a seated position into heparinized vacutainers between 9:30 – 10:00 am and kept at room temperature. All provided a written informed consent. The protocol was approved by the Institutional Review Board of University of California, San Diego. Given the consistent and large effects of β-agonists and antagonists on TNF production by monocytes in the literature, we anticipated that a sample size of six would result in a power of 0.8 or greater to achieve the effect size (Cohen’s d) of around 1.5.
2.3.2. Blood cell stimulation and treatment with AR agonists and antagonists
Washed whole blood cells were used to clearly determine the effects of given concentrations of β-agonists in BARIC. Blood was diluted by 10-fold with X-VIVO medium (Lonza, Walkersville, MD) upon drawing, and the supernatant was removed following centrifugation (5 min at 500 × g) to bring existing catecholamines to approximately 3 × 10−11M Epi, and 2.4 × 10−10M NE. To investigate the effects of the β- and α-agonists on the LPS-stimulated TNF production, cells were incubated with LPS in the absence or presence of (−)-Iso (10−11 - 10−6M), (−)-Epi (10−11 - 10−6M), or (−)-NE (10−11 - 10−5M) (Sigma-Aldrich). To investigate the effect of subtype-specific β-blockers on the LPS-stimulated cytokine production, cells were pre-incubated with CGP 12177A (0–10−5M, approximately 500-fold β1/β2 selectivity (Baker, 2005)), ICI 118551 (0–10−5M, approximately 500-fold β2/β1 selectivity (Baker, 2005)), or betaxolol (0–10−5M, approximately 7-fold β1/β2 selectivity (Baker, 2005)) (all from Sigma-Aldrich), for 20 min prior to incubation with LPS and β-agonist Iso (10−8M). To investigate the effect of α-blockers on the LPS-stimulated cytokine production, cells were pre-incubated with ICI 118551 (0–10−5M), phentolamine (Sigma-Aldrich) or both, for 20 min prior to incubation with LPS and β-, α-agonists Epi (10−8M) or NE (10−6M).
2.4. Statistical analysis
The % TNF+ monocytes and MFI are presented as means ± standard error of the mean (SEM). Normal distribution was confirmed by the Shapiro-Wilk test for all variables of interest, and the statistical significance was determined at p ≤ .05. In order to test the effect of exercise on TNF production and catecholamine release, two-tailed Student’s paired t-tests were performed prFe- to post-exercise, in the presence or absence of a β2-antagonist. As an exploratory analysis, Pearson correlations were performed to examine the associations between the degrees of exercise-induced inhibition of TNF production and the increase in catecholamine levels pre- to post-exercise. As initial analyses showed that the exercise-induced inhibition of TNF production was not associated with age, race, BMI and VO2 peak of the participants in this sample, no further analyses including these measures were pursued.
For in vitro data, fitted standard curves were calculated by nonlinear regression using SigmaPlot, and the EC50 was determined as the concentration of catecholamine required to obtain 50 % inhibition of TNF production. Two-tailed Student’s paired t-tests were used to assess the reversal effect of α- and β-blockers on catecholamine-induced TNF inhibition in samples treated with catecholamine alone vs. samples treated and catecholamine plus α- and β-blockers. Cohen’s d effect size was calculated by dividing the mean difference by the standard deviation of the difference.
3. Results
3.1. Ex vivo exercise experiments
3.1.1. Demographic characteristics and metabolic responses and monocyte numbers during exercise
The demographic data and metabolic responses during exercise of 47 participants in the exercise study are presented in Table 1. Circulating monocyte numbers increased from 420 ± 25 cells/µl pre-exercise to 607 ± 38 cells/µl post-exercise (t (37) = −6.0; d = −0.99; p < .001). This increase in total monocytes also resulted in an increase of the absolute numbers of monocytes producing TNF upon LPS stimulation (197 ± 14 cells per µl pre-exercise to 261 ± 20 post-exercise, t (37) = −4.2; d = −0.69; p < .001).
Table 1.
Demographic characteristics and metabolic responses of the study participants (n= 47)
| Age (years) | 40.9 (1.4) |
| Gender (No. male/female) | 26/21 |
| Race (No. Caucasian/others) | 27/20 |
| Body mass index (kg/m2) | 29.3 (0.9) |
| VO2 peak (ml/kg/min) | 32.5 (1.4) |
| Borg’s RPE during 20 min exercise | 12.1 (0.3) |
| Heart rate (beats/min) at rest | 80.2 (2.1) |
| Heart rate (beats/min) during exercise | 133.3 (2.5) |
Values are presented as mean (SEM); VO2 peak, peak oxygen consumption; RPE, ratings of perceived exertion
3.1.2. 20-min moderate exercise attenuates LPS-stimulated TNF production by monocytes
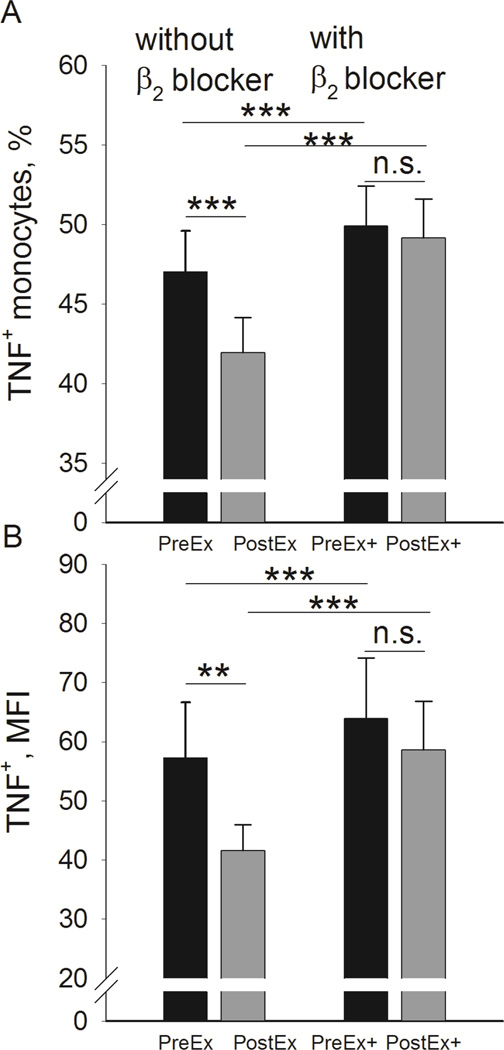
A bout of 20-min moderate treadmill exercise caused a significant decrease in % monocytes producing TNF stimulated by LPS from 47.1 ± 2.5 % pre-exercise to 42.0 ± 2.2 % post-exercise (t (47) = 5.2; d = 0.76; p < .001; Fig. 2A, left). This exercise effect of decreased % TNF-producing monocytes disappeared when the cells were pre-incubated with a highly specific β2 adrenergic antagonist ICI 118551 prior to LPS stimulation; similar values of approximately 49 % TNF+ monocytes were observed pre- and post-exercise (Fig. 2A, right). Interestingly, pre-incubation with ICI 118551 resulted in a significant increase in % TNF+ monocytes for both pre-and post-exercise samples (p’s < .001, as compared to the condition without ICI 118551). The inhibition of monocytic TNF production by exercise was also observed for the MFI of TNF expression levels, indicating reduced average per-cell levels of TNF production post exercise (t (47) = 3.0; d = 0.43; p < .01); Fig. 2B, left). This MFI suppression also disappeared after the pre-incubation with ICI 118551 (Fig. 2B, right). ICI 118551 again, led to a significant increase in MFI for both pre and post exercise samples (p’s < .001). Although absence of LPS stimulation resulted in minimal production of TNF by monocytes (Fig. 1B), exercise led to a significant decrease in % monocytes spontaneously producing TNF from 0.41 ± 0.09 % pre-exercise to 0.34 ± 0.09 % post-exercise (t (47) = 5.6; d = 0.82; p < .001). The absolute number of total monocytes spontaneously producing TNF did not change significantly pre- to post-exercise by the fact that the % decrease was offset by the absolute monocytes number increase as reported above.
Figure 2.
Effect of exercise on LPS-stimulated TNF production in monocytes. (A) Acute exercise results in inhibition of % TNF+ monocytes (left). The effect disappears when cells are incubated with β2 blocker ICI 118551 (right). (B) Inhibition of monocytic TNF production by exercise is also observed for the MFI of TNF (left). The effect, again, disappears when cells are incubated with β2 blocker ICI 118551 (right). **p < .01 for a pre- and post-exercise comparison. ***p < .01 for a pre- and post-exercise comparison or for a comparison between blood cells treated in the absence vs. presence of ICI 118551.
3.1.3. 20-min moderate exercise induces no change in TNF but significant increase in IL-6 and catecholamine levels in plasma
As expected, exercise induced marked SNS activation, as indicated by significantly elevated circulating catecholamine levels. Epi increased by 40 % from 3.0 × 10−10M (55.3 ± 2.1 pg/ml) pre-exercise to 4.1 × 10−10M (74.6 ± 2.5 pg/ml) post-exercise (t (47) = −9.8; d = −1.5; p < .001). NE increased by 30 % from 2.4 × 10−9M (414 ± 16.4 pg/ml) pre-exercise to 3.0 × 10−9M (508 ± 20.4 pg/ml) post-exercise (t (47) = −4.0; d = −0.59; p < .001). Plasma TNF levels did not change pre- to post-exercise (8.6 ± 0.6 pg/ml to 8.6 ± 0.5 pg/ml). Plasma IL-6 levels increased from 1.47 ± 0.27 pg/ml pre-exercise to 1.61 ± 0.29 pg/ml post-exercise (t (47) = −2.0; d = −0.29; p = .05).
3.2. In vitro experiments
3.2.1. β-AR agonists inhibit LPS-stimulated TNF production by monocytes in vitro
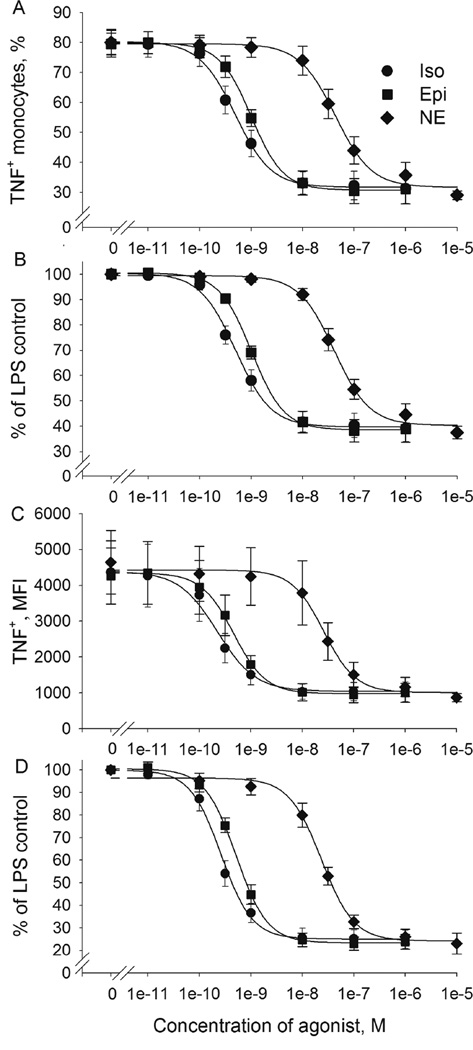
To determine the relative potency of Epi, NE, and Iso in regulation of TNF production (“BARIC”) we challenged washed whole blood cells with LPS in the presence or absence of a β-AR agonist Iso and β- and α-agonists Epi and NE in broad range of concentrations (10−11 - 10−5M) in vitro. The % TNF+ monocytes was reduced by all AR agonists in a dose-dependent and agonist-specific manner (Fig. 1C for Iso effects of a representative subject). The effective concentration generating 50 % of the effect (EC50) was 4.7 × 10−10M for Iso, 1 × 10−9M for Epi −2 times higher than the EC50 of Iso, and 4.3 × 10−8M for NE - 100 times higher than the EC50 of Iso (Fig. 3A). The maximum suppression of TNF production was reached at Iso and Epi concentrations of 10−8M, which reduced the % TNF+ monocytes to 42 % of those in the absence of Iso or Epi (Fig. 3B). In the presence of Iso and Epi at the concentration of 3.16 × 10−10M the % TNF+ monocytes was 76 % and 90 % of those in the absence of Iso (t (6) = 6.3; d = 2.6; p < .01) and Epi (t (6) = 5.6; d = 2.3; p < .01), respectively (Fig. 3B). NE also reduced the % TNF+ monocytes, although with significantly less potency. The inhibition produced by NE alone first reached significance at the concentration of 10−8M, and the maximum inhibition was achieved at 10−6M, which reduced the % TNF+ monocytes to 45 % of those without NE (t (6) = 10.5; d = 4.3; p < .001).
Figure 3.
Effect of the β-AR agonists isoproterenol (Iso), epinephrine (Epi) and norepinephrine (NE) on LPS-stimulated TNF production by monocytes. Washed whole blood cells from 6 healthy donors were stimulated with LPS (200 pg/mL) in the absence or presence of isoproterenol (10−11 - 10−6M), epinephrine (10−11 - 10−6M) or norepinephrine (10−10 - 10−5M). Data are presented as mean values ± SEM. (A) The % TNF+ monocytes, (B) the % TNF+ monocytes expressed as % of the LPS only control (without β-AR agonists), (C) the MFI of monocytic TNF expression and (D) the MFI as % of the LPS only control. The fitted standard curves are calculated by nonlinear regression using SigmaPlot.
The inhibition of monocyte TNF production by Iso, Epi and NE was also observed for the MFI in a dose-dependent and agonist-specific manner. Compared to the % TNF+ monocytes, MFI values appeared more reflective of the inhibitory effect of catecholamines: the EC50 was 2.1 × 10−10M for Iso, 4.4 × 10−10M for Epi - 2 times higher than the EC50 of Iso, and 2.6 × 10−8M for NE −100 times higher than the EC50 of Iso (Fig. 3C). Reduction of TNF first became significant at a concentration of 1 × 10−10M for both Iso and Epi and at 10−8M of NE (t (6) = 3.6; d = 1.5; p < .05, t (6) = 4.4; d = 1.8; p < .01, t (6) = 2.9; d = 1.2; p < .05, for Iso, Epi and NE, respectively). Maximum inhibition was achieved at Iso and Epi concentrations of 10−8M, and a NE concentration of 10−6M, which reduced MFI to 25 %, 25 % and 26 %, respectively, of those without β-AR agonists (Fig. 3D).
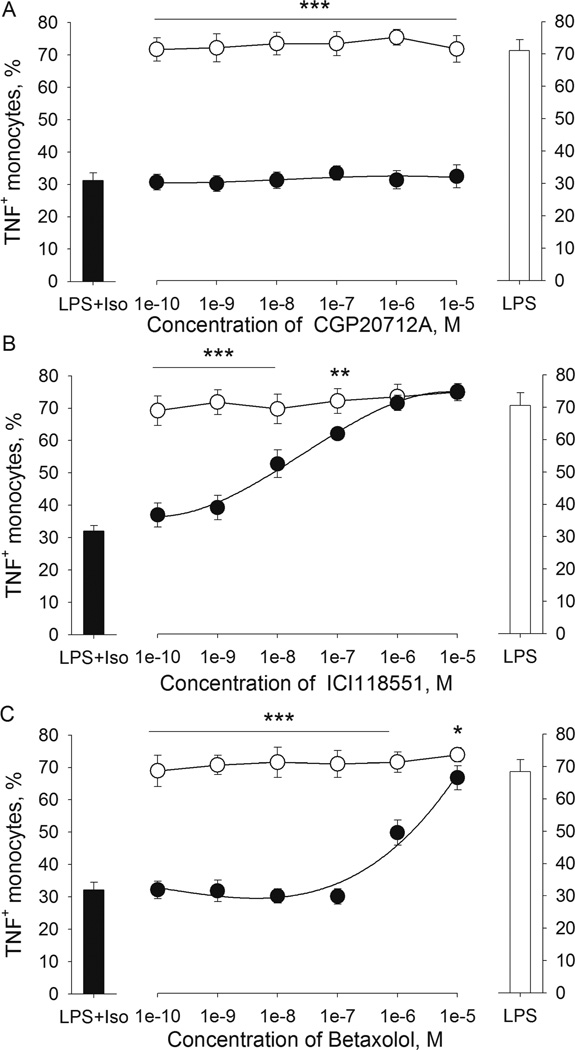
3.2.2. β-AR antagonists block the inhibitory effect of Iso on LPS-induced TNF production by monocytes in a β-AR subtype-specific manner
With the use of β-AR antagonists with varying specificity, the β-AR subtypes mediating the TNF inhibition by Iso was investigated via competition assays. Washed whole blood was pre-incubated with increasing amounts of either CGP 12177A (highly selective β1 blocker, in 10−10 - 10−5M), ICI 118551 (highly selective β2-blocker, in 10−10 - 10−5M) or betaxolol (β1-blocker with relatively low β1/β2 selectivity, in 10−10 - 10−5M), prior to the addition of Iso (10−8M) and LPS (200 pg/mL). There was no difference in % TNF+ monocytes between cells treated with LPS only (without Iso or antagonists) and the cells treated with LPS and CGP 12177A, ICI 118551 or betaxolol without Iso (Fig. 4). In the presence of Iso, the previously seen suppression of % TNF+ monocytes was gradually reversed in accordance with increasing amounts of a β2-blocker, ICI 118551, and reached the levels of those without Iso at the concentration of 10−6M (Fig. 4B). In contrast, a β1-blocker CGP 12177A did not reverse the effect of Iso (Fig. 4A). Betaxolol inhibited the effect of Iso only when used in very high concentration at 10−5M, an effect most likely achieved through its β2-blocking activities (Fig. 4C). The same results for all β-blockers were observed for the MFI values (results are not shown). These findings suggest that β-agonist inhibition of LPS-induced TNF production in monocytes is mediated by ARs of the β2 subtype.
Figure 4.
β2-AR but not β1-AR antagonists reverse the inhibitory effect of Isoproterenol (Iso) on LPS-induced TNF production by monocytes. Washed whole blood cells from 5 healthy donors were pre-incubated with (A) CGP 12177A (highly selective β1-blocker, 0–10−5M), (B) ICI 118551 (highly selective β2-blocker, 0–10−5M) or (C) betaxolol (β-blocker with low β1/β2 selectivity, 0–10−5M) for 20 min prior to incubation with LPS (200 pg/mL) and isoproterenol (10−8M). Results are expressed as mean ± SEM of % TNF+ monocytes. Black bars (left) indicate cells treated with LPS plus isoproterenol, and clear bars (right) indicate cells treated with LPS only as references (without blockers). ICI 118551 and high concentrations of betaxolol, but not CGP20712A (black circles) reversed the inhibitory effect of Iso (*p < .05; **p < .01; ***p < .001). Blockers did not affect LPS-stimulated TNF expression by themselves (clear circles).
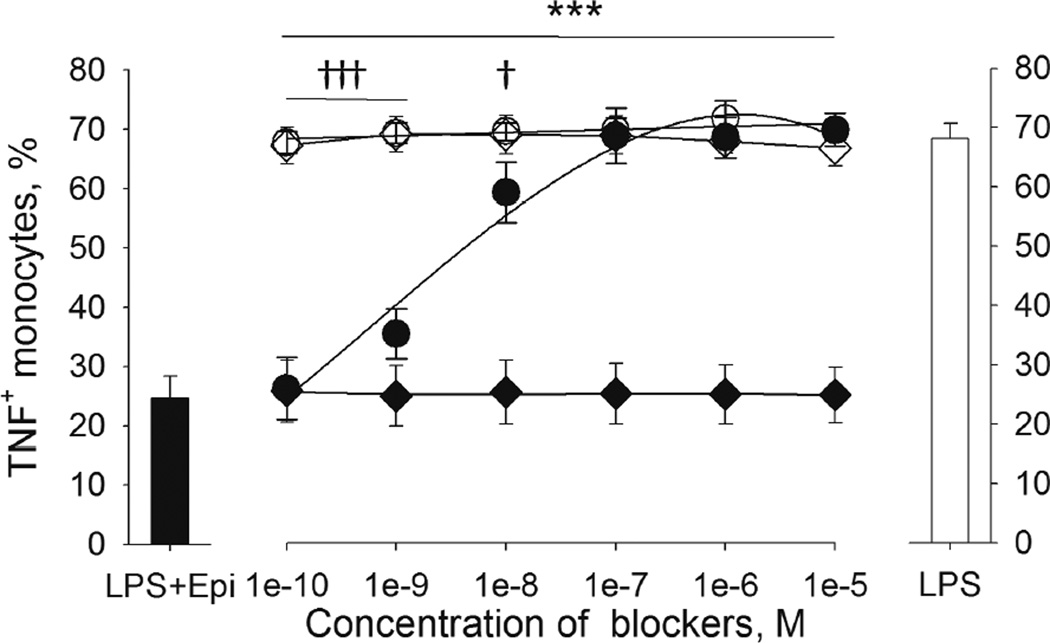
3.2.3. ICI 118551, but not α-AR antagonist phentolamine reverses the inhibitory effect of Epi and NE on LPS-induced TNF production in monocytes
In addition to their β-adrenergic effects, Epi and NE exert α-adrenergic activity. Thus, the potential role of α-adrenergic activity of Epi and NE in the modulation of TNF production was examined using ICI 118551 and α-AR antagonist phentolamine in competition assays. Washed whole blood cells were pre-incubated with increasing amounts of either ICI 118551 (10−10 - 10−5M), phentolamine (10−10 - 10−5M) or both, prior to the addition of 10−8M Epi or 10−6M NE and 200 pg/mL LPS. There was no difference in the % TNF+ monocytes between blood treated with LPS only (without Epi or antagonists) and the blood treated with LPS and ICI 118551 or phentolamine (Fig. 5). In the presence of Epi, the previously seen suppression of the % TNF+ monocytes was gradually reversed in accordance with increasing amounts of ICI 118551, and reached the levels of those without Epi at the ICI 118551 concentration of 10−7M (Fig. 5). In contrast, phentolamine did not affect the inhibitory effect of Epi (Fig. 5). The addition of ICI 118551 together with phentolamine to cells incubated with LPS and Epi resulted in the % TNF+ monocytes similar to those found after the addition of ICI 118551 alone. The same results were also found when NE was used instead of Epi. These findings show that α-adrenergic activity of Epi and NE did not play a role in modulation of monocytic TNF production.
Figure 5.
β2-AR antagonist, ICI 118551, but not α-AR antagonist, phentolamine, reversed the inhibitory effect of epinephrine (Epi) on LPS-induced TNF production by monocytes. Washed whole blood cells from 4 healthy donors were pre-incubated with ICI 118551 (0–10−5M, circles) or phentolamine (0–10−5M, diamonds) for 20 min prior to incubation with LPS (200 pg/mL) and epinephrine (10−8M). Results are expressed as mean ± SEM of % TNF+ monocytes. The black bar (left) indicates cells treated with LPS plus epinephrine, and the clear bar (right) indicates cells treated with LPS only as references (without blockers). ICI 118551 (black circles) reversed the inhibitory effect of Epi when compared to the control without Epi (clear circles; †p < .05; †††p < .001). Whereas, phentolamine treatment (black diamonds) did not reverse the inhibitory effect of Epi compared to the control without Epi (clear diamonds; ***p < .001). There was no effect of phentolamine on TNF expression. Of note, the values of LPS plus ICI 118551 (clear circles) and LPS plus phentolamine (clear diamonds) treatments overlap greatly.
3.2.4. Association between exercise-induced inhibition of monocytic TNF expression and increased β-AR agonists in vivo and β-AR sensitivity in vitro
To explore whether the degree of exercise-induced inhibition of % TNF+ monocytes was directly associated with the increases in catecholamine levels, we examined Pearson correlation coefficients between the difference in % TNF+ monocyte and increases in catecholamine levels pre- to post-exercise. Exercise-induced inhibition of % TNF+ monocytes did not correlate with the pre- to post-exercise change in Epi or NE levels (r = .12, p = .44; r = −.20, p = .19, for Epi and NE, respectively). Additional analyses showed that the magnitude of exercise-induced inhibition of % TNF+ monocytes was positively correlated with BARIC at rest (r = .49, p < .001).
4. Discussion
Previous in vivo studies report inconsistent results in the effects of acute exercise on TNF production, and in vitro studies demonstrate inhibitory effects of catecholamines on TNF production only in concentrations well beyond physiological levels. We show that a short single bout of 20-min moderate treadmill exercise has an inhibitory effect on both LPS-stimulated and spontaneous % monocytic TNF production and per cell production ex vivo with moderate to large effects. We also conclude that this inhibitory effect of exercise on TNF production is mediated by increased catecholamine levels via β2-ARs since the exercise effect disappears when cells are treated with β2-adrenergic antagonists. These findings further the current knowledge of the anti-inflammatory function of acute exercise and its mechanisms. Our investigation also corroborate previous findings that the primary cytokine released in the circulation during and after acute moderate exercise is IL-6 (Mathur and Pedersen, 2008; Petersen and Pedersen, 2006; Starkie et al., 2003), whereas circulating levels of TNF are often unaffected. It is unclear if this discrepancy in cellular production of TNF and its levels that are detectable in circulating blood pre- to post-exercise is indicative of differential effects of Epi on stimulated intracellular production versus endogenous release of TNF, or indicative of differing Epi effects on various cellular sources. In addition, it should be also noted that in spite of decreased % TNF-producing monocytes and per-cell TNF production levels pre to post exercise the absolute numbers of TNF+ monocytes unchanged or increased due to exercise-induced monocytosis and hemoconcentration. Nonethelss, the results of a decreased proportion and per-cell based TNF-production by monocytes upon an immunological challenge support anti-inflammatory effects of exercise.
Regular exercise has a protective role against chronic metabolic and cardiovascular diseases, in part due to its anti-inflammatory effect. These disorders have been associated with chronic low-grade systemic inflammation, which includes the increase of TNF production, for example by activated macrophages in adipose tissue. Inflammation is also key pathophysiology in insulin resistance and atherosclerosis. The present evidences for the anti-inflammatory role of regular exercise has been recently reviewed (Gleeson et al., 2011). Furthermore, now it is understood that each bout of exercise induces anti-inflammatory environment, including increased production of IL-6 by myokines that is able to suppress TNF (Petersen and Pedersen, 2005; Starkie, 2003); inhibition of adipose tissue infiltration by monocytes and macrophages; reduced expression of Toll-like receptors on monocytes and macrophages; phenotypic switching of macrophages within adipose tissue; and an increase in the circulating numbers of T regulatory cells (Gleeson et al., 2011). Our current findings add catecholamines to this list of acute exercise-induced mediators that have anti-inflammatory effect and thereby might have a protective role against chronic inflammatory diseases.
The magnitude of the exercise effect on TNF was comparable to those seen in our in vitro experiments with 3.16 × 10−10M Epi or 1 × 10−8M NE. Given that the exercise raised levels of Epi from 3 × 10−10 to 4.7 × 10−10M (~ 55 to 90 pg/mL) and NE from 2.6 × 10−9 to 3.2 × 10−9M (~ 450 to 550 pg/mL) among our participants, endogenous Epi, released by the adrenal medulla during SNS activation, supposedly contributed to the acute exercise-induced suppression of the LPS-stimulated TNF production by peripheral blood monocytes. However, the exercise-induced inhibition of the TNF production did not correlate to the increase in Epi levels pre- to post-exercise, which may be attributed to the individual variability in the sensitivity of the β-ARs on monocytes. It can be speculated that the overall SNS over-activation indicated by greater Epi release during exercise may have contributed to attenuated sensitivity to β-ARs. Indeed, our exploratory analysis showed greater β-AR sensitivity to 10−8M Iso at rest, resulting in greater inhibition of TNF production, was positively correlated with the degree of inhibition in TNF production by exercise. These data also imply the promising utility of β-adrenergic agonist-stimulated monocytic TNF production as a method to assess AR sensitivity. Particularly, it would be interesting to evaluate AR sensitivity in physically fit individuals, which have a decreased sympathetic tone and low catecholamine levels at rest (Mueller, 2007) and therefore might have an increased AR responsiveness.
Although elevated NE levels were also found in blood during acute exercise, NE is less likely to be a contributor in the inhibition of circulating monocytes’ TNF production we observed, as our in vitro data indicate the effectiveness of NE only in concentrations well beyond those found in blood. At the same time, the overall role of NE cannot be disregarded, as the spleen, which is richly innervated by sympathetic nerves (Bellinger et al., 2008; Elenkov et al., 2000; Madden, 2003) and has been recently identified as a reservoir of activated monocytes (Mebius and Kraal, 2005), appears to be one of the major compartments from which immune cells traffic to peripheral blood during acute stress (Dhabhar et al., 2012) and exercise (Nielsen et al., 1997). We have also shown that non-classical monocyte subset (identified by CD14dim cells) more readily traffic to the circulation during moderate exercise (Hong and Mills, 2008), while intracellular TNF levels in these monocytes significantly decreased pre- to post-exercise (Dimitrov et al., 2013). The demargination response during acute stress is likely to be mediated by high splenic levels of NE released from sympathetic nerves via β2-ARs in spite of low affinity. In addition, production of Epi from NE by immune cells themselves may be plausible, given the evidence of extra-adrenal production of Epi (Kennedy et al., 1995). Thus, the independent or combined role of Epi and NE in the inhibition of monocytic cytokine production via β2-ARs during acute stress remains to be further clarified.
The impact of exercise on immune cells and its biological implications should be evaluated with both cell function and redistribution in consideration. Catecholamines inhibit cytokine production in monocytes on a per cell basis, while it leads to cellular mobilization into the blood, hence increased cell numbers (Dimitrov et al., 2010). Both effects are mediated via signaling cascade that involves β2-ARs and increase in cyclic AMP production. On a functional level the exercise effect is inhibitory, on a cellular the effect depends largely on the post-exercise traffic of monocytes, e.g., in the wounded tissue, back to the marginal pool, in the adipose tissue, in the bone marrow, etc. Human studies on the post-exercise redistribution of immune cells to different tissue compartments are largely lacking. Animal studies suggest that exercise may limit the traffic of immune cells into inflamed adipose tissue via different mechanisms, which would have an anti-inflammatory effect (Gleeson et al., 2011). The cells instead probably traffic post-exercise back to the marginal pool and place of infections and injury (Dhabhar et al., 2012).
Systematic comparisons of differing Epi and NE effects on TNF production by monocytes in relation to AR subtypes is lacking in the literature. The relative degrees of binding affinity and cyclase stimulation are Iso > NE = Epi for β1 receptors, Iso ≈ 2xEpi >> NE for β2 receptors, and Iso > NE > Epi for β3 receptors in CHO cells transfected with β1, β2 or β3 human AR subtypes (Hoffmann et al., 2004). Our in vitro findings in differing potency of agonists and antagonists (Iso = 2xEpi >> NE) further confirm our conclusion that the effects of catecholamines on monocytic TNF production during exercise are mediated by β2-ARs. Studies using highly specific antagonists report findings that are in agreement with ours (Farmer and Pugin, 2000) yet, the regulatory role of β1-AR in TNF production is reported in studies that employed high doses of β1-antagonists (i.e. metoprolol, bisoprolol) with poor β1/β2 specificity, thus presumably blocking β2-ARs (van der Poll et al., 1994). Our findings of betaxolol, a β1-blocker with greatly diminished β1/β2 specificity (Baker, 2005) strongly suggest such by exhibiting its effect against Iso in TNF inhibition only at a very high concentration, 10−5M. But, a highly selective β1-antagonist CGP 12177A had no effect even at the highest concentration, 10−5M. In addition to their β-AR mediated activity, Epi and NE are shown to potentiate LPS-stimulated TNF production in macrophages via binding to α-ARs (Spengler et al., 1990). However, our results of little difference in TNF production upon β2- and a combination of α- and β2-blockers demonstrate that acute exercise effects on TNF production by peripheral blood monocytes via α-ARs is unlikely. This might be explained by the fact that in contrast to macrophages, monocytes don’t express substantial numbers of α-ARs (Rouppe, V et al., 1999).
The lowest concentrations of Epi found to inhibit LPS-induced TNF production in previous studies were 10−5M (Rontgen et al., 2004), 10−7M (van der Poll et al., 1996), or 10−7 to 10−8M (for THP-1 cells and whole blood cells, respectively) (Severn et al., 1992). The minimum effective concentration reported for NE varies from 10−5 to 10−8M, depending on the method and the concentration of LPS used (Rontgen et al., 2004; van der Poll et al., 1994; von Haehling et al., 2005). In contrast we have found that Iso and Epi in concentrations of 1 × 10−10M and NE at 10−8M already inhibit monocytic TNF. The maximum inhibition in our study was achieved at relatively low concentrations of 10−8M Epi and 10−6M NE. This discrepancy in effective agonist concentrations may be in part attributed to a smaller dose of LPS we used for stimulating the cells, 0.2 ng/mL compared to 1 to 1000 ng/mL typically used in previous studies. The greatest inhibitory effect of NE on TNF production is also observed in studies using low concentrations of LPS of 0.2 ng/mL (Rontgen et al., 2004) or 1 ng/mL (van der Poll et al., 1994) compared to higher doses. Given that the inhibitory effects of catecholamines on monocytic TNF production are less pronounced when high concentrations of LPS are used and that levels of 200 pg/mL are more likely to be clinically relevant (Kiechl et al., 2001; Wiedermann et al., 1999), optimal antigen concentrations should be used when designing such experiments.
Findings from our study together provide comprehensive evidence that the elevation of circulating levels of Epi during 20-min moderate exercise inhibits spontaneous and LPS-stimulated production of TNF by blood monocytes mediated by β2-ARs. Given the suggested anti-inflammatory effects of exercise to combat many inflammatory diseases, the evidence of cytokine modulatory effect of relatively brief and moderate-intensity exercise is promising. It should be noted that our study is not to disregard the anti-inflammatory role of other hormones or cytokines such as glucocorticoids/cortisol (Petrovsky and Harrison, 1998), IL-6 and IL-10, which are also elevated during acute exercise (Mastorakos et al., 2005; Starkie et al., 2003). Furthermore, because our investigation was in healthy individuals clinical implications of our evidence of the inhibitory effect of catecholamines on TNF production for individuals with chronic infections or inflammatory disease should be considered with caution. Given the pivotal role of TNF in low-grade inflammation in various pathological conditions (Branen et al., 2004; Groeneveld et al., 2006; Mathur and Pedersen, 2008; Petersen and Pedersen, 2006), future investigation of how and to what extent exercise-induced catecholamine release leads to potential down-modulation of TNF production in chronic inflammatory diseases is warranted.
Highlights.
We investigated sympathoadrenergic activation effects on monocytic TNF production.
Acute exercise suppresses monocytic TNF production via β2-ARs.
Physiological epinephrine levels observed during exercise inhibit TNF in vitro.
Exercise-induced catecholamine release may have an anti-inflammatory effect.
Exercise effects on monocytic production of vs. plasma TNF differed.
Acknowledgments
We are grateful to Farah Shaikh, Jonathan Reynolds, Kathleen Wilson and Michael Green for their assistance with exercise tests and coordinating blood specimen acquisition. We also express our gratitude to Drs. Paul J. Mills, Michael G. Ziegler and Tanja Lange for their helpful comments on this manuscript. This work was supported by the research grants R01HL090975 and HL090975S1 (American Recovery and Reinvestment Act grant) to SH and UL1RR031980 for the UCSD Clinical and Translational Science Awards from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Reference List
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br. J. Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D. Sympathetic modulation of immunity: relevance to disease. Cell Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones--Curt Richter Award winner. Psychoneuroendocrinology. 2012;37:1345–1368. doi: 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 2010;184:503–511. doi: 10.4049/jimmunol.0902189. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Shaikh F, Pruitt C, Green M, Wilson K, Beg N, Hong S. Differential TNF production by monocyte subsets under physical stress: blunted mobilization of pro-inflammatory monocytes in prehypertensive individuals. Brain Behav. Immun. 2013;27C:101–108. doi: 10.1016/j.bbi.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE, Moss J. Plasma catecholamines in stress and exercise. JAMA. 1980;243:340–342. [PubMed] [Google Scholar]
- Drenth JP, Krebbers RJ, Bijzet J, Van der Meer JW. Increased circulating cytokine receptors and ex vivo interleukin-1 receptor antagonist and interleukin-1beta production but decreased tumour necrosis factor-alpha production after a 5-km run. Eur. J. Clin. Invest. 1998;28:866–872. doi: 10.1046/j.1365-2362.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Farmer P, Pugin J. beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am. J. Physiol Lung Cell Mol. Physiol. 2000;279:L675–L682. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Mills PJ, Irwin MR, Ziegler MG. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom. Med. 2000;62:591–598. doi: 10.1097/00006842-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Grisanti LA, Evanson J, Marchus E, Jorissen H, Woster AP, DeKrey W, Sauter ER, Combs CK, Porter JE. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol. Immunol. 2010;47:1244–1254. doi: 10.1016/j.molimm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld AB, Beishuizen A, de Jong MF. Catecholamines, parasympathetic stimuli, or cortisol for overwhelming sepsis? Crit Care Med. 2006;34:1549–1550. doi: 10.1097/01.CCM.0000216174.22321.AD. [DOI] [PubMed] [Google Scholar]
- Haahr PM, Pedersen BK, Fomsgaard A, Tvede N, Diamant M, Klarlund K, Halkjaer-Kristensen J, Bendtzen K. Effect of physical exercise on in vitro production of interleukin 1, interleukin 6, tumour necrosis factor-alpha, interleukin 2 and interferon-gamma. Int. J. Sports Med. 1991;12:223–227. doi: 10.1055/s-2007-1024672. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN. Comparative pharmacology of human beta-adrenergic receptor subtypes--characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:151–159. doi: 10.1007/s00210-003-0860-y. [DOI] [PubMed] [Google Scholar]
- Hong S, Dimitrov S, Cheng T, Redwine L, Pruitt C, Mills PJ, Ziegler MG, Green JM, Shaikh F, Wilson K. Beta-adrenergic receptor mediated inflammation control by monocytes is associated with blood pressure and risk factors for cardiovascular disease. Brain Behav. Immun. 2015;50:31–38. doi: 10.1016/j.bbi.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Johnson TA, Farag NH, Guy HJ, Matthews SC, Ziegler MG, Mills PJ. Attenuation of T-lymphocyte demargination and adhesion molecule expression in response to moderate exercise in physically fit individuals. J. Appl. Physiol. 2005;98:1057–1063. doi: 10.1152/japplphysiol.00233.2004. [DOI] [PubMed] [Google Scholar]
- Hong S, Mills PJ. Effects of an exercise challenge on mobilization and surface marker expression of monocyte subsets in individuals with normal vs. elevated blood pressure. Brain Behav. Immun. 2008;22:590–599. doi: 10.1016/j.bbi.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavelaars A, van de PM, Zijlstra J, Heijnen CJ. Beta 2-adrenergic activation enhances interleukin-8 production by human monocytes. J. Neuroimmunol. 1997;77:211–216. doi: 10.1016/s0165-5728(97)00076-3. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Bigby TD, Ziegler MG. Nonadrenal epinephrine-forming enzymes in humans. Characteristics, distribution, regulation, and relationship to epinephrine levels. J. Clin. Invest. 1995;95:2896–2902. doi: 10.1172/JCI117996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–2153. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Egger G, Mayr M, Wiedermann CJ, Bonora E, Oberhollenzer F, Muggeo M, Xu Q, Wick G, Poewe W, Willeit J. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation. 2001;103:1064–1070. doi: 10.1161/01.cir.103.8.1064. [DOI] [PubMed] [Google Scholar]
- Kollias G, Douni E, Kassiotis G, Kontoyiannis D. On the role of tumor necrosis factor and receptors in models of multiorgan failure, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Immunol. Rev. 1999;169:175–194. doi: 10.1111/j.1600-065x.1999.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Madden KS. Catecholamines, sympathetic innervation, and immunity. Brain Behav. Immun. 2003;(17 Suppl 1):S5–S10. doi: 10.1016/s0889-1591(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Maisel AS, Fowler P, Rearden A, Motulsky HJ, Michel MC. A new method for isolation of human lymphocyte subsets reveals differential regulation of beta-adrenergic receptors by terbutaline treatment. Clin. Pharmacol. Ther. 1989;46:429–439. doi: 10.1038/clpt.1989.161. [DOI] [PubMed] [Google Scholar]
- Maisel AS, Harris T, Rearden CA, Michel MC. Beta-adrenergic receptors in lymphocyte subsets after exercise. Alterations in normal individuals and patients with congestive heart failure. Circulation. 1990;82:2003–2010. doi: 10.1161/01.cir.82.6.2003. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Pavlatou M, amanti-Kandarakis E, Chrousos GP. Exercise and the stress system. Hormones. (Athens. ) 2005;4:73–89. [PubMed] [Google Scholar]
- Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators. Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nat. Rev. Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Moldoveanu AI, Shephard RJ, Shek PN. Exercise elevates plasma levels but not gene expression of IL-1beta, IL-6, and TNF-alpha in blood mononuclear cells. J. Appl. Physiol. 2000;89:1499–1504. doi: 10.1152/jappl.2000.89.4.1499. [DOI] [PubMed] [Google Scholar]
- Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin. Exp. Pharmacol. Physiol. 2007;34:377–384. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Nielsen HB, Secher NH, Kristensen JH, Christensen NJ, Espersen K, Pedersen BK. Splenectomy impairs lymphocytosis during maximal exercise. Am. J. Physiol. 1997;272:R1847–R1852. doi: 10.1152/ajpregu.1997.272.6.R1847. [DOI] [PubMed] [Google Scholar]
- O’Brien DP, Briles DE, Szalai AJ, Tu AH, Sanz I, Nahm MH. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect. Immun. 1999;67:595–601. doi: 10.1128/iai.67.2.595-601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J. Appl. Physiol (1985) 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J. Physiol Pharmacol. 2006;(57 Suppl 10):43–51. [PubMed] [Google Scholar]
- Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol. Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Harrison LC. The chronobiology of human cytokine production. Int. Rev. Immunol. 1998;16:635–649. doi: 10.3109/08830189809043012. [DOI] [PubMed] [Google Scholar]
- Rontgen P, Sablotzki A, Simm A, Silber RE, Czeslick E. Effect of catecholamines on intracellular cytokine synthesis in human monocytes. Eur. Cytokine Netw. 2004;15:14–23. [PubMed] [Google Scholar]
- Rouppe vdV, Kavelaars A, van de PM, Heijnen CJ. Neuroendocrine mediators up-regulate alpha1b- and alpha1d–adrenergic receptor subtypes in human monocytes. J. Neuroimmunol. 1999;95:165–173. doi: 10.1016/s0165-5728(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Straub RH. Norepinephrine, the beta-adrenergic receptor, and immunity. Brain Behav. Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- Sekut L, Champion BR, Page K, Menius JA, Jr, Connolly KM. Anti-inflammatory activity of salmeterol: down-regulation of cytokine production. Clin. Exp. Immunol. 1995;99:461–466. doi: 10.1111/j.1365-2249.1995.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J. Immunol. 1992;148:3441–3445. [PubMed] [Google Scholar]
- Simpson RJ, Kunz H, Agha N, Graff R. Exercise and the Regulation of Immune Functions. Prog. Mol. Biol. Transl. Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Smart NA, Larsen AI, Le Maitre JP, Ferraz AS. Effect of exercise training on interleukin-6, tumour necrosis factor alpha and functional capacity in heart failure. Cardiol. Res. Pract. 2011;2011:532620. doi: 10.4061/2011/532620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J. Immunol. 1990;145:1430–1434. [PubMed] [Google Scholar]
- Starkie R. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. 2003 doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Starkie RL, Hargreaves M, Rolland J, Febbraio MA. Heat stress, cytokines, and the immune response to exercise. Brain Behav. Immun. 2005;19:404–412. doi: 10.1016/j.bbi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-alpha levels after prolonged running. Am. J. Physiol Cell Physiol. 2001;280:C769–C774. doi: 10.1152/ajpcell.2001.280.4.C769. [DOI] [PubMed] [Google Scholar]
- Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, Zink A. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301:737–744. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J. Clin. Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect. Immun. 1994;62:2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haehling S, Genth-Zotz S, Bolger AP, Kalra PR, Kemp M, Adcock IM, Poole-Wilson PA, Dietz R, Anker SD. Effect of noradrenaline and isoproterenol on lipopolysaccharide-induced tumor necrosis factor-alpha production in whole blood from patients with chronic heart failure and the role of beta-adrenergic receptors. Am. J. Cardiol. 2005;95:885–889. doi: 10.1016/j.amjcard.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J. Am. Coll. Cardiol. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- Zaldivar F, Wang-Rodriguez J, Nemet D, Schwindt C, Galassetti P, Mills PJ, Wilson LD, Cooper DM. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J. Appl. Physiol. 2006;100:1124–1133. doi: 10.1152/japplphysiol.00562.2005. [DOI] [PubMed] [Google Scholar]