Abstract
Rapidly growing mycobacteria have become increasingly recognized as pathogens implicated in surgical site infections that can be both difficult to diagnose and treat with an evolving understanding of both intrinsic and acquired resistance patterns. As common environmental commensal organisms that can colonize water supplies, they are of particular concern in the setting of a growing medical tourism industry. We present a case of a 49-year-old woman who acquired a highly multidrug-resistant Mycobacterium abscessus skin and soft-tissue infection after cosmetic abdominoplasty that required radical surgical debridement and 6 months of intravenous therapy to eradicate. This case highlights the challenges in the management of M. abscessus infections including delay to diagnosis and resistance patterns that are likely to become more common despite antibiotic stewardship efforts.
Keywords: Lipotourism, medical tourism, multidrug resistance, Mycobacterium abscessus skin and soft-tissue infection, plastic surgery
INTRODUCTION
Rapidly growing mycobacteria (RGM) are environmental organisms found worldwide and defined by their ability to grow in subculture within 1 week. Mycobacterium abscessus is known to be the most pathogenic of the RGM group and has been identified in recent outbreaks of wound infections related to medical tourism in the Caribbean islands. Reports of patients with M. abscessus infections from the Dominican Republic have been described since the early 2000s and recently culminated in a US Centers for Disease Control and Prevention outbreak investigation that identified 21 cases in six states from 2013 to 2014.[1] Despite a growing awareness of these infections, they nonetheless remain rare and there is often a delay in diagnosis. We present a case of lipotourism-related M. abscessus surgical site infection notable for significant multidrug resistance.
CASE REPORT
A 49-year-old woman underwent abdominoplasty with breast implant exchange, mastopexy, and upper back liposuction in the Dominican Republic in May 2016. She reportedly had significant seromas after these procedures that required daily aspirations for 2 weeks with drain placement. Three months postoperatively and after returning to the United States, she developed erythematous indurated areas over her abdomen, particularly near the former aspiration sites. She denied fevers, chills, night sweats, or gastrointestinal symptoms but did have progressive malaise and abdominal pain. She was evaluated by plastic surgery and multiple superficial subcutaneous abscesses were drained at bedside in clinic. The largest collection was 5.5 cm and cultures had no growth. Despite this, she was treated with cefadroxil for 10 days. She had a normal white blood cell count and C-reactive protein. She returned several times for bedside incision and drainage and culture data were frequently without growth, although one culture grew sensitive Klebsiella pneumoniae and another showed coagulase-negative Staphylococcus treated with 7-day courses of trimethoprim-sulfamethoxazole. After a month of procedures, a wound culture eventually isolated Mycobacterium abscessus. She was referred to the infectious disease clinic and empirically started on moxifloxacin and clarithromycin, pending sensitivity data on the isolate.
After 3 weeks of therapy, she redeveloped openly draining superficial periumbilical abscesses. Her isolate's susceptibilities returned with resistance to all oral antibiotics tested including moxifloxacin (minimum inhibitory concentration [MIC] >8), clarithromycin (MIC >16), doxycycline, minocycline, trimethoprim-sulfamethoxazole, and ciprofloxacin as well as identification of clarithromycin-inducible resistance. Given the severity of infection, she eventually agreed to radical surgical debridement 4 months after commencement of care. Intraoperatively, a large amount of unhealthy granulation tissue with fat necrosis was observed, and 15–20 individual pockets of infection were addressed over an area of 25 cm × 25 cm [Figure 1]. The surgery team postulated that the liposuction cannula may have been contaminated as most of the infections were above Scarpa's layer with some involvement of deeper fat and fascia where the cannula may have passed.
Figure 1.
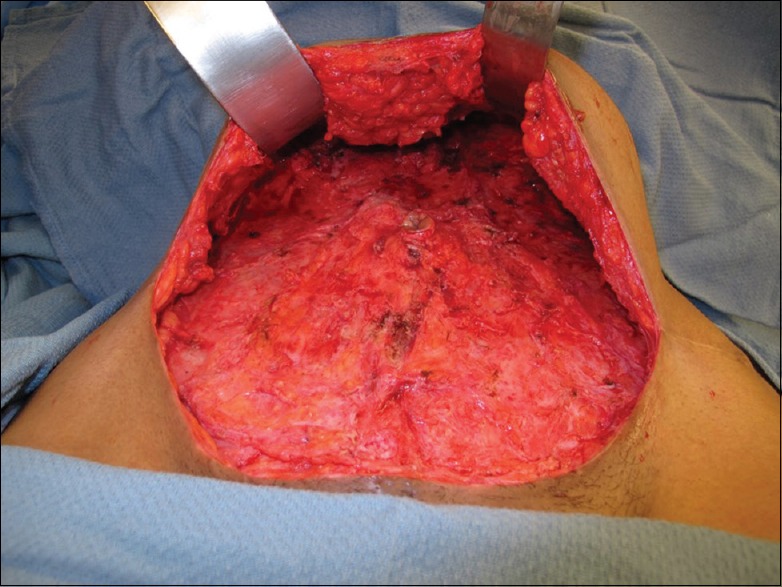
Initial radical surgical debridement
Postoperatively, she was started on amikacin 15 mg/kg daily (MIC 16 sensitive) and imipenem-cilastatin 500 mg every 6 h (MIC 8 intermediate). She underwent baseline auditory testing and completed a 6-month course with good overall cosmesis [Figure 2] and without significant adverse side effects. While she managed to complete this course without a port (long-term central venous catheter), she did undergo three exchanges of her short-term peripherally inserted cutaneous catheter and faced significant financial instability including loss of home.
Figure 2.
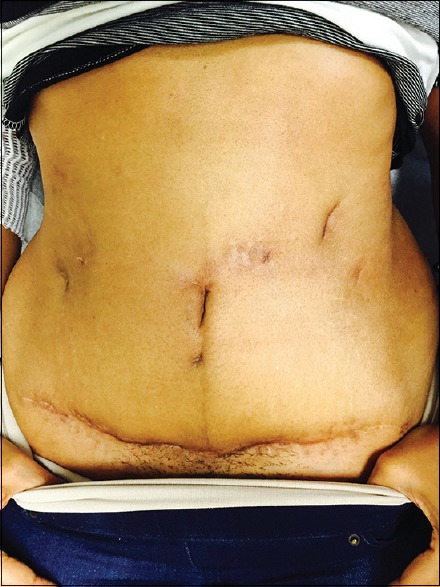
After completion of both surgical and antibiotic therapy
DISCUSSION
This case is notable in that it highlights two major challenges: (1) the growth of medical tourism and its possible complications and (2) the treatment of multidrug-resistant M. abscessus skin and soft-tissue infections (SSTIs).
In an era of globalization, there have been predictions of ten-fold increases in medical tourism from the United States growing from 750,000 individuals estimated to have traveled abroad in 2007.[2] While accurate estimates of medical tourists are sparse in current literature, there is an increasing number of reported cases of medical tourism-related mycobacterial complications.[3,4,5,6] While care in low- and middle-income countries is not necessarily substandard, providers should be aware of associated infections that may be less commonly seen in the United States. RGMs are known environmental pathogens that can form tenacious biofilms in water systems due to their hydrophobic fatty acid cell wall that makes eradication difficult without rigorous disinfection.[7] RGM surgical site infections are often attributable to medical center tap water supplies and inadequate disinfection of surgical instruments and liposuction cannulas.[8]
M. abscessus SSTIs are considered uncommon and frequently difficult-to-treat infections for which the American Thoracic Society/Infectious Diseases Society of America guideline on nontuberculous mycobacterial (NTM) infections offers minimal guidance beyond the recommendation to treat severe infections with a minimum of 4 months and consideration of combination therapy.[9] These recommendations are based on expert opinion and case series experience as there are no published randomized clinical trials. Much of our therapeutic strategies for mycobacterial SSTI are extrapolated from pulmonary infections that represent a separate clinical entity.
The discovery of an erythromycin methylase (erm) gene was a major development in this field which elucidated mechanisms of innate macrolide resistance that have important implications for treatment. Macrolides were described in NTM guidelines to be the only drug class with reliable in vitro activity; however, clinically had high rates of treatment failure despite predicted susceptibility. Nash et al. demonstrated that the presence of the erm gene conferred inducible macrolide resistance demonstrable by a rising clarithromycin MIC when isolates were held for an extended incubation period of 14 days.[10] As such, as in this case, M. abscessus infections continue to be a challenging clinical scenario with limited antibiotic options.
CONCLUSION
It is important for clinicians to have a high index of suspicion for atypical surgical site infections including RGM in the setting of lipotourism. Especially, in areas of the world with evolving antibiotic stewardship, multidrug-resistant infections may become more common and is especially important for mycobacteria which already have significant innate resistance mechanisms.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work would not have been possible without the support of the Boston Children's Hospital and Beth Israel Deaconess Medical Center. I am especially indebted to Drs. Peter Weller, Chair of the Infectious Diseases Division at Beth Israel Deaconess Medical Center, and Dr. Michael Wessels, Chair of Infectious Diseases at Boston Children's Hospital. I am also indebted to the plastic surgeons involved in the care of this patient, in particular, Dr. Eugene Fukodome.
REFERENCES
- 1.Schnabel D, Esposito DH, Gaines J, Ridpath A, Barry MA, Feldman KA, et al. Multistate US outbreak of rapidly growing mycobacterial infections associated with medical tourism to the Dominican Republic, 2013-2014(1) Emerg Infect Dis. 2016;22:1340–7. doi: 10.3201/eid2208.151938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keckley PH, Underwood HR. Medical Tourism: Consumers in Search of Value. Washington: Deloitte Center for Health Solutions; 2008. [Last accessed on 2017 Nov 10]. Available from: http://www.medicaltourisminturkey.org/panel/Panel1/3Deloitte.pdfError! Hyperlink reference not valid . [Google Scholar]
- 3.Klein HJ, Simic D, Fuchs N, Schweizer R, Mehra T, Giovanoli P, et al. Complications after cosmetic surgery tourism. Aesthet Surg J. 2017;37:474–82. doi: 10.1093/asj/sjw198. [DOI] [PubMed] [Google Scholar]
- 4.Ross KM, Moscoso AV, Bayer LR, Rosselli-Risal L, Orgill DP. Plastic surgery complications from medical tourism treated in a U.S. Academic medical center. Plast Reconstr Surg. 2018;141:517e–23e. doi: 10.1097/PRS.0000000000004214. [DOI] [PubMed] [Google Scholar]
- 5.Cusumano LR, Tran V, Tlamsa A, Chung P, Grossberg R, Weston G, et al. Rapidly growing Mycobacterium infections after cosmetic surgery in medical tourists: The Bronx experience and a review of the literature. Int J Infect Dis. 2017;63:1–6. doi: 10.1016/j.ijid.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Adabi K, Stern CS, Weichman KE, Garfein ES, Pothula A, Draper L, et al. Population health implications of medical tourism. Plast Reconstr Surg. 2017;140:66–74. doi: 10.1097/PRS.0000000000003459. [DOI] [PubMed] [Google Scholar]
- 7.van Ingen J, Blaak H, de Beer J, de Roda Husman AM, van Soolingen D. Rapidly growing nontuberculous mycobacteria cultured from home tap and shower water. Appl Environ Microbiol. 2010;76:6017–9. doi: 10.1128/AEM.00843-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). Rapidly growing mycobacterial infection following liposuction and liposculpture – Caracas, Venezuela, 1996-1998. MMWR Morb Mortal Wkly Rep. 1998;47:1065–7. [PubMed] [Google Scholar]
- 9.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 10.Nash KA, Brown-Elliott BA, Wallace RJ., Jr A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53:1367–76. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


