Abstract
Fosfomycin is a bactericidal antibiotic that interferes with cell wall synthesis. The drug therefore has a broad spectrum of activity against a wide range of Gram-positive and Gram-negative bacteria. Both the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have started review processes of the accumulated information on the use of fosfomycin and on information from new clinical trials. The intent is to establish usage terms in Europe and to authorize the sale of fosfomycin in the US. This monograph reviews the most current aspects of the compound. From the microbiological point of view, fosfomycin’s single mechanism of action can provide a synergistic effect to other classes of antibiotics, including β-lactams, aminoglycosides, lipopeptides and fluoroquinolones. The resistance mechanisms include the reduced intracellular transport of the antibiotic, the change in target and the direct inactivation of the antibiotic by metalloenzymes and kinases; however, the clinical impact of some of these mechanisms has not yet been elucidated. The lack of agreement in determining the sensitivity cutoffs between the Clinical and Laboratory Standards Institute (CLSI) (≤64 mg/L) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (≤32 mg/L), the fact that a number of microorganisms require a higher MIC (Klebsiella spp., Enterobacter spp., Serratia spp., Pseudomonas aeruginosa) and the drug’s different effective concentrations against Gram-positive and Gram-negative bacteria have resulted in recommended dosages for treating multiresistant microorganism infections that vary between 8 and 12 g/day for Gram-positive bacteria and 16 and 24 g/day for Gram-negative bacteria. Fosfomycin has 3 presentations (intravenous with disodium salt, oral with calcium salt and combined with tromethamine), has good distribution in tissues and abscesses and is well tolerated. The pharmacodynamic ratio of dosage production for fosfomycin is AUC/MIC. However, the pharmacokinetics/pharmacodynamic ratio could be optimized in daily practice based on the pathogen, the patient’s clinical profile or the infection model. Fosfomycin is the treatment of choice for cystitis in immunocompetent patients, patients with transplants, pregnant women and in pediatric settings. The drug is especially useful due to its microbiological activity and oral posology in cystitis caused by ESBL bacteria. Administer intravenously at high doses and combined with other antimicrobial agents. Fosfomycin has been useful in treating infections by multiresistant Gram-negative bacteria, such as Enterobacteriaceae, carbapenemase carriers and P. aeruginosa, extensively resistant or panresistant in urinary infections and in skin and soft tissue. Fosfomycin has also been shown active in combination with daptomycin or imipenem in osteoarticular infections by methicillin-resistant Staphylococcus aureus. Fosfomycin is an old antibiotic that still has much to reveal.
Keywords: Fosfomycin, resistance, pharmacodynamic, treatment, multiresistant microorganisms
BACKGROUND
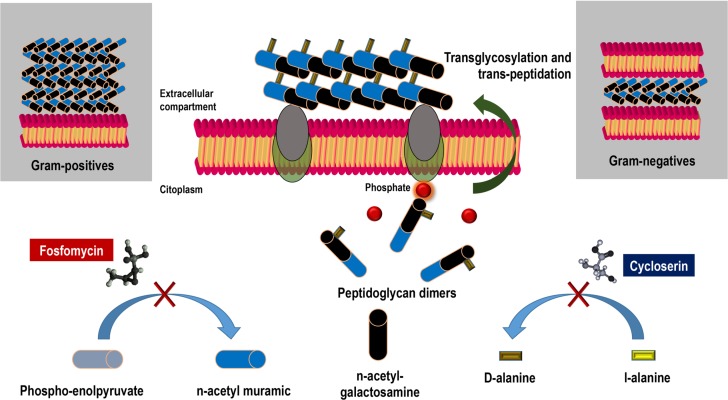
Fosfomycin was discovered and synthesized in the Medina Foundation (Fundación Medina, Granada, Spain) from Streptomyces fradiae and Pseudomonas syringae. The drug acts by inhibiting UDP-N-acetylglucosamine enolpyruvyl transferase (MurA), an enzyme responsible for catalyzing the formation of N-acetylmuramic acid, a precursor of peptidoglycan, through the binding of N-acetylglucosamine and phosphoenolpyruvate, resulting in bacterial lysis (figure 1). Gram-positive and Gram-negative bacteria require the formation of N-acetylmuramic acid for peptidoglycan synthesis, which means that fosfomycin’s spectrum of action is very broad, presenting activity against the main genera in clinical practice, such as Staphylococcus spp., Enterococcus spp., Enterobacteriaceae, Pseudomonas spp. and Acinetobacter spp. Fosfomycin is water-soluble, has a low molecular weight (138 g/mol) and has low protein binding, which provides it with high tissue dissemination (volume of distribution of 0.3 L/kg). Fosfomycin also disseminates in experimental biofilm models in concentrations greater than or equal to those of ciprofloxacin and cotrimoxazole [1].
Figure 1. Mechanism of action of fosfomycin. Impact on synthesis of bacterial wall.
Both the European Medicines Agency and the US Food and Drug Administration have started reviewing the accumulated information on the use of fosfomycin and the information from new clinical trials. The intent is to establish common usage criteria in Europe and to authorize the sale of fosfomycin in the US [2, 3]. In its various formulations (both intravenous [disodium salt] and oral [calcium salt or trometamol]), the prescription of fosfomycin has increased spectacularly due to the considerable incidence of multidrug-resistant microorganisms in which fosfomycin constitutes, alone or in combination, a treatment option [4, 5].
NEW MICROBIOLOGICAL DATA
Fosfomycin’s mechanisms of resistance include the reduction in intracellular transport of the antibiotic (mutation in transporter genes, regulator genes or ampC for glpT), the change in target due to changes in the expression of murA and the direct inactivation of the antibiotic by metalloenzymes (fosA, fosB and fosX) or by kinases (formA and formB) [6].
Despite the considerable ease of selecting fosfomycin-resistant mutations, their clinical repercussion has not been sufficiently tested. In some cases, resistance reduces the bacteria’s fitness; in others, resistance reduces its virulent nature (such as its ability to adhere to epithelial cells and synthetic materials such as catheters) [7, 8]. A more limiting aspect is the mechanism of direct inactivation of the antibiotic by metalloenzymes (FosA, FosB and FosX), which are transmissible and frequently found in ESBL enterobacteria and carriers of carbapenemases, especially Escherichia coli [9].
There have been recent reports of the presence of mutations with a loss of uhpT expression, which phenotypically cause the growth of E. coli colonies in the halo of inhibition, with no correlation with the symptoms [10]. Given that the rate of concentration of mutations depends on the concentration of fosfomycin being above the microorganism’s minimum inhibitory concentration (MIC) (1 of every 5.5x105 with concentrations 5 times the MIC and 1.2x109 with concentrations 256 times the MIC), this resistant mutant selection window can be prevented with high doses of the drug, especially if prescribed in monotherapy [11]. A recent meta-analysis [12] found a 3.4% (95% CI 1.8-5.1%) rate of resistances in treatments with fosfomycin in monotherapy, which, coupled with the synergistic activity with other antimicrobials, establishes attractive prescription scenarios, such as the therapeutic combination against multidrug-resistant microorganisms.
The aforementioned meta-analysis established the benefit of employing fosfomycin in combination with another antibiotic over monotherapy. In an extensive review, Falagas et al. described fosfomycin’s synergistic in vitro effect, combined with any antimicrobial, against sensitive and resistant Gram-positive and Gram-negative microorganisms [4]. The combination of fosfomycin and meropenem is synergistic and prevents the onset of drug resistance in severe infections caused by strains of ESBL-producing enterobacteria and P. aeruginosa [13]. The combination of fosfomycin and tobramycin has recently been studied in biofilm models of P. aeruginosa, observing a significant reduction of the biofilm at 24 h compared with monotherapy [14].
The lack of agreement in determining the sensitivity cutoffs between the Clinical and Laboratory Standards Institute (CLSI) (≤64 mg/L) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (≤32 mg/L), the fact that a number of microorganisms require a higher MIC (Klebsiella spp., Enterobacter spp., Serratia spp., P. aeruginosa) and the drug’s differing effective concentrations against Gram-positive and Gram-negative bacteria have resulted in recommended dosages for treating multidrug-resistant microorganism infections that vary between 8 and 12 g/day for Gram-positive bacteria and 16 and 24 g/day for Gram-negative bacteria [5, 15].
PHARMACOKINETICS/PHARMACODYNAMICS APPROACH
There are 3 fosfomycin formulations: a disodium formulation for intravenous infusion and 2 oral presentations (one calcium and one trometamol). The first formulation consists of 1-8 g of fosfomycin disodium powder with succinic acid as the only excipient. The second formulation is fosfomycin in calcium salt, marketed in a few countries as 500-mg hard gelatin capsules. The third, fosfomycin trometamol, is a derivative of phosphonic acid, available as (1R,2S)-(1,2-epoxypropyl) phosphonic acid with 2-amino-2-(hydroxymethyl)-1,3-propanediol. The formulation is presented in a 3-g packet with white granules of fosfomycin-trometamol.
The pharmacodynamic (PD) ratio of dosage effectiveness for fosfomycin is AUC/MIC. However, the pharmacokinetics PK/PD ratio could be optimized in daily practice based on the pathogen, the patient’s clinical profile and the infection model. Fosfomycin exhibits concentration-dependent bactericidal activity against strains of E. coli, P. mirabilis and Streptococcus pneumonie and time-dependent bactericidal activity against S. aureus and P. aeruginosa [16.17]. By optimizing fosfomycin in Monte Carlo simulations, the PK/PD ratios with which an effective therapeutic objective could be reached (probability of target attainment [PTA] >40%) against enterobacteria are T>MIC over 70% and AUC/MIC >23 [18]. Fosfomycin’s molecular stability at room temperature could allow for continuous infusions in complex infection models, alone or combined with other antimicrobials. For example, Asuphon et al. provided the results of the continuous infusion of 16-g fosfomycin combined with an extended infusion of meropenem (1-2 g of infusion for 3 h every 8 h) against clinical isolates of multidrug-resistant P. aeruginosa, achieving a cumulative fraction of response (CFR) greater than 88% [19]. A PTA and CFR ≥ 90% are considered optimal against a bacterial population, while a CFR or PTA between 80% and 90% is associated with a moderate chance of success.
Fosfomycin is a fairly safe antimicrobial. Exceptional cases of intolerance have been reported due to the saline overload that fosfomycin can generate. A gram of fosfomycin sodium provides 0.33 g (14.4 meq) of sodium [20], such that a treatment of 12-24 g of fosfomycin provides between 4 and 8 g of salt to the extracellular compartment. Cases of dyspnea and intolerance to decubitus have been reported in patients undergoing treatment with fosfomycin, even with normal ejection fractions, which have required withdrawal of the drug [21-23]. Monitoring the response of the extracellular compartment when faced with saline overload during high-dosage treatments (16-24 g/day) could be useful for patients with comorbidities and water balance disorders (hepatic cirrhosis, heart failure or renal failure) to avoid precipitating an episode of clinical heart failure [24]. The continuous infusions enabled by fosfomycin’s molecular stability at room temperature could in turn allow for lower prescribed dosages (12-16 g/day), ensuring plasma concentrations above 32 mg/L, decreasing the total saline overload that would require a fractionated dose. These lower dosages could be especially beneficial for patients with the aforementioned dyscrasias.
A recent review by Falagas et al. [4] examined the kinetics of various formulations of fosfomycin. The oral bioavailability of fosfomycin trometamol ranged from 34% to 58%. Absorption occurs mainly in the small intestine. Although evidence suggests that joint administration with food delays the absorption, renal recovery of the drug does not vary (50-60%) and is not affected by age. The trometamol formulation is absorbed 6-fold more than the calcium formulation during the first 2 h after dosing and approximately 3 to 4-fold more than the calcium formulation during the 12-h period after dosing. The concentrations of a single 2-g dose of fosfomycin trometamol are 2 to 4-fold higher than those of a one 3-g dose of the calcium formulation. The explanation lies in the fact that fosfomycin calcium is hydrolyzed and inactivated by gastric juices [4].
The serum elimination half-life (t1/2) of fosfomycin trometamol is approximately 5 h. A study with healthy volunteers showed serum fosfomycin disodium concentrations of 10 mg/L and 4 mg/L 4 h and 8 h, respectively, after administering a dose of 40 mg/kg. The same fosfomycin doses administered orally (trometamol) presented similar serum concentrations [25]. Further pharmacokinetic studies are needed, given the potential utility of this oral drug in sequential therapy for various infection models, especially in the urinary tract, where the drug concentration is high [4].
APPROACH TO CURRENT CLINICAL PRACTICE
Fosfomycin has been employed for treating urinary and respiratory infections, meningitis, otitis, neurosurgical infections, endocarditis, bacteremia, cardiac surgery, nosocomial infections by extensively drug-resistant P. aeruginosa and Acinetobacter baumannii and carbapenemase-carrying enterobacteria. Fosfomycin has also been employed for gynecological infections, as well as for device-related and osteoarticular infections by methicillin-resistant and methicillin-susceptible S. aureus, among others.
In terms of urinary tract infections, 93-99% of fosfomycin is excreted unaltered in urine and barely binds to plasma proteins, disseminating widely in the renal parenchyma, bladder and uninflamed prostate [6]. Thus, for example, maximum concentrations in urine are reached 2 h after administering a 3-g dose of fosfomycin trometamol orally, with concentrations varying between 1,053 mg/L and 3,749 mg/L, maintaining a mean concentration above 128 mg/L.
A systematic review showed that orally administered fosfomycin trometamol achieved 80% microbiological eradication in cystitis in treated patients, with clinical healing that exceeded 90%, even for those infections caused by ESBL strains [26]. A 3-g dose of fosfomycin-trometamol on days 1, 3 and 5 was active in uncomplicated cystitis, even when caused by ESBL strains, with clinical success of 78-91% [26, 27]. However, for immunosuppressed (transplantation) or catheterized (urethral stent, double J) patients, the eradication rate decreased to 59% [28].
Fosfomycin is recommended for cystitis in immunocompetent patients, according to the guidelines of the Infectious Diseases Society of America [29], even in conditions with ESBL, as are nitrofurantoin and cotrimoxazole [30]. In Spain, fosfomycin is the empiric treatment of choice for acute cystitis, immunocompetent patients and patients with transplants, according to the recommendations of the Spanish Society of Infectious Diseases and Clinical Microbiology [31, 32]. Oral fosfomycin is also employed in asymptomatic bacteriuria and cystitis for pregnant woman [33]. In the pediatric setting, fosfomycin has numerous advantages for use in urinary tract infections: It is easy to dose, it reaches high concentrations in urine, its adverse effects are uncommon, and it does not affect the intestinal flora. Due to its excellent sensitivity pattern against E. coli and other enterobacteria, fosfomycin is also considered one of the treatments of choice for afebrile pediatric cystitis, especially in its trometamol form [34].
Fosfomycin has a synergistic effect in combination with other antimicrobials, especially daptomycin and imipenem, against multidrug-resistant Gram-positive strains [35] and has shown greater dissemination than other antibiotics through biofilms [36, 37]. These two characteristics could be useful for treating osteoarticular infections.
There is evidence of the clinical benefit of fosfomycin in combination with daptomycin and imipenem in bacteremia and endocarditis caused by methicillin-resistant S. aureus (MRSA) [22, 38]. There is a study underway comparing the activity of fosfomycin in monotherapy versus that of fosfomycin combined with daptomycin in treating MRSA infection [39]. In Spain, the combination of fosfomycin and daptomycin is recommended for treating persistent or complicated MRSA infection in the management guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology [40]. In the pediatric setting, an alternative could be considered for patients with acute MRSA-induced hematogenous osteomyelitis or for those with beta-lactam allergies [41].
The benefit of combined therapies for multidrug-resistant Gram-negative bacteria has been reinforced by the results of the recent INCREMENT study, which showed that the therapies had less impact on mortality in patients with the most severe conditions (scores >7) with bacteremia caused by carbapenemase-producing enterobacteriaceae [42]. High-dose intravenous fosfomycin and fosfomycin in combination with other antimicrobials have been shown to be useful for treating infections by multidrug-resistant Gram-negative bacteria such as extensively drug-resistant or pan-resistant carbapenemase-carrying enterobacteria and P. aeruginosa, especially in urinary tract infections, as well as abdominal, skin and soft tissue infections [43-45]. These formulations are recommended as alternative treatments in combination against urinary tract infections caused by carbapenemase-carrying enterobacteria with an MIC greater than 8 mg/L [46] and for immunosuppressed patients with solid organ transplants [47].
Lastly, the first results of the ZEUS study were presented in March 2019. The study compared fosfomycin against piperacillin-tazobactam for treating complicated urinary tract infections, including pyelonephritis. The randomized study included 465 patients, 233 treated with fosfomycin and 231 treated with piperacillin-tazobactam. In the microbiologically eligible population, fosfomycin fulfilled the primary objective of noninferiority compared with piperacillin-tazobactam, with overall success rates of 64.7% (119/184 patients) and 54.5% (97/178 patients), respectively. The clinical cure rates in the test of cure (TOC) on days 19 to 21 were high and similar between the two treatments (90.8% for fosfomycin [167/184] versus 91.6% for piperacillin-tazobactam [163/178]). In the post-hoc analysis with pathogens typified through pulsed-field gel electrophoresis, the overall success rates in the TOC by modified intent-to-treat were 69.0% (127/184) for fosfomycin versus 57.3% (102/178) for piperacillin-tazobactam (difference of 11.7%; 95% CI 1.3, 22.1) [48].
The new challenges that fosfomycin must address for its implementation in clinical practice include sequential orally administered therapy (once the focus of infection has been controlled and the bacteremia cleared) and optimization of the dosage and galenical oral formulation to achieve these objectives from the pharmacodynamic standpoint (effective concentration in the focus and in blood), with minimal gastrointestinal intolerance. Being able to include fosfomycin in oral sequential therapy for other infection models (beyond urinary) would be enthusiastically welcomed in the stewardship programs.
We are therefore dealing with a compound that, although it has been known for some time, has much left to be discovered. The more we know of this compound, the more potential benefits will be encountered. The most attractive therapeutic model at this time, given its safety and activity, is probably that of urinary tract infection. However, there is increasing in vitro and in vivo evidence of fosfomycin’s usefulness in synergistic combination with other antimicrobials for treating complex infections by resistant microorganisms.
REFERENCES
- 1.Rodríguez-Martínez JM, Ballesta S, Pascual A. Activity and penetration of fosfomycin, ciprofloxacin, amoxicillin/clavulanic acid and co-trimoxazole in Escherichia coli and Pseudomonas aeruginosa biofilms. Int J Antimicrob Agents 2007; 30: 366-368. DOI: 10.1016/j.ijantimicag.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 2. https://www.ema.europa.eu/en/medicines/human/referrals/fosfomycin-containing-medicinal-products.
- 3. https://www.nabriva.com/pipeline-research.
- 4.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev 2016; 29: 321–47. doi: 10.1128/CMR.00068-15 DOI: 10.1128/CMR.00068-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candel FJ, Cantón R. Current approach to fosfomycin: From bench to bedside. Enferm Infecc Microbiol Clin 2018. doi: 10.1016/j.ijggc.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 6.Dijkmans AC, Zacarías NVO, Burggraaf J et al. . Fosfomycin: pharmacological, clinical and future perspectives. Antibiotics 2017; 6: 24 DOI: 10.3390/antibiotics6040024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson AI, Otto B, Aspevall O, Kahlmeter G, Andersson DI. Biological Costs and Mechanisms of Fosfomycin Resistance in Escherichia coli. Antimicrob Agents Chemother 2003; 47:2850–8. doi: 10.1128/AAC.47.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falagas ME, Athanasaki F, Voulgaris GL, Triarides NA, Vardakas KZ. Resistance to fosfomycin: Mechanisms, Frequency and Clinical Consequences. Int J Antimicrob Agents 2018. doi: 10.1103/PhysRevB.83.075123 [DOI] [PubMed] [Google Scholar]
- 9.Benzerara Y, Gallah S, Hommeril B, Genel N, Decré D, Rottman M, et al. . Emergence of plasmid-mediated fosfomycin-resistance genes among Escherichia coli isolates, France. Emerg Infect Dis 2017; 23: 1564–7. doi: 10.3201/eid2309.170560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas A, Ito R, Mustapha MM et al. . Frequency and mechanisms of spontaneous fosfomycin non-susceptibility observed upon disk diffusion testing of Escherichia coli. J Clin Microbiol. 2017; 56 pii: DOI: 10.1128/JCM.01368-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Scoy BD, Mc Cauley J, Ellis-Grosse EJ et al. . Exploration of the pharmacokinetic-pharmacodynamic relationships for fosfomycin efficacy using an in vitro infection model. Antimicrob Agents Chemother 2015; 59:7170-7. DOI: 10.1128/AAC.04955-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grabein B, Graninger W, Rodríguez Baño J. Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature. Clin Microbiol Infect 2017; 23:363-372. DOI: 10.1016/j.cmi.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 13.Docobo-Pérez F, Drusano GL, Johnson A et al. . Pharmacodynamics of fosfomycin: Insights into clinical use for antimicrobial resistance. Antimicrob Agents Chemother 2015; 59: 5602-10. DOI: 10.1128/AAC.00752-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díez-Aguilar M, Morisini MI, Köksal E et al. . Use of Calgary and microfluidic BioFlux systems to test the activity of fosfomycin and tobramycin alone and in combination against cystic fibrosis Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 2017; 62(1). DOI: 10.1128/AAC.01650-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez-Baño J, Cisneros JM, Cobos-Trigueros N, Fresco G, Navarro-San Francisco C, Gudiol C, et al. . on behave of Study Group of Nosocomial Infections (GEIH) of the Spanish Society of Infectious Diseases, Infectious Diseases (SEIMC). Executive summary of the diagnosis and antimicrobial treatment of invasive infections due to multidrug-resistant Enterobacteriaceae. Guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enferm Infecc Microbiol Clin. 2015; 33: 338-41. DOI: 10.1016/j.eimc.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 16.Walsh CC, McIntosh MP, Peleg AY, Kirkpatrick CM, Bergen PJ. In vitro pharmacodynamics of fosfomycin against clinical isolates of Pseudomonas aeruginosa. J Antimicrob Chemother 2015; 70: 3042-50. DOI: 10.1093/jac/dkv221 [DOI] [PubMed] [Google Scholar]
- 17.Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents 2009; 34: 506-15. DOI: 10.1016/j.ijantimicag.2009.08.013 [DOI] [PubMed] [Google Scholar]
- 18.Lepak AJ, Zhao M, VanScoy B, Taylor DS, Ellis-Grosse E, Ambrose PG et al. . In vivo pharmacokinetics and pharmacodynamics of ZTI-01 (Fosfomycin for Injection) in the neutropenic murine thigh infection model against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2017;61 pii: . doi: 10.1128/AAC.00476-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asuphon O, Montakantikul P, Houngsaitong J, Kiratisin P, Sonthisombat P. Optimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulation. Int J Infect Dis 2016;50: 23-9. DOI: 10.1016/j.ijid.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 20.Spanish agency for medicines and health products. Available at: http://www.ern.es/wp-content/uploads/2013/01/FT-Fosfocina-IV-IM.pdf. [accessed 29.01.2019].
- 21.Coronado-Alvarez MN, Parra D, Parra-Ruiz J. Clinical efficacy of fosfomycin combinations against a variety of gram-positive cocci. Enferm Infecc Microbiol Clin. 2019; 37(1):4-10. DOI: 10.1016/j.eimc.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 22.Del Rio A, Gasch O, Moreno A, et al. . Efficacy and safety of fosfomycin plus imipenem as rescue therapy for complicated bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: a multicenter clinical trial. Clin Infect Dis. 2014; 59: 1105–1112. DOI: 10.1093/cid/ciu580 [DOI] [PubMed] [Google Scholar]
- 23.Cañamares-Orbis I, Silva JT, López-Medrano F, Aguado JM. Is high-dose intravenous fosfomycin safe for the treatment of patients prone to heart failure?. Enferm Infecc Microbiol Clin. 2015; 33: 294 DOI: 10.1016/j.eimc.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 24.Candel FJ, Matesanz M, Martín-Sánchez FJ, González Del Castillo JM. Monitoring of high-dose fosfomycin guided by NT-proBNP. Int J Cardiol. 2016; 209: 131-132. DOI: 10.1016/j.ijcard.2016.02.037 [DOI] [PubMed] [Google Scholar]
- 25.Goto M, Sugiyama M, Nakajima S, Yamashina H. Fosfomycin kinetics after intravenous and oral administration to human volunteers. Antimicrob Agents Chemother 1981; 20: 393–397. PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE. Fosfomycin for the treatment of multidrug-resistant, including extended-spectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. 2010; 10: 43-50. DOI: 10.1016/S1473-3099(09)70325-1 [DOI] [PubMed] [Google Scholar]
- 27.Qiao LD, Zheng B, Chen S, et al. . Evaluation of three-dose fosfomycin tromethamine in the treatment of patients with urinary tract infections: an uncontrolled, open-label, multicentre study. BMJ Open 2013; 3:e004157 DOI: 10.1136/bmjopen-2013-004157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–8. DOI: 10.1128/AAC.00402-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta K, Hooton TM, Naber KG, et al. . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011; 52:e103-20. DOI: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 30.Walker E, Lyman A, Gupta K, Mahoney MV, Snyder GM, Hirsch EB. Clinical Management of an Increasing Threat: Outpatient Urinary Tract Infections Due to Multidrug-Resistant Uropathogens. Clin Infect Dis. 2016; 63: 960-965. DOI: 10.1093/cid/ciw396 [DOI] [PubMed] [Google Scholar]
- 31.de Cueto M, Aliaga L, Alós JI, Canut A, Los-Arcos I, Martínez JA et al. . Executive summary of the diagnosis and treatment of urinary tract infection: Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm Infecc Microbiol Clin. 2017; 35: 314-320. DOI: 10.1016/j.eimc.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 32.Vidal E, Cervera C, Cordero E, Armiñanzas C, Carratalá J, Cisneros JM, et al. . “Management of urinary tract infection in solid organ transplant recipients: Consensus statement of the Group for the Study of Infection in Transplant Recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Spanish Network for Research in Infectious Diseases (REIPI)”. Enferm Infecc Microbiol Clin. 2015. December;33(10): 679.e1-679.e21. doi: 10.1016/j.eimc.2015.03.020 [DOI] [PubMed] [Google Scholar]
- 33.Keating GM. Fosfomycin trometamol: a review of its use as a single-dose oral treatment for patients with acute lower urinary tract infections and pregnant women with asymptomatic bacteriuria. Drugs. 2013. November;73(17):1951-66. DOI: 10.1007/s40265-013-0143-y [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Lozano J, de Malet A, Cano ME, de la Rubia L, Wallmann R, Martínez-Martínez L, et al. . Antimicrobial susceptibility of microorganisms that cause urinary tract infections in pediatric patients. Enferm Infecc Microbiol Clin 2018;36:417–22. doi: 10.1016/j.eimc.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 35.Coronado-Alvarez MN, Parra D, Parra-Ruiz J. Clinical efficacy of fosfomycin combinations against a variety of gram-positive cocci. Enferm Infecc Microbiol Clin. 2018. June 12 pii: . doi: 10.1016/j.eimc.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 36.Monzón M, Oteiza C, Leiva J et al. . Biofilm testing of Staphylococcus epidermidis clinical isolates: low performance of vancomycin in relation to other antibiotics. Diagn Microbiol Infect Dis. 2002; 44: 319-324. PMID: [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Martínez JM, Ballesta S, Pascual A. Activity and penetration of fosfomycin, ciprofloxacin, amoxicillin/clavulanic acid and co-trimoxazole in Escherichia coli and Pseudomonas aeruginosa biofilms. Int J Antimicrob Agents 2007; 30: 366-368. DOI: 10.1016/j.ijantimicag.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 38.Miró JM, Entenza JM, Del Río A, Velasco M, Castañeda X, Garcia de la Mària C, et al. . High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 2012; 56 (8): 4511-4515. DOI: 10.1128/AAC.06449-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw E, Miró JM, Puig-Asensio M, Pigrau C, Barcenilla F, Murillas J, et al. . Daptomycin plus fosfomycin versus daptomycin monotherapy in treating MRSA: protocol of a multicentre, randomised, phase III trial. BMJ Open. 2015; 5 (3): e006723 DOI: 10.1136/bmjopen-2014-006723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gudiol F, Aguado JM, Almirante B, Bouza E, Cercenado E, Domínguez MA, et al. . Diagnosis and treatment of bacteremia and endocarditis due to Staphylococcus aureus. A clinical guideline from the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm Infecc Microbiol Clin. 2015; 33(9): 625.e1-625.e23. doi: 10.1016/j.eimc.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Corti N, Sennhauser FH, Stauffer UG, Nadal D. Fosfomycin for the initial treatment of acute haematogenous osteomyelitis. Arch Dis Child 2003; 88: 512–516. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, et al. , REIPI/ESGBIS/INCREMENT Investigators. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 2017; 17:726 –734. DOI: 10.1016/S1473-3099(17)30228-1 [DOI] [PubMed] [Google Scholar]
- 43.Pontikis K, Karaiskos I, Bastani S et al. . Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Intern J Antimicrob Agents 2014; 43: 52-59. DOI: 10.1016/j.ijantimicag.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 44.Papst L, Beović B, Pulcini C, Durante-Mangoni E, Rodríguez-Baño J, Kaye KS, et al. ; ESGAP, ESGBIS, ESGIE and the CRGNB treatment survey study group. Antibiotic treatment of infections caused by carbapenem-resistant Gram-negative bacilli: an international ESCMID cross-sectional survey among infectious diseases specialists practicing in large hospitals. Clin Microbiol Infect. 2018; 24(10):1070-1076. doi: 10.1016/j.cmi.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Apisarnthanarak A, Mundy LM. Carbapenem-resistant Pseudomonas aeruginosa pneumonia with intermediate minimum inhibitory concentrations to doripenem: combination therapy with high-dose, 4-h infusion of doripenem plus fosfomycin versus intravenous colistin plus fosfomycin. Int J Antimicrob Agents. 2012; 39: 271-2. DOI: 10.1016/j.ijantimicag.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 46.Bassetti M, Peghin M, Pecori D. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis 2016, 29:583–594. DOI: 10.1097/QCO.0000000000000314 [DOI] [PubMed] [Google Scholar]
- 47.Silva JT, Fernández-Ruiz M, Aguado JM. Multidrug-resistant Gram-negative infection in solid organ transplant recipients: implications for outcome and treatment. Curr Opin Infect Dis. 2018; 31(6):499-505. doi: 10.1097/QCO.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 48.Kaye KS, Rice LB, Dane A, Stu V, Sagan O, Fedosiuk E, et al. . Fosfomycin for injection (ZTI-01) vs Piperacillin-Tazobactam (PIP-TAZ) for the Treatment of Complicated Urinary Tract Infection (cUTI) Including Acute Pyelonephritis (AP): ZEUS, A Phase 2/3 Randomized Trial. Clin Infect Dis 2019. doi: 10.1093/cid/ciz181/5370034. [DOI] [PMC free article] [PubMed] [Google Scholar]