Abstract
Inhalation therapy is likely to continue to dominate asthma and chronic obstructive pulmonary disease (COPD) treatment. The pressurised metered-dose inhaler (pMDI) accounts for most of the global inhaler market, but this kind of device is difficult to use properly. Dry powder inhalers (DPIs) have several advantages over pMDIs: they are breath-activated, easy and convenient to use, and environmentally friendly. The Easyhaler® (Orion; Finland) is a multidose reservoir-type DPI developed to efficiently deliver a wide range of medications, including fixed-dose combinations of bronchodilators and corticosteroids. Easyhaler shares a similar shape with the pMDI, and its performance is unaffected by moisture, dropping, vibration, and freezing/thawing. For these reasons, Easyhaler may be considered one of the most convenient inhalers for daily use in patients with asthma or COPD.
Keywords: asthma, chronic obstructive pulmonary disease, DPI performance, dry powder inhalers, Easyhaler®
Background
Inhaled bronchodilators and corticosteroids are the cornerstone of pharmacological treatment for asthma and chronic obstructive pulmonary disease (COPD). These medications are delivered to the airways by inhalers such as pressurised metered-dose inhalers (pMDIs) and dry powder inhalers (DPIs).1 In the 1950s, the first pMDI was introduced as a portable, multidose delivery system for bronchodilator drugs, and the pMDIs are still the most often recommended inhalers for asthma and COPD treatment.1,2 This inhaler utilises a propellant under pressure to generate a metered dose of an aerosol, through an atomisation nozzle. However, the pMDI is renowned to be difficult to use, as it requires considerable patient co-ordination between inhalation and drug delivery from the canister. Thus, physicians need to continuously warn patients about correct inhalation.1–4 Additionally, subjects often fail device activation timing, before or at the end of inhalation, or by initiating inhaler actuation whilst breath-holding.1–4
In the 1970s, the first DPI was launched; it was a single-dose device, having the active substance in a gelatine capsule that was loaded into the device prior to use.5 Since the late 1980s, multidose DPIs became accessible. These had the same level of suitability as the pMDIs. DPI doses can be pre-metered in the form of single capsules or foil blisters or as multiple single-dose unit disks. Alternatively, device metering of bulk powder can be achieved using a reservoir apparatus.5 In contrast to pMDIs, DPIs are activated and run by the patient’s inspiratory flow, which means they do not need a propellant to generate the aerosol, thus bypassing the requirement to synchronise inhaler actuation with inhalation.5 However, a powerful and profound inhalation through the DPI is required to deaggregate the powder formulation into breathable-sized particles as efficiently as possible and, consequently, to guarantee drug delivery to the lungs.5 The inhalation manoeuvre covers a range of inhalation techniques that the patient will use for achieving the correct procedure, as recommended by product-specific instruction. This is important for DPIs, because if the patient does not make sufficient inspiratory efforts to inhale the dose, then the dose is not received at all.
Evidence suggests that both pMDIs and DPIs show an equivalent effectiveness, if correctly used.6 Nevertheless, each device presents specific characteristics that deserve attention in terms of reaching an efficacious delivery of the drugs. Concerning this matter, Molimard and colleagues found that 76% of patients made errors with pMDIs and that these were considered ‘critical’ in 28% of cases.7 According to the device, at least one error was made with DPIs in 49–55% of patients, which were considered crucial in 11–32% of patients.7 Literature reviews reported very high error rates in the use of pMDIs and DPIs.8,9 The challenge of poor inhaler performance was not limited to primary care: a significant percentage of patients with asthma or COPD attending chest clinics produce errors when using inhaler devices.6 Above all, errors remain often unknown, because the patient did not explain the inhalation method to the healthcare team, and were associated with poor asthma control and COPD outcomes.6
Technological innovations in pMDIs and DPIs have improved their efficiency in lung deposition up to 40% of the nominal dose, compared with 10–15% that was achieved in the past.4 However, the ‘perfect’ inhaler has yet to be designed. Hypothetically, it should be user-friendly, convenient to carry, and robust; it should not require priming or coordination between triggering and inhalation; it should supply dose consistency, independently of inhalation manoeuvres, and it must have a dose counter. Moreover, it should provide the patient with feedback to confirm that the dose has been inhaled and that the correct process has been used; finally, it should be appealing for the patient.10 To date, none of the available inhalers can be considered ‘ideal’ regarding these characteristics, because prescribers still need to train patients on how to use them and also check their inhalation technique regularly.
In the panorama of currently available devices, this review will focus on Easyhaler® (Orion Pharma, Finland), a DPI delivering corticosteroids and bronchodilators, either alone or in a fixed-dose combination. The aim is to provide an overview of the scientific literature on Easyhaler, with the purpose of emphasising features that make it close to the concept of an ‘ideal’ inhaler for daily use in patients with asthma or COPD. The article search was conducted using online databases (PubMed, EMBASE, and GOOGLE SCHOLAR), limiting articles to those published in English language, from the date of inception to September 2018. The keywords were Easyhaler, dry powder inhaler, and inhaler.
Easyhaler
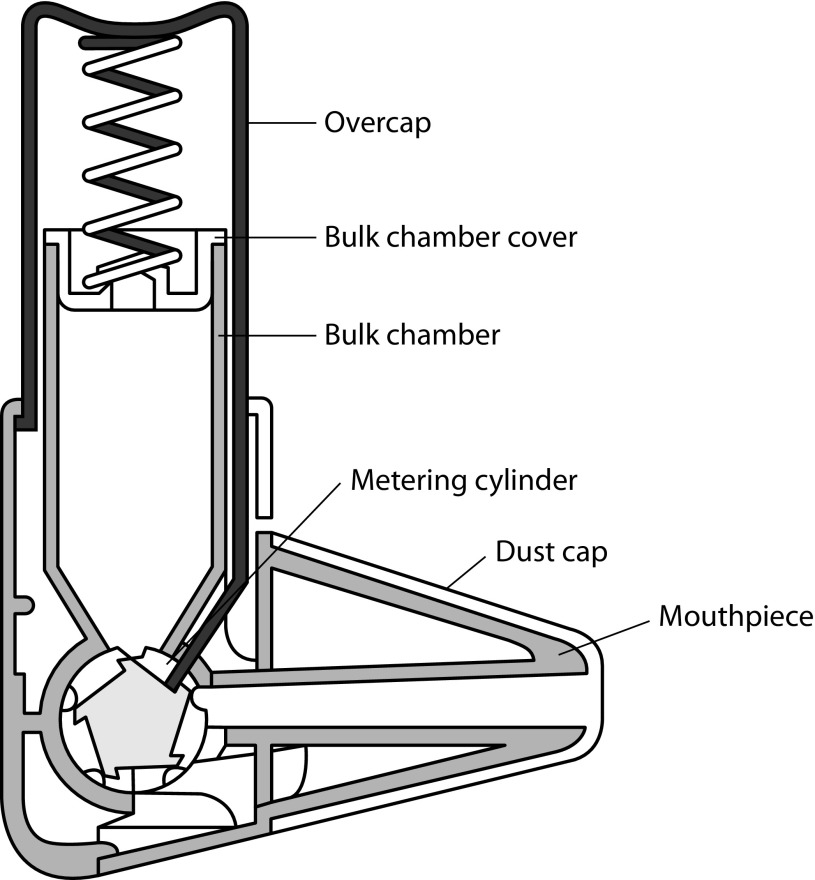
Easyhaler (Figure 1) is a multidose reservoir-type DPI that shares a comparable shape with the pMDI.11 In particular, Easyhaler has an innovative design, which combines simplicity of use with effective performance, resulting in guaranteed drug delivery.11 It consists of seven plastic parts, a metering cylinder spring, and plastic components.11 Easyhaler delivers a broad range of medications, the latest being formoterol/budesonide (4.5/80, 4.5/160, and 9/320 μg/inhalation) and salmeterol/fluticasone propionate combinations (50/250 and 50/500 μg/inhalation). Although Easyhaler has been available in Europe since early 1990s, its benefits continue to be important in comparison with newer DPIs.
Figure 1.
Schematic representation of the Easyhaler® dry powder inhaler operation. Reproduced with permission from Vidgren et al.24
Drug delivery by the Easyhaler
Any inhaler device used for the treatment of airway diseases should systematically deliver an expectable and reproducible drug dose during repeated use (i.e. from the first to the last labelled dose).12 Depending on the particle size distribution, lung deposition may be more central or peripheral. The inhaled particles should have a size making them breathable (i.e. particles with a mass median aerodynamic diameter <5 μm).12,13 Particles with an aerodynamic diameter <1 μm are exhaled to a large degree.12,13 The released dose enters the systemic circulation both via the lungs and through being swallowed.13,14 It is crucial that both the emitted dose and the fine particle dose are consistent each time the inhaler is used.13,14 The pMDI is generally known for its uniformity,15 whereas, due to the differences in manufacture and powder formulation, great variations in particle sizes have been reported for DPIs.16 For example, an in vitro study showed that there is large inter- and intrainhaler variation in dose emission with the Turbuhaler®, particularly with respect to the fine particle dose.17 At variance with the Turbuhaler, in vitro studies showed that Easyhaler steadily delivers drug doses,18 also under simulated real-life conditions, such as moisture (30°C and 75% relative humidity), fall of the device from a height of 1 metre, vibration simulating inhaler carrying, and freezing/thawing (from −20 to 25°C).19,20 Of note, Easyhaler delivered doses, taken at the beginning (doses 1–3), at the middle, and at the end of the device lifespan, setting the first one as 100%, only varied from 94 to 103%, for all measures, both at the middle and at the end of the Easyhaler drug content.19
Lung deposition, clinical effect, and safety of the Easyhaler
Total and regional lung budesonide deposition, obtained using the Easyhaler, the Turbuhaler, and a pMDI with a Nebuhaler large-volume spacer attached, was quantified by gamma scintigraphy in patients with mild-to-moderate asthma.21 The drug distribution pattern mainly involved the lung central area for all three devices. Mean total lung deposition, expressed as a percentage of the metered dose, was similar for Easyhaler and Turbuhaler (18.5 and 21.8%, respectively) but significantly (p<0.01) higher for the pMDI plus Nebuhaler (44.1%). The comparable lung deposition obtained with the Easyhaler and Turbuhaler translates into equivalent therapeutic responses.21–23 Indeed, inhalation from Easyhaler revealed no differences in clinical and safety outcome measures for the reliever22 and preventer23 formulations, when compared with those obtained using a pMDI, accompanied by a large-volume spacer. Moreover, the comparison of the salbutamol Easyhaler with the pMDI without spacer in asthmatic patients who had been instructed in the inhalation technique for each device, showed (as reported) no difference in spirometric responses.24 In 29 asthmatic subjects, salbutamol 100 μg delivered via Easyhaler was also found to provide equivalent clinical effectiveness to terbutaline 250 μg delivered via Turbuhaler.25 Clinical equivalence has also been demonstrated between budesonide inhaled with Easyhaler and with Turbuhaler in asthmatic children26 as well as in corticosteroid-naive asthmatic adults, who were trained to use the devices.27 In particular, the improvement in morning peak flow was numerically higher for the Easyhaler than the Turbuhaler, even if not statistically significant.27 This difference could be ascribed to the optimal inhalation rate (60 L/min) requested with the Turbuhaler, an inhalation rate that is difficult to achieve for most patients even after practice.
Studies in healthy male adults have also shown no difference in the safety profile of drugs delivered by the Easyhaler when compared with the same drugs administered using other inhalers. For instance, after inhalation of budesonide with the Easyhaler and the Turbuhaler, in single-dose28 and multiple-dose29 studies, serum budesonide concentrations did not significantly differ. The similar delivery of budesonide was confirmed through no differences in the suppression of serum cortisol levels30 and local effects.31 Taken together, these studies highlight the pharmacological equivalence between the Easyhaler reliever or preventer and its equivalent device, the Turbuhaler.
How patients use Easyhaler in a real-life setting
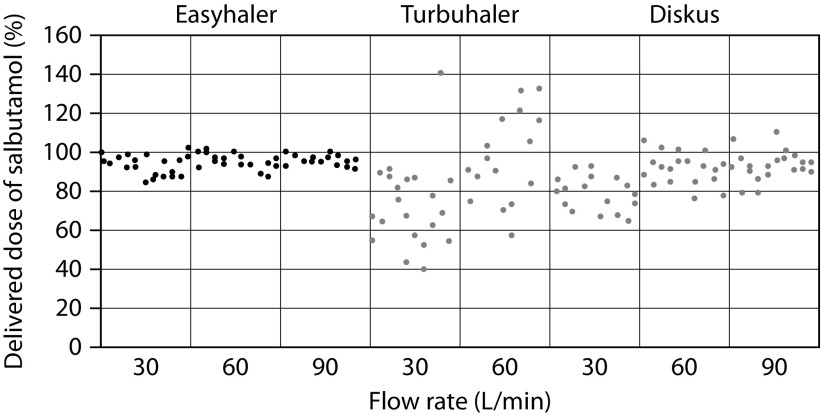
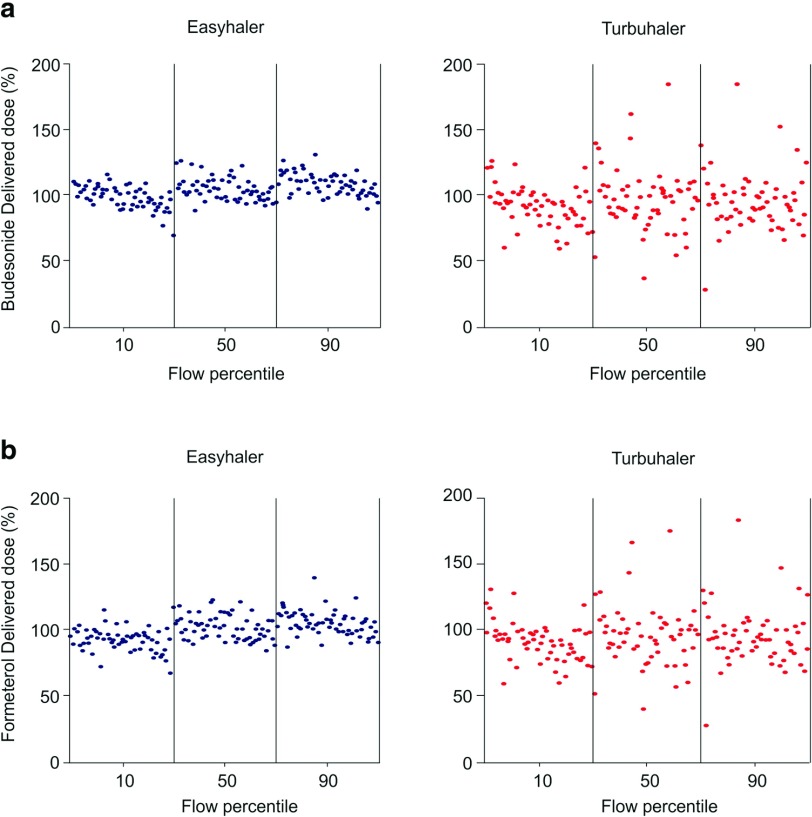
Studies have shown that, depending on the type of inhaler and method of assessment, 20–80% of patients use DPIs improperly.3,7,9 Common patient errors include lack of exhalation before inhalation, improper inhaler positioning and loading, failure to inhale forcefully and deeply through the device, and failure to breath-hold after inhalation.3,7,9 All these missteps may lead to poor drug delivery, which negatively influences its efficacy, causing inadequate disease control. Dose preparation with the Easyhaler requires shaking, with the inhaler kept in an upright position; this manoeuvre is needed to aid the dose being deposited into the dosing cup.11 The dose is delivered by pressing once on the overcap of the inhaler.11 In the Easyhaler device, the dosing cup deposits the drug and its carrier into the inhalation channel where turbulent airflow, generated by patient inhalation, breaks the inhaled product into breathable particles, which are then deposited into the lungs (Figure 1). As for all DPIs, it is important that the dose delivered is minimally affected by changes in the patient inhalation rate. However, studies have shown large delivered doses inter- and intrapatient variability due to different inhalation rates in using DPIs.32,33 At variance with the Turbuhaler, which shows a flow-dependent dose emission, the delivered dose from the Easyhaler is consistent within a set of different, clinically relevant flow rates (Figure 2).18 Indeed, the variability in dose delivery and fine particle dose of fixed-combination budesonide/formoterol from the Easyhaler is significantly smaller than that from the Turbuhaler, for both budesonide and formoterol, at all tested flow rates (Figure 3).19,20 Overall, dose delivery performance is more consistent with the Easyhaler than the Turbuhaler, independent of the inhalation rate. The latter finding suggests that patients are more likely to receive the same drug dose every time by using the Easyhaler than the Turbuhaler. Studies have shown that children34 and patients with severe airway obstruction35 have problems achieving the minimum required inhalation rate through DPIs, particularly those with high internal resistance. This stresses that, even though the subjects are instructed on how to use an inhaler device, if they do not reach the required inhalation flow rate, then the dose will not be as expected (and could even be zero). Malmström and coworkers measured peak inspiratory flow (PIF) in 120 children, aged 4–16 years, using Easyhaler.36 Bronchodilator response to salbutamol via Easyhaler was also compared with that obtained when the drug was inhaled by a pMDI with a spacer. Only 4 of the 120 children were unable to achieve PIF <28 L/min, but even in these patients it was possible to produce bronchodilatation almost equal to that obtained with a pMDI attached to a spacer.36 Therefore, the delivery of the required response by Easyhaler was confirmed by this study’s results, even at the low inhalation flow rates. More recently, Azouz and coworkers evaluated the inhalation profile (i.e. PIF, acceleration rate and pressure change) of asthmatic children and patients with COPD using the Easyhaler, Turbuhaler, Aerolizer®, or Diskus® DPI.32 They found that, in both asthmatic children and COPD patients, the use of Easyhaler provided higher acceleration rate and pressure change values than all other DPIs, thus suggesting that Easyhaler produces the most favourable set of inhalation characteristics, for formulation, deaggregation, and delivery of the emitted dose into the lungs.32 With Easyhaler, optimal drug delivery has been reported at PIF levels of 30 L/min and above.37 Furthermore, the vast majority of children, adolescents, adults, and elderly patients with asthma, as well as patients with COPD, are capable of achieving a PIF of 30 L/min or higher via the Easyhaler.38,39
Figure 2.
Consistency of the delivered dose by the Easyhaler®, the Diskus®, and the Turbuhaler®. Reproduced with permission from Chrystyn.44
Figure 3.
Dose delivery of budesonide (a) and formoterol (b) from two budesonide/formoterol multidose DPIs, the Easyhaler® and the Turbuhaler®, at three different flow rates. Reproduced with permission from Haikarainen et al.19
Differences in handling errors and device mastery (defined as an absence of healthcare professional-observed errors) between the Easyhaler, Spiromax, and Turbuhaler, have been investigated in healthy adult volunteers.40 The authors found that nearly all participants were able to reach short-term device mastery after healthcare professional instruction, demonstrating the value of face-to-face interaction during inhaler training.
Real-world evidence has demonstrated the clinical effectiveness of the Easyhaler device in patients with asthma.41 More recently, Tamási and coworkers42 have shown that, in a real-world setting, most patients with obstructive airway disease treated with fixed combinations of inhaled bronchodilators and steroids, and who switched from their current inhaler to the Easyhaler, achieved better disease control after 3 months from switching. Of note, most patients considered the Easyhaler to be portable and easy to learn to use and keep clean, during daily activities.42
Patient preference in the choice of Easyhaler
Patient opinions about a variety of inhaler devices have been obtained in numerous studies, involving approximately 800 patients. A meta-analysis of these studies stated that Easyhaler was more accepted by patients than the pMDI (with or without spacer) and Diskhaler.31 In comparison with Turbuhaler, Easyhaler was shown to be more accepted overall, even though the difference in one of the nine studies was not statistically significant. In studies comparing Easyhaler with the Turbuhaler, the first was found to be easier to use. Overall, 60% of patients chose the Easyhaler compared with 35% who favoured the Turbuhaler (p<0.01).
Conclusion
Many DPIs are currently available on the market. As already mentioned, to be considered as ‘ideal’, an inhaler device should be (1) effective (i.e. able to guarantee inhalation of an adequate fraction of drug, in breathable-size particles, independently, as much as possible, of changes in patient inspiratory flow); (2) reproducible (i.e. able to allow inhalation of the same drug amount, also in terms of its breathable fraction); (3) precise (i.e. able to consent to know, at any moment, the number of doses in the device, and whether inhalation was correctly performed); (4) stable (i.e. able to protect the drug(s) from the effects of temperature and/or humidity variation); (5) comfortable (i.e. easy to use in different circumstances and including several doses of the drug(s) for long-term use); (6) versatile (i.e. permit the administration of different drugs); (7) environmentally friendly (e.g. absence of chemical contaminants).
At present, none of the commercially available DPIs fulfil all the above-mentioned criteria. However, some inhaler devices have features that closely meet the concept of the best possible inhaler device in a ‘real-life’ setting. Amongst these, Easyhaler shows many of the characteristics of an ideal ‘real-life’ inhaler. Easyhaler performs consistently, irrespective of inspiration rate and at PIF rates as low as 30 L/min, thus offering healthcare teams the possibility of prescribing bronchodilators, corticosteroids, or their combination to children or adults whose inspiration rates are reduced. Pharmacological equivalence with the Turbuhaler establishes the potential for seamless switching to an Easyhaler. In this respect, a recent retrospective study demonstrated that asthma patients may be switched from other inhaled corticosteroid devices to Easyhaler with no reduction in clinical effectiveness or cost increase.43
Easyhaler has also exhibited high patient preference. As reported, when given the choice, patient preference for the Easyhaler is high, because it is easy to learn and use. It is very similar to the pMDI, except for the fact that drug release is breath-actuated with the Easyhaler, bypassing the need to coordinate canister pressing with starting an inhalation, an essential step with pMDI use. Moreover, in contrast to Turbuhaler, upright shaking does not result in declined performance of the Easyhaler.
Overall, the Easyhaler closely reflects many criteria that could be considered to be associated with an ideal inhaler in real-life conditions. It has added design features, such as a protective case and dose counter. At the same time, proven patient acceptance of Easyhaler should improve adherence to therapeutic regimens. Although additional efforts are desirable to obtain better inhalers, the Easyhaler certainly represents an advance in the way asthma and COPD therapies are delivered to patients.
Acknowledgements
Editorial assistance was provided by Content Ed Net, with the helpful support of David Figgitt, PhD. Easyhaler, Turbuhaler and Nebuhaler are registered trademarks. The trademark symbol has been used on first occasion in the article but omitted after that for easier reading.
Footnotes
Contributions: The author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, takes responsibility for the integrity of the work as a whole, and has given their approval for this version to be published.
Disclosure and potential conflicts of interest: In the last 3 years, Federico Lavorini has received grants for research, as well as fees for lectures and advisory boards, from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini International, Novartis, Orion Pharma, Teva, and Trudell. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at http://www.drugsincontext.com/wp-content/uploads/2019/05/dic.212596-COI.pdf
Funding declaration: Editorial assistance was supported by The Menarini Group.
Correct attribution: Copyright © 2019 Lavorini F. https://doi.org/10.7573/dic.212596. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
Peer review comments to author: 2 May 2019
References
- 1.Lavorini F. Inhaled drug delivery in the hands of the patient. J Aerosol Med Pulm Drug Deliv. 2014;27:414–418. doi: 10.1089/jamp.2014.1132. [DOI] [PubMed] [Google Scholar]
- 2.Lavorini F, Corrigan CJ, Barnes PJ, et al. Retail sales of inhalation devices in European countries: so much for a global policy. Respir Med. 2011;105(7):1099–1103. doi: 10.1016/j.rmed.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL Study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(4):1071–1081.e9. doi: 10.1016/j.jaip.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Lavorini F, Fontana GA, Usmani OS. New inhaler devices - the good, the bad and the ugly. Respiration. 2014;88(1):3–15. doi: 10.1159/000363390. [DOI] [PubMed] [Google Scholar]
- 5.Lavorini F, Pistolesi M, Usmani OS. Recent advances in capsule-based dry powder inhaler technology. Multidiscip Respir Med. 2017;12:11. doi: 10.1186/s40248-017-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolovich MB, Ahrens RC, Hess DR, et al. American College of Chest Physicians; American College of Asthma, Allergy, and Immunology. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy and Immunology. Chest. 2005;127(1):335–371. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 7.Molimard M, Raherison C, Lignot S, Depont F, Abouelfath A, Moore N. Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary care. J Aerosol Med. 2003;16(3):249–254. doi: 10.1089/089426803769017613. [DOI] [PubMed] [Google Scholar]
- 8.Crompton GK, Barnes PJ, Broeders M, et al. The need to improve inhalation technique in Europe: a report from the Aerosol Drug Management Improvement Team. Respir Med. 2006;100(9):1479–1494. doi: 10.1016/j.rmed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102(4):593–604. doi: 10.1016/j.rmed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Borgström L, Asking L, Thorsson L. Idealhalers or realhalers? A comparison of Diskus and Turbuhaler. Int J Clin Pract. 2005;59(12):1488–1495. doi: 10.1111/j.1368-5031.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 11.Vidgren P, Silvasti M, Poukkula A, et al. Easyhaler powder inhaler: a new alternative in the anti-inflammatory treatment of asthma. Acta Ther. 1994;20:117–131. doi: 10.1080/02786829408959751. [DOI] [Google Scholar]
- 12.Laube BL, Janssens HM, Jongh FHC, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331. doi: 10.1183/09031936.00166410. [DOI] [PubMed] [Google Scholar]
- 13.Lavorini F, Pedersen S, Usmani OS Aerosol Drug Management Improvement Team (ADMIT) Dilemmas, confusion, and misconceptions related to small airways directed therapy. Chest. 2017;151(6):1345–1355. doi: 10.1016/j.chest.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Chrystyn H. Methods to identify drug deposition in the lungs following inhalation. Br J Clin Pharmacol. 2001;51(4):289–299. doi: 10.1046/j.1365-2125.2001.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cripps A, Riebe M, Schulze M, Woodhouse R. Pharmaceutical transition to non-CFC pressurized metered dose inhalers. Respir Med. 2000;94(Suppl 2):S3–S9. doi: 10.1016/S0954-6111(00)80143-2. [DOI] [PubMed] [Google Scholar]
- 16.de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143–151. doi: 10.1016/j.ejpb.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 17.Tarsin W, Assi KH, Chrystyn H. In-vitro intra- and inter-inhaler flow rate-dependent dosage emission from a combination of budesonide and eformoterol in a dry powder inhaler. J Aerosol Med. 2004;17(1):25–32. doi: 10.1089/089426804322994433. [DOI] [PubMed] [Google Scholar]
- 18.Palander A, Mattila T, Karhu M, Muttonen E. In vitro comparison of three salbutamol-containing multidose dry powder inhalers. Clin Drug Investig. 2000;20(1):25–33. doi: 10.2165/00044011-200020010-00004. [DOI] [Google Scholar]
- 19.Haikarainen J, Selroos O, Loytana T, Metsarinne S, Happonen A, Rytila P. Budesonide/Formoterol easyhaler: performance under simulated real-life conditions. Pulm Ther. 2017;3:125–138. doi: 10.1007/s41030-016-0025-z. [DOI] [Google Scholar]
- 20.Haikarainen J, Rytil ä P, Roos S, Metsärinne S, Happonen A. Dose uniformity of budesonide Easyhaler under simulated real-life conditions and with low inspiration flow rates. Chron Respir Dis. 2018;15(3):265–271. doi: 10.1177/1479972317745733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirst PH, Bacon RE, Pitcairn GR, Silvasti M, Newman S. A comparison of the lung deposition of budesonide from Easyhaler®, Turbuhaler® and pMDI plus spacer in asthmatic patients. Respir Med. 2001;95(9):720–727. doi: 10.1053/rmed.2001.1107. [DOI] [PubMed] [Google Scholar]
- 22.Koskela T, Malmström K, Sairanen U, Peltola S, Keski-Karhu J, Silvasti M. Efficacy of salbutamol via Easyhaler® unaffected by low inspiratory flow. Respir Med. 2000;94(12):1229–1233. doi: 10.1053/rmed.2000.0959. [DOI] [PubMed] [Google Scholar]
- 23.Koskela T, Hedman J, Ekroos H, et al. Equivalence of two steroid-containing inhalers: Easyhaler multidose powder inhaler compared with conventional aerosol with large-volume spacer. Respiration. 2000;67(2):194–202. doi: 10.1159/000029486. [DOI] [PubMed] [Google Scholar]
- 24.Vidgren M, Silvasti M, Korhonen P, Kinkelin A, Frischer B, Stern K. Clinical equivalence of a novel multiple dose powder inhaler versus a conventional metered dose inhaler on bronchodilating effects of salbutamol. Arzneimittelforschung. 1995;45(1):44–47. [PubMed] [Google Scholar]
- 25.Malinen A, Hedman J, Koskela T. Salbutamol via Easyhaler® produces equivalent bronchodilation to terbutaline via Turbuhaler® following inhalation of a single dose of equipotent β2-sympathomimetic. Clin Drug Investig. 2000;20(3):165–171. doi: 10.2165/00044011-200020030-00004. [DOI] [Google Scholar]
- 26.Vanto T, Hamalainen KM, Vahteristo M, Wille S, Njå F, Hyldebrandt N. Comparison of two budesonide dry powder inhalers in the treatment of asthma in children. J Aerosol Med. 2004;17(1):15–24. doi: 10.1089/089426804322994424. [DOI] [PubMed] [Google Scholar]
- 27.Schweisfurth H, Malinen A, Koskela T, Toivanen P, Ranki-Pesonen M German Study Group. Comparison of two budesonide powder inhalers, Easyhaler ® and Turbuhaler®, in steroid naive asthmatic patients. Respir Med. 2002;96(8):599–606. doi: 10.1053/rmed.2002.1311. [DOI] [PubMed] [Google Scholar]
- 28.Lähelmä A, Kirjavainen M, Kela M, et al. Equivalent lung deposition of budesonide in vivo: a comparison of dry powder inhalers using a pharmacokinetic method. Br J Clin Pharmacol. 2005;59(2):167–173. doi: 10.1111/j.1365-2125.2004.02238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hämäläinen KM, Granander M, Toivanen P, Malinen A. Assessment of the systemic effects of budesonide inhaled from Easyhaler® and from Turbuhaler® in healthy male volunteers. Respir Med. 2001;95(11):863–869. doi: 10.1053/rmed.2001.1157. [DOI] [PubMed] [Google Scholar]
- 30.Tukiainen H, Rytilä P, Hämäläinen KM, Silvasti MS, Keski-Karhu J Finnish Study Group. Safety, tolerability and acceptance of two dry powder inhalers in the administration of budesonide in steroid-treated asthmatic patients. Respir Med. 2002;96(4):221–229. doi: 10.1053/rmed.2001.1261. [DOI] [PubMed] [Google Scholar]
- 31.Ahonen A, Leinonen M, Ranki-Pesonen M. Patient satisfaction with Easyhaler ® compared with other inhalation systems in the treatment of asthma: a meta-analysis. Curr Ther Res. 2000;61(2):61–73. doi: 10.1016/S0011-393X(00)88529-X. [DOI] [Google Scholar]
- 32.Azouz W, Chetcuti P, Hosker HS, Saralaya D, Stephenson J, Chrystyn H. The inhalation characteristics of patients when they use different dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2015;28(1):35–42. doi: 10.1089/jamp.2013.1119. [DOI] [PubMed] [Google Scholar]
- 33.Azouz W, Chrystyn H. Clarifying the dilemmas about inhalation techniques for dry powder inhalers: integrating science with clinical practice. Prim Care Respir J. 2012;21(2):208–213. doi: 10.4104/pcrj.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedersen S, Hansem OR, Fuglsang G. Influence of inspiratory flow rate upon the effect of a Turbuhaler. Arch Dis Child. 1990;65(3):308–310. doi: 10.1136/adc.65.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nsour WM, Alldred A, Corrado J, Chrystyn H. Measurement of peak inhalation rates with an in-check meter to identify an elderly patient’s ability to use a turbuhaler. Respir Med. 2001;95(12):965–968. doi: 10.1053/rmed.2001.1190. [DOI] [PubMed] [Google Scholar]
- 36.Malmström K, Sorva R, Silvasti M. Application and efficacy of the multi-dose powder inhaler, Easyhaler, in children with asthma. Pediatr Allergy Immunol. 1999;10(1):66–70. doi: 10.1034/j.1399-3038.1999.101002.x. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381–387. doi: 10.1089/jamp.2017.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malmberg P, Rytilä P, Happonen P, Haahtela T. Inspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: age and gender rather than severity matters. Int J Chron Obstruct Pulmon Dis. 2010;5:257–262. doi: 10.2147/COPD.S11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malmberg LP, Everard ML, Haikarainen J, Lähelmä S. Evaluation of In Vitro and In Vivo Flow Rate Dependency of Budesonide/Formoterol Easyhaler. J Aerosol Med Pulm Drug Deliv. 2014;27(5):329–340. doi: 10.1089/jamp.2013.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandler N, Holländer J, Långström D, Santtila P, Saukkonen A, Torvinen S. Evaluation of inhaler handling-errors, inhaler perception and preference with Spiromax, Easyhaler and Turbuhaler devices among healthy Finnish volunteers: a single site, single visit crossover study (Finhaler) BMJ Open Respir Res. 2016;31:e000119. doi: 10.1136/bmjresp-2015-000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirozynski M, Hantulik P, Almgren-Rachtan A, Chudek J. Evaluation of the efficiency of single-inhaler combination therapy with budesonide/formoterol fumarate in patients with bronchial asthma in daily clinical practice. Adv Ther. 2017;34(12):2648–2660. doi: 10.1007/s12325-017-0641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamási L, Szilasi M, Gálffy G. Clinical effectiveness of budesonide/formoterol fumarate Easyhaler® for patients with poorly controlled obstructive airway disease: a real-world study of patient-reported outcomes. Adv Ther. 2018;35(8):1140–1152. doi: 10.1007/s12325-018-0753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price D, Thomas V, von Ziegenweidt J, Gould S, Hutton C, King C. Switching patients from other inhaled corticosteroid devices to the Easyhaler®: historical, matched-cohort study of real-life asthma patients. J Asthma Allergy. 2014;7:31–51. doi: 10.2147/JAA.S59386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chrystyn H. Closer to an ‘ideal inhaler’ with the Easyhaler: an innovative dry powder inhaler. Clin Drug Investig. 2006;26(4):175–183. doi: 10.2165/00044011-200626040-00001. [DOI] [PubMed] [Google Scholar]