Abstract
The alarming increase in antibiotic resistance rates reported for various pathogens has resulted in the use of alternative treatment policies. Given the fairly limited availability of new antimicrobial drugs, the reassessment of older antibiotics is now an interesting option. Fosfomycin, a bactericidal analog of phosphoenolpyruvate that has been previously been employed as an oral treatment for uncomplicated urinary tract infection, has recently raised interest among physicians worldwide. In general, the advanced resistance described in Gram-negative bacteria suggests that fosfomycin can be an appropriate treatment option for patients with highly resistant microbial infections. This review, which refers to key available data, focuses on the possibility of extending the use of fosfomycin beyond urinary tract infections and against multidrug-resistant Gram-negative bacteria.
Keywords: Fosfomycin, Gram-negative bacteria, Multiresistance, Antibiotic treatment
FOSFOMYCIN’S PLACE IN THE CURRENT PANORAMA OF RESISTANCE IN GRAM-NEGATIVE PATHOGENS
With the increased worldwide prevalence of bacterial resistance, a need has emerged for developing new antibiotics and recovering old substances when sufficient options are not available. Fosfomycin is a derivative of phosphonic acid, initially described and isolated at the end of the 1960s from cultures of Streptomyces species. Fosfomycin behaves as a bactericidal antibiotic analog of phosphoenolpyruvate and has a low molecular weight, broad spectrum and putative activity against various bacteria, including many multidrug-resistant Gram-negative microorganisms. Fosfomycin acts by irreversibly inhibiting cell wall synthesis in an early stage, blocking the first step in this synthesis in UDP-GlcNAc enolpyruvyl-transferase. This single mechanism of action means that cross-resistance with other classes of antibiotics is less likely and enables fosfomycin to retain significant in vitro activity against numerous Gram-positive and Gram-negative bacteria, including multidrug-resistant strains. Based on this action, interest in fosfomycin has increased among clinicians and microbiologists worldwide for all potential facets of use.
Resistances in Gram-negative bacteria: treatment possibilities. Over the past decade, the resistances of Gram-negative bacteria have become one of the largest threats to public health worldwide. The severity of infections generated by these bacteria, their considerable capacity for transmission and dispersion through the environment, the difficulty in employing empiric treatment (and even appropriately targeted treatment) and the scarcity of new antibiotics against some Gram-negative bacilli (GNB), such as Acinetobacter baumannii, Pseudomonas aeruginosa, Stenotrophomonas maltophilia and certain enterobacteria with numerous mechanisms of resistance, has raised enormous concern in healthcare systems worldwide [1]. In addition to the attributable complications, morbidity and mortality that multidrug resistance entails, studies have shown the repercussion of this disease burden on quality of life, disability, induction of dependence and, consequently, on the sustainability of the social and healthcare system.
Multidrug resistance is the most important problem in antibiotic resistance due to the difficulty in treating multidrug-resistant microorganisms and the exponential increase in multidrug resistance over the last decade, not to mention AmpC production and the emergence and dissemination of extended-spectrum beta-lactamases (ESBL) and carbapenemases; these ESBL-producing and carbapenemase-producing strains are the main pathogens involved in nosocomial or healthcare-associated infections. A considerable majority of these strains are characterized by the loss of activity against beta-lactam agents, as well as marked resistance to other families of commonly employed antibiotics, such as quinolones and aminoglycosides, due to the accumulation of numerous resistance mechanisms or the transmission of plasmids that transport genes with additional resistance [2-4].
The limited new options against these types of bacterial strains has meant that, over the last decade, antibiotics such as fosfomycin have gained considerable importance as rescue strategies or as combined therapy options for treating infections caused by these multidrug-resistant bacteria [5]. Recovering these old antibiotics for managing complex infections requires, however, an understanding of and an update on their pharmacokinetic and pharmacodynamic characteristics to optimize the antibiotics’ efficacy and minimize the significant adverse events occasionally associated with these agents.
Fosfomycin’s spectrum of action against Gram-negative bacteria
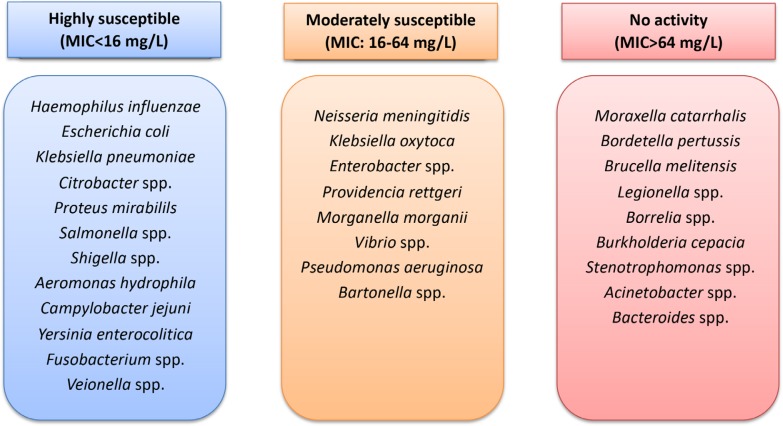
Fosfomycin presents good activity against Gram-negative bacteria such as Haemophilus influenzae and most enterobacteria (figure 1), including Citrobacter spp., Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, Serratia marcescens and Shigella spp. [6-8], with a minimum inhibitory concentration (MIC) of 0.25-16 mg/L in most isolates. However, a number of these isolates have been observed to reach MIC values of 64 mg/L. Other enterobacteria such as Klebsiella oxytoca, Enterobacter spp. and Morganella morganii have lower susceptibility to this antibiotic, with an MIC of 16-64 mg/L.
Figure 1.
Susceptibility to fosfomycin of Gram-negative bacteria
Among the nonfermenting GNB, P. aeruginosa and A. baumannii present moderate susceptibility to fosfomycin, with similar MIC values of 16-64 mg/L [9]. Fosfomycin itself presents activity against strains of Aeromonas hydrophila, Campylobacter jejuni and Yersinia enterocolitica. Against species of the genera Bordetella, Legionella, Pasteurella and Vibrio, fosfomycin’s activity is moderate [10, 11]. Species such as Burkholderia cepacia, S. maltophilia and a number of species of the genus Acinetobacter are not susceptible to fosfomycin (figure 1) [9].
Fosfomycin has also shown good activity for penetrating the interior of biofilms of Gram-negative bacteria, both in monotherapy and in combined therapy, showing excellent eradication activity [12-14].
Fosfomycin activity against multidrug-resistant Gram-negative bacteria. In recent years, we have witnessed a marked increase in the resistance to drugs commonly employed for managing various infections by Gram-negative bacteria, such as quinolones, beta-lactams and aminoglycosides. In this context of increasing resistances, classical antibiotics including fosfomycin, chloramphenicol, cephamycins, temocillin, polymyxins (colistin), tetracyclines (minocycline) and glycylcyclines (tigecycline) are still some of the few available options.
Numerous studies have demonstrated fosfomycin’s good activity in vitro against ESBL-producing enterobacteria. The MIC to inhibit 90% (MIC90) of ESBL E. coli strains is typically 2-4 mg/L, although Asian countries have observed greater resistance (MIC90 of up to 128 mg/L) [15]. Other enterobacteria present a more obvious reduction in their fosfomycin susceptibility after acquiring ESBL. Thus, strains of ESBL-producing K. pneumoniae have an MIC90 that varies from 32 to >1,024 mg/L [16], greater than that of strains without this resistance mechanism. However, it is worth noting that an increase has been observed in fosfomycin resistance among enterobacteria, with increasing multidrug resistance, in certain geographical regions in recent years. In their study, Rodríguez-Avial et al. showed a significant reduction in fosfomycin susceptibility from 2005 to 2011 in more than 16,000 strains of ESBL-producing E. coli. Nevertheless, fosfomycin activity during the last period remained above 80% [17], while ciprofloxacin resistance was 78.2%, cotrimoxazole resistance was 62.3%, and amoxicillin-clavulanate-resistance was 55.3%. In other studies, fosfomycin showed good activity against strains of ESBL-producing E. coli, with resistance rates of 2.6% [18] to 10%. Fosfomycin is therefore still a good treatment option in these cases. The impression given by these data and those of other similar studies is that the phenomenon of co-resistance in ESBL-producing enterobacteria related to quinolones and cotrimoxazole is greater and more common and to a much lower degree in combination with fosfomycin.
In terms of carbapenemase-producing enterobacteria (CPE), most of the data come from studies conducted with class A carbapenemase-producing strains of K. pneumoniae, known as KPC. The fosfomycin susceptibility of these strains varies between 39% and 100% according to various studies [15, 19, 20]. It is worth noting that fosfomycin also maintains activity against strains of enterobacteria that present the mcr-1 plasmid, a mobile genetic element known for creating colistin resistance. A study that identified 19 strains carrying this plasmid, among 390 enterobacteria with colistin resistance, showed that they all maintained fosfomycin susceptibility [21].
Fosfomycin activity against nonfermenting GNB such as P. aeruginosa and A. baumannii in conditions of multidrug resistance is less predictable and varies widely depending on the phenotypes present in the various epidemiological environments [15, 16]. This antibiotic’s particular mechanism of action makes it a highly attractive option for use in combination with other agents based on the synergy or addition observed in in vitro studies. In fact, there are numerous studies that have demonstrated the clinical efficacy of the combination with antibiotics such as carbapenems and colistin [22-24]. Combined therapy with fosfomycin for managing infections caused by multidrug-resistant Gram-negative bacteria is consistent with the current trends in managing infections caused by these strains [25, 26].
CLINICAL USE OF FOSFOMYCIN IN THE MANAGEMENT OF INFECTIONS BY MULTIDRUG-RESISTANT GRAM-NEGATIVE BACTERIA
Given its pharmacokinetic characteristics (table 1), its particular mechanism of action and its preserved spectrum against multidrug-resistant strains, interest in using fosfomycin has grown significantly and beyond its classical application in managing uncomplicated urinary tract infection.
Table 1.
Pharmacokinetic properties and tissue penetration of fosfomycin and other antibiotics employed in managing infections by multidrug-resistant Gram-negative bacteria
| Fosfomycin | Meropenem | Tigecycline | Amikacin | |
|---|---|---|---|---|
| VD, L/kg | 0.4-0.5 | 0.2-0.4 | 7-9 | 0.2-0.4 |
| Protein binding | <5% | <5% | 75-85% | <5% |
| Renal clearance | 35-50% | 75-80% | 30% | >95% |
| Lungs | 30-50% | 20-40% | 5-30% | 10-15% |
| Subcutaneous tissue | 40% | 70-80% | 80-100% | 20-30% |
| Aqueous humor | 15% | 5-8% | 10% | 8-10% |
| Bone | 20% | 15-20% | 350-450% | 10% |
| CSF | 65% | 5-20% | 10-52% | 10-20% |
CSF, cerebrospinal fluid; VD, volume of distribution; (%) percentage of the property, parameter or degree of tissue penetration.
Infection by multidrug-resistant bacteria. Over the past decade, numerous guidelines and consensuses on managing infections by multidrug-resistant bacteria have been published, which have established alternatives to the use of classical antibiotics. The Spanish guidelines on managing infections by multidrug-resistant enterobacteria include fosfomycin as a relevant option for treating this type of infection, at the same drug level as colistin, tigecycline and aminoglycosides (Level C-III) [27]. Despite limited available experience, the guidelines’ authors concluded that fosfomycin could be an appropriate option, at high dosages (4-6 g/6 h or 8 g/8 h) and always in combination with other antibiotics.
A review published by the US Society of Infectious Diseases Pharmacists in 2014 concluded that fosfomycin should be a valid option for managing infections by multidrug-resistant strains, having shown good tolerance in published studies. The review re-emphasized the need to use fosfomycin in combined therapy due to its synergistic effect with other antibiotics and for minimizing the creation of resistances [28].
Two guidelines on managing infections caused by P. aeruginosa have recently been published. The guidelines of the Spanish Society of Chemotherapy (Sociedad Española de Quimioterapia, SEQ) consider that fosfomycin could be an option for combined targeted therapy against strains resistant to other antibiotics, in dosages of 16 to 24 g/day [29]. The review published by Bassetti et al. went a step further, indicating that fosfomycin is a possible empiric therapy along with a potentially active beta-lactam for patients with a high suspicion of P. aeruginosa infection [30].
Urinary tract infection. Urinary tract infection is the most widely extended indication for fosfomycin, which has been employed since its commercial launch for managing acute and chronic complicated urinary tract infections, both in adults and children. For treating uncomplicated cystitis, the fosfomycin-trometamol formulation constitutes a first-line treatment, along with nitrofurantoin, in 3-g doses in adults and 1-g doses in children [31, 32].
Fosfomycin has gained special importance in recent years in managing complicated urinary tract infections caused by multidrug-resistant strains of Gram-negative bacteria, both intravenously and intramuscularly, at dosages of 12-18 g/day. The use of fosfomycin has been successfully applied in monotherapy and combined therapy with other agents, including aminoglycosides, tigecycline, colistin, piperacillin/tazobactam and carbapenems [33, 34], and shows high response rates against enterobacteria and Pseudomonas spp. In fact, a number of authors recommend the use of fosfomycin in sepsis of urinary origin caused by ESBL-producing enterobacteria in which the use of carbapenems is not indicated [32]. Nevertheless, the clinical practice guidelines have still not included the use of fosfomycin among the options for empirically managing urinary sepsis with a high suspicion of ESBL [35-38]. Results are still pending from the FOREST study [39], an interesting clinical trial that is comparing the use of fosfomycin in monotherapy versus meropenem for managing bacteremia of urinary origin caused by enterobacteria. The results will more clearly position fosfomycin in the management of urinary tract infections caused by multidrug-resistant Gram-negative bacteria.
Pulmonary infection. Fosfomycin has shown good penetration (32-53%) in the lung tissue (table 1) of patients with pneumonia compared with the administered dose and the blood concentration reached [40]. Fosfomycin has therefore been proposed as an option for managing pneumonia, predominantly nosocomial, with resistances to the commonly employed antibiotics. It is worth noting that in the published cases of pulmonary infection by multidrug-resistant Gram-negative bacteria successfully treated with intravenous fosfomycin, this antibiotic has been administered in combination with other antibiotics, including carbapenems, tigecycline and colistin [41]. There is also experience in the use of intravenous fosfomycin in combination with other drugs for managing exacerbations caused by P. aeruginosa in patients with cystic fibrosis, observing good responses and tolerance to treatment [42]. Fosfomycin is not currently included in the guidelines as empiric treatment for managing nosocomial pneumonia [43], although the limited published experiences might make fosfomycin a consideration in centers with high rates of pneumonia by Gram-negative bacteria and high resistances to beta-lactam when good susceptibility to this antibiotic is maintained.
In terms of adjuvant nebulized therapy, there are several active studies to determine its efficacy in managing pneumonia, primarily in conjunction with aminoglycosides. In a recently published, randomized clinical trial on pneumonia by Gram-negative bacteria associated with mechanical ventilation, the joint administration of fosfomycin and amikacin through a special inhalation system showed no benefits in terms of clinical curing and mortality, compared with the placebo arm and intravenous antibiotic treatment, despite a reduction in bacterial load [44]. Therefore and given the limited and conflicting experience with this pathway, the use of this combination should be reserved for conditions in which there is a suspicion of high pulmonary inoculum and there are no other available options.
Osteoarticular infection. Although Gram-positive microorganisms represent the largest number of cases of osteoarticular infection, infections by Gram-negative microorganisms have experienced a marked increase over the last decade [45], representing an added difficulty for antibiotherapy due to the microorganisms’ faculty for developing resistances during extended treatment and the difficulties in selecting active antibiotics with good penetration in osteoarticular tissue (table 1). Sirot et al. [46] measured fosfomycin’s capacity for penetrating bone tissue in 20 patients and found that the concentrations reached 1 and 3 h after administering 4-g doses were 19.6 and 10 mg/mL, respectively, representing 15% of the concentrations reached in blood. Other authors have also measured high fosfomycin concentrations in bone and interstitial fluid [47], revealing the treatment option with this drug. In addition, we have fosfomycin’s activity against bacteria that form and live in biofilms, which constitute the main mechanism of bacterial persistence in prosthetic joints and the cause of failure in antimicrobial therapy.
In vitro studies have demonstrated fosfomycin’s superior eradication activity to other antibiotics such as gentamicin, tigecycline and colistin against strains of ESBL-producing E. coli in prosthetic materials [48]. Fosfomycin could therefore be considered a good option in managing infections associated with osteoarticular prosthetics caused by multidrug-resistant strains, although more clinical evidence is needed to recommend its use.
Endocarditis. As with other drugs, information regarding the use of fosfomycin in endocarditis caused by Gram-negative microorganisms is limited, with its activity demonstrated only in animal models [49]. The guidelines of the European Society of Cardiology [50] and Infectious Diseases Society of America [51] therefore do not include this drug as a candidate for managing these types of bacteremic infections of endovascular origin. Nevertheless, this drug has recognized activity against Gram-positive microorganisms such as methicillin-resistant S. aureus, where its synergistic activity with antibiotics such as imipenem has been confirmed [52]. This fact means that this combination could be a basis for managing endocarditis by Gram-negative bacteria.
Central nervous system infections. Despite the limited published experience with fosfomycin in managing central nervous system (CNS) infections, 2 of the antibiotic’s characteristics make it an attractive option for managing nosocomial CNS infections, in which GNB predominates. Firstly and as mentioned earlier, fosfomycin presents good eradication activity against biofilms, which play a relevant role in persistent infections in patients with ventricular drainage. In a recent study with 1,642 samples of cerebrospinal fluid (CSF) obtained through ventricular drainage, approximately 7.5% showed a positive result for Gram-negative bacilli isolates, with half of the study strains producing biofilms [53]. Fosfomycin exhibits a good capacity for passing through the blood-brain barrier, with approximately 30% penetration [54], which is higher than that of glycopeptides, aminoglycosides and many beta-lactams. Thus, fosfomycin has good diffusion in CSF and CSF collections, both with inflamed and noninflamed meninges; fosfomycin’s CCSF is therefore greater than the MIC of the susceptible bacteria (table 1).
Despite the limited reported experience, there are case series of CNS infections by ESBL-producing enterobacteria successfully treated with fosfomycin [55]. Fosfomycin could therefore be considered an option for managing these infections when there are few therapeutic alternatives.
Gastrointestinal infections. Fosfomycin presents good activity against the main Gram-negative pathogens involved in gastroenterocolitis, including isolates of Campylobacter, E. coli, Salmonella spp. and Shigella [56]. Moreover, fosfomycin’s structure facilitates good diffusion in the gastrointestinal tissue after its systemic administration; fosfomycin has therefore been widely employed for decades for treating these infections [48]. A study of 118 children showed that fosfomycin was able to effectively eradicate strains of Shiga toxin-producing E. coli O157:H7 and, consequently, enterohemorrhagic conditions; therefore, the use of fosfomycin in the first 5 days of the disease could reduce the risk and onset of hemolytic uremic syndrome [57]. This protective nature of fosfomycin assumes even greater value when we consider the current controversy regarding the undefined role of antibiotic treatment in this infection and that the previous use of antibiotics could be a significant risk factor for developing the aforementioned syndrome.
In terms of its application for managing secondary or tertiary intraabdominal infection, fosfomycin’s activity against ESBL-producing and carbapenemase-producing enterobacteria makes this drug an attractive option, despite the limited experience described to date [58, 59].
FOSFOMYCIN AND STRATEGIES FOR COMBINED THERAPY
In a recent survey conducted within a European study of expert opinions to explore the contemporary antibiotic management of hospital infections caused by carbapenem-resistant, Gram-negative bacteria, the combination of a polymyxin and a carbapenem was the most widely used combination in most cases, although combinations of polymyxin and tigecycline, an aminoglycoside, fosfomycin and rifampicin were also common [60]. Combination therapy was prescribed at least occasionally in 99.1% of the participating hospitals (114 of 115) and was considered more frequently when treating bacteremia, pneumonia and CNS infections, in a similar manner among enterobacteria, P. aeruginosa and A. baumannii. Monotherapy was employed for treating complicated urinary tract infections, typically with an aminoglycoside or a polymyxin and less frequently with fosfomycin. The aim of combined therapy is to improve treatment effectiveness and prevent the development of resistance. In general, those surveyed shared the erroneous idea that combined therapy (the preferred strategy) was supported by solid, high-quality scientific evidence [60].
In treating intra-abdominal, skin and soft tissue infections caused by carbapenemase-producing enterobacteria, the double combinations of tigecycline and a carbapenem or an aminoglycoside were the most common; for complicated urinary tract infections, the combination of an aminoglycoside and fosfomycin was the most common (34/105, 32.4%). For infections caused by P. aeruginosa with carbapenem resistance, the combined treatment bound a carbapenem (54.7% in the case of bacteremia), an aminoglycoside or fosfomycin to the polymyxin (colistin). In triple combination therapy, the polymyxin is bound to a carbapenem and typically more to fosfomycin than to an aminoglycoside to avoid resulting in renal toxicity [60].
The benefit of combined therapies for multidrug-resistant Gram-negative bacteria has been reinforced by the results of the recent retrospective INCREMENT cohort study, which investigated the effect of more appropriate therapy and more appropriate combined therapy on the mortality of patients with bacteremia caused by carbapenemase-producing enterobacteria [61]. Appropriate therapy was associated with a protective effect on mortality, and combined therapy was related to improved survival but only in the patient subgroup classified with a high mortality score at baseline. The authors therefore concluded that to manage bacteremia by carbapenemase-producing enterobacteria, patients should undergo early appropriate therapy as soon after the diagnosis as possible and that monotherapy should be reserved for episodes classified as low mortality using the scale [61]. The most commonly employed combinations were those of colistin plus tigecycline (31%), aminoglycosides plus tigecycline (35%) and colistin plus carbapenem (44%). The overall mortality of the monotherapies was 41% and that of the combined therapies as a whole was 35%, with 33% for the combinations that included fosfomycin, although this antibiotic was used in only 10 of the 343 episodes of bacteremia with appropriate treatment (78% of the series; 22% underwent inadequate treatment). The mortality in the cases of combined therapy with fosfomycin was higher (40%) among the patients with high-risk scores (8-15) than among the patients with low mortality risk scores (0-7). It should be noted that, in this study, the most common microorganism was K. pneumoniae (85% of the cases of bacteremia) and that the most common type of carbapenemase was KPC (in approximately 75%).
A recent comprehensive review of treatment for infections caused by AmpC-producing, ESBL-producing and carbapenemase-producing enterobacteria included highly detailed assessments and positioning statements on the role of fosfomycin for managing these infections [62]. Once again, treatment in monotherapy was a possible option in only one series of infectious syndromes, such as urinary tract infections; however, the authors also warned that until the results of a series of studies currently underway are made available [39, 63], firm recommendations cannot be made regarding the treatment of ESBL-producing or AmpC-producing enterobacteria with fosfomycin alone.
For carbapenemase-producing enterobacteria, combined therapies are recommended, given that the efficacy in monotherapy is questionable for a number of the drugs active in vitro, including polymyxins, tigecycline and fosfomycin. Thus, the importance of exploring combined therapies to find a potential synergistic or additive effect between some of these antibiotics. Due to the lack of relevant information, fosfomycin is not a first option against severe infections by carbapenemase-producing enterobacteria when there are other active drugs available (even less so in monotherapy) but might be necessary in some patients with few options. Among the explicit recommendations, fosfomycin is included among the accompanying drugs to be added to double or triple combinations, both in combinations where a beta-lactam is the main antibiotic and in those that involve colistin, depending on whether susceptibility is maintained to various beta-lactam agents of potential use (ceftazidime-avibactam or meropenem-vaborbactam; alternatively, meropenem [if the MIC is ≤8 mg/L], ceftazidime or aztreonam). In the case of resistance to beta-lactam and colistin, fosfomycin would accompany an aminoglycoside and tigecycline [62]. In such cases, the recommendation is high dosages of fosfomycin (16 to 24 g per day) in combination.
The usage possibilities for fosfomycin in combined regimens has also been contemplated and included in other recent guidelines on managing infections by multidrug-resistant GNB in recipients of solid organ transplants [64]. In particular, fosfomycin is preferred for use in triple combination therapies, combined with 2 other active antibiotics (a carbapenem only when the MIC is ≤8 mg/L, administered at high dosages and in extended infusions) and especially in urinary tract infections, although it can be used in other infectious syndromes and bacteremia of diverse origin [65]. Only in cases of less invasive or less severe infection, especially urinary, patients could benefit from a carbapenem-free treatment with colistin, aminoglycosides or fosfomycin in monotherapy.
CONCLUSIONS
In an environment of increasing resistance among Gram-negative bacteria, fosfomycin has been positioned as an option to consider in treating infection by these bacteria, due to fosfomycin’s sustained activity against these strains and its pharmacokinetic properties and activity against biofilms [66]. The risk of emerging resistant subpopulations under monotherapy should always be considered and thereby prevented. Although susceptibility rates vary by region, the resistances seem to increase in settings with a high use of fosfomycin and along with exposure when faced with multidrug-resistant pathogens [67]. Beyond the urinary infections as the main focus of prescription [68, 69], fosfomycin’s excellent capacity for diffusion to various tissues grants it considerable versatility for managing infections by Gram-negative microorganisms in various other types of infectious syndromes [70]. All of this makes fosfomycin one of the key wildcards of combined therapy according to the various guidelines and recommendation documents.
REFERENCES
- 1.Curcio D. Multidrug-resistant Gram-negative bacterial infections: are you ready for the challenge? Curr Clin Pharmacol 2014; 9(1):27-38. PMID: . [DOI] [PubMed] [Google Scholar]
- 2.Cubero M, Cuervo G, Dominguez MÁ, Tubau F, Martí S, Sevillano E, et al. . Carbapenem-resistant and carbapenem-susceptible isogenic isolates of Klebsiella pneumoniae ST101 causing infection in a tertiary hospital. BMC Microbiol 2015; 15:177. doi: 10.1186/s12866-015-0510-9. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front Cell Infect Microbiol 2017; 7:39. doi: 10.3389/fcimb.2017.00039. eCollection 2017. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaidya VK. Horizontal Transfer of Antimicrobial Resistance by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae. J Lab Physicians 2011; 3(1):37-42. doi: 10.4103/0974-2727.78563. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sastry S, Doi Y. Fosfomycin: Resurgence of An Old Companion. J Infect Chemother 2016; 22(5):273-80. doi: 10.1016/j.jiac.2016.01.010. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samonis G, Maraki S, Rafailidis PI, Kapaskelis A, Kastoris AC, Falagas ME. Antimicrobial susceptibility of Gram-negative nonurinary bacteria to fosfomycin and other antimicrobials. Future Microbiol 2010; 5(6):961-70. doi: 10.2217/fmb.10.47. PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Stock I, Wiedemann B. Natural antibiotic susceptibility of Escherichia coli, Shigella, E. vulneris, and E. hermannii strains. Diagn Microbiol Infect Dis 1999; 33(3):187-99. PMID: . [DOI] [PubMed] [Google Scholar]
- 8.Fukuyama M, Furuhata K, Oonaka K, Hara T, Sunakawa K. [Antibacterial activity of fosfomycin against the causative bacteria isolated from bacterial enteritis]. Jpn J Antibiot 2000; 53(7):522-31. PMID: . [PubMed] [Google Scholar]
- 9.Falagas ME, Kastoris AC, Karageorgopoulos DE, Rafailidis PI. Fosfomycin for the treatment of infections caused by multidrug-resistant non-fermenting Gram-negative bacilli: a systematic review of microbiological, animal and clinical studies. Int J Antimicrob Agents 2009; 34(2):111-20. DOI: 10.1016/j.ijantimicag.2009.03.009. PMID: . [DOI] [PubMed] [Google Scholar]
- 10.Reparaz J, Fernández C. Sensitivity of Vibrio spp. to fosfomycin. Chemotherapy 1977; 23 Suppl 1:58-62. PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez Martin CB, Rodríguez Ferri EF. In vitro susceptibility of Pasteurella multocida subspecies multocida strains isolated from swine to 42 antimicrobial agents. Zentralblatt Bakteriol Int J Med Microbiol 1993; 279: 387-93. PMID: . [DOI] [PubMed] [Google Scholar]
- 12.Cai Y, Fan Y, Wang R, An M-M, Liang B-B. Synergistic effects of aminoglycosides and fosfomycin on Pseudomonas aeruginosa in vitro and biofilm infections in a rat model. J Antimicrob Chemother 2009; 64(3):563-6. DOI: 10.1093/jac/dkp224. PMID: . [DOI] [PubMed] [Google Scholar]
- 13.Anderson GG, Kenney TF, Macleod DL, Henig NR, O’Toole GA. Eradication of Pseudomonas aeruginosa biofilms on cultured airway cells by a fosfomycin/tobramycin antibiotic combination. Pathog Dis 2013; 67(1):39-45. DOI: 10.1111/2049-632X.12015. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corvec S, Furustrand Tafin U, Betrisey B, Borens O, Trampuz A. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-β-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob Agents Chemother 2013; 57(3):1421-7. DOI: 10.1128/AAC.01718-12. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vardakas KZ, Legakis NJ, Triarides N, Falagas ME. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents 2016; 47(4): 269-85. DOI: 10.1016/j.ijantimicag.2016.02.001. PMID: . [DOI] [PubMed] [Google Scholar]
- 16.Sastry S, Clarke LG, Alrowais H, Querry AM, Shutt KA, Doi Y. Clinical Appraisal of Fosfomycin in the Era of Antimicrobial Resistance. Antimicrob Agents Chemother 2015; 59 (12): 7355-61. doi: 10.1128/AAC.01071-15. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-Avial C, Rodríguez-Avial I, Hernández E, Picazo JJ. [Increasing prevalence of fosfomycin resistance in extended-spectrum-beta-lactamase-producing Escherichia coli urinary isolates (2005-2009-2011)]. Rev Esp Quimioter 2013; 26(1):43-6. PMID: . [PubMed] [Google Scholar]
- 18.De Cueto M, López L, Hernández JR, Morillo C, Pascual A. In vitro activity of fosfomycin against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: comparison of susceptibility testing procedures. Antimicrob Agents Chemother 2006; 50(1):368-70. DOI: 10.1128/AAC.50.1.368-370.2006. PMID: ; PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev 2016; 29(2): 321-47. DOI: 10.1128/CMR.00068-15. PMID: ; PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Shen P, Wei Z, Liu L, He F, Shi K, et al. . Dissemination of a clone carrying a fosA3-harbouring plasmid mediates high fosfomycin resistance rate of KPC-producing Klebsiella pneumoniae in China. Int J Antimicrob Agents 2015; 45(1):66-70. DOI: 10.1016/j.ijantimicag.2014.08.010. PMID: . [DOI] [PubMed] [Google Scholar]
- 21.Castanheira M, Griffin MA, Deshpande LM, Mendes RE, Jones RN, Flamm RK. Detection of mcr-1 among Escherichia coli Clinical Isolates Collected Worldwide as Part of the SENTRY Antimicrobial Surveillance Program in 2014 and 2015. Antimicrob Agents Chemother 2016; 60(9):5623-4. DOI: 10.1128/AAC.01267-16. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apisarnthanarak A, Mundy LM. Carbapenem-resistant Pseudomonas aeruginosa pneumonia with intermediate minimum inhibitory concentrations to doripenem: combination therapy with high-dose, 4-h infusion of doripenem plus fosfomycin versus intravenous colistin plus fosfomycin. Int J Antimicrob Agents 2012; 39(3):271-2. DOI: 10.1016/j.ijantimicag.2011.11.012. PMID: . [DOI] [PubMed] [Google Scholar]
- 23.Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2014; 58(9): 5598-601. doi: 10.1128/AAC.02435-13; PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinh A, Salomon J, Bru JP, Bernard L. Fosfomycin: efficacy against infections caused by multidrug-resistant bacteria. Scand J Infect Dis. 2012; 44(3):182-9. doi: 10.3109/00365548.2011.616221; PMID: . [DOI] [PubMed] [Google Scholar]
- 25.Karaiskos I, Antoniadou A, Giamarellou H. Combination therapy for extensively-drug resistant gram-negative bacteria. Expert Rev Anti Infect Ther 2017; 15(12): 1123-40. doi: 10.1080/14787210.2017.1410434; PMID: . [DOI] [PubMed] [Google Scholar]
- 26.Perez F, El Chakhtoura NG, Papp-Wallace KM, Wilson BM, Bonomo RA. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply «precision medicine» to antimicrobial chemotherapy? Expert Opin Pharmacother 2016; 17(6):761-81. doi: 10.1517/14656566.2016.1145658. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodríguez-Baño J, Cisneros JM, Cobos-Trigueros N, Fresco G, Navarro-San Francisco C, Gudiol C, et al. . Executive summary of the diagnosis and antimicrobial treatment of invasive infections due to multidrug-resistant Enterobacteriaceae. Guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enferm Infecc Microbiol Clin 2015; 33(5):338-41. doi: 10.1016/j.eimc.2014.11.015. PMID: . [DOI] [PubMed] [Google Scholar]
- 28.Reffert JL, Smith WJ. Fosfomycin for the Treatment of Resistant Gram-Negative Bacterial Infections: Insights from the Society of Infectious Diseases Pharmacists. Pharmacother J Hum Pharmacol Drug Ther 2014; 34(8):845-57. doi: 10.1002/phar.1434. PMID: . [DOI] [PubMed] [Google Scholar]
- 29.Mensa J, Barberán J, Soriano A, Llinares P, Marco F, Cantón R, et al. . Antibiotic selection in the treatment of acute invasive infections by Pseudomona aeruginosa: Guidelines by the Spanish Society of Chemotherapy. Rev Esp Quimioter 2018; 31(1):78-100. PMID: ; PMCID: . [PMC free article] [PubMed] [Google Scholar]
- 30.Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage Pseudomonas aeruginosa infections. Drugs in Context 2018; 7: 212527. doi: 10.7573/dic.212527. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidal E, Cervera C, Cordero E, Armiñanzas C, Carratalá J, Cisneros JM, et al. . Management of urinary tract infection in solid organ transplant recipients: Consensus statement of the Group for the Study of Infection in Transplant Recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Spanish Network for Research in Infectious Diseases (REIPI). Enferm Infecc Microbiol Clin 2015; 33(10):679.e1-679.e21. doi: 10.1016/j.eimc.2015.03.024. PMID: . [DOI] [PubMed] [Google Scholar]
- 32.De Cueto M, Aliaga L, Alós J-I, Canut A, Los-Arcos I, Martínez JA, et al. . Executive summary of the diagnosis and treatment of urinary tract infection: Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm Infecc Microbiol Clin. 2017; 35(5):314-20. doi: 10.1016/j.eimc.2016.11.005. PMID: . [DOI] [PubMed] [Google Scholar]
- 33.Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56(11):5744-8. doi: 10.1128/AAC.00402-12. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giancola SE, Mahoney MV, Hogan MD, Raux BR, McCoy C, Hirsch EB. Assessment of Fosfomycin for Complicated or Multidrug-Resistant Urinary Tract Infections: Patient Characteristics and Outcomes. Chemotherapy 2017; 62(2):100-4. doi: 10.1159/000449422. PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, et al. . Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med 2013; 41(2):580-637. doi: 10.1097/CCM.0b013e31827e83af. PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Naber KG, Bergman B, Bishop MC, Bjerklund-Johansen TE, Botto H, Lobel B, et al. . EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur Urol 2001; 40(5):576-88. PMID: . [DOI] [PubMed] [Google Scholar]
- 37.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC. Diagnosis, Prevention, and Treatment of Catheter Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010; 50(5):625-63. PMID: . [DOI] [PubMed] [Google Scholar]
- 38.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, el al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52(5): e103-20. doi: 10.1093/cid/ciq257. PMID: . [DOI] [PubMed] [Google Scholar]
- 39.Rosso-Fernández C, Sojo-Dorado J, Barriga A, Lavín-Alconero L, Palacios Z, López-Hernández I, et al. . Fosfomycin versus meropenem in bacteraemic urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli (FOREST): study protocol for an investigator-driven randomised controlled trial. BMJ Open. 2015; 5(3):e007363. 10.1136/bmjopen-2014-007363. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matzi V, Lindenmann J, Porubsky C, Kugler SA, Maier A, Dittrich P, et al. . Extracellular concentrations of fosfomycin in lung tissue of septic patients. J Antimicrob Chemother 2010; 65(5):995-8. doi: 10.1093/jac/dkq070. PMID: . [DOI] [PubMed] [Google Scholar]
- 41.Pontikis K, Karaiskos I, Bastani S, Dimopoulos G, Kalogirou M, Katsiari M, et al. . Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int J Antimicrob Agents 2014; 43(1): 52-9. doi: 10.1016/j.ijantimicag.2013.09.010. PMID: . [DOI] [PubMed] [Google Scholar]
- 42.Mirakhur A, Gallagher MJ, Ledson MJ, Hart CA, Walshaw MJ. Fosfomycin therapy for multiresistant Pseudomonas aeruginosa in cystic fibrosis. J Cyst Fibros 2003; 2(1):19-24. DOI: 10.1016/S1569-1993(02)00143-1. PMID: . [DOI] [PubMed] [Google Scholar]
- 43.Wilke M, Grube R. Update on management options in the treatment of nosocomial and ventilator assisted pneumonia: review of actual guidelines and economic aspects of therapy. Infect Drug Resist 2013; 7:1-7. doi: 10.2147/IDR.S25985. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kollef MH, Ricard J-D, Roux D, Francois B, Ischaki E, Rozgonyi Z, et al. . A Randomized Trial of the Amikacin Fosfomycin Inhalation System for the Adjunctive Therapy of Gram-Negative Ventilator-Associated Pneumonia: IASIS Trial. Chest 2017; 151(6):1239-46. doi: 10.1016/j.chest.2016.11.026. PMID: . [DOI] [PubMed] [Google Scholar]
- 45.Murillo O, Grau I, Lora-Tamayo J, Gomez-Junyent J, Ribera A, Tubau F, et al. . The changing epidemiology of bacteraemic osteoarticular infections in the early 21st century. Clin Microbiol Infect 2015; 21(3):254.e1-8. doi: 10.1016/j.cmi.2014.09.007. PMID: . [DOI] [PubMed] [Google Scholar]
- 46.Sirot J, Lopitaux R, Dumont C, Rampon S, Cluzel R. [Diffusion of fosfomycin into bone tissue in man]. Pathol Biol (Paris) 1983; 31(6):522-4. PMID: . [PubMed] [Google Scholar]
- 47.Meissner A, Haag R, Rahmanzadeh R. Adjuvant fosfomycin medication in chronic osteomyelitis. Infection 1989; 17(3):146-51. PMID: . [DOI] [PubMed] [Google Scholar]
- 48.Corvec S, Furustrand Tafin U, Betrisey B, Borens O, Trampuz A. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-β-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob Agents Chemother 2013; 57(3):1421-7. doi: 10.1128/AAC.01718-12. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bugnon D, Potel G, Xiong YQ, Caillon J, Navas D, Gras C, et al. . Bactericidal effect of pefloxacin and fosfomycin against Pseudomonas aeruginosa in a rabbit endocarditis model with pharmacokinetics of pefloxacin in humans simulated in vivo. Eur J Clin Microbiol Infect Dis 1997; 16(8):575-80. PMID: . [DOI] [PubMed] [Google Scholar]
- 50.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. . 2015. ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075-128. doi: 10.1093/eurheartj/ehv319. PMID: . [DOI] [PubMed] [Google Scholar]
- 51.Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, et al. . Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015; 132(15):1435-86. doi: 10.1161/CIR.0000000000000296. PMID: . [DOI] [PubMed] [Google Scholar]
- 52.Del Río A, Gasch O, Moreno A, Peña C, Cuquet J, Soy D, et al. . Efficacy and safety of fosfomycin plus imipenem as rescue therapy for complicated bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: a multicenter clinical trial. Clin Infect Dis 2014; 59(8):1105-12. doi: 10.1093/cid/ciu580. PMID: . [DOI] [PubMed] [Google Scholar]
- 53.Benachinmardi KK, Ravikumar R, Indiradevi B. Role of Biofilm in Cerebrospinal Fluid Shunt Infections: A Study at Tertiary Neurocare Center from South India. J Neurosci Rural Pract 2017; 8(3):335-41. doi: 10.4103/jnrp.jnrp_22_17. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfausler B1, Spiss H, Dittrich P, Zeitlinger M, Schmutzhard E, Joukhadar C. Concentrations of fosfomycin in the cerebrospinal fluid of neurointensive care patients with ventriculostomy-associated ventriculitis. J Antimicrob Chemother 2004; 53(5):848-52. DOI: 10.1093/jac/dkh158. PMID: . [DOI] [PubMed] [Google Scholar]
- 55.Tseng Y-C, Kan L-P, Huang L-Y, Yin T, Yang Y-S, Lin J-C, et al. . Successful treatment of a patient with ventriculoperitoneal shunt-associated meningitis caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Tohoku J Exp Med 2014; 233(4):301-5. PMID: . [DOI] [PubMed] [Google Scholar]
- 56.Gobernado M. Fosfomycin. Rev Española Quimioter 2003; 16(1):15-40. PMID: . [PubMed] [Google Scholar]
- 57.Tajiri H, Nishi J, Ushijima K, Shimizu T, Ishige T, Shimizu M, et al. . A role for fosfomycin treatment in children for prevention of haemolytic-uraemic syndrome accompanying Shiga toxin-producing Escherichia coli infection. Int J Antimicrob Agents 2015; 46(5):586-9. doi: 10.1016/j.ijantimicag.2015.08.006. PMID: . [DOI] [PubMed] [Google Scholar]
- 58.Tobudic S, Matzneller P, Stoiser B, Wenisch JM, Zeitlinger M, Vychytil A, et al. . Pharmacokinetics of intraperitoneal and intravenous fosfomycin in automated peritoneal dialysis patients without peritonitis. Antimicrob Agents Chemother 2012; 56(7):3992-5. doi: 10.1128/AAC.00126-12. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gallardo A, Sáez JM, Enriquez G, Cobacho AR, Torronteras R, Recordan C, et al. . Surgical suppurating infections and surgical abdominal infections treated with fosfomycin. Chemotherapy 1977; 23 Suppl 1:392-8. DOI: 10.1159/000222080. PMID: . [DOI] [PubMed] [Google Scholar]
- 60.Papst L, Beović B, Pulcini C, Durante-Mangoni E, Rodríguez-Baño J, Kaye KS, et al.; ESGAP, ESGBIS, ESGIE and the CRGNB treatment survey study group. Antibiotic treatment of infections caused by carbapenem-resistant Gram-negative bacilli: an international ESCMID cross-sectional survey among infectious diseases specialists practicing in large hospitals. Clin Microbiol Infect 2018; 24(10):1070-1076. doi: 10.1016/j.cmi.2018.01.015. PMID: . [DOI] [PubMed] [Google Scholar]
- 61.Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, et al., REIPI/ESGBIS/INCREMENT Investigators. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 2017; 17: 726-34. doi: 10.1016/S1473-3099(17)30228-1. PMID: . [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. Treatment of infections caused by extended-spectrum-betalactamase-, AmpC-, and carbapenemase producing Enterobacteriaceae. Clin Microbiol Rev 2018; 31:e00079-17. doi: 10.1128/CMR.00079-17. PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaye KS, Rice LB, Dane A, Stus V, Sagan O, Fedosiuk E, Das A, Skarinsky D, Eckburg PB, Ellis-Grosse EJ. 2017. Intravenous fosfomycin (ZTI-01) for the treatment of complicated urinary tract infections (cUTI) including acute pyelonephritis (AP): results from a multi-center, randomized, double-blind phase 2/3 study in hospitalized adults (ZEUS), abstr 1845. IDWeek. [Google Scholar]
- 64.Aguado JM, Silva JT, Fernández-Ruiz M, Cordero E, Fortún J, Gudiol C, et al.; Spanish Society of Transplantation (SET); Group for Study of Infection in Transplantation of the Spanish Society of Infectious Diseases and Clinical Microbiology (GESITRA-SEIMC); Spanish Network for Research in Infectious Diseases (REIPI) (RD16/0016). Management of multidrug resistant Gram-negative bacilli infections in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant Rev (Orlando) 2018; 32(1):36-57. doi: 10.1016/j.trre.2017.07.001. PMID: . [DOI] [PubMed] [Google Scholar]
- 65.Silva JT, Fernández-Ruiz M, Aguado JM. Multidrug-resistant Gram-negative infection in solid organ transplant recipients: implications for outcome and treatment. Curr Opin Infect Dis 2018; 31(6):499-505. doi: 10.1097/QCO.0000000000000488. PMID: . [DOI] [PubMed] [Google Scholar]
- 66.Alrowais H, McElheny CL, Spychala CN, Sastry S, Guo Q, Butt AA, et al. . Fosfomycin Resistance in Escherichia coli, Pennsylvania, USA. Emerg Infect Dis 2015; 21(11): 2045-7. doi: 10.3201/eid2111.150750. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Falagas ME, Athanasaki F, Voulgaris GL, Triarides NA, Vardakas KZ. Resistance to fosfomycin: Mechanisms, Frequency and Clinical Consequences. Int J Antimicrob Agents 2019; 53(1): 22-28. doi: 10.1016/j.ijantimicag.2018.09.013. PMID: . [DOI] [PubMed] [Google Scholar]
- 68.Avent ML, Rogers BA, Cheng AC, Athan E, Francis JR, Roberts MJ, et al. . Fosfomycin: what was old is new again. Intern Med J 2018; 48(12): 1425-1429. doi: 10.1111/imj.14122. PMID: . [DOI] [PubMed] [Google Scholar]
- 69.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis 2008; 46(7): 1069-77. doi: 10.1086/527442. Re PMID: . [DOI] [PubMed] [Google Scholar]
- 70.Dijkmans AC, Zacarías NVO, Burggraaf J, Mouton JW, Wilms EB, van Nieuwkoop C, et al. . Fosfomycin: Pharmacological, Clinical and Future Perspectives. Antibiotics (Basel). 2017; 6(4). pii: . doi: 10.3390/antibiotics6040024. PMID: ; PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]